Abstract
This report describes 5 cases of fatal Lawsonia intracellularis-associated ulcerative and necro-hemorrhagic enteritis in weanling Thoroughbred and Standardbred foals. The lesions are similar to those of the L. intracellularis-associated ulcerative and necro-hemorrhagic enteritis syndrome in pigs. Two foals had concurrent severe typhlo-colitis as a result of a large burden of encysted cyathostomes. The clinical, diagnostic, and therapeutic challenges, and the potential complications encountered during the management of such cases are discussed.
Résumé
Entérite ulcérative et nécro-hémorragique associée àLawsonia intracellularischez 5 poulains sevrés. Ce rapport décrit 5 cas mortels d’entérite ulcérative et nécro-hémorragique associée à Lawsonia intracellularis chez des poulains Thoroughbred et Standardbred. Les lésions sont semblables à celles du syndrome de l’entérite ulcérative et nécro-hémorragique associée à L. intracellularis chez les porcs. Deux poulains étaient atteints d’une typhlo-colite grave concomitante en raison d’une charge importante de cyathostomes enkystés. Les difficultés cliniques, diagnostiques et thérapeutiques ainsi que les complications potentielles rencontrées durant la gestion de ces cas sont analysées.
(Traduit par Isabelle Vallières)
Case descriptions
Case 1
A 7-month-old 250-kg Thoroughbred colt was submitted to the Animal Health Laboratory (AHL) at the University of Guelph for postmortem examination on September 27, 2012 after being found dead in the paddock. The colt was last seen alive approximately 8 h earlier. Two days previously, the colt had a mild fever (38.9°C) and poor appetite. Treatment with flunixin meglumine (Flunixin Injection; Pfizer Canada, Kirkland, Quebec), 1.1 mg/kg body weight (BW), IV, and trimethoprim-sulfadoxine (Trivetrin; Schering-Plough, Pointe Claire, Quebec), 24 mg/kg BW, IV, were commenced. On the farm, a horse with equine proliferative enteropathy (EPE) due to Lawsonia intracelluraris and a horse with Potomac horse fever (Neoricketsia risticii) were diagnosed and treated successfully.
Case 2
A 7-month-old 241-kg Standardbred filly was referred to the Ontario Veterinary College-Veterinary Teaching Hospital (OVC-VTH) on December 13, 2011 for suspected EPE but was dead on arrival and was submitted for postmortem examination at the AHL. The filly had shown signs of lethargy, anorexia, fever, decreased gastrointestinal sounds, and hypoproteinemia. On initial physical examination by the referring veterinarian the filly was bright and alert, and mildly febrile (38.9°C). The gastrointestinal sounds were decreased. The filly was administered an anthelmintic (ivermectin) and treated with flunixin meglumine (Flunixin Injection; Pfizer Canada), 1.1 mg/kg BW, IV. The following day, the filly was dull and reluctant to move. On physical examination the filly was pyrexic (40.5°C), dehydrated (approximately 10%), moderately tachycardic (60 beats/ min), and had purple mucous membranes with a prolonged capillary refill time (4 s). Serum biochemistry abnormalities included severe panhypoproteinemia [17 g/L, reference range (RR): 56 to 79 g/L], characterized by hypoalbuminemia (5 g/L, RR: 29 to 37 g/L) and hypoglobulinemia (12 g/L, RR: 24 to 47 g/L). A complete blood (cell) count (CBC) showed a mild leukopenia (5.50 × 109/L, RR: 6 to 12.5 × 109/L), characterized by neutropenia. From the same farm, another foal (9-month-old) with similar clinical signs was referred to the OVC-VTH on the same day. This foal had a positive polymerase chain reaction (PCR) for L. intracellularis in a fecal sample and had a 1:120 titer (> 1:60 is seropositive) using the serum immunoperoxidase monolayer assay (IPMA) (1). This foal responded to treatment and was discharged after 8 days of hospitalization.
Case 3
An 8-month-old 277-kg Thoroughbred colt was referred to the OVC-VTH on November 16, 2009 with a history of lethargy and neurological signs, which had developed 24 h prior to presentation. On physical examination the colt was severely depressed, sweating profusely, hypothermic (36.5°C), tachycardic (64 beats/min), and tachypneic (32 breaths/min). Ataxia, head tilt, tongue paralysis, and medial bilateral strabismus were found on a detailed neurological examination. Blood-gas analysis showed a severe metabolic acidosis (pH 7.18; RR: 7:35 to 7.45), low bicarbonate (13.5 mmol/L; RR: 23 to 30 mmol/L), hyponatremia (122 mmol/L; RR: 136 to 144 mmol/L), hypokalemia (2.8 mmol/L; RR: 3.1 to 4.3 mmol/L), hypochloremia (89 mmol/L; RR: 95 to 104 mmol/L), and hyperlactatemia (15.0 mmol/L; RR: 0.28 to 1.72 mmol/L). During the physical examination, the foal became recumbent, unresponsive, unable to rise and began to exhibit seizures. As a result of the rapid deterioration and poor prognosis, the colt was euthanized and postmortem examination was conducted at the AHL.
Case 4
A 6-month-old 280-kg Thoroughbred filly was noted to be lethargic, inappetent, and exhibited mild colic signs. The foal was in a group of 14 weanlings of which 8 other foals exhibited similar clinical signs. This outbreak occurred over a 10- to 12-day period and was characterized by pasty to profuse, watery diarrhea, lethargy, inappetence, and mild colic. Serum biochemistry on these foals showed severe hypoproteinemia and hypoalbuminemia. Fecal PCR for L. intracellularis was positive in 7/10 foals tested. Based on these findings, EPE due to L. intracellularis infection was diagnosed (2). All foals with a positive PCR test were treated with erythromycin (Apo-Erythro; Apotex, Toronto, Ontario) 15 mg/kg BW, PO, q8h, and rifampin (Rofact; Valeant, Montreal, Quebec), 5 mg/kg BW, PO, q12h. Hypoproteinemic foals were treated with 2 to 3 L of polyimmune plasma (Plasvacc USA, Templeton, California, USA). This filly was treated with intravenous fluids lactated Ringer’s solution (LRS); however, despite this treatment, the filly deteriorated rapidly, collapsed, and died. The filly was submitted to the AHL for postmortem examination on November 18, 2011.
Case 5
An 8-month-old 263-kg Thoroughbred filly was referred to the OVC-VTH on November 18, 2011 with a history of fever, depression, and hypoproteinemia of 3 days duration. Three years previously, a weanling on the same farm had been diagnosed with EPE due to L. intracellularis. Since that time, bi-monthly plasma total protein (TP) measurement by refractometer had become part of the routine weanling management. Normal TP values were measured at 3 wk (66 g/L) and 1 wk (63 g/L) prior to presentation. Three days prior to presentation, the filly became febrile (41.0°C) and anorexic. This prompted the farm manager to repeat the TP measurement, which was 40 g/L. Given the farm history and the clinical-pathological findings, a presumptive diagnosis of EPE was made and treatment commenced with doxycycline (Apo-Doxy; Apotex), 10 mg/kg BW, PO, q12h. The filly became lethargic and anorexic, prompting referral to the OVC-VTH. Regular fecal flotation tests and rotation of anthelmintics was performed under supervision of the referring veterinarian. The filly had been administered an anthelmintic every 6 wk since 2 months of age. Anthelmintics administered were pyrantel, oxibendazole, and fenbendazole (10 mg/kg BW, PO). Ivermectin and praziquantel were administered 3 wk prior to presentation.
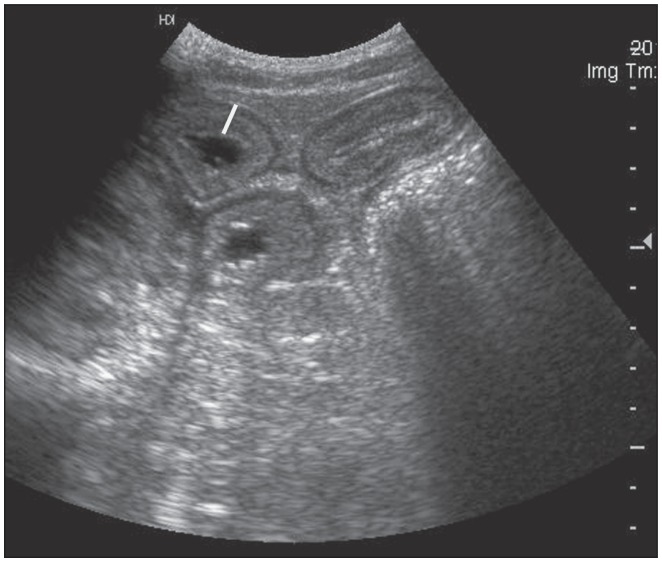
On physical examination the filly was depressed, mildly tachycardic (52 beats per min), and had congested oral mucous membranes with a prolonged capillary refill time (4 s). Dehydration was assessed to be 6% to 8%. The initial blood gas and electrolyte analysis showed hemoconcentration (packed cell volume 48%), hypoproteinemia (32 g/L), hyponatremia (125 mmol/L), and hypochloremia (93 mmol/L). On abdominal ultrasound examination, small intestinal loops with increased wall thickness (5 to 6 mm) were observed, and the colonic wall also appeared thickened and fluid-filled (Figure 1).
Figure 1.
Ultrasonographic image of an 8-month-old
Thoroughbred filly (case 5), showing small intestinal loops with severely thickened walls (white bar = 9 mm; normal < 3 mm).
Supportive care with intravenous fluid therapy (LRS 4 mL/kg BW/h), anti-inflammatory therapy (flunixin meglumine 0.5 mg/kg BW, IV q8h), oxytetracycline (Oxyvet; Vétoquinol, Lavaltrie, Quebec), 6.6 mg/kg BW, IV, q12h (3), and anti-ulcer medication, sucralfate (Nova-Sucralfate; Novopharm), 20 mg/kg BW, PO, q8h was commenced.
The CBC showed leukocytosis (14.0 × 109/L; RR: 5.1 to 11.0 × 109/L) due to mild neutrophilia (7.98 × 109/L; RR: 2.8 to 7.7 × 109/L) showing moderate toxicity. Serum biochemistry confirmed hypoproteinemia (26 g/L), hypoalbuminemia (12 g/L), hypoglobulinemia (14 g/L), and hyponatremia (120 mmol/L). A fecal sample collected on admission was PCR-positive for L. intracellularis, supporting the clinical diagnosis of EPE.
The filly responded poorly to supportive care with intravenous crystalloid and colloid fluids, partial parenteral nutrition, and anti-inflammatory and anti-microbial therapy directed at L. intracellularis. This response was atypical for L. intracellularis-associated enteritis, and the possible involvement of encysted cyathostomes was considered. Repeated abdominal ultrasound examinations showed that the small intestinal wall thickness had decreased (5 to 6 mm), but the colonic mural edema appeared more extensive. In addition, multiple hyperechoic small spots were visible within the colonic wall. Based on the suspicion of cyathostomasis, treatment with fenbendazole (Safe-Guard; Intervet, Whitby, Ontario), 5 mg/kg BW, PO, q24h, for 5 days was instituted. At this stage, the owner elected to continue the medical treatment at the farm under the supervision of the referring veterinarian; however, the filly died within 24 h after returning to the farm, and postmortem examination was conducted at the AHL on November 24, 2011.
Necropsy findings
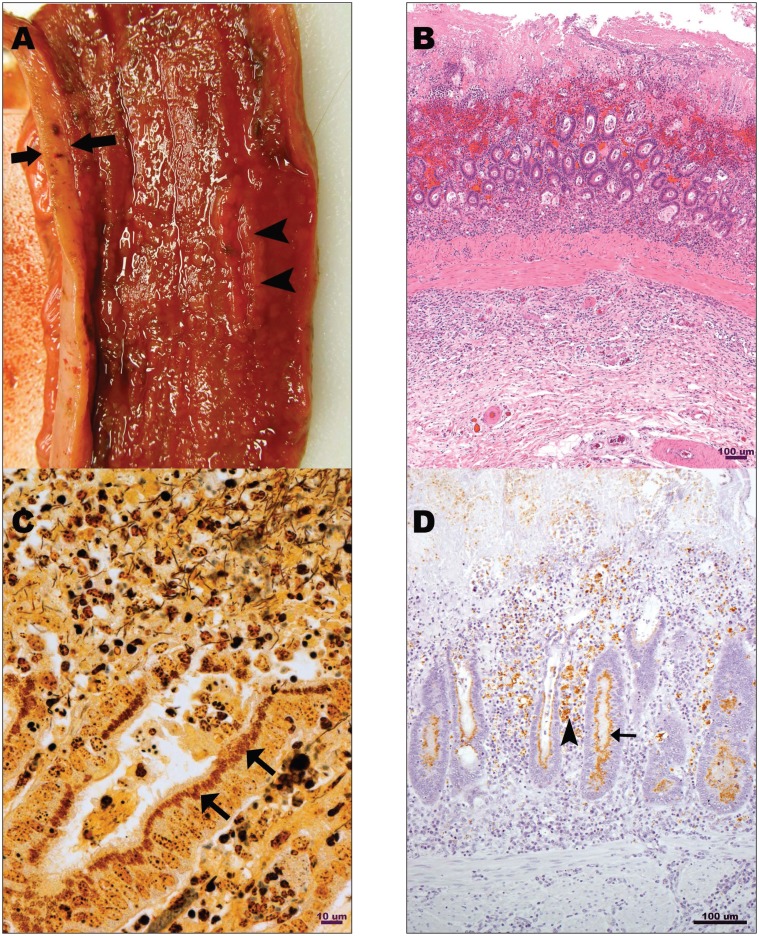
The gross lesions in the small intestine were similar in all foals and involved up to 80% of the length of the small intestine, including the distal 50% to 60% of jejunum and the entire ileum. The intestinal lumen was filled with bloody fluid, and mucosal surfaces were diffusely ulcerated and covered by fibrino-necrotic debris. The mucosa was thickened and corrugated in 2 foals (cases 1 and 4), and the submucosa in 2 foals was expanded by edema (case 5), or edema and hemorrhage (case 1) (Figure 2A).
Figure 2.
A — Ulcerative and necro-hemorrhagic enteritis. Terminal ileum of an 8-month-old Thoroughbred filly (case 5). Irregularly thickened (between arrows), ulcerated (arrowheads), and hyperemic mucosa covered by a thin layer of necrotic debris. B — Ulcerative and necro-hemorrhagic enteritis. Ileum, 8-month-old Thoroughbred colt (case 3). Necrosis and hemorrhage of the lumenal 50% of mucosa, with crypt epithelial hyperplasia and dysplasia in residual deep mucosa. Hematoxylin and eosin stain. Bar = 100 μm. C — Ulcerative and necro-hemorrhagic enteritis. Ileum, 8-month-old Thoroughbred colt (case 3). Numerous short argentophyllic bacilli are present in apical cytoplasm of epithelial cells lining mucosal crypts (arrows). Warthin-Starry stain. Bar = 10 μm. D — Ulcerative and necro-hemorrhagic enteritis. Ileum, 8-month-old Thoroughbred colt (case 3). Abundant staining for Lawsonia intracellularis antigen in enterocytes (arrow) and in lamina proprial macrophages (arrowhead). DAB chromogen. Bar = 100 μm.
In all 5 foals, the cecum and colon were thickened by mucosal and submucosal edema, and colonic lumens were filled with green opaque fluid. Colonic lymph nodes were edematous in 1 foal (case 3), and the tip of the cecum was infarcted in 1 foal (case 2). Numerous encysted cyathostome larvae were visible as pinpoint black foci within colonic mucosa of 2 foals (cases 4 and 5).
Histopathology
Mucosal necrosis with variably extensive ulceration was the predominant histopathologic lesion in the small intestine of all 5 foals, and a mixture of fibrino-hemorrhagic and necrotic debris covered the affected mucosa. Necrosis extended to involve the submucosa and Peyer’s patches in 1 foal (case 1). Proliferative crypts lined by hyperplastic, slightly dysplastic columnar epithelium were present within mucosal remnants at the base of the ulcerated foci and in the intact mucosa adjacent to the foci of ulceration in 3 foals (cases 1, 2, 3) (Figure 2B). These lesions were also present but subtle and sparse in the ileum of 1 foal (case 2). Intestinal sections were stained with Warthin-Starry (silver) stain and in 3 foals (cases 1, 2, 3), numerous short, slightly curved argentophyllic bacilli consistent with L. intracellularis were present in apical cytoplasm of epithelium lining proliferative mucosal crypts (Figure 2C). No similar bacilli were identified in silver-stained small intestinal sections from the remaining 2 foals (cases 4 and 5). Immunohistochemical staining for L. intracellularis was positive in 3 foals (cases 1, 2, 3) (Figure 2D). Fibrin thrombi filled the lumens of numerous lamina proprial capillaries and fewer submucosal capillaries and venules in 2 foals (cases 1, 2). Similar thrombi were limited to lamina proprial capillaries in 1 foal (case 3), and involved submucosal blood vessels in 1 foal (case 4). Submucosal or transmural edema was present in 4 foals (cases 1, 2, 3, 4). Localized peritonitis was evident in 1 foal (case 4), in which small intestinal serosa was covered by a thick layer of fibrinocellular debris.
Colonic mucosal necrosis and ulceration in 2 foals (cases 1 and 2) was similar to the lesion in the small intestine. Submucosal edema was evident in the colon of all 5 foals, and encysted cyathostome larvae with multiple small aggregates of mixed inflammatory cells (neutrophils, eosinophils, macrophages) were present in colonic submucosa in 2 foals (cases 4, 5).
Microbiological findings
Polymerase chain reaction (PCR) for L. intracellularis performed on ileal mucosal scrapings was positive in 3 foals (cases 1, 2, 4), negative in 1 foal (case 5), and not performed on case 3. Modified acid-fast (MAF) staining of ileal mucosal smears was negative for organisms consistent with L. intracellularis in the 3 foals for which this test was performed (cases 2, 4, 5). No significant, potentially pathogenic bacteria (specifically Salmonella spp. and Clostridium perfringens) were isolated from small intestinal or colonic mucosa of any of the foals, with the exception of moderate numbers of C. perfringens isolated from the small intestine of 1 foal (case 2). Clostridium difficile toxin enzyme-linked immunosorbent assay (ELISA) and C. perfringens enterotoxin ELISA were performed using intestinal content from 3 foals (cases 1, 2, and 5), and the results were negative in all animals.
Discussion
Equine proliferative enteropathy (EPE) due to L. intracellularis infection and typhlo-colitis associated with encysted cyathostome larva have been previously described in weanling foals (4,5). Equine proliferative enteropathy caused by L. intracellularis has been increasingly recognized in weanling foals as sporadic cases (6,7) as well as in outbreaks (8,9). L. intracellularis is an obligate intracellular, Gram-negative, argyrophilic, non-membrane-bound bacterium that typically causes a proliferative enteropathy in foals, clinically manifest as malabsorption and protein-losing enteropathy (4,10). Clinical and hematological findings include poor body condition with or without diarrhea and marked hypoproteinemia, which is characterized by severe hypoalbuminemia (11). The histological lesions found in EPE cases include proliferation and immaturity of epithelium lining small intestinal mucosal crypts. Using silver-based histochemical stains, a myriad of curved intracellular bacteria can be visualized within infected enterocytes (12).
In pigs, chronic proliferative enteropathy is the most common manifestation of L. intracellularis-associated disease (12). Other forms of L. intracellularis-associated disease that have been identified in this species include necrotic enteritis and proliferative hemorrhagic enteropathy (PHE), as well as hemorrhagic ulcerative enteritis (12). Typical porcine proliferative enteropathy (PPE) occurs in growing pigs between 2 and 4 months of age and the clinical findings, gross and histologic lesions in the intestine are similar to those reported in other species (13). Proliferation of mucosal crypts in distal small intestine, cecum, and colon results in profound mucosal thickening and ensuing clinical signs of malabsorption and intestinal protein loss. A related syndrome of necrotic enteritis (NE) develops in some PPE-affected pigs, presumably as a result of superimposed infection by other pathogenic enteric bacteria (14,15). Acute hemorrhagic enteritis (PHE) represents another clinically distinct syndrome typically affecting a slightly older cohort of young adult pigs (4 to 12 mo) (15). This syndrome is frequently fatal due to rapid, severe enteric blood loss. Histologic lesions in the intestine are disproportionate to the severity of lamina proprial and luminal hemorrhage, with small foci of mucosal epithelial degeneration and mild inflammatory infiltrates superimposed on lesions of proliferative enteropathy (16). Although clinically well-recognized, the pathogenesis of PHE remains undetermined; however, contributions of immune dysregulation, hypersensitivity, and vascular instability have been speculated (16). Regardless of the specific clinical and histopathologic features of PPE syndromes, lesions of mucosal crypt proliferation are invariably identified in small intestine. These lesions are particularly prominent in the ileum and are associated with numerous intracellular bacilli consistent with L. intracellularis. In pigs, the necrotizing and ulcerative form of L. intracellularis infection is uncommon. Recently anecrotizing enteritis associated with L. intracellularis was reported in 4 Thoroughbred weanling horses in Kentucky (17). These weanlings were of the same age (6 to 8 mo), had the same acute onset of clinical signs prior to referral (5 to 78 h), and despite intensive supportive care and specific therapy for L. intracellularis none survived beyond 48 h. At necropsy, the lesions were characterized as a necro-ulcerative enteritis. The ileal mucosal scrapings from all 4 horses were PCR-positive for L. intracellularis and L. intracellularis-specific IHC performed on 2 horses directly identified the bacterium in the intestinal lesions of both cases (17). The cases in the present report had very similar histologic lesions, with the addition of a distinct hemorrhagic component in all 5 cases. The rapidly progressive clinical deterioration and the lack of response to treatment in these cases appear to be a common feature of this disease.
The necro-hemorrhagic enteritis described in the 5 weanling foals in this report has some clinical and pathological features of both the NE and PHE syndromes of L. intracellularis infection in swine. Of particular note in these cases is the lack of significant intestinal crypt proliferation despite histologic evidence of infection with L. intracellularis and the presence of the organism within enteric lesions in 3 of the 5 cases. Unlike the clinical presentation in pigs, the necro-hemorrhagic form of L. intracellularis enteritis in foals has been identified in similarly aged foals as the proliferative form of the disease (6 to 8 mo). In older foals and adult horses the possibility of L. intracellularis-associated necro-hemorrhagic enterocolitis warrants further investigation. The lesions may be overlooked at necropsy due to minimal intestinal crypt proliferation which may be obscured by superimposed mucosal necrosis.
Two foals (cases 4 and 5) were negative for L. intracellularis on IHC analysis and WS staining. These cases were, however, PCR positive on ileal contents (case 4) and PCR positive on an antemortem fecal sample (case 5). Both cases were treated with antimicrobials for L. intracellularis for a few days prior to death. Only a small number of intestinal samples were collected from cases 4 and 5 at necropsy and as a result of the severe ulceration there were few residual crypts that could be evaluated. We hypothesize that the previous treatment and limited histopatho-logical analysis may explain the negative L. intracellularis IHC and WS results. The gross and histologic lesions in the intestines observed in the 5 weanlings could also occur in salmonellosis or clostridial enterocolitis (18). The results of bacteriological cultures and ELISA tests for clostridial toxins were negative in all 5 foals, and Clostridia were considered unlikely to be the etio-logical agents; however, they could not be completely excluded.
The subtle mucosal crypt proliferation indicates that the lesion could easily be overlooked, especially if affected by mucosal necrosis. As a result, L. intracellularis would often not be logically included among the etiologic differential diagnoses in cases of equine necro-hemorrhagic enteritis. Routine evaluation of equine enteritis cases for presence of L. intracellularis may be warranted for identification of such atypical cases.
Two of the foals included in this report were also infected with a large number of encysted cyathostomes, causing severe typhlocolitis. Cyathostome larval infiltration of the large intestine has previously been recognized in this region (5). The stage 3 larvae (L3) may cause serious damage to the intestinal mucosa as thousands of encysted larvae can cover the entire mucosa, submucosa, or both, of the large intestine and cecum, causing severe damage and impairing function. Conventional anthelmintic therapy may be ineffective as the wall of the cyst protects the larva. This severe parasite burden can cause an inflammatory protein-losing enteropathy and alterations in intestinal motility. The cyathostomin larval stages are frequently resistant to anthelmintics and severe cyathostomin infestation has been associated with death in horses that were regularly dewormed (19–21). The real challenge with encysted larvae lies in their antemortem diagnosis as affected horses will often have low or negative fecal egg counts (22). Transabdominal ultrasonographic findings in case 5 were suggestive of intramural larval cysts, but it is likely that by the time these ultrasound changes become evident, the edema and damage to the colonic wall are too severe to respond to anthelmintics and supportive medical therapy.
In summary, we report L. intracellularis-associated ulcerative necro-hemorrhagic enteritis in 5 weanling foals, with a concurrent severe encysted cyathostome infection in 2 of these cases. The presumptive diagnosis of EPE in these cases was based on the typical signalment, clinical signs, biochemical abnormalities and diagnostic testing (PCR in feces, IPMA in serum). Medical therapy for such cases has been associated with a good response and favorable prognosis (5). However, despite the medical management of these 5 cases, the horses rapidly deteriorated and died. The refractory nature of the disease and the rapid deterioration of these horses may be suggestive of this variant form of L. intracellularis-associated enteritis. Recently a very similar case series was reported involving 4 Thoroughbred weanling horses (17).
Acknowledgment
Immunostaining for L. intracellularis was performed at the Veterinary Diagnostic Laboratory, Iowa State University, Ames, Iowa USA and interpreted by Dr. Kent J. Schwartz. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Page AE, Slovis NM, Gebhart CJ, Wolfsdorf K, Mapes SM, Pusterla N. Serial use of serologic assays and fecal PCR assays to aid in identification of subclinical Lawsonia intracellularis infection for targeted treatment of Thoroughbred foals and weanlings. J Am Vet Med Assoc. 2011;238:1482–1489. doi: 10.2460/javma.238.11.1482. [DOI] [PubMed] [Google Scholar]
- 2.Frazer ML. Lawsonia intracellularis infection in horses: 2005–2007. J Vet Int Med. 2008;22:1243–1248. doi: 10.1111/j.1939-1676.2008.0160.x. [DOI] [PubMed] [Google Scholar]
- 3.Sampieri F, Hinchcliff KW, Toribio RE. Tetracycline therapy of Lawsonia intracellularis enteropathy in foals. Equine Vet J. 2006;38:89–92. doi: 10.2746/042516406775374270. [DOI] [PubMed] [Google Scholar]
- 4.Lavoie J-P, Drolet R. Lawsonia intracellularis proliferative enteropathy. In: Robinson NE, editor. Current Therapy in Equine Medicine. 5th ed. St. Louis, Missouri: Saunders; 2003. pp. 164–166. [Google Scholar]
- 5.Peregrine AS, McEwen B, Bienzle D, Koch TG, Weese JS. Larval cyathostominosis in horses in Ontario: An emerging disease? Can Vet J. 2006;47:80–82. [PMC free article] [PubMed] [Google Scholar]
- 6.Duhamel GE, Wheeldon EB. Intestinal adenomatosis in a foal. Vet Pathol. 1982;19:447–450. doi: 10.1177/030098588201900410. [DOI] [PubMed] [Google Scholar]
- 7.Williams NM, Harrison LR, Gebhart CJ. Proliferative enteropathy in a foal caused by Lawsonia intracellularis-like bacterium. J Vet Diagn Invest. 1996;8:254–256. doi: 10.1177/104063879600800220. [DOI] [PubMed] [Google Scholar]
- 8.Lavoie JP, Drolet R, Parsons D, et al. Equine proliferative enteropathy: A cause of weight loss, colic, diarrhoea and hypoproteinaemia in foals on three breeding farms in Canada. Equine Vet J. 2000;32:418–425. doi: 10.2746/042516400777591110. [DOI] [PubMed] [Google Scholar]
- 9.McGurrin MK, Vengust M, Arroyo LG, Baird JD. An outbreak of Lawsonia intracellularis infection in a standardbred herd in Ontario. Can Vet J. 2007;48:927–930. [PMC free article] [PubMed] [Google Scholar]
- 10.McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820–825. doi: 10.1099/00207713-45-4-820. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie JP. Lawsonia intracellularis infection in foals. In: Lavoie J-P, Hinchcliff KW, editors. Blackwell’s Veterinary Five-minute Consult: Equine. 2nd ed. Ames, Iowa: Wiley-Blackwell; 2008. p. 451. [Google Scholar]
- 12.Lawson GH, Gebhart CJ. Proliferative enteropathy. J Comp Pathol. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- 13.McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999. pp. 521–534. [Google Scholar]
- 14.Rowland AC, Lawson GHK. Porcine intestinal adenomatosis: A possible relationship with necrotic enteritis, regional ileitis and proliferative haemorrhagic enteropathy. Vet Rec. 1975;97:178–181. doi: 10.1136/vr.97.10.178. [DOI] [PubMed] [Google Scholar]
- 15.Rowland AC, Rowntree PGM. A haemorrhagic bowel syndrome associated with intestinal adenomatosis in the pig. Vet Rec. 1972;91:235–241. doi: 10.1136/vr.91.10.235. [DOI] [PubMed] [Google Scholar]
- 16.McOrist S, Roberts L, Jasni S, et al. Developed and resolving lesions in porcine proliferative enteropathy: Possible pathogenetic mechanisms. J Comp Pathol. 1996;115:35–45. doi: 10.1016/s0021-9975(96)80026-0. [DOI] [PubMed] [Google Scholar]
- 17.Page AE, Fallon LH, Bryant UK, Horohov DW, et al. Acute deterioration and death with necrotizing enteritis associated with Lawsonia intracellularis in 4 weanling horses. J Vet Intern Med. 26:1476–1480. doi: 10.1111/j.1939-1676.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- 18.Feary DJ, Hassel DM. Enteritis and colitis in horses. Vet Clin North Am Equine Pract. 2006;22:437–479. doi: 10.1016/j.cveq.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Lyons ET, Drudge JH, Tolliver SC. Larval cyathostomiasis. Vet Clin North Am Equine Pract. 2000;16:501–513. doi: 10.1016/s0749-0739(17)30092-5. [DOI] [PubMed] [Google Scholar]
- 20.Mair TS. Outbreak of larval cyathostomiasis among a group of yearling and two-year-old horses. Vet Rec. 1994;135:598–600. [PubMed] [Google Scholar]
- 21.Van Loon G, Deprez P, Muylle E, Sustronck B. Larval cyathostomiasis as a cause of death in two regularly dewormed horses. Zentralbl Veterinarmed A. 1995;42:301–316. doi: 10.1111/j.1439-0442.1995.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 22.Dowdall SMJ, Matthews JB, Mair T, Murphy D, Love S, Proudman CJ. Antigen-specific IgG(T) responses in natural and experimental cyathostominae infection in horses. Vet Parasitol. 2002;106:225–242. doi: 10.1016/s0304-4017(02)00085-7. [DOI] [PubMed] [Google Scholar]