Abstract
One of the four transmembrane receptors that belong to the erB family, is the HER2/neu oncoprotein. It forms heterodimers by binding to specific ligands, enhancing cell signaling and assisting in cell growth and differentiation. A variety of human epithelial tumors are characterised by an overexpression and gene amplification of the HER2/neu oncoprotein. This is the case of breast tumors, in which the receptor’s overexpression and its gene have been studied extensively and its overexpression has been associated with unfavorable prognosis.
In addition, HER2/neu plays a major role in understanding the oncogenesis of prostate adenocarcinoma. For this reason, clarifying the HER2/neu expression is particularly important in androgen independent prostate cancer (PCa), due to the increasing interest in using anti-HER2 targeted therapies for advanced disease treatment. On the other hand, the overexpression of HER2/neu has been reported to release soluble extracellular domain (ECD) in the serum of PCa patients. For this reason, the present review focuses only on studies referring to Serum HER2/neu levels in PCa patients. Serum levels of HER2/neu generally increase with advanced disease state and higher levels have been associated with recurrent or metastatic PCa and a clinically worse outcome. Therefore, it may be concluded that since there is a correlation between increased HER2/neu levels and a poor prognosis in prostate adenocarcinoma, serum HER2/neu could be used in clinical practice and follow up of patients with advanced PCa.
Keywords: HER2/neu oncoprotein, prostate cancer, serum, ECD, metastatic, advanced disease
Introduction
Prostate cancer (PCa) is the most common malignancy among men in most of Western countries1. The incidence of PCa varies among different countries with the highest rates reported in North America, Australia and northern and central Europe while the lowest rates are reported in south-eastern and south central Asia and northern Africa.
Usually, increased initial PSA levels present a worse cause-specific survival rate for PCa patients and many of them, with increasing PSA values, develop extra capsular disease or evidence of bone metastatic involvement2. For this reason, serial PSA measurements can assist in monitoring disease progression.
On the other hand, conventional pre-treatment modalities, such as bone scan, computed tomography (CT) and magnetic resonance imaging (MRI) often fail to detect early dissemination of microscopic metastatic disease which unfortunately, leads to biochemical recurrence of PCa3-4. For most patients with locally advanced or metastatic PCa, hormonal therapy remains the primary and most important treatment, resulting in PSA reduction and symptomatic improvement5. However, in almost all of these cases, PCa finally progress to hormone independence6-7. The mutations and androgen receptor’s overexpression are among the most important mechanisms involved in the growth of androgen-independent PCa8. In one third of advanced prostate tumors however, no discernible androgen-receptor mutation or amplification has been reported, therefore implying a potential role of non-androgenic growth factors9-10. On the other hand, the main factors affecting PCa disease outcome are the stage, Gleason score and PSA levels. But, PSA as an independent tumor marker for PCa, has limitations in its sensitivity and specificity11-13. For these reasons, the necessity of identifying improved prognostic biomarkers becomes obvious, in order to recognize disease progression and optimize therapeutic decisions.
The human epidermal growth factor receptor 2 (HER2/neu) is an oncoprotein, which belongs to the EGFR family and plays a major role in proliferation, cell growth and differentiation14. It consists of an extracellular ligand-binding domain, a transmembrane and an intracellular tyrosine kinase domain. Binding of specific ligands to the extracellular domain of HER2/neu forms hetero-dimers and that way initiates cell signaling, resulting in inhibition of apoptosis and activation of tumor cell growth and invasion. Over expression of HER2/neu protein and its gene amplification have been related with the progression of many types of tumors15. About 30% of breast and ovarian cancers overexpress HER2/neu, and anti-HER2/neu treatment has been shown to be very effective strategy when co-administered with other chemotherapeutic agents16-20. Especially, trastuzumab, a monoclonal antibody with high specificity for a HER2/neu epitope, in combination with chemotherapy, presents encouraging results and prolongs survival20.
Despite the receptor’s clinical importance for breast cancer, the role of HER2/neu in PCa is still controversial21-23. Evidence, however, suggests that it may contribute to androgen independence and in identifying patients more likely to present disease progression24-29. Nishio Y, et al considered that “HER2/neu overexpression, may be useful as a marker of an unfavorable prognosis by predicting the interval until relapse and the outcome in bone metastatic PCa patients after endocrine therapy’’30. Consistent with that, Morote J, et al agreed that “in cases with disease relapse HER2/neu overexpression was associated with poor prognosis’’ and their results suggest that HER2/neu causes cancer cells to proliferate more aggressively31.
In PCa, steroid hormones and growth factors play a regulatory role in cell proliferation and HER2/neu has been associated with activation of androgen-receptor pathways. So, it has been proposed as a survival factor for PCa cells during hormone-refractory disease progression32-34. Indeed, several studies reported that HER2/neu (as measured by immunohistochemistry) is associated with lower survival rates21,30,35. According to a large review, Neto AS, et al reported that in PCa patients with high HER2/neu overexpression, the recurrence rate and risk of death was increased (p<0,0001)36.
Therefore, HER-2 inhibition becomes a possible treatment strategy for hormone-refractory PCa patients37-39. Unfortunately, its efficacy has yet to be proven in clinical practice.
HER2/neu in serum of PCa patients
As previously mentioned, HER-2/neu consists of an intracellular, a transmembrane and an extracellular domain (ECD). HER-2 ECD can be detected in the serum of PCa patients. Increased ECD values usually correlate with a clinical outcome, suggesting a more aggressive tumor growth and metastasis40-41. Taking these facts into account, the current review was limited to studies referring to HER2 ECD levels in the serum of patients with advanced PCa, thus enhancing its prognostic implications.
As far as early prostate cancer stages are concerned, the correlation between PCa and BPH, is without statistical significance (p>0.05) and ECD values do not appear to be elevated42-43. More specifically, in a recent study with 227 untreated patients with localized PCa, Shariat SF, et al reported that “plasma HER2/neu levels were elevated in those patients with higher Gleason scores (p=0.028)’’44. In the same study, a preoperative multivariable analysis showed that HER2/neu values were associated with PSA levels and disease progression (p<0.001 and p=0.023 respectively). Consistent with the above in the review of Neto AS, et al, it was agreed that the percent of patients with Gleason score >7 was higher in the HER2/neu over expression group (p=0.01) and it varied with different antibodies used36. Moreover, a trend of increasing HER-2 ECD was observed in correlation with higher cancer stages.
Indeed, as Okegawa, et al described, HER-2 was uncommon in the serum of primary untreated PCa45. However, serum HER-2 ECD was significantly higher in PCa patients with bone metastatic disease compared with non-metastatic. Their data are in agreement with other published reports showing that serum HER-2 is indeed elevated in metastatic PCa (Table 1). Moreover, higher levels have been associated with hormone-refractory disease. It seems that overexpression of HER-2 not only activates cell proliferation, but stimulates androgen receptor signaling as well, and renders PCa cells refractory to androgen receptor inhibition 28-29, 46.
Table 1. Association between serum HER2/neu levels with other pathological features.
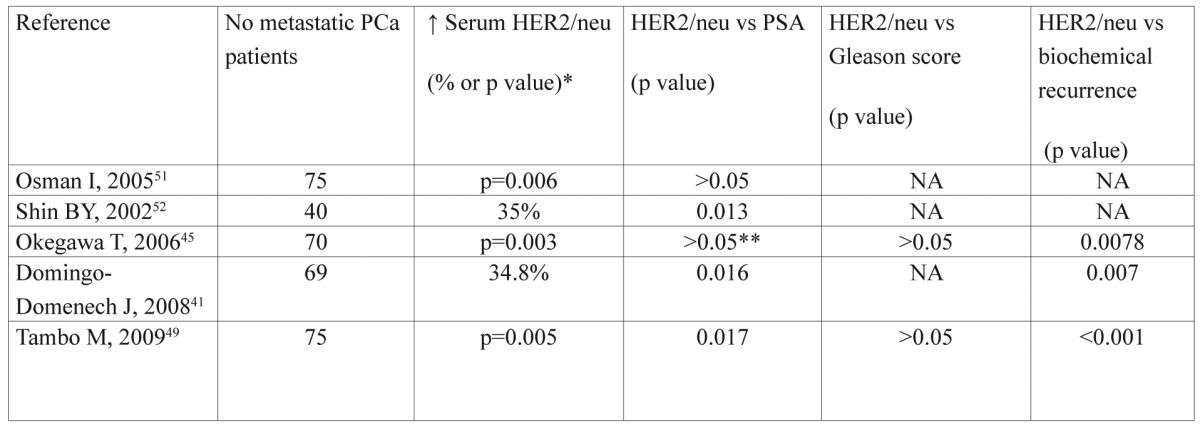
NA: not available, *: Correlation between PCa patients with vs without metastases, **: Only when PSA range ≤20ng/ml, there was no correlation
Correlations with ECD HER2/neu
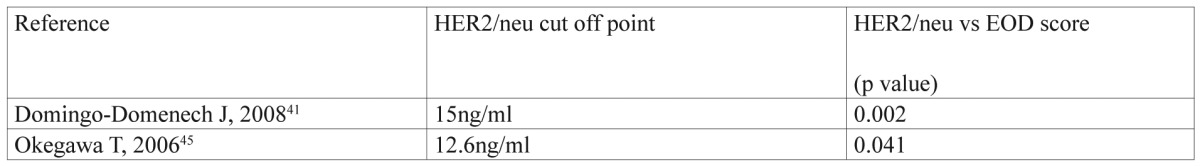
As shown in Table 1, patients with HER2 ECD values above the cut-off point in each study were at increased risk for recurrence and PSA progression. In addition, HER-2 ECD levels were significantly associated with the extent of bone disease (EOD), according to Soloway’s et al method47 (Table 2). In that case, it is possible that matrix metalloproteinases are involved in proteolytic cleavage of HER2 ECD into the blood stream, leading to tumor invasion and metastasis48. Arai Y, et al reported that “serum HER-2 levels were increased in patients with advanced disease and these patients had a significantly shorter interval to disease progression than those with a normal level’’46. So, HER-2 ECD might be a factor of unfavorable prognosis in advanced metastatic PCa patients.
Table 2. Correlation of serum HER2/neu with EOD score.
The survival rate in relation to HER2 ECD level has been reported only in two studies, and according to Neto AS, et al, on meta-analysis, there seems to be a significant correlation of elevated HER2 ECD values with earlier recurrence and risk of death (p<0.007), (Table 3).
Table 3. Increased risk of cause specific death in PCa patients with serum HER2/neu above the cut off values.

HER2/neu level evaluation included any method, or kit and overexpression in PCa has been associated with a worse clinical outcome. In most of the studies, HER2/neu expression was assessed using immunohistochemistry (IHC). IHC staining was classified into a 0-3+ scale. PCa patients with IHC score of 2+ or above were considered positive to have HER2/neu overexpression. Currently, HER2 ECD expression in PCa (measured by the method of ELISA) has been the focus of many researchers, but there is limited published data on the association between HER2/neu tissue and serum expression, with conflicting results (Table 4). HER2/neu amplification was not detected in any of them.
Table 4. Association between serum HER2/neu (ELISA) and its tissue expression (IHC, FISH) in PCa.
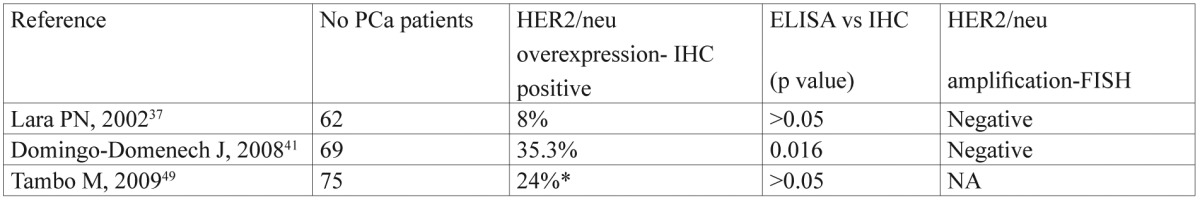
NA: not available, *: Score 1+ or above counted positive
According to Tambo M, et al, possible explanations for such discrepancies may be the variability in fixation and staining techniques and the fact that tissue samples collected by biopsy might not reflect the whole tumor status in IHC49. In patients with multiple metastases, the heterogeneity of HER2/neu expression may lead to false-negative results. On the other hand, PSA progression may occur as a result of local residual disease or occult nodal disease or present distant metastases during prostatectomy or even a combination of the above. Thus, assays which rely on tissue sampling are inadequate for assessing HER2/neu changes after tumor removal, while ELISA blood sampling techniques allow for real-time effective monitoring of post-treatment HER2/neu status.
In PCa, the prognostic value of HER2 ECD level may reflect the biological behaviour of hormone refractory tumors. So, these patients should be included into anti-HER2/neu clinical trials. But, anti-HER2/neu agents as monotherapy proved to be ineffective in the management of androgen-independant PCa patients, probably because of the interference of monoclonal antibodies with elevated soluble HER2 ECD, or because the introduction of the therapy was too late in the natural history of the disease36, 49. It therefore becomes obvious, that additional research is required in order to prove the effectiveness of chemotherapy, as combined with anti-HER2 agents for hormone refractory PCa50.
Conclusions
HER2/neu is mainly overexpressed in more aggressive disease and hormone-independent PCa. Increased HER2 ECD values in the serum correlate with the presence of metastatic disease and may indicate patients with increased risk of death. Therefore, detecting HER2 in serum, as opposed to tumor tissue sampling, is a minimally invasive alternative method of identifying disease progression in PCa patients. More extensive research studies and longer follow ups are required to optimize this assay for application in the clinical setting.
Conflict of Interest
Authors declare that there is no conflict of interest and no funding was received.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Yu KK, Hawkins RA. The prostate: diagnostic evaluation of metastatic disease. Radiol Clin North Am. 2000;38:139–157, ix. doi: 10.1016/s0033-8389(05)70153-6. [DOI] [PubMed] [Google Scholar]
- 3.Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–1184. doi: 10.1016/j.eururo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Sandler HM, Eisenberger MA. Assessing and treating patients with increasing prostate specific antigen following radical prostatectomy. J Urol. 2007;178:S20–S24. doi: 10.1016/j.juro.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Ross RW, Oh WK, Xie W, Pomerantz M, Nakabayashi M, Sartor O, et al. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2008;26:842–847. doi: 10.1200/JCO.2007.13.6804. [DOI] [PubMed] [Google Scholar]
- 6.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 7.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 8.Feldman BJ, Feldman D. The development of androgen independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 9.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 10.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Loeb S, Makarov DV, Schaeffer EM, Humphreys EB, Walsh PC. Prostate specific antigen at the initial diagnosis of metastasis to bone in patients after radical prostatectomy. J Urol. 2010;184:157–161. doi: 10.1016/j.juro.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Shariat SF, Karam JA, Roehrbom CG. Blood biomarkers for prostate cancer detection and prognosis. Future Oncol. 2007;3:449–461. doi: 10.2217/14796694.3.4.449. [DOI] [PubMed] [Google Scholar]
- 13.Bickers B, Aukim-Hastie C. New molecular biomarkers for the prognosis and management of prostate cancer--the post PSA era. Anticancer Res. 2009;29:3289–3298. [PubMed] [Google Scholar]
- 14.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signalling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 17.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 18.Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11:117–127. doi: 10.1007/BF00918679. [DOI] [PubMed] [Google Scholar]
- 19.Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of 20 human breast cancers. Oncogene. 1999;18:2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 20.Pegram MD, Slamon DJ. Combination therapy with trastuzumab (Herceptin) and cisplatin for chemoresistant metastatic breast cancer: evidence for receptor-enhanced chemosensitivity. Semin Oncol. 1999;26:89–95. [PubMed] [Google Scholar]
- 21.Carles J, Lloreta J, Salido M, Font A, Suarez M, Baena V, et al. Her-2/neu expression in prostate cancer: a dynamic process? Clin Cancer Res. 2004;10:4742–4745. doi: 10.1158/1078-0432.CCR-04-0115. [DOI] [PubMed] [Google Scholar]
- 22.Mass R, Sanders C, Kasian C, Johnson L, Everett T, Anderson S. The concordance between the clinical trials assay (CTA) and fluorescence in situ hybridization (FISH) in the Herceptin pivotal trials. Proc Am Soc Clin Oncol. 2000;19:75a. [Google Scholar]
- 23.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 24.Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, Autorino R, et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- 25.Zellweger T, Ninck C, Bloch M, Mirlacher M, Koivisto PA, Helin HJ, et al. Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer. 2005;113:619–628. doi: 10.1002/ijc.20615. [DOI] [PubMed] [Google Scholar]
- 26.Osman I, Scher HI, Drobnjak M, Verbel D, Morris M, Agus D, et al. HER-2/neu (p185neu) protein expression in the natural or treated history of prostate cancer. Clin Cancer Res. 2001;7:2643–2647. [PubMed] [Google Scholar]
- 27.Reese DM, Small EJ, Magrane G, Waldman FM, Chew K, Sudilovsky D. HER2 protein expression and gene amplification in androgen-independent prostate cancer. Am J Clin Pathol. 2001;116:234–239. doi: 10.1309/VXKK-YVRH-9B11-YDPT. [DOI] [PubMed] [Google Scholar]
- 28.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signalling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 29.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 30.Nishio Y, Yamada Y, Kokubo H, Nakamura K, Aoki S, Taki T, et al. Prognostic significance of immunohistochemical expression of the Her-2/neu oncoprotein in bone metastatic prostate cancer. Urology. 2006;68:110–115. doi: 10.1016/j.urology.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Morote J, de Torres I, Caceres C, Vallejo C, Schwartz S Jr, Reventos J. Prognostic value of immunohistochemical expression of the c-erbB-2 oncoprotein in metastatic prostate cancer. Int J Cancer. 1999;84:421–425. doi: 10.1002/(sici)1097-0215(19990820)84:4<421::aid-ijc16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Berger R, Lin DI, Nieto M, Sicinska E, Garraway LA, Adams H, et al. Androgen-dependent regulation of Her-2/neu in prostate cancer cells. Cancer Res. 2006;66:5723–5728. doi: 10.1158/0008-5472.CAN-05-3928. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Brands FH, Chatterjee S, Feng AC, Groshen S, Schewe J, et al. Her-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001;166:1514–1519. doi: 10.1016/s0022-5347(05)65822-3. [DOI] [PubMed] [Google Scholar]
- 34.Shi XB, Ma AH, Tepper CG, Xia L, Gregg JP, Gandour-Edwards R, et al. Molecular alterations associated with LNCaP cell progression to androgen independence. Prostate. 2004;60:257–271. doi: 10.1002/pros.20039. [DOI] [PubMed] [Google Scholar]
- 35.Hernes E, Fosså SD, Berner A, Otnes B, Nesland JM. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br J Cancer. 2004;90:449–454. doi: 10.1038/sj.bjc.6601536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neto AS, Tobias-Machado M, Wroclawski ML, Fonseca FL, Teixeira GK, Amarante RD, et al. Her-2/neu expression in prostate adenocarcinoma: a systematic review and meta-analysis. J Urol. 2010;184:842–850. doi: 10.1016/j.juro.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 37.Lara PN Jr, Chee KG, Longmate J, Ruel C, Meyers FJ, Gray CR, et al. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer. 2004;100:2125–2131. doi: 10.1002/cncr.20228. [DOI] [PubMed] [Google Scholar]
- 38.Ziada A, Barqawi A, Glode LM, Varella-Garcia M, Crighton F, Majeski S, et al. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate. 2004;60:332–337. doi: 10.1002/pros.20065. [DOI] [PubMed] [Google Scholar]
- 39.de Bono JS, Bellmunt J, Attard G, Droz JP, Miller K, Flechon A, et al. Open-label phase II study evaluating the efficacy and safety of two doses of pertuzumab in castrate chemotherapy-naive patients with hormone-refractory prostate cancer. J Clin Oncol. 2007;25:257–262. doi: 10.1200/JCO.2006.07.0888. [DOI] [PubMed] [Google Scholar]
- 40.Yuan CX, Lasut AL, Wynn R, Neff NT, Hollis GF, Ramaker ML, et al. Purification of Her-2 extracellular domain and identification of its cleavage site. Protein Expr Purif. 2003;29:217–222. doi: 10.1016/s1046-5928(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 41.Domingo-Domenech J, Fernandez PL, Filella X, Martinez-Fernandez A, Molina R, Fernandez E, et al. Serum HER2 extracellular domain predicts an aggressive clinical outcome and biological PSA response in hormone-independent prostate cancer patients treated with docetaxel. Ann Oncol. 2008;19:269–275. doi: 10.1093/annonc/mdm490. [DOI] [PubMed] [Google Scholar]
- 42.Myers RB, Srivastava S, Oelschlager DK, Grizzle WE. Expression of p160erbB-3 and p185erbB-2 in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. J Natl Cancer Inst. 1994;86:1140–1145. doi: 10.1093/jnci/86.15.1140. [DOI] [PubMed] [Google Scholar]
- 43.Croal BL, Mitchell IDC, Mutch WJ, Dickie A, Cohen N, Ross IS. Serum HER-2/neu extracellular domain levels in men presenting with suspected prostate cancer. UroOncology. 2002;2:99–102. [Google Scholar]
- 44.Shariat SF, Bensalah K, Karam JA, Roehrborn CG, Gallina A, Lotan Y, et al. Preoperative plasma HER2 and epidermal growth factor receptor for staging and prognostication in patients with clinically localized prostate cancer. Clin Cancer Res. 2007;13:5377–5384. doi: 10.1158/1078-0432.CCR-07-0330. [DOI] [PubMed] [Google Scholar]
- 45.Okegawa T, Kinjo M, Nutahara K, HIigashihara E. Pretreatment serum level of HER2/neu as a prognostic factor in metastatic prostate cancer patients about to undergo endocrine therapy. Int J Urol. 2006;13:1197–1201. doi: 10.1111/j.1442-2042.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- 46.Arai Y, Yoshiki T, Yoshida O. C-erbB-2 oncoprotein: a potential biomarker of advanced prostate cancer. Prostate. 1997;30:195–201. doi: 10.1002/(sici)1097-0045(19970215)30:3<195::aid-pros8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 47.Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 48.Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res., 9: 00-00, 2003. Clin Cancer Res. 2003;9:551–554. [PubMed] [Google Scholar]
- 49.Tambo M, Higashihara E, Terado Y, Nutahara K, Okegawa T. Comparison of serum HER2/neu with immunohistochemical HER2/neu expression for the prediction of biochemical progression in metastatic prostate cancer. Int J Urol. 2009;16:369–374. doi: 10.1111/j.1442-2042.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 50.Wu JT. C-erbB2 oncoprotein and its soluble ectodomain: a new potential tumor marker for prognosis early detection and monitoring patients undergoing Herceptin treatment. Clin Chim Acta. 2002;322:11–19. doi: 10.1016/s0009-8981(02)00134-1. [DOI] [PubMed] [Google Scholar]
- 51.Osman I, Mikhail M, Shuch B, Clute M, Cheli CD, Ghani F, et al. Serum levels of shed Her2/neu protein in men with prostate cancer correlate with disease progression. J Urol. 2005;174:2174–2177. doi: 10.1097/01.ju.0000181205.23233.65. [DOI] [PubMed] [Google Scholar]
- 52.Shin BY, Leitzel K, Ali SM, et al. Elevated serum HER-2/neu in metastatic prostate cancer. Proc Am Soc Clin Oncol. 2002;21:429. [Google Scholar]


