Abstract
Aim: To study possible ocular surface and lacrimal drainage changes in women being on adjuvant chemotherapy with 5-Fluorouracil 600 mg/m2, Epirubicin 60-90 mg/m2, Cyclophosphamide 600 mg/m2 (FEC) regimen for breast cancer.
Methods: Sixty one consecutive women with early stage breast cancer (median age 58 years - interquartile range 22) were included in this study. They all underwent mastectomy followed by 6 cycles of tri-weekly administration of FEC regimen and were free of ocular surface, eyelid and tear film symptomatic disease at baseline. None of them had pre- or coexisting treatment with other chemotherapeutic agent or radiotherapy. Slit lamp examination of the ocular surface, Schirmer test I (without topical anesthesia) and tears Break up Time test (BUT) were performed before the initiation of treatment and immediately after the third therapeutic cycle.
Results: From 61 women 39.34% had significant conjunctival hyperemia, 41.0% lid margin abnormalities, 4.92% blepharitis, 6.56% madarosis, 3.28% punctate epithelial keratopathy and 4.92% oedema of the lower punctum mucosal opening after three chemotherapeutic cycles. Mean BUT measures were found lower after the third chemotherapeutic cycle (p=0.001) but mean Schirmer test I values were higher after the third chemotherapeutic cycle (p=0.001).
Conclusion: Women on chemotherapy with FEC regimen are more susceptible to develop ocular surface and tear film alterations, within the first three cycles of chemotherapy for breast cancer, and thus, prompt ophthalmological evaluation may be proven beneficial for early diagnosis and management of the induced ocular disease.
Keywords: Breast cancer, chemotherapy, 5-fluorouracil, epirubicin, cyclophosphamide, ocular surface, tear film
Introduction
Breast cancer is the most common cancer in women1. Widespread screening tests and effective surgical procedures combined with chemotherapy, have led to significant reduction of mortality during the last two decades2.Moreover, the use of adjuvant systemic chemotherapy (monotherapy or polychemotherapy) in the management of breast cancer has led to major improvements in overall survival3,4.
New chemotherapy agents are being developed and new combinations are being tested. However, ocular toxicity is not uncommon and new regiments can also be associated with ocular side effects. The antitumor agents may cause ocular complications by interfering with normal cellular processes (i.e. DNA replication, division). They express their efficacy by activating DNA cross linking, strand breakage, interfering with DNA/RNA synthesis, competing with normal metabolites for the catalytic or regulatory site of a key enzyme, or substituting for a metabolite that is normally incorporated into DNA and RNA5.
Consequently, cytotoxic chemotherapy may lead to various ophthalmic complications and therefore it is important for the ophthalmologist to be aware of these possible complications in order to prevent, diagnose and treat them. There are reports referring to the effect of 5-Fluorouracil (5FU) on lacrimal gland fibrosis, canalicular edemaand on Meibomian gland Dysfunction (MGD)6-8. Non-infectious conjunctivitis is also related with administration of Doxorubicin9,10, which is an anthracycline and also with Epirubicin11.
The purpose of this study is to investigate possible early ocular surface and tear film abnormalities in women treated with adjuvant chemotherapy with the 5-Fluorouracil 600 mg/m2, Epirubicin 60-90 mg/m2, Cyclophosphamide 600 mg/m2 (FEC) regimen for breast cancer.
Materials and methods
This is a prospective, observational case series study of women who underwent mastectomy for early stage breast cancer and fulfilled the following criteria. After the operation, all of them were placed on adjuvant chemotherapy with the FEC regimen. This treatment consists of three anticancer drugs: 5FU, Epirubicin and Cyclophosphamide. The dose of the regimen was 600 mg/m2, 60-90 mg/m2 and 600 mg/m2, respectively. The regimen was administered intravenously every 21 days, for 6 therapeutic cycles. Past medical and ocular history were obtained before the initiation of the chemotherapy and all patients underwent a complete eye examination before the initiation of treatment and were rendered free of signs and symptoms of ocular surface tear film disease. None of the patients had prior history of ocular surgery, other ocular diseases or use of topical medications or contact lens wearing that might affect the ocular surface. Also, patients were free of previous radiotherapy.
Outcome measures were selected symptoms and signs of ocular surface disease. In order to detect and quantify the patients’ symptoms of the possible ocular surface alterations, we applied the Ocular Surface Disease Index (OSDI)12.
Patients were evaluated before the initiation of chemotherapy and immediately after the third chemotherapeutic cycle. The eyelids, eyelid margins, conjunctiva and puncti as well as the cornea and the whole ocular surface were carefully evaluated for possible changes. More emphasis was given on induced abnormalities of the eyelid margin including posterior blepharitis and signs of MGD including morphological lid margin changes.
Conjunctival hyperemia, as the main sign of ocular surface disease was evaluated with the Efron scale13,14. We considered the classes 3 to 4 as indications of significant hyperemia.
The inferior lacrimal puncti were evaluated for inflammation and edema of the surrounding conjunctival and also the mucosa of the orifice. Stenosis or obstruction of the punctum and canaliculus was assessed with the use of a fine Nettleship dilator and a double 0 Bowman probe. Free insertion of the dilator into the ampulla and subsequent unobstructed passage of the probe into the sac, followed by successful syringing, rendered the system patent. Resistance on the insertion of the dilator into the punctum was sign of stenosis. Similarly, obstruction of the probe towards the sac was sign of canalicular obstruction.
For purpose of statistical analysis only the right eyes of the patients were included. The rates of occurrence of each ocular surface and tear film abnormality detected in women under three cycles of chemotherapy with the FEC regimen were calculated by dividing the number of right eyes with each condition by the total number of patients (61) included in our study.
Tear film evaluation included Break Up Time test (BUT) and Schirmer Test I (with no anesthesia). BUT evaluates tear film stability. For the purpose of the test we instilled 2% fluorescein into the subject’s lower fornix and calculated the time to tear brake after three blinks with abnormal BUT values <10 sec. Immediately after this, we recorded possible fluorescein staining areas on the corneal and conjunctival epithelial surface using cobalt blue exciter filter and a complementary yellow barrier filter. Grading was calculated according to the Oxford staining scheme (range 0-15) 15. We also performed Schirmer test I (basic and reflex secretion). We examined both eyes simultaneously. As abnormal (impaired tear secretion) were considered Schirmer I test values <10 mm in 5 minutes.
All the above evaluations were performed before the initiation of chemotherapy and immediately after the third chemotherapeutic cycle.
Statistical analysis was performed using SPSS version 17.0 software (SPSS Inc., Chicago, IL,USA); p<0.05 was considered statistically significant. Using the Kolmogorov-Smirnov test, we rejected the zero hypothesis for 0.05 significance level that variables follow normal distribution. Consequently, non-parametric tests were used (Wilcoxon signed rank test, Chi-Square tests, Mann-Whitney test and Spearman’s Correlation Coefficient).
Results
Sixty-one women with early stage of breast cancer were recruited for this study. The median age was 58 and age interquartile range was 22 years. Before the initiation of chemotherapy, median value of OSDI was 0.00 and interquartile range was 2.10. After the 3rd chemotherapeutic cycle, median value of OSDI was 10.40 and interquartile range was 29.20. Also, 34/61women (55.74%) developed ocular surface disease. These patients had median OSDI value 33.30 and interquartile range 12.50 and they mainly complained of ocular discomfort, burning, itching and foreign body sensation. Furthermore, 24 women developed significant conjunctival hyperemia, which was a bilateral finding.
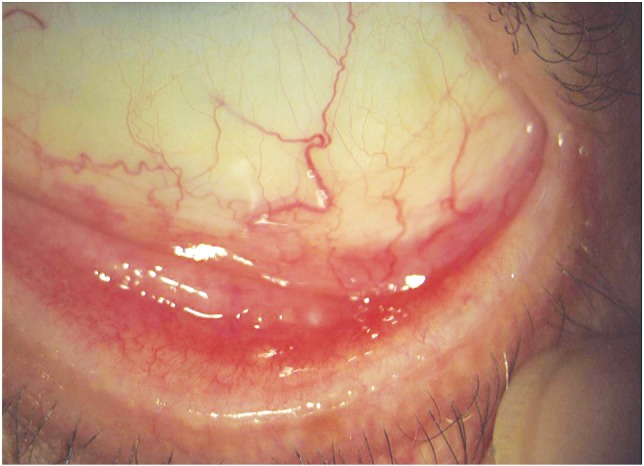
Abnormalities of the eyelid margin were identified in 25 women, consisting of posterior blepharitis (15 women) and/or occluded MG orifices (14 women), thickening of the MG (3 women), posterior dragging of the MG (4 women), scarring and keratinization of the eyelid margin (1 woman). All these findings were detected bilaterally. Fifteen women who developed abnormalities of the eyelid margin had also significant conjunctival hyperemia (Figure 1). Also, bilateral and partial madarosis occurred in four women (6.56%) after the third chemotherapeutic cycle.
Figure 1. Palpebral conjunctival hyperemia with obstruction of the Meibomian gland orifices.
Three women (4.92%) developed anterior blepharitis, which coexisted with hyperemia and abnormalities of the eyelid margin. These findings were bilateral and the patients complained for ocular discomfort, burning, itching and foreign body sensation.
Punctate epithelial keratopathy was observed in two patients (3.28%) and these findings were bilateral. In addition, these patients developed abnormalities of the eyelid margin and BUT values were particularly low after the third chemotherapeutic cycle. They complained mainly of foreign body sensation.In three patients (4.92%) oedema of the lower punctum mucosa was observed. Inflammation and consequent stenosis of the lower punctum were observed (Figure 2). Complete punctal occlusion was not observed. All complications are summarized in Table 1.
Figure 2. Severe abnormality of the eyelid margin. Palpebral hyperemia, obstruction of the Meibomian gland orifices and oedema of the lower punctum mucosa.
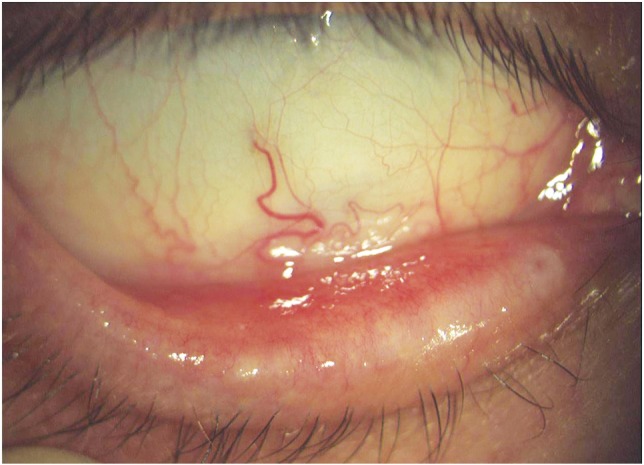
Table 1. Rates of ocular surface and lacrimal complications in women under three cycles of chemotherapy with the FEC regimen.
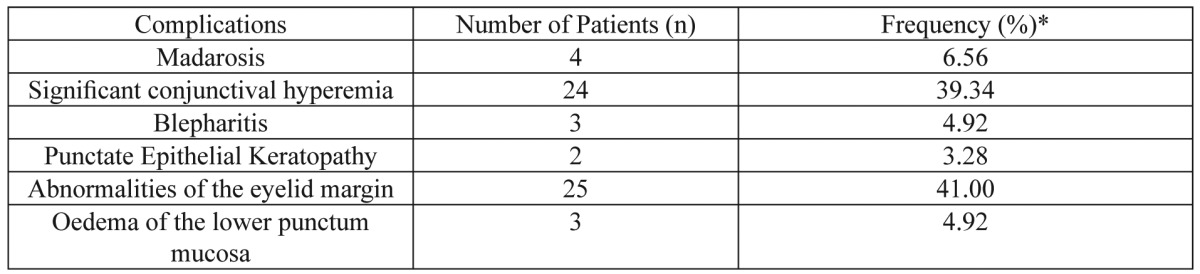
*The rates of ocular surface and lacrimal complications in women under three cycles of chemotherapy with the FEC regimen were calculated by dividing the number of right eyes with each abnormality by the total number of examined patients in our study (61 patients)
Regarding the tear tests, before the initiation of chemotherapy median value of BUT was 14.00 sec and interquartile range was 7.00 sec and after the 3rd chemotherapeutic cycle median BUT was 10.00 sec and interquartile range was 7.00 sec. Twenty three out of 61 patients had pathological BUT values (BUT<10 sec) after the third chemotherapeutic cycle. The median value of Schirmer test I was 15.00 mm and the interquartile range was 8.00 mm, before the initiation of the chemotherapy. After the third chemotherapeutic cycle, the median value for Schirmer test I was 18.00 mm and the interquartile range 10 mm.
Statistical Analysis
Mann Whitney test was conducted to assess the relationship between patients having ocular surface disease and the OSDI score after the third chemotherapeutic cycle (Table 2). It was found that women having developed ocular surface disease after the third chemotherapeutic cycle are more likely to develop symptoms of the ocular surface and this was statistically significant (p=0.001). Also, the same test was conducted to assess the relationship between ocular surface disease and BUT values after the third chemotherapeutic cycle (Table 2). It was found that women with ocular surface disease had statistically significantly lower BUT values after the third chemotherapeutic cycle (p=0.005). No statistically significant relation was found between ocular disease and Schirmer test I values after the third chemotherapeutic cycle (p=0.572) (Table 2).
Table 2. Mann Whitney U tests. Relationship between patients having ocular surface disease and OSDI, BUT and Schirmer values after the third chemotherapeutic cycle. Values presented are the median and the interquartile ranges (IQR).
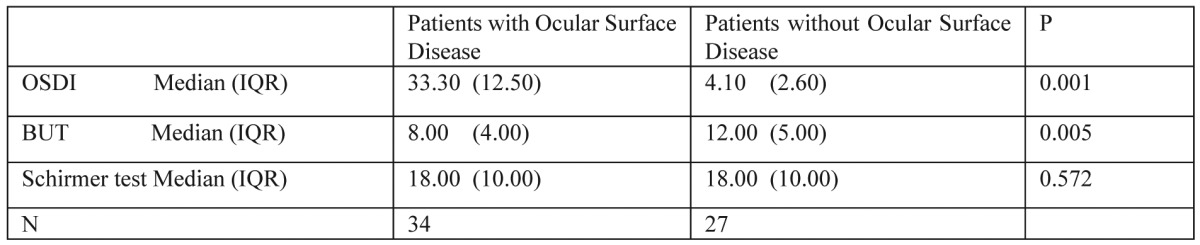
Patients with ocular surface disease have statistically significant different OSDI score and BUT values comparing to patients without ocular surface disease but patients with ocular surface disease have no statistically significant different Schirmer values comparing to patients without ocular surface disease
In order to assess the relationship between patients having significant conjunctival hyperemia and patients with abnormalities of the eyelid margin after the third chemotherapeutic cycle, Chi-Square tests were conducted (Table 3). It was found that women having developed significant conjunctival hyperemia are more likely to develop abnormalities of the eyelid margin (p=0.013). Also, statistically significant relation was found between age and abnormalities of the eyelid margin after conducting Mann-Whitney test (p=0.008) and it is more likely older women treated with the FEC regimen to develop abnormalities of the eyelid margin after the third chemotherapeutic cycle (Table 4). No statistically significant relation was found between age and significant conjunctival hyperemia (p=0.255) (Table 4).
Table 3. Relationship between patients having significant conjunctival hyperemia and patients with abnormalities of the eyelid margin after the third chemotherapeutic cycle was assessed by Chi-Square test.
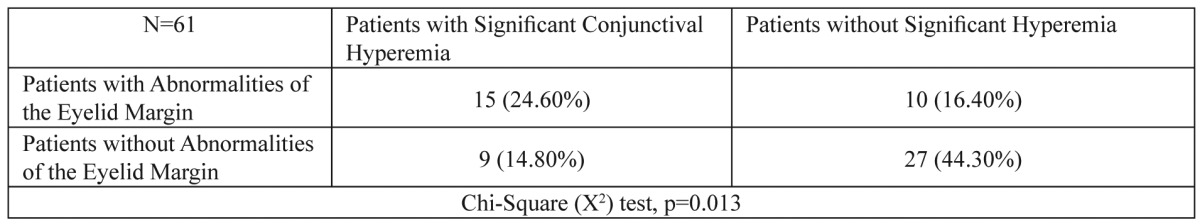
Statistically significant relation was found between patients having significant conjunctival hyperemia and patients who developed abnormalities of the eyelid margin after the third chemotherapeutic cycle
Table 4. Mann Whitney U tests. Relationship between age and patients having abnormalities of the eyelid margin and patients with significant conjunctival hyperaemia.
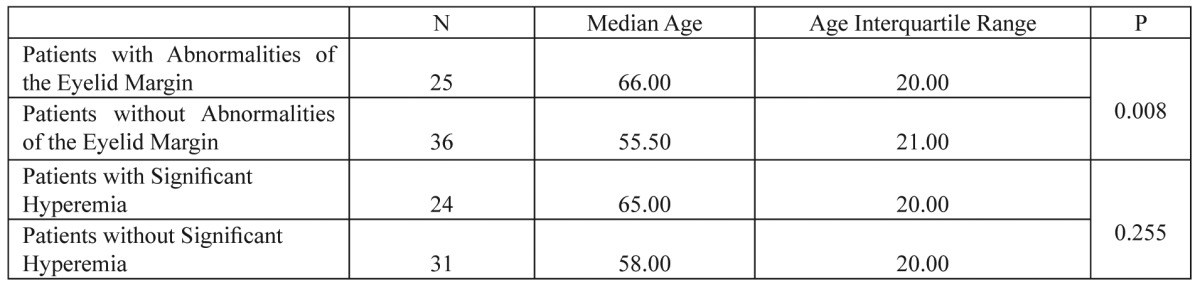
Patients with abnormalities of the eyelid margin have statistically significant different age compared to patients without abnormalities of the eyelid margin but patients with significant conjunctival hyperemia have no statistically significant different age compared to patients without significant conjunctival hyperemia
With regards to the tear tests, Wilcoxon signed rank test was conducted to assess the relationship between BUT values before the initiation of chemotherapy and after the 3rd chemotherapeutic cycle. BUT values after chemotherapy were lower than BUT values before chemotherapy and this difference was statistically significant (p=0.001). The same test was also conducted to assess the relationship between Schirmer test I values before the initiation of chemotherapy and after the 3rd chemotherapeutic cycle. Schirmer test I values after the third chemotherapeutic cycle were statistically significantly higher (p=0.001).
Also, Spearman Correlation Coefficient was conducted to assess the relationship between age and the continuous variables of BUT and Schirmer test I before the initiation of the chemotherapy and after the third chemotherapeutic cycle but no statistically significant correlation was found between age and any of the above continuous variables (Table 5).
Table 5. Spearman Correlation Coefficient provide the relationship between age and BUT (before and after the 3rd chemotherapeutic cycle) and between age and Schirmer test I (before and after the 3rd chemotherapeutic cycle).
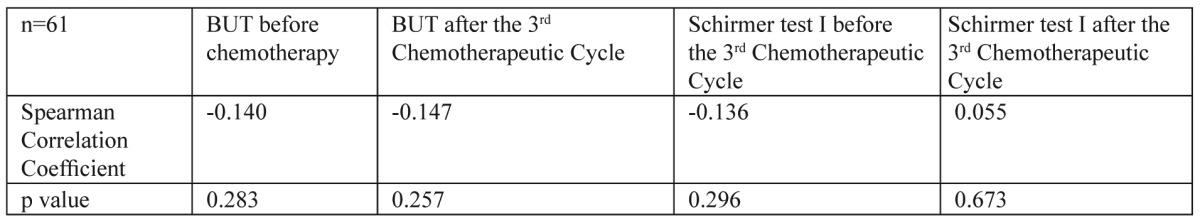
No statistically significant difference was found between age and any of the variables
Discussion
FEC is a chemotherapeutic regimen that is widely used as adjuvant therapy in non-metastatic breast cancer. The prevalence of survival rate is high16. 5FU is a pyramidine analogue and a potent inhibitor of thymidylate synthetase and so it can block DNA synthesis. Epirubicin is an anthracycline antibiotic that position itself between base parts, thereby uncoiling the DNA helix. Cyclophosphamide is an alkylating agent that substitutes hydrogen atoms in certain organic compounds by alkyl groups. Tissues with rapidly proliferating cellular agents are most affected by the FEC regimen and this fact explains the ability of the regimen to diminish neoplastic growth. Systemic side effects of the regimen are usually nausea, vomiting, diarrhea, mucositis, bone marrow suppression, dermatitis and alopecia17-19.
Researchers also investigated ocular surface complications induced by topical application of 5FU for glaucoma surgery. They observed that corneal wound healing rate was reduced after topical application of 5FU20,21. Others investigated the mitotic rate of conjunctiva and cornea in rabbits. They concluded that when 5FU is topically applied, the mitotic rate of both tissues was diminished22. Consequently, cell proliferation of conjunctiva, cornea and eyelid margin might be inhibited after the initiation of the chemotherapy with 5FU. Other researchers investigating ocular surface, ocular adnexal, and lacrimal complications associated with the use of systemic 5FU, observed that conjunctivitis, ocular irritation, blepharitis, keratitis, eyelid dermatitis, tearing and punctal-canalicular stenosis occur after the systemic use of 5FU in a rate of 3.8%, 5.8%, 3.8%, 3.8%, 5.8%, 26.9% and 5.8%, respectively23. In another study, researchers found out that conjunctivitis occurred in a rate of 25% in women receiving Cyclophosphamide, Methotrexate and 5FU19. Others found that 5FU is secreted in tears after intravenous administration and consequently ocular surface tissues bathe in 5FU6,24,25.
There are also a few reports that anthracyclines, mainly Doxorubicin, induce conjunctivitis in some patients10,11. Some researchers, by comparing the efficacy of the 5FU 500 mg/m2, Doxorubicin 50 mg/m2 and Cyclophosphamide 500 mg/m2 (FAC) regimen with the Cyclophosphamide 600 mg/m2, Methotrexate 60 mg/m2 and 5FU 500 mg/m2 (CMF) regimen given every 3 weeks for 6 cycles as adjuvant chemotherapy for operable breast cancer, found that conjunctivitis occurred at a rate of 8.5% and 12.0%, respectively26. In another comparative study, researchers evaluated the feasibility and the tolerability of two sequential regimens (Doxorubicin 75 mg/m2 - Docetaxel 100 mg/m2 and CMF) in which the sequence of drug administration was reversed. Conjunctivitis occurred at a rate of 25% and 57% respectively27.
In our series with the FEC regimen, significant conjunctival hyperemia occurred at a rate of 39.34%, blepharitis 4.92% and punctate epithelial keratopathy 3.28%. Also, Schirmer test I (basic and reflex tear secretion) values were statistically significantly higher after the third chemotherapeutic cycle. One explanation might be that irritation caused by 5FU secreted in tears and consequent ocular surface complications may cause an increase of tear production, though no statistically significant relation was found between symptoms of ocular surface disease, according to OSDI, and Schirmer test I.
Apart from the tear production, the tear drainage may also be affected by the 5FU. There are several case reports in the literature suggesting that systemic use of 5FU can cause chronic inflammation of the canaliculi and also in many cases fibrosis and stenosis7,28-30. Other researchers in a case report of a patient with canalicular obstruction after weekly administration of 5FU for colon cancer found out that severe squamous metaplasia and stenosis of the canaliculi’s lumen occurred31.
The BUT values in our study were statistically significantly lower after the third chemotherapeutic cycle. Other researchers reported a small number of patients (n=10) with ocular symptoms who were receiving merely oral treatment with S-1 regimen, an anti-cancer drug containing tegafur (5FU pro-drug), gimeracil and oteracil. On slit-lamp examination, they found pigmentary depositions around the MG orifices as well as complete occlusion in many of them. By using transillumination meibography, they noted dropout of more than half of the grandular structures and by in vivo confocal microscopy, they observed diminish of mean density of acinar units of MG in the patients’ group. Mean BUT value was lower (8.0±3.2 sec) and Schirmer test I (without topical anesthesia) was higher (15.9±8.9) compared to the control group (healthy individuals)8.
Conjunctival inflammation and mucin production was studied in an experimental model by researchers using the IκBζ gene disrupted mice. They found that as inflammatory symptoms of the conjunctiva progressed, inflammatory cells infiltrated the submucosa of the conjunctival epithelia, with a loss of goblet cells32. The decrease of mucin production can cause tear film instability and decrease BUT values33. In our material, 39.34% of the examined women had significant conjunctival hyperemia and the BUT values were statistically significantly lower after the third chemotherapeutic cycle.
As reported by other authors who investigated the Cyclophosphamide-related ocular complications in a series of 90 patients who received high doses of chemotherapy, dry eye syndrome was diagnosed in 40 patients. The diagnosis was established on the criteria of abnormal Schirmer test, BUT and rose Bengal staining test. Almost all 90 patients underwent total body irradiation and chemotherapy consisted of Cyclophosphamide alone or in combination with other agents34. However, total body irradiation may induce lacrimal gland damage35. It is not clear whether Cyclophosphamide alone has serious effect on Schirmer test I and BUT values.
Considering all 61 patients with early breast cancer in our study, 39.34% had conjunctival hyperemia, 41% abnormalities of the eyelid margin, three women (4.92%) developed bilateral blepharitis coexisting with conjunctival hyperemia and abnormalities of the eyelid margin, two women (3.28%) developed punctate epithelial keratopathy, three patients (4.92%) developed oedema of the lower punctum mucosa. BUT values were statistically significantly lower and Schirmer test I values were statistically significantly higher after the third chemotherapeutic cycle. No statistically significant relation was found between age, conjunctival hyperemia, BUT and Schirmer test I but statistically significant relation was found between age and abnormalities of the eyelid margin. Thus, older women under treatment with the FEC regimen were more likely to develop abnormalities of the eyelid margin after the third chemotherapeutic cycle.
In conclusion, breast cancer patients under treatment with the FEC regimen, may develop ocular surface and tear film abnormalities at an early therapeutic stage, mainly conjunctivitis and lid margin abnormalities with MGD. It is recommended that treated patients should have consultation thorough ocular surface evaluation, in order prevent, early diagnose and efficiently treat these established ocular complications of the chemotherapy treatment.
Financial support
No financial support was received for this submission.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6:391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 2.Benson JR, Jatoi I, Keisch M, Esteva FJ, Makris A, Jordan VC. Early Breast Cancer. Lancet. 2009;373:1463–1479. doi: 10.1016/S0140-6736(09)60316-0. [DOI] [PubMed] [Google Scholar]
- 3.Tamoxifen for early breast cancer: an overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 4.Polychemotherapy for early breast cancer: an overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 5.Schmid KE, Korneck GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51:19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Loprinzi CL, Love RR, Garrity JA, Ames MM. Cyclophosphamide, methotrexate and 5-fluorouracil (CMF)-induced ocular toxicity. Cancer Invest. 1990;8:459–465. doi: 10.3109/07357909009012068. [DOI] [PubMed] [Google Scholar]
- 7.Lee V, Bentley CR, Olver JM. Sclerosing canaliculitis after 5-fluorouracil breast cancer chemotherapy. Eye (Lond) 1998;12:343–349. doi: 10.1038/eye.1998.83. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Dogru M, Sato EA, Ibrahim OM, Tatematsu Y, Ogawa Y, et al. S-1 induces meibomian gland dysfunction. Ophthalmology. 2010;117: 1275:e4–7. doi: 10.1016/j.ophtha.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 9.Hazin R, Abuzetun JY, Daoud YJ, Abu-Khalaf MM. Ocular complications of cancer therapy: a primer for the ophthalmologist treating cancer patients. Curr Opin Ophthalmol. 2009;20:308–317. doi: 10.1097/ICU.0b013e32832c9007. [DOI] [PubMed] [Google Scholar]
- 10.Curran CF, Luce JK. Ocular adverse reactions with adriamycin (doxorubicin) Am J Ophthalmol. 1989;108:709–711. doi: 10.1016/0002-9394(89)90866-0. [DOI] [PubMed] [Google Scholar]
- 11.Wickremasinghe S, Dansingani KK, Tranos P, Liyanage S, Jones A, Davey C. Ocular presentations of breast cancer. Acta Ophthalmol Scand. 2007;85:133–142. doi: 10.1111/j.1600-0420.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 12.Ocular Surface Disease Index© (OSDI©) Allergan. 1995 http://www.dryeyezone.com/documents/osdi.pdf, last accessed 10/4/2012. [Google Scholar]
- 13.Shiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 14.Efron N. Grading scales for contact lens complications. Ophthalmic Physiol Opt. 1998;18:182–186. doi: 10.1016/s0275-5408(97)00066-5. [DOI] [PubMed] [Google Scholar]
- 15.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Coombes RC, Bliss JM, Wils J, Morvan F, Espié M, Amadori D, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node-positive operable breast cancer: results of a randomized trial. The International Collaborative Cancer Group. J Clin Oncol. 1996;14:35–45. doi: 10.1200/JCO.1996.14.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Vizel M, Oster MW. Ocular side effects of cancer chemotherapy. Cancer. 1982;49:1999–2002. doi: 10.1002/1097-0142(19820515)49:10<1999::aid-cncr2820491009>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Griffin JD, Garnick MB. Eye toxicity of cancer chemotherapy: a review of the literature. Cancer. 1981;48:1539–1549. doi: 10.1002/1097-0142(19811001)48:7<1539::aid-cncr2820480713>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 20.Fluorouracil Filtering Surgery Study one-year follow-up. The Fluorouracil Filtering Surgery Study Group. Am J Ophthalmol. 1989;108:625–635. doi: 10.1016/0002-9394(89)90853-2. [DOI] [PubMed] [Google Scholar]
- 21.Knapp A, Heuer DK, Stern GA, Driebe WT Jr. Serious corneal complications of glaucoma filtering surgery with postoperative 5-fluorouracil. Am J Ophthalmol. 1987;103:183–187. doi: 10.1016/s0002-9394(14)74224-2. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro MS, Thoft RA, Friend J, Parrish RK, Gressel MG. 5-Fluorouracil toxicity to the ocular surface epithelium. Invest Ophthalmol Vis Sci. 1985;26:580–583. [PubMed] [Google Scholar]
- 23.Eiseman AS, Flanagan JC, Brooks AB, Mitchell EP, Pemberton CH. Ocular surface, ocular adnexal, and lacrimal complications associated with the use of systemic 5-fluorouracil. Ophthalm Plast Reconstr Surg. 2003;19:216–224. doi: 10.1097/01.iop.0000066648.33513.3d. [DOI] [PubMed] [Google Scholar]
- 24.Christophidis N, Lucas I, Vajda FJ, Louis WJ. Lacrimation and 5-Fluorouracil. Ann Intern Med. 1978;89:574. doi: 10.7326/0003-4819-89-4-574_1. [DOI] [PubMed] [Google Scholar]
- 25.Christophidis N, Vajda FJ, Lucas I, Louis WJ. Ocular side effects with 5-fluorouracil. Aust N Z J Med. 1979;9:143–144. doi: 10.1111/j.1445-5994.1979.tb04317.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin M, Villar A, Sole-Calvo A, Gonzalez R, Massuti B, Lizon J, et al. Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol. 2003;14:833–842. doi: 10.1093/annonc/mdg260. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso F, Ferreira Filho AF, Crown J, Dolci S, Paesmans M, Riva A, et al. Doxorubicin followed by docetaxel vs docetaxel followed by doxorubicin in the adjuvant treatment of node positive breast cancer: results of a feasibility study. Anticancer Res. 2001;21:789–795. [PubMed] [Google Scholar]
- 28.Esmaeli B, Golio D, Lubecki L, Ajani J. Canalicular and nasolacrimal duct blockage: an ocular side effect associated with the antineoplastic drug S-1. Am J Ophthalmol. 2005;140:325–327. doi: 10.1016/j.ajo.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 29.Brink HM, Beex LV. Punctual and canalicular stenosis associated with systemic fluorouracil therapy. Report of five cases and review of the literature. Doc Ophthalmol. 1995;90:1–6. doi: 10.1007/BF01203288. [DOI] [PubMed] [Google Scholar]
- 30.Prasad S, Kamath GG, Phillips RP. Lacrimal canalicular stenosis associated with systemic 5-fluorouracil therapy. Acta Ophthalmol Scand. 2000;78:110–113. doi: 10.1034/j.1600-0420.2000.078001110.x. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal MR, Esmaeli B, Burnstine MA. Squamous metaplasia of the canaliculi associated with 5-fluorouracil: a clinicopathologic case report. Ophthalmology. 2002;109:2359–2361. doi: 10.1016/s0161-6420(02)01290-3. [DOI] [PubMed] [Google Scholar]
- 32.Ueta M, Hamuro J, Yamamoto M, Kaseda K, Akira S, Kinoshita S. Spontaneous ocular surface inflammation and goblet cell disappearance in I kappa B zeta gene-disrupted mice. Invest Ophthalmol Vis Sci. 2005;46:579–588. doi: 10.1167/iovs.04-1055. [DOI] [PubMed] [Google Scholar]
- 33.Lemp MA. The mucin-deficient dry eye. Int Ophthalmol Clin. 1973;13:185–189. doi: 10.1097/00004397-197301310-00013. [DOI] [PubMed] [Google Scholar]
- 34.Jack MK, Hicks JD. Ocular complications in high dose chemoradiotherapy and marrow transplantation. Ann Ophthalmol. 1981;13:709–711. [PubMed] [Google Scholar]
- 35.Thomas O, Mahé M, Campion L, Bourdin S, Milpied N, Brunet G, et al. Long-term complications of total body irradiation in adults. Int J Radiat Oncol Biol Phys. 2001;49:125–131. doi: 10.1016/s0360-3016(00)01373-0. [DOI] [PubMed] [Google Scholar]


