Abstract
Nine juvenile mink with hind-limb paresis/paralysis from 2 Ontario farms were submitted for necropsy. Diagnostic tests revealed spinal compression and severe thoracic diskospondylitis with intralesional Gram-positive coccoid bacterial colonies. Streptococcus canis, Streptococcus dysgalactiae subsp. equisimilis, and hemolytic Staphylococcus spp. were isolated from vertebral lesions.
Résumé
Discospondylite bactérienne chez des jeunes visons provenant de 2 fermes de visons de l’Ontario. Neuf jeunes visons atteints d’une parésie/paralysie des membres postérieurs provenant de 2 fermes de l’Ontario ont été soumis à une nécropsie. Les tests diagnostiques ont révélé une compression médullaire et une discospondylite thoracique grave avec des colonies de bactéries coccoïdes à Gram positif. Les bactéries Streptococcus canis, Streptococcus dysgalactiae subsp. equisimilis, et Staphylococcus spp. hémolytiques ont été isolés des lésions vertébrales.
(Traduit par Isabelle Vallières)
Despite ongoing advances in nutrition, housing, biosecurity, and animal welfare, morbidity and mortality associated with naturally occurring disease continue to negatively impact the efficiency and productivity of Ontario mink farms. Historically, sporadic reports of hind-limb paresis/paralysis in juvenile mink from Ontario farms have been associated with botulism, Aleutian mink disease virus infection, and urolithiasis (1,2). In recent years, reports in the United States and Spain have described diskospondylitis as a cause for hind-limb paresis/paralysis in farmed mink (3,4); however, this disease, to our knowledge, has never been described in Canada. In this report, we describe bacterial diskospondylitis, affecting juvenile mink on 2 Ontario farms.
Case description
Five dead 12-week-old pastel and black male mink (Mustela vison) from Ontario farm A (#1, #2, #3, #4, #5) and 4, 14-week-old pastel and black male and female mink from Ontario farm B (#6, #7, #8, #9), including 1 live mink were submitted for necropsy to the Animal Health Laboratory of the University of Guelph (AHL), in July 2008 and August 2011, respectively. All animals had a clinical history of bilateral hind-limb paresis or paralysis and urinary incontinence of 3 to 4 wk duration prior to submission.
There were 24 000 juvenile mink on farm A, and approximately 50 animals (0.21%) were affected; most of the affected mink were pastel males. Animals had been weaned at 8 weeks of age and separated into same gender pairs. One to two weeks later they began to show clinical signs of hind-limb paresis/paralysis and urinary incontinence. An on-farm prepared ration, containing 80% to 90% mixture of meat and meat and/or fish-by-products (excluding seal meat) and 10% to 20% cereal-based portion, was initially fed to the growing mink twice per day for the first 4 wk following weaning and then feeding frequency was reduced to once a day.
On farm B, 6500 juvenile mink were reared and by 8 weeks of age, when the weaning and separation of kits into mixed gender pairs was carried out, approximately 3% of the mink kits had lost the distal portions of the tails from tail chewing. One week after weaning, approximately 50 animals (0.77%), both male and female of black and pastel color phases, began to show signs of hind-end paralysis/paresis and new cases continued to develop until the mink were 14 wk old. The growing mink were fed once per day, with a ration produced on-farm that incorporated fishmeal in the cereal mix and used a higher proportion of chicken by-products and no additional fish by-products.
Approximately 1 to 3 wk after the onset of clinical signs, animals on both farms had been vaccinated with a single subcutaneous dose of a 4-way combination aluminum-adjuvanted vaccine containing a modified live canine distemper virus, inactivated mink enteritis virus, Clostridium botulinum Type C bacterin-toxoid, and inactivated Pseudomonas aeruginosa bacterin (Distox-Plus; Merck Animal Health, Intervet Canada, Kirkland, Quebec). Both farms reported no history of Aleutian mink disease virus infection, and none of the mink had received treatments prior to submission to the laboratory.
The dead mink submitted from both farms had been euthanized on-farm by carbon monoxide inhalation, and the live mink from farm B had been euthanized at the laboratory by carbon dioxide inhalation prior to necropsy.
Orthogonal radiographs (dorsoventral and lateral views) of whole body mink centered at the spine were obtained from 3 dead mink from each farm, without individual identification of the animals. Radiographs were taken using a computerized radiography system (Agfa CR Systems; Agfa HealthCare, Albuquerque, New Mexico, USA).
Complete necropsies were performed within 24 h post-euthanasia and tissue samples including skin, heart, lung, liver, spleen, kidney, stomach, intestine, mesenteric lymph node, adrenal gland, urinary bladder, brain, and longitudinal sections of affected vertebrae, were fixed in 10% neutral-buffered formalin and routinely processed for histology. Vertebral samples were demineralized in Surgipath Decalcifier II (Leica Microsystems, Richmond, Illinois, USA). Paraffin-embedded, 4-μm sections were stained with hematoxylin and eosin (H&E) and selected sections were stained with either Brown and Brenn or Brown and Hopps Gram stains.
At necropsy, samples of facial skin of mink #1 and mink #2, swab of the subcutaneous hindlimb abscess of mink #2, swabs of the vertebral lesions of mink #4, #5 and #8, and swab and tissue of the vertebral lesion of mink #6 were submitted for bacterial culture.
All vertebral lesions and the abscess were cultured to detect aerobic and anaerobic bacteria, whereas skin was cultured for the presence of aerobic bacteria only. For aerobic culture, swabs and skin samples were plated on blood agar (BA) and MacConkey agar (MAC) plates. Blood agar plates were incubated at 35°C in the presence of 5% CO2, whereas MAC plates were incubated at the same temperature in air. Plates were checked for the presence of bacterial growth at 24 and 48 h of incubation. For anaerobic culture, Brucella and phenylethyl alcohol (PEA) agar plates were used. These plates were incubated in an anaerobic jar at 35°C in the presence of 94% N2 and 6% CO2. Initial bacterial identification was done using a range of biochemical reactions following standard procedures used in the laboratory (5,6). Bacterial identification was confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (7–9).
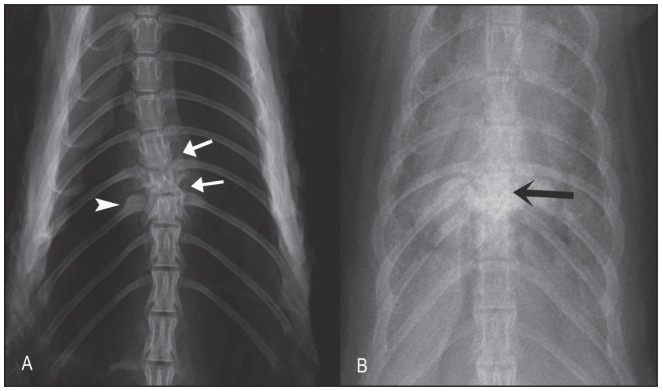
The radiographic findings included lesions that varied from mild to severe diskospondylitis in thoracic (5 animals) and cervical vertebrae (1 animal) (Figure 1). Mild lesions were characterized by areas of bone lysis and sclerosis at the end plates of the affected adjacent vertebral bodies as well as mild widening and irregular intervertebral disk spaces. Severe lesions were characterized by marked osteolysis of the adjacent affected end plates and also irregular amorphous bony proliferation with severe bone sclerosis of the adjacent vertebral bodies (spondylitis component). In the animals with diskospondylitis in the thoracic spine area, there was evidence of extension of the inflammatory process to the adjacent ribs characterized by mild irregular bony proliferation and bilateral mild moth-eaten lysis of the proximal aspect of the ribs. Two animals with severe diskospondylitis also had mild kyphosis suspected to be due to pathologic fracture.
Figure 1.
Ventrodorsal radiographic projections of dead mink with diskospondylitis. A — There is lysis at the intervertebral disk spaces at T10–T11 and T11–T12 (white arrows). The body of T11 is misshapen due to secondary pathological fracture. There is mild periosteal reaction of the adjacent rib (arrowhead). B — Advanced diskospondylitis is characterized by marked irregular periosteal reaction at T9–T10–T11 (black arrow). The body of T10 is not clearly seen due to marked pathological fracture.
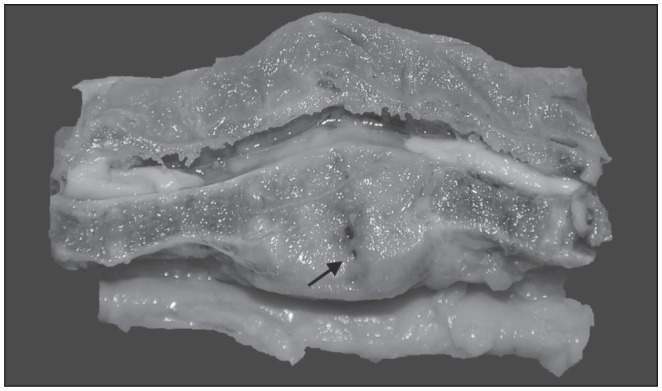
Eight animals had grossly identifiable enlargement and deformation of 1 or 2 thoracic (#1, #3, #4, #5, #6, #8, #9) or cervical vertebrae (#7) (Table 1). These animals had variable degrees of ventrodorsal or lateral deviation of the spinal column. On sagittal section, there was thickening of the ventral aspect of the vertebral bone, collapse of the vertebral joint and/or fusion with the adjacent vertebrae (Figure 2). In other cases, fracture at the level of the vertebral joint with dorsal displacement and spinal cord compression was observed. Four animals (#6, #7, #8, #9) had shortened tails with ulcerated, swollen, hemorrhagic tips crusted with exudate and protruding coccygeal vertebrae. One mink (#6) had hind feet with abraded skin of the toes and missing nails from dragging the feet following the onset of the hind-limb paralysis and another one (#7) had similar lesions on the front feet likely related to the cervical vertebral lesion. Two mink (#1, #2) had single 2-cm round, crusted alopecic areas on their cheeks, and 1 animal (#2) had dermatitis of the plantar aspect of the foot and a large abscess in the right thigh. Two mink (#1, #2) also had mild gingivitis.
Table 1.
Lesions and bacterial isolates from 9 mink from Farms A & B
| Lesion | |||||
|---|---|---|---|---|---|
|
|
|||||
| Animal | Farm | Diskospondylitisa | Tail/foot mutilation | Pyodermab | Abscessc |
| 1 | A | T4–T5 Not cultured |
− | + (S. canis, Staph. pseudintermedius) |
− |
| 2 | A | Not present | − | + (S. canis, Staph. pseudintermedius) |
+ (S. canis) |
| 3 | A | T4–T5 Not cultured |
− | − | − |
| 4 | A | T10–T11 Streptococcus canis |
− | − | − |
| 5 | A | T4–T5 S. canis |
− | − | − |
| 6 | B | T9–T10 S. canis, S. dysgalactiae subsp. equisimilis, Staph. muscae, Staph. delphini |
+ | − | − |
| 7 | B | C7–T1 Not cultured |
+ | − | − |
| 8 | B | T9–T10 S. canis, S. dysgalactiae subsp. equisimilis |
+ | − | − |
| 9 | B | T9–T11 Not cultured |
+ | − | − |
Location of lesion, T — Thoracic vertebra, C — Cervical vertebra.
Pustular dermatitis and furunculosis in the face.
Thigh abscess. Staph — Staphylococcus; S. — Streptococcus.
Figure 2.
Sagittal section of the spine of mink #6 between T9–T10. Swelling and replacement of intervertebral disk and adjacent vertebral bone by a whitish tissue can be observed (arrow). There is collapse of the vertebral joint, loss of the intervertebral disk, shortening of the adjacent vertebral bodies, and ventral compression of the spinal cord.
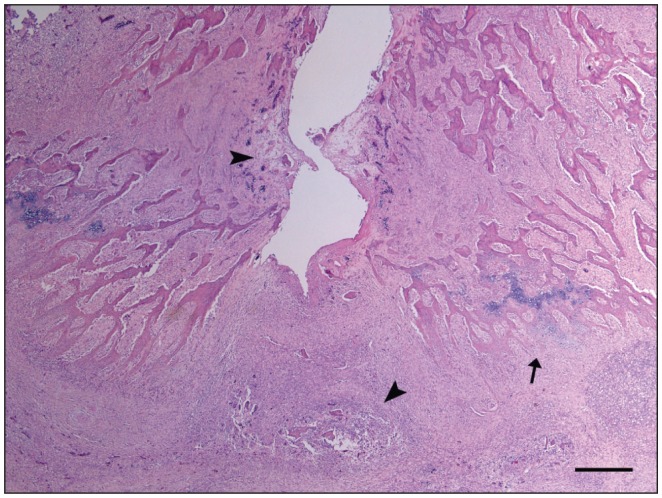
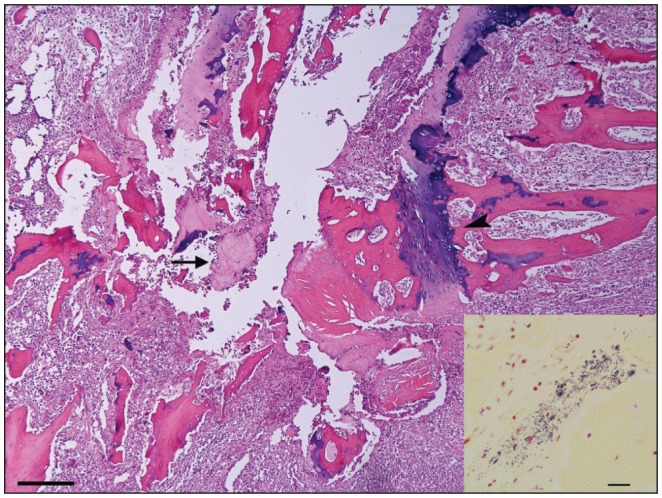
Histologically, the intervertebral disk and cartilage end plates were lost and marked inflammation was observed extending into adjacent growth plates and soft tissue around vertebral bodies resulting in compression of the spinal cord (Figure 3). Intralesionally, small fragments of fibrocartilage from the disk and necrotic trabecular bone with occasional bacterial colonies were surrounded by marked infiltration of neutrophils, fewer macrophages with fibrin deposition, and extensive amounts of granulation tissue (Figure 4). There was also marked remodeling of the trabecular bone with increased numbers of osteoclasts and osteoblasts, and periosteal new bone formation by cartilage formation and subsequent endochondral ossification. In the spinal cord, hemorrhages and thrombosis were observed in the leptomeninges. In the white matter, axons were swollen in some areas, with some of them removed by macrophages; a few chromatolytic neurons were present in the gray matter. With Gram’s stain, multiple coccoid bacterial colonies were observed within the vertebral necrotic bone (Figure 4).
Figure 3.
Sagittal section of the spine of mink #6 between T9–T10, showing the central and ventral area of the intervertebral joint. There is loss of the intervertebral disk with necrosis of adjacent trabecular bone (arrowheads) and marked inflammation extending to the ventral aspect of the vertebra. Periosteum shows new bone formation by endochondral ossification (arrow). Hematoxylin and eosin stain. Bar = 200 μm.
Figure 4.
Sagittal section of the spine of mink #8 between T9–T10. There is necrosis and fragmentation of intervertebral disk cartilage (arrow) surrounded by pyogranulomatous inflammation affecting trabecular bone and vertebral growth plate (arrowhead). Hematoxylin and eosin stain. Bar = 100 μm. Inset: Multiple coccoid bacterial colonies within vertebral necrotic bone. Brown and Brenn Gram stain. Bar = 20 μm.
In the facial skin of 2 animals (#1, #2), there was severe pyoderma and furunculosis. This was consistent with necrosis of epidermal cells and serocellular crust accumulation on the superficial epidermis intermingled with abundant coccoid bacterial colonies. There was also marked infiltration of neutrophils and fewer macrophages extending also into the dermis and producing follicular destruction, hemorrhage, and fibrosis.
Streptococcus canis was consistently isolated from all the lesions in the farm A submission including the facial pyoderma (#1, #2), the subcutaneous abscess (#2) and the vertebral lesions (#4, #5); Staphylococcus pseudintermedius was isolated from the facial pyoderma only (#1, #2). In the submission from farm B, S. canis and Streptococcus dysgalactiae subsp. equisimilis were isolated from affected vertebral columns in 2 animals (#6, #8), whereas Staphylococcus muscae and Staphylococcus delphini were isolated from lesions in 1 animal only (#6). No anaerobes were isolated from any of the samples cultured anaerobically.
Discussion
The present work describes cases of posterior paresis/paralysis occurring on 2 mink farms in Ontario during the summers of 2008 and 2011. Although this clinical presentation has been seen sporadically on Ontario mink farms in the past, this is the first time it has been linked with bacterial diskospondylitis. Bacterial diskospondylitis in mink has been previously reported in the United States and, as in our present findings, appeared during summer time mostly affecting animals between 2 and 4 months of age (3). Diskospondylitis, but without an established bacterial etiology, was also reported in 2- to 5-month-old mink in Spain (4). It is not known why young mink are more commonly affected. It remains to be determined if onset of disease could be related to farm management practices and to the stresses associated with weaning and growing. During summer, mink kits are weaned and handled multiple times for separating, pairing, vaccinating, implanting and are subjected to changing diets. Moreover, during this period, high ambient temperatures and humidity favor the proliferation of bacteria. All of these events, in addition to the decline in maternal antibodies (3), could be predisposing factors for the development of bacterial diskospondylitis.
Beta-hemolytic Streptococcus spp., namely S. canis and S. dysgalactiae subsp. equisimilis, together with species of hemolytic Staphylococcus were isolated from vertebral lesions in our cases. While S. canis was isolated from all vertebral lesions, there was no consistency in the isolation of S. dysgalactiae subsp. equisimilis and Staphylococcus spp. Although these data need to be interpreted with caution, given the small number of samples in our cases, another study also showed that β-hemolytic Streptococcus spp. may play a role in mink diskospondylitis with β-hemolytic Streptococcus spp. in pure culture isolated from 8 of 8 intervertebral disk cultures and 6 of 8 heart blood cultures in affected mink (3). However, unlike in our study, these isolates were not further speciated and therefore it remains to be established if both species of β-hemolytic Streptococcus play a role in the disease or if it is S. canis predominantly. Based on AHL data, S. canis appears to be the main streptococcal pathogen isolated from mink cases. From 54 mink cases submitted between May 2007 and August 2012, S. canis was isolated from 24, whereas S. dysgalactiae subsp. equisimilis was isolated from only 2 cases including the 1 case described in this report. Mainly S. canis has been associated with septicemia, pneumonia, metritis, osteomyelitis, diskospondylitis, subcutaneous abscesses, and footpad dermatitis in our cases (data not shown). Our data indirectly support a role of S. canis in diskospondylitis, but a larger study with full speciation of β-hemolytic Streptococcus spp. is needed to confirm that notion. In general, β-hemolytic Streptococcus spp. are common opportunistic pathogens in many animal species (6). As with other opportunistic pathogens, it is likely that predisposing factors are necessary to cause the disease in mink, including bite wounds, trauma, perinatal infection, bacteremia (3), and high feed bacterial contamination (4). In the present study, some animals had lost the distal portions of their tail tips from chewing; others had subcutaneous abscesses, facial and foot dermatitis, and mild gingivitis from which S. canis and hemolytic Staphylococcus spp. were isolated. Most of these lesions could be derived from trauma and could serve as entry points for the bacteria with subsequent bacteremia and localization in the thoracic vertebrae. These latter findings were in contrast with other reported cases of bacterial diskospondylitis in which traumatic injuries, cutaneous lesions, or other possible sources of bacterial infection were not observed (3). Further support for the role of traumatic wounds in the development of bacterial diskospondylitis came from husbandry changes on farm A subsequent to the diagnosis. At the time of weaning, separating, and pairing, juvenile mink were moved into double-partitioned side-by-side pens to minimize animal contact (chewing and biting) and the occurrence of traumatic injuries between mink in adjacent cages resulting in a reduction in the number of cases.
The reason for the apparent predilection of streptococci for localization within bone is unknown, but probably reflects the fact that certain bacteria possess receptors for bone surface proteins, such as sialoproteins and collagen (10). Other authors (3) suggested that the highly specific proclivity of these bacteria for the thoracic vertebrae and, possibly, the narrow age window during which disease usually occurs in mink, were related to the vascular supply to the vertebral column. Based on the human literature (11,12), it was suggested that the thoracic intervertebral disks in young mink may have a blood supply that atrophies as the animals mature, differing from that of the cervical and lumbar vertebrae (3).
In conclusion, this report describes outbreaks of paresis/paralysis in juvenile mink on 2 Ontario mink farms associated with chronic diskospondylitis likely due to S. canis infections. Traumatic injuries might have played a role in the development of the vertebral lesions as some of the mink from both farms also had cutaneous, subcutaneous, or footpad lesions or tail tip wounds prior to disease outbreak. Although this clinical presentation has already been reported in other countries, the authors are unaware of this disease being previously described in Canada. With the inclusion of this disease in the list of differential diagnoses for cases of hind-limb paresis/paralysis in juvenile mink, the approach to laboratory testing in the future should be modified to include radiography for identification of vertebral lesions.
Acknowledgments
We are grateful to the 2 Ontario mink farmers for providing background information that assisted us in the preparation of this manuscript. We thank Dr. Grant Maxie, from the Animal Health Laboratory (University of Guelph), for the revision of the manuscript. We dedicate this article to our colleague, the late Dr. D. Bruce Hunter of the Ontario Veterinary College; he unselfishly shared his enthusiasm for mink health, disease, and management with us. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Aulerich R, Bursian S. Toxicology in mink. In: Hunter B, Lemieux N, editors. Mink: Biology, Health and Disease. Guelph, Ontario: University of Guelph; 1996. pp. 18-1–18-34. [Google Scholar]
- 2.Dyer NW, Ching B, Bloom ME. Nonsuppurative meningoencephalitis associated with Aleutian mink disease parvovirus infection in ranch mink. J Vet Diagn Invest. 2000;12:159–162. doi: 10.1177/104063870001200212. [DOI] [PubMed] [Google Scholar]
- 3.Olson EJ, Parker JB, Carlson CS. Bacterial diskospondylitis associated with posterior paresis/paralysis in North American farmed mink (Mustela vison) Vet Pathol. 2005;42:125–131. doi: 10.1354/vp.42-2-125. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Antonio R, López-Peña M, Faílde LD, et al. Diskospondylitis in mink (Neovison vison): A preliminary study in Spanish mink farms. Proceedings of the IX International Scientific Congress in Fur Animal Production; Halifax, Nova Scotia. 2008. p. 212. [Google Scholar]
- 5.Quinn PJ, Carter ME, Markey B, et al. Staphylococcus species. In: Clinical Veterinary Microbiology. Edinburgh, Scotland: Mosby-Wolfe; 1994. pp. 118–136. [Google Scholar]
- 6.Songer JG, Post KW. Veterinary Microbiology: Bacterial and Fungal agents of Animal Disease. St. Louis, Missouri: Elsevier Saunders; 2005. pp. 35–51. [Google Scholar]
- 7.Carpaij N, Willems RJL, Bonten MJM, et al. Comparison of the identification of coagulase-negative staphlylococci by matrix assisted laser desorption ionization time-of-flight mass spectrometry and tuf sequencing. Eur J Clin Microbiol Infect Dis. 2011;30:1169–1172. doi: 10.1007/s10096-011-1204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherkaoui A, Emonet S, Fernández J, et al. Evaluation of matrix-assisted laser desorption ionization time-of-flight mass spectrometry for rapid identification of beta-hemolytic streptococci. J Clin Microbiol. 2011;49:3004–3005. doi: 10.1128/JCM.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decristophoris P, Fasola A, Benagali C, et al. Identification of Staphylococcus intermedius group by MALDI-TOF MS. Sys Appl Microbiol. 2011;34:45–51. doi: 10.1016/j.syapm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Thompson K. Bones and joints. In: Maxie G, editor. Pathology of Domestic Animals. 5th ed. Vol. 1. Edinburgh, Scotland: Saunders Elsevier; 2007. pp. 1–180. [Google Scholar]
- 11.Ratcliffe JF. An evaluation of the intra-osseous arterial anastomoses in the human vertebral body at different ages. A microarteriographic study. J Anat. 1982;134:373–382. [PMC free article] [PubMed] [Google Scholar]
- 12.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: Update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11–24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]