Abstract
Eight healthy dogs undergoing elective ovariohysterectomy were anesthetized with a standard protocol and received a low-dose medetomidine constant rate infusion during surgery. Cardiorespiratory parameters, including non-invasive cardiac output, were measured at various times. This protocol resulted in acceptable and stable cardiovascular performance, allowed low isoflurane concentrations, and provided smooth recoveries.
Résumé
Utilisation clinique d’une infusion d’une faible dose de médétomidine chez les chiennes en santé subissant une ovariohystérectomie. Huit chiennes en santé subissant une ovariohystérectomie non urgente ont été anesthésiées à l’aide d’un protocole standard et ont reçu une infusion constante d’une faible dose de médétomidine durant la chirurgie. Les paramètres cardiorespiratoires, incluant le débit cardiaque non effractif, ont été mesurés à divers moments. Ce protocole a produit un rendement cardiovasculaire acceptable et stable et a permis de faibles concentrations d’isoflurane et des réveils sans problème.
(Traduit par Isabelle Vallières)
Balanced anesthesia consists of the administration of more than 1 anesthetic throughout anesthesia. This allows administering lower doses of the individual drugs in order to achieve an appropriate plane of anesthesia, while minimizing the adverse effects associated with each drug (1). Medetomidine is an alpha-2 adrenoceptor agonist drug with sedative, analgesic, muscle relaxant, and anxiolytic effects (2). Medetomidine decreases the requirements of injectable anesthetics (3) as well as of inhalant agents (4–6) in a dose-dependent fashion. These features make medetomidine an attractive drug to be used intraoperatively as part of balanced anesthetic protocols. Medetomidine also has the advantage of blunting the stress response associated with surgery (7,8). However, despite all these potential intraoperative advantages, the use of medetomidine seems to be limited to the premedication and recovery periods, possibly because of fear of adverse cardiovascular effects.
The negative cardiovascular effects associated with medetomidine and its active isomer dexmedetomidine are significant, characterized by intense bradycardia, with possible atrio-ventricular blocks, increased systemic vascular resistance, with initial hypertension followed by normotension, and pronounced decrease in cardiac output (2,5,9–12). These effects are of similar magnitude at doses of medetomidine ranging from 5 to 20 μg/kg body weight (BW) in conscious dogs (10). Lower doses (1 and 2 μg/kg BW) induced similar but less pronounced adverse cardiovascular effects (10).
The aim of this prospective case series was to describe the quality of maintenance of and recovery from anesthesia, as well as some cardiovascular variables, including cardiac output (CO) using a non-invasive cardiac output (NiCO) monitor, during a low-dose constant rate infusion (CRI) of medetomidine in healthy dogs undergoing ovariohysterectomy.
Case description
Eight healthy adult dogs, American Society of Anesthesiologists classification 1, weighing > 15 kg and scheduled for elective ovariohysterectomy were anesthetized with a predefined, routine anesthetic protocol commonly used at our institution. Dogs were client-owned and determined to be healthy based on pre-operative physical examination and blood work, including hematocrit (Ht), total serum protein (TP), and blood urea nitrogen (BUN). The dogs were maintained in accordance with the Canadian Council of Animal Care guidelines.
The dogs received hydromorphone (Sandoz Canada, Boucherville, Quebec), 0.05 mg/kg BW, IM, and acepromazine (Atravet; Boehringer Ingelheim, Burlington, Ontario), 0.05 mg/kg BW, IM, mixed in the same syringe for pre-medication. Approximately 20 min later, a 20G cephalic catheter was placed and anesthesia was induced with diazepam (Sandoz Canada), 0.2 mg/kg BW, IV, and propofol (Novopharm, Toronto, Ontario), 2 mg/kg BW, IV. After orotracheal intubation with an appropriate size cuffed tube, the dogs were connected to an F-circuit and isoflurane (Aerrane; Baxter, Mississauga, Ontario) in oxygen was administered to maintain anesthesia. Immediately after induction, a Doppler flowmeter probe (Doppler Medical Electronics 811-B; Parks Medical Electronics, Aloha, Oregon, USA) for measurement of systolic arterial blood pressure (SBP) (13) and electrocardiography leads were placed. For CO measurements, a NiCO monitor (NICO; Novametrix Medical Systems, Wallingford, Connecticut, USA) was used. The flow sensor, mainstream capnograph and rebreathing loop from the NiCO monitor were placed between the endotracheal tube and the F-circuit and the pulse oximeter, also from the NiCO monitor, was placed on the tongue.
Once all monitors had been connected, mechanical ventilation (Multiflow 2002 Anesthesia Ventilator; Hallowell EMC, Pittsfield, Massachusetts, USA) was instituted using a tidal volume of 10 to 20 mL/kg BW and a respiratory frequency of 10 to 12 breaths per min in order to maintain end-tidal carbon dioxide tension (EtCO2) between 35 and 40 mmHg. Five minutes after mechanical ventilation was started, an arterial blood sample was obtained from the dorsal pedal artery for arterial blood gases, lactate and hemoglobin concentration, which were measured within 5 min of collection (ABL 700 Series analyzer; Radiometer, Copenhagen, Denmark). Values of partial pressure of arterial oxygen (PaO2) and carbon dioxide (PaCO2) obtained from the arterial blood gas analysis were entered in the NiCO monitor as well as values for hemoglobin concentration and inspired oxygen fraction. The values for tidal volume, respiratory rate and peak airway pressure were recorded from the spirometer included in the NiCO monitor. The cardiac index (CI) (mL/kg BW/min) was calculated dividing NiCO (L/min) by body weight (kg).
A medetomidine bolus (Domitor; Novartis Animal Health Canada, Mississauga, Ontario), 1 μg/kg BW, IV followed by a CRI (1 μg/kg BW per hour, IV) was administered after the first set of cardiovascular variables was recorded, before the start of surgery. The medetomidine CRI was continued until the end of surgery, when it was stopped once the last set of cardiovascular variables had been recorded.
Cardiovascular variables including NiCO, SBP, and heart rate (HR), as well as expired gases (Cardiocap/5; Datex-Ohmeda, GE Healthcare Finland Oy, Helsinki, Finland) including EtCO2 and end-tidal isoflurane concentration (EtIso) were recorded at different times during anesthesia: after induction, before medetomidine administration (T1), 5 to 10 min after the administration of the medetomidine bolus, before start of surgery (T2), during skin incision (T3), during removal of the first ovary (T4), during removal of the second ovary (T5), during closure of the abdominal wall (T6), and immediately after the skin was sutured (T7). An arterial blood sample was obtained again at the end of surgery (T7) for arterial blood gases, lactate, Ht and TP, which were measured within 5 min of blood collection.
A balanced electrolyte solution (Plasma-lyte A; Baxter) was administered IV for the duration of anesthesia at a rate of 10 mL/kg BW per hour. Anesthesia was monitored by final year veterinary students under the supervision of an experienced anesthetist (ER, GG, or AV). The students were aware of the anesthetic protocol and kept the dogs at an ideal surgical plane of anesthesia (absence of palpebral reflexes, relaxed jaw tone, no movement in response to surgery, eyes positioned ventromedially, and presence of mild sympathetic responses to surgical stimuli), while also recording the EtIso. Surgery was performed by final year veterinary students under the supervision of a surgery resident. If the dogs became light during the surgical procedure a propofol bolus was administered (0.5 mg/kg BW, IV) and recorded, and the isoflurane level was increased to achieve a surgical plane of anesthesia. Total duration of medetomidine CRI, anesthesia time, surgery time, total amount of fluids and total amount of propofol administered were recorded. A dose of hydromorphone (0.025 mg/kg BW, IV) was administered 3 h after the premedication dose and a dose of meloxicam (Metacam; Boehringer Ingelheim), 0.1 mg/kg BW, IV, was administered during the closure of the abdominal wall (T6).
The quality of recovery was scored using a descriptive scale from 1 to 5 (1 being excellent and 5 being very poor recovery) during the first 15 min after tracheal extubation.
The recovery quality scoring system is as follows:
Quiet, calm, breathing normally, good analgesia during at least 15 min after extubation (no response when the incision was palpated).
Some vocalization but no excitement during the first 15 min after extubation, not in pain, relaxes in response to human contact, no medetomidine or acepromazine needed.
Some vocalization and/or mild but not painful excitement during 15 min after extubation, requires medetomidine or acepromazine to relax.
Vocalizing, thrashing, seems very excited but not painful, at some point during the first 15 min after extubation, needs medetomidine or acepromazine to calm down.
Same as 4 but seems to have pain and additional hydromorphone top-up is needed to relax.
If the dog showed signs of excitement during recovery, sedation was provided with a dose of medetomidine (1 μg/kg BW, IV) or acepromazine, 0.01 mg/kg BW, IV, and if it seemed to have pain, a dose of hydromorphone (0.025 mg/kg BW, IV) was administered.
Commercial statistical software (SAS, version 9.1.3; SAS Institute, Cary, North Carolina, USA) was used to analyze the data. All variables were tested for normality using a Shapiro-Wilk test. Normally distributed variables measured over time (CI, SBP, HR, EtCO2, and EtIso) were analyzed using an analysis of variance (ANOVA) for repeated measures and when the overall effect of time was significant (F test ≤ 0.05), multiple pair-wise comparisons were performed using post-hoc Tukey adjustments. Normally distributed variables measured only twice (Ht, TP, PaO2, PaCO2, and lactate) were analyzed using paired t-tests, and non-normally distributed variables (pH) were analyzed using Signed Rank Sum test to compare the values pre- and post-surgery. Descriptive statistics were performed for variables measured only once. Significance level was set at P < 0.05.
The mean ± standard deviation (SD) age and weight of the dogs were 2 ± 1.7 y and 18.7 ± 6.5 kg, respectively. The total amount of fluids administered was 31.2 ± 10.7 mL/kg BW (mean ± SD). Mean (± SD) total anesthesia and surgery times were 3.2 ± 1 h and 2 ± 0.8 h, respectively. Three dogs received intraoperative propofol (0.8, 1.0, and 1.5 mg/kg BW) in order to deepen anesthesia during ovarian pedicle manipulation or abdominal closure. Two dogs received the additional dose of hydromorphone during abdominal closure and the other 6 during skin closure. The median (range) recovery score was 1 (1–3). Only 2 dogs required sedation during recovery (1 received acepromazine and the other medetomidine).
Mean ± SD values of EtIso and EtCO2 did not significantly differ over time and were 1.06% ± 0.23% and 37.5 ± 5.6 mmHg, respectively, during anesthesia. Mean ± SD tidal volumes used for mechanical ventilation were 14 ± 2 mL/kg BW.
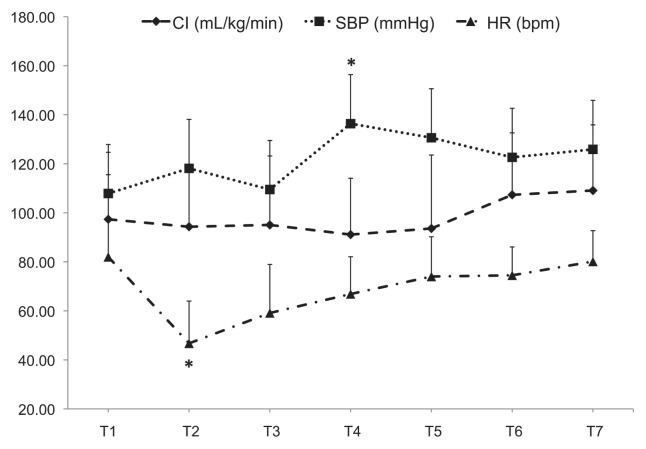
Results of the cardiovascular variables are summarized in Figure 1. Heart rate decreased after the administration of the medetomidine bolus (T2) to mean ± SD values of 47 ± 17 beats/min and these values were significantly different from T1 (P < 0.001). Immediately after the medetomidine bolus, 1 dog developed second-degree atrio-ventricular blocks and 2 other dogs had a loss of Doppler signal quality for a few seconds. Systolic blood pressure increased significantly at T4 compared with T1 (P = 0.016). Cardiac index did not significantly vary over time (P = 0.2), with mean ± SD values of 98 ± 26 mL/kg BW/min throughout anesthesia.
Figure 1.
Mean ± SD values of cardiac index (CI), systolic arterial blood pressure (SBP), and heart rate (HR) obtained at various times during anesthesia in dogs undergoing ovariohysterectomy that were anesthetized with isoflurane and received medetomidine (1 μg/kg BW bolus followed by 1 μg/kg BW per hour, IV). T1 — after induction, before medetomidine bolus; T2 — 5 to 10 min after medetomidine bolus and start of medetomidine CRI; T3 — skin incision; T4 and T5 — removal of first and second ovaries, respectively; T6 — closure of abdominal wall; T7 — after skin sutures. * Value significantly different from T1 (P < 0.05).
Results of the blood gases, Ht, TP, and lactate concentrations are summarized in Table 1. The PaO2, pH, and lactate did not change significantly after surgery. The PaCO2 increased (P = 0.03), whereas Ht and TP decreased (P < 0.001) significantly after surgery.
Table 1.
Mean ± SD values of Ht and TP obtained before anesthesia (sample 1) and immediately after surgery (sample 2), and of arterial blood gases (pH, PaO2, and PaCO2) and lactate concentrations obtained during anesthesia before surgery (sample 1) and after surgery (sample 2), in dogs undergoing ovariohysterectomy that were anesthetized with isoflurane and received medetomidine (1 μg/kg BW bolus followed by 1 μg/kg BW per hour, IV)
| Sample | PaO2 (mmHg) | PaCO2 (mmHg) | pH | Ht (Vol%) | TP (g/L) | Lactate (mmol/L) |
|---|---|---|---|---|---|---|
| 1 | 506 ± 62.2 | 36.6 ± 4 | 7.4 ± 0.0 | 47.6 ± 3.5 | 68 ± 5 | 1.1 ± 0.5 |
| 2 | 451.4 ± 55.6 | 43.3 ± 6.5* | 7.3 ± 0.1 | 36.9 ± 2.2* | 55 ± 05* | 0.9 ± 0.4 |
Value significantly different from sample 1 (P < 0.05). PaO2 — partial pressure of arterial oxygen, PaCO2 — partial pressure of arterial carbon dioxide, Ht — hematocrit, TP — total serum protein.
Discussion
This report describes the use of a low-dose medetomidine CRI during ovariohysterectomy in healthy dogs and its associated cardiovascular effects, which were considered acceptable. Heart rate is the parameter that most markedly decreased following administration of medetomidine, but CO was maintained at acceptable values, similar to those obtained at baseline, throughout anesthesia. This report also shows the clinical application of a NiCO monitor in dogs undergoing surgery.
A dexmedetomidine CRI of 0.5 μg/kg BW per hour (which is equipotent to 1 μg/kg BW per hour of medetomidine) decreased the minimum alveolar concentration (MAC) of isoflurane by 18% ± 12% in dogs (14). In the present report, the isoflurane was maintained at levels approximately 17% below the MAC described for dogs (1.28%) (15) throughout the procedure. Surgical anesthesia is usually obtained at doses of at least 1.5 times the MAC of an inhalant anesthetic used as the sole agent, which corresponds to 1.92% of isoflurane. The dogs in the present report also received other drugs (acepromazine, hydromorphone, and diazepam) in addition to the medetomidine CRI, which likely also contributed to the decrease in isoflurane requirements during surgery.
The negative cardiovascular effects associated with medetomidine and its active isomer dexmedetomidine are significant, and limit their clinical use mostly to healthy patients. In the dogs of the present report, CO, which is the parameter together with the arterial oxygen content that determines the adequacy of blood and oxygen supply to the tissues, remained constant throughout anesthesia and surgery. Adequate tissue oxygenation was supported by the low plasma lactate levels after surgery, which has also been observed in dogs undergoing surgery receiving a CRI of dexmedetomidine at low doses (16). In contrast, CO markedly decreased following intravenous medetomidine and dexmedetomidine administration in dogs in other studies (5,10–12,16–18). The decrease in CO associated with medetomidine is pronounced and independent of the dose at medium to high doses (≥ 5 μg/kg BW) (10), but it is less severe and dose-dependent at low doses (≤ 3 μg/kg BW) (10,19,20). Administration of low doses of medetomidine (≤ 1 μg/kg BW/h) (20) or dexmedetomidine (0.5 μg/kg BW/h) (17), as CRI during isoflurane anesthesia in research dogs decreased the CO by 27%, but this decrease was short-lived and well-tolerated by the animals (20).
In the present report, CO was determined by a non-invasive technique that uses partial re-breathing of CO2 and calculates CO by using a differential form of the Fick equation (21). This technique only requires measurement of EtCO2 and hemoglobin saturation to derive the CO and has been validated previously in isoflurane-anesthetized dogs (22). In this technique, a re-breathing loop is attached between the endotracheal tube and the breathing circuit, which temporarily adds a portion of the expired CO2 to the breathing circuit so that the animal inhales only a portion of the exhaled gases. The readout is obtained intermittently at 3-minute intervals, which allows nearly continuous monitoring of the CO. This NiCO method is an ideal method of CO determination in the clinical setting as it is non-invasive and, thus, can be easily applied to clinical cases in which small increases in CO2 are not contraindicated. The drawbacks of this NiCO method include that the patient needs to be mechanically ventilated to ensure constant elimination of CO2 and that the required tidal volume is at least 200 mL; therefore, limiting its application to medium- to large-sized dogs, small equids, and ruminants.
Some of the dogs in this report showed immediate and pronounced bradycardia after the administration of the medetomidine bolus, with associated atrio-ventricular blocks observed in 1 animal and loss of the Doppler signal quality in another 2 animals. Bradycardia with first and second degree atrio-ventricular blocks is common following the administration of alpha-2 adrenoceptor agonists (2,16,23). The loss of Doppler signal quality could have been due to the immediate and pronounced bradycardia and/or intense peripheral vasoconstriction, decreasing peripheral blood flow, as a consequence of the rapid rise in plasma concentration of medetomidine. It is possible that these immediate effects could have been partially blunted or avoided by slow intravenous or intramuscular administration of medetomidine; therefore, the authors recommend these routes over fast intravenous bolus administration. Nonetheless, this effect was short-lived and the Doppler signal quality improved within a few seconds. The bradycardic effect was also short lasting and the HR returned to values similar to baseline once surgery commenced, which is in agreement with previous studies in dogs using similar low CRI doses of medetomidine (20) or dexmedetomidine (16).
Alpha-2 adrenoceptor agonists decrease plasma levels of circulating catecholamines and attenuate the stress response to surgery (7,8,24). Cardiovascular responses to surgery were not totally abolished with the use of a low-dose medetomidine CRI in these dogs during ovariohysterectomy since blood pressure increased significantly during surgical manipulation of the ovarian pedicles. It is possible that the increase in SBP and HR in response to surgery would have been more pronounced if no medetomidine had been used. Unfortunately, this study did not include a control group (i.e., no medetomidine administered), which would have helped answer this question. However, in a previous study comparing the use of medetomidine or acepromazine for premedication in dogs undergoing ovariohysterectomy, the blood pressure remained higher in the medetomidine group throughout surgery, but the increase in blood pressure in response to ovarian pedicle manipulation was much greater in the dogs that had received acepromazine (7).
In conclusion, the use of a low-dose CRI of medetomidine during ovariohysterectomy in healthy dogs induced minimal and acceptable changes in CO, as determined with a non-invasive monitor, allowed the use of low concentrations of isoflurane and provided smooth recoveries when administered as part of a balanced anesthetic protocol including acepromazine, hydromorphone, and diazepam.
Acknowledgment
The authors thank Gabrielle Monteith for her help with the statistical analysis. CVJ
Footnotes
Presented in abstract form as a poster at the autumn meeting of the European College of Veterinary Anesthesia and Analgesia and Association of Veterinary Anesthetists, Santorini, Greece, September 2010.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Reference
- 1.Ilkiw JE. Balanced anesthetic techniques in dogs and cats. Clin Tech Small Anim Pract. 1999;14:27–37. doi: 10.1016/S1096-2867(99)80024-3. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair MD. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J. 2003;44:885–897. [PMC free article] [PubMed] [Google Scholar]
- 3.Sano T, Nishimura R, Mochizuki M, Sasaki N. Effects of midazolam-butorphanol, acepromazine-butorphanol and medetomidine on an induction dose of propofol and their compatibility in dogs. J Vet Med Sci. 2003;65:1141–1143. doi: 10.1292/jvms.65.1141. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Villamandos RJ, Palacios C, Benitez A, et al. Effect of medetomidine infusion on the anaesthetic requirements of desflurane in dogs. Res Vet Sci. 2008;84:68–73. doi: 10.1016/j.rvsc.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Vickery RG, Sheridan BC, Segal IS, Maze M. Anesthetic and hemodynamic effects of the stereoisomers of medetomidine, an alpha 2-adrenergic agonist, in halothane-anesthetized dogs. Anesth Analg. 1988;67:611–615. [PubMed] [Google Scholar]
- 6.Ewing KK, Mohammed HO, Scarlett JM, Short CE. Reduction of isoflurane anesthetic requirement by medetomidine and its restoration by atipamezole in dogs. Am J Vet Res. 1993;54:294–299. [PubMed] [Google Scholar]
- 7.Vaisanen M, Raekallio M, Kuusela E, et al. Evaluation of the perioperative stress response in dogs administered medetomidine or acepromazine as part of the preanesthetic medication. Am J Vet Res. 2002;63:969–975. doi: 10.2460/ajvr.2002.63.969. [DOI] [PubMed] [Google Scholar]
- 8.Benson GJ, Grubb TL, Neff-Davis C, et al. Perioperative stress response in the dog: Effect of pre-emptive administration of medetomidine. Vet Surg. 2000;29:85–91. doi: 10.1111/j.1532-950x.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 9.Murrell JC, Hellebrekers LJ. Medetomidine and dexmedetomidine: A review of cardiovascular effects and antinociceptive properties in the dog. Vet Anaesth Analg. 2005;32:117–127. doi: 10.1111/j.1467-2995.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 10.Pypendop BH, Verstegen JP. Hemodynamic effects of medetomidine in the dog: A dose titration study. Vet Surg. 1998;27:612–622. doi: 10.1111/j.1532-950x.1998.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 11.Bloor BC, Frankland M, Alper G, Raybould D, Weitz J, Shurtliff M. Hemodynamic and sedative effects of dexmedetomidine in dog. J Pharmacol Exp Ther. 1992;263:690–697. [PubMed] [Google Scholar]
- 12.Weitz JD, Foster SD, Waugaman WR, Katz RL, Bloor BC. Anesthetic and hemodynamic effects of dexmedetomidine during isoflurane anesthesia in a canine model. Nurse Anesth. 1991;2:19–27. [PubMed] [Google Scholar]
- 13.Chalifoux A, Dallaire A, Blais D, Lariviere N, Pelletier N. Evaluation of the arterial blood pressure of dogs by two noninvasive methods. Can J Comp Med. 1985;49:419–23. [PMC free article] [PubMed] [Google Scholar]
- 14.Pascoe PJ, Raekallio M, Kuusela E, McKusick B, Granholm M. Changes in the minimum alveolar concentration of isoflurane and some cardiopulmonary measurements during three continuous infusion rates of dexmedetomidine in dogs. Vet Anaesth Analg. 2006;33:97–103. doi: 10.1111/j.1467-2995.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 15.Steffey EP, Howland D., Jr Isoflurane potency in the dog and cat. Am J Vet Res. 1977;38:1833–1836. [PubMed] [Google Scholar]
- 16.Uilenreef JJ, Murrell JC, McKusick BC, Hellebrekers LJ. Dexmedetomidine continuous rate infusion during isoflurane anesthesia in canine surgical patients. Vet Anaesth Analg. 2008;35:1–12. doi: 10.1111/j.1467-2995.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 17.Pascoe PJ. The cardiovascular effects of dexmedetomidine given by continuous infusion during isoflurane anesthesia in dogs. Vet Anaesth Analg. 2005;32:9. [Google Scholar]
- 18.Grimm KA, Tranquilli WJ, Gross DR, et al. Cardiopulmonary effects of fentanyl in conscious dogs and dogs sedated with a continuous rate infusion of medetomidine. Am J Vet Res. 2005;66:1222–1226. doi: 10.2460/ajvr.2005.66.1222. [DOI] [PubMed] [Google Scholar]
- 19.Carter JE, Campbell NB, Posner LP, Swanson C. The hemodynamic effects of medetomidine continuous rate infusions in the dog. Vet Anaesth Analg. 2010;37:197–206. doi: 10.1111/j.1467-2995.2009.00522.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaartinen J, Pang D, Moreau M, et al. Hemodynamic effects of an intravenous infusion of medetomidine at six different dose regimens in isoflurane-anesthetized dogs. Vet Ther. 2010;11:E1–E16. [PubMed] [Google Scholar]
- 21.Haryadi DG, Orr JA, Kuck K, McJames S, Westenskow DR. Partial rebreathing indirect Fick technique for non-invasive measurement CO2 of cardiac output. J Clin Monit Comput. 2000;16:361–374. doi: 10.1023/a:1011403717822. [DOI] [PubMed] [Google Scholar]
- 22.Gunkel CI, Valverde A, Morey TE, Hernandez J, Robertson SA. Comparison of non-invasive cardiac output measurement by partial carbon dioxide rebreathing with the lithium dilution method in anesthetized dogs. J Vet Emerg Crit Care (San Antonio) 2004;14:187–195. [Google Scholar]
- 23.Ko JC, Fox SM, Mandsager RE. Effects of preemptive atropine administration on incidence of medetomidine-induced bradycardia in dogs. J Am Vet Med Assoc. 2001;218:52–58. doi: 10.2460/javma.2001.218.52. [DOI] [PubMed] [Google Scholar]
- 24.Ambrisko TD, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in beagle dogs. Can J Vet Res. 2002;66:42–49. [PMC free article] [PubMed] [Google Scholar]