Abstract
Background: To analyze the pattern of clinical expression and the 5-year disease course in Caucasian patients with late onset of systemic lupus erythematosus (SLE) and to compare the findings with an early onset SLE group.
Methods: Medical records of 551 patients who presented with SLE at hospitals of the region of Thessaloniki between 1989 and 2007 were studied. Patients who developed SLE at or after the age of 50 years were classified as the late onset group, while younger patients served as the early onset group. Data on clinical manifestations and damage accrual at disease onset and at 5 years was obtained and compared between the two groups.
Results: In 121 patients, the disease started after the age of 50 years. Elderly patients showed less pronounced female predominance and less often presented with malar rash, nephropathy, fever and lymphadenopathy, while lung involvement, pericarditis and sicca syndrome were more frequent. Damage accrual was similar in both groups. The main causes of damage at 5 years differed, with the elderly exhibiting more cardiovascular damage. They also had a higher incidence of hypertension and osteoporosis at 5 years.
Conclusions: Caucasian SLE patients with late onset of the disease present with different clinical manifestations, suggesting that age affects the expression of SLE. Damage accrual at 5 years is similar in the elderly and the younger patients. However, the causes of this damage and the occurrence of other comorbidities follow a different pattern, possibly reflecting the disease process and the effects of aging.
Keywords: fish oil, omega 3 fatty acids, rats, aging, brain, oxidative stress
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease considered to affect predominantly women of reproductive age1. However, the occurrence of SLE in older subjects is well described and is being reported to occur in the range of 10-20% of the lupus populations studied2,3.
Several authors suggest that age at onset influences disease expression, so that patients with late onset SLE may constitute a separate subgroup, with distinct clinical features, disease course and outcome3-10. Studies have reported that these patients present commonly with an insidious onset and have less neurologic and renal involvement7-8. However, others were not able to detect differences in disease expression between early and late onset SLE11. On the other hand, regarding the disease course and its outcome, there are conflicting results, with some supporting that late onset lupus is milder with better prognosis7,9, whereas others concluding that is not a benign entity, with more damage accrual and higher rate of mortality4,6,12. The multiracial background of subjects being studied may constitute a reason for these contradictory results, since race is a known factor which affects disease expression13,14. Another limitation of some studies, possibly influencing the results, isthe small number of late onset lupus patients included.
The aim of this study is to analyze the prevalence of the main clinical manifestations at diagnosis of SLE, as well as the incidence of the main causes of morbidity in a 5-year period after the diagnosis, in a large sample of Caucasian lupus patients, with disease onset after the age of 50 years and to compare the results with a group of earlier onset SLE.
Methods
Patients
This is a retro - prospective epidemiological study, in which data was collected from the medical records of SLE patients being followed up, either as in- or outpatients, at the hospitals in the region of Thessaloniki, Greece. These hospitals are Hippokration, Papanikolaou, Papageorgiou, AHEPA and Agios Pavlos. A total of 551 lupus patients were identified from 1989 to 2007. All were Caucasian, fulfilling at least 4 of the 1997 ACR classification criteria for SLE15.
Variables
The variables included in this protocol are (1) gender of the patient, (2) age at the onset of the disease, defined as the age at which the patient presented the initial manifestations clearly attributable to SLE, (3) age at diagnosis, defined as the age at which the patient fulfilled 4 or more of the 1997 revised ACR criteria for the classification of SLE15, (4) clinical manifestations at diagnosis, (5) damage at the time of diagnosis and at 5 years, assessed retrospectively, using the Systemic Lupus International Collaborating Clinics/ACR (SLICC/ACR) Damage Index (SDI)16, (6) associated medical problems at 5 years, the diagnoses of which were based on clinical grounds and confirmed by appropriate complementary techniques.
Patients with diagnosis of SLE at the age of 50 or later were classified as the late onset lupus group, while those in whom the diagnosis took place before 50 years served as the early onset control group. This arbitrary age limit was based on previous literature1,4,5,7-9.
Statistical analysis
Descriptive data are expressed as mean +/- standard deviation or median (with range), according to the distribution or absolute values (with percentage). The incidence of comorbidities during the 5-year follow-up is reported as the cumulative incidence proportion.
To examine associations between late onset of the disease (independent variable) and the occurrence of symptoms (dependent variables) and to rule out possible confounding factors when another independent variable (gender) appeared to have statistical significance in the univariate analysis, a multivariate logistic regression is performed. Only those variables showing statistical significance in the multivariate analysis were considered as significant in the results of the study. All reported p values are two-tailed, and are considered significant when less than 0.05. The adjusted odds ratio (OR) were calculated for assessing the risk of appearance of each variable. A lower limit of 95% confidence interval (C.I.) >1.0 was taken to indicate statistical significance in the case of positive association and an upper limit <1.0 in the case of negative association. The statistical analysis was performed by means of the SPSS software (version 12.0, IBM SPSS Inc, Chicago, IL, USA).
Results
From the total of 551 SLE patients, included in this study, all being Caucasians, in 121 (20.4%) the diagnosis of the disease took place after the age of 50 (median 59.5 years, range 51-74). In the remaining 430 (79.6%), SLE was diagnosed before this age limit and served as the early onset control group (median 30 years, range 2-49). In the late onset group, there were 107 (88.4%) females and 14 (11.6%) males, giving a female: male ratio of 7.6:1, while in the early onset group this ratio was 10:1 (391 females and 39 males). Median age of onset of symptoms in the elderly was 57 years (range 50-73) and in the control group 29 years (range 6-48). The interval between disease onset and diagnosis was 2.5 years for the older patients, compared with 1 year of interval in the younger SLE population. This difference was not statistically significant.
Clinical manifestations at diagnosis in both groups are summarized in Table 1. Late onset lupus subjects showed a significantly decreased prevalence of fever, and rash, especially malar rash, lymphadenopathy and nephropathy. On the contrary, lung involvement, pericarditis and sicca syndrome tended to occur more commonly in these patients.
Table 1. Clinical manifestations at the diagnosis of SLE in late (n=121) and early (n=430) onset patients (logistic regression analysis).
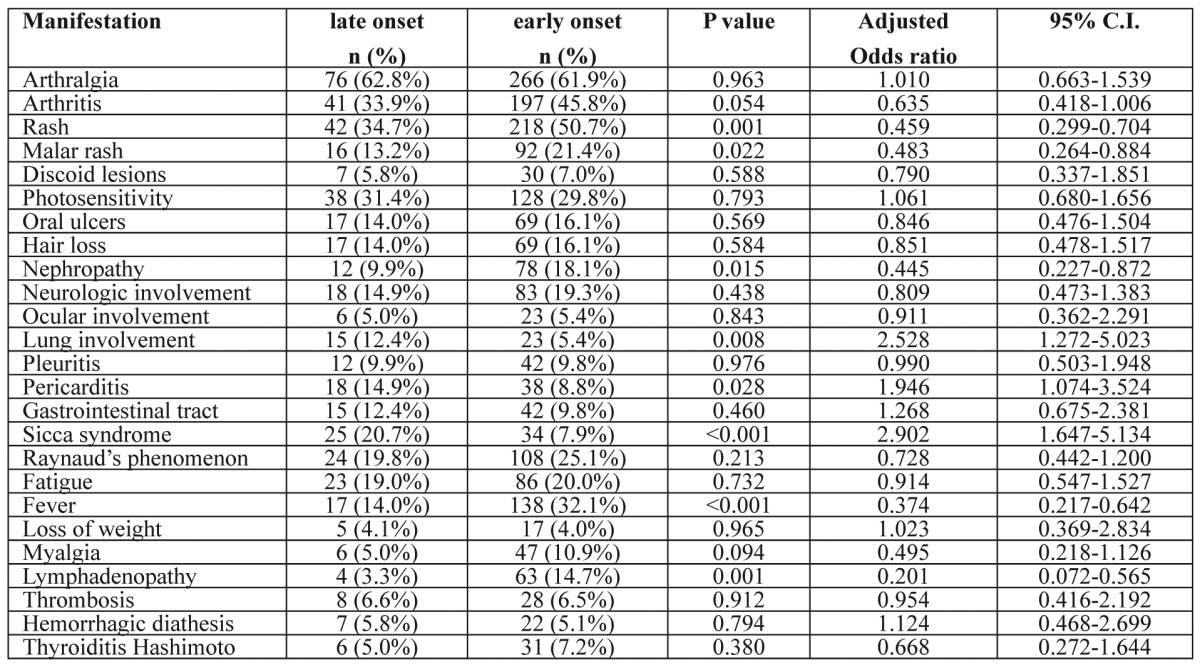
The independent variables were the age (late onset group) and the gender. The regression coefficients presented above are for the age independent variable
From the 121 patients with onset of SLE after the age of 50, 79 had completed a 5-year period of follow-up at the time of collection of the sample. In these patients, SDI at diagnosis was 0.65 (0.51-0.80) and at 5 years was 0.99 (0.74-1.25). In the control group, data of 5-year follow-up was available in 356 patients. SDI was 0.67 (0.59-0.74) and 1.04 (0.90-1.18) at diagnosis and at 5 years, respectively. Comparing the damage accrual between the two groups, no statistical difference was demonstrated either at the time of diagnosis (p=0.845) or after 5 years (p=0.859). Musculoskeletal, cardiovascular and neuropsychiatric damage were the leading causes of damage at 5 years in the elderly, whereas younger patients showed more frequently musculoskeletal, renal and neuropsychiatric damage.
Table 2 summarizes the main comorbidities that these patients developed during the 5-year period of follow-up. The late onset of lupus was associated with increased incidence of hypertension and osteoporosis.
Table 2. Other medical problems that occurred during the 5-year follow-up after the diagnosis in late (n=79) and early (n=356) onset patients (logistic regression analysis).
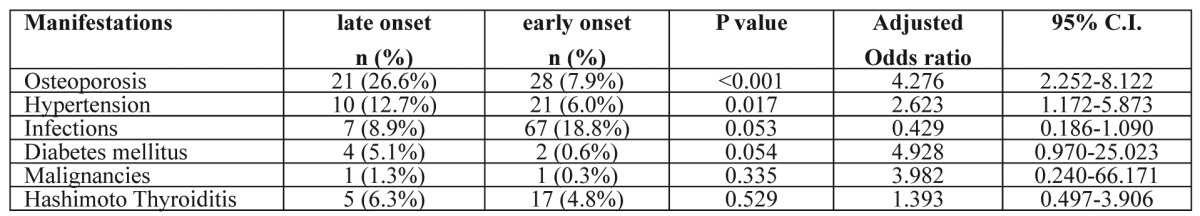
The independent variables were the age (late onset group) and the gender. The regression coefficients presented above are for the age independent variable
Discussion
Although SLE has been traditionally considered to be a disease of young women, several studies have described SLE in older patients2-12. In this study, 121 out of 551 lupus patients (20.4%) developed the disease after the age of 50. This figure is one of the highest reported2,3,12. A possible explanation for this finding is that the population of this study was composed solely of Caucasians. Existing literature supports that Caucasians tend to be older at diagnosis of SLE4. Our observations also suggest that the female predominance is not as pronounced in the group of late onset lupus (female: male ratio 7.6:1), which is in agreement with many7-9,12.
Most authors agree that age at onset of SLE influences the clinical expression of the disease, so that late onset lupus has a different profile, compared with younger patients3-10. In our sample, typical symptoms of SLE, like malar rash and renal involvement tended to occur less commonly in older than in younger patients and these differences reached statistical significance. The less frequent renal involvement in the elderly has being reported by the majority of authors1,4,7,8,12. The same stands for cutaneous involvement1,7,8,10,12. On the contrary, sicca syndrome, pericarditis and lung involvement were more common as initial manifestations, findings which are coherent to those reported by other studies5,7. The findings of the present study come to support the view that late onset SLE is a distinct subgroup, in which typical manifestations like rash or renal involvement are less prevalent. On the other hand, the more frequent presentation of these patients with sicca syndrome, pericarditis or lung involvement can make sometimes the diagnosis of SLE challenging. However, the clinical manifestations described in studies of SLE in the elderly vary and some even failed to detect any differences at the expression of the disease11.
Another observation is that the time between onset of symptoms and final diagnosis of SLE was longer in the elderly (2.5 years) compared with younger ones (1 year), although not statistically significant. Similar results have been reported by others5,7,11. This longer interval may be explained by the commonly atypical picture and the less frequent prevalence of SLE in the elderly, making physicians reluctant to make such a diagnosis. Thus, high clinical awareness is necessary in order to diagnose lupus timely in this group of patients.
Regarding the damage accrual, we found no difference in the SDI scores between the two groups, either at the time of diagnosis or after 5 years. This is contrast with other reports, which concluded that the occurrence of organ damage is greater in patients with late onset SLE than in younger ones6,10. However, in these studies patients included were not strictly Caucasians. While in younger and in older patients musculoskeletal and neuropsychiatric damage occurred with similar frequency, the late onset group suffered from more cardiovascular damage. This might reflect the effects of the disease process or the effects of age per se in the elderly, as also noted by other authors4,6.
Osteoporosis and hypertension were significantly increased in the elderly after 5 years of follow-up. Diabetes mellitus was also more frequent, but not statistically significant. Although these findings were expected, they underline the need for close and multifaceted monitoring of this special group of lupus patients.
The explanation for this age-related variability in disease expression is still unclear. Altered responsiveness of an aging immune system may be implicated. It has been speculated that older and younger patients may have different genetic determinants of disease and respond to different triggering mechanisms. Alternatively, the less exuberant expression of SLE both clinically and immunologically in older patients may reflect senescence of the immune system1.
Although the size of our sample is quite big, this study is not without limitations. First, it is representative only for the population of Thessaloniki, since the collection of the sample was made exclusively from this region. Furthermore, the results of the study represent mainly moderate or severe cases of SLE and there has been probably an underestimation of mild SLE. This results from the fact that the revised criteria of SLE, though they are generally accepted, are not very sensitive in diagnosis of mild SLE and at early stages of the disease. Finally, the validity of the study is also limited by the retro-prospective nature of it, as the collection of the data may not be most accurate.
In conclusion, this study in a Caucasian SLE population showed that patients with disease onset after the age of 50 present with different clinical manifestations, with classical lupus features such as rash or nephritis occurring less frequently, supporting the overall conclusion of previous investigators that age affects the expression of SLE. Damage accrual at 5 years is similar in the elderly and the younger patients. However, the causes of this damage and the occurrence of other comorbidities follow a different pattern, possibly reflecting the disease process and the effects of aging.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1993;72:113–124. [PubMed] [Google Scholar]
- 2.Lazaro D. Elderly-onset systemic lupus erythematosus: prevalence, clinical course and treatment. Drugs Aging. 2007;24:701–715. doi: 10.2165/00002512-200724090-00001. [DOI] [PubMed] [Google Scholar]
- 3.Rovenský J, Tuchynová A. Systemic lupus erythematosus in the elderly. Autoimmun Rev. 2008;7:235–239. doi: 10.1016/j.autrev.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Bertoli AM, Alarcón GS, Calvo-Alén J, Fernández M, Vilá LM, Reveille JD. LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort. XXXIII. Clinical [corrected] features, course, and outcome in patients with late-onset disease. Arthritis Rheum. 2006;54:1580–1587. doi: 10.1002/art.21765. [DOI] [PubMed] [Google Scholar]
- 5.Mak SK, Lam EK, Wong AK. Clinical profile of patients with late-onset SLE: not a benign subgroup. Lupus. 1998;7:23–28. doi: 10.1191/096120398678919723. [DOI] [PubMed] [Google Scholar]
- 6.Maddison P, Farewell V, Isenberg D, Aranow C, Bae SC, Barr S, et al. Systemic Lupus International Collaborating Clinics. The rate and pattern of organ damage in late onset systemic lupus erythematosus. J Rheumatol. 2002;29:913–917. [PubMed] [Google Scholar]
- 7.Ho CT, Mok CC, Lau CS, Wong RW. Late onset systemic lupus erythematosus in southern Chinese. Ann Rheum Dis. 1998;57:437–440. doi: 10.1136/ard.57.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boddaert J, Huong DL, Amoura Z, Wechsler B, Godeau P, Piette JC. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore) 2004;83:348–359. doi: 10.1097/01.md.0000147737.57861.7c. [DOI] [PubMed] [Google Scholar]
- 9.Formiga F, Moga I, Pac M, Mitjavila F, Rivera A, Pujol R. Mild presentation of systemic lupus erythematosus in elderly patients assessed by SLEDAI. SLE Disease Activity Index. Lupus. 1999;8:462–465. doi: 10.1177/096120339900800609. [DOI] [PubMed] [Google Scholar]
- 10.Appenzeller S, Pereira DA, Costallat LT. Greater accrual damage in late-onset systemic lupus erythematosus: a long-term follow-up study. Lupus. 2008;17:1023–1028. doi: 10.1177/0961203308089695. [DOI] [PubMed] [Google Scholar]
- 11.Padovan M, Govoni M, Castellino G, Rizzo N, Fotinidi M, Trotta F. Late onset systemic lupus erythematosus: no substantial differences using different cut-off ages. Rheumatol Int. 2007;27:735–741. doi: 10.1007/s00296-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 12.Lalani S, Pope J, de Leon F, Peschken C. Members of CaNIOS/1000 Faces of Lupus. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol. 2010;37:38–44. doi: 10.3899/jrheum.080957. [DOI] [PubMed] [Google Scholar]
- 13.Alarcón GS, Calvo-Alén J, McGwin G Jr, Uribe AG, Toloza SM, Roseman JM, et al. LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis. 2006;65:1168–1174. doi: 10.1136/ard.200X.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper GS, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Sociodemographic associations with early disease damage in patients with systemic lupus erythematosus. Arthritis Rheum. 2007;57:993–999. doi: 10.1002/art.22894. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]


