Abstract
Introduction:
One model for neurological assessment in chiropractic pertains to autonomic variability, tested commonly with heart rate variability (HRV). Since HRV may not be convenient to use on all patient visits, more user-friendly methods may help fill-in the gaps. Accordingly, this study tests the association between manual pulse rate and heart rate variability. The manual rates were also compared to the heart rate derived from HRV.
Methods:
Forty-eight chiropractic students were examined with heart rate variability (SDNN and mean heart rate) and two manual radial pulse rate measurements. Inclusion criteria consisted of participants being chiropractic students. Exclusion criteria for 46 of the participants consisted of a body mass index being greater than 30, age greater than 35, and history of: a) dizziness upon standing, b) treatment of psychiatric disorders, and c) diabetes. No exclusion criteria were applied to the remaining two participants who were also convenience sample volunteers. Linear associations between the manual pulse rate methods and the two heart rate variability measures (SDNN and mean heart) were tested with Pearson’s correlation and simple linear regression.
Results:
Moderate strength inverse (expected) correlations were observed between both manual pulse rate methods and SDNN (r = −0.640, 95% CI −0.781, −0.435; r = −0.632, 95% CI −0.776, −0.425). Strong direct (expected) relationships were observed between the manual pulse rate methods and heart rate derived from HRV technology (r = 0.934, 95% CI 0.885, 0.962; r = 0.941, 95% CI 0.897, 0.966).
Conclusion:
Manual pulse rates may be a useful option for assessing autonomic variability. Furthermore, this study showed a strong relationship between manual pulse rates and heart rate derived from HRV technology.
Keywords: heart rate, chiropractic, pulse rate, adjustment, manipulation
Abstract
Introduction :
Un des modèles d’évaluation neurologique en chiropratique est lié à la variabilité autonome, testée habituellement avec la variabilité de la fréquence cardiaque (VFC). Puisque l’usage de la VFC n’est pas toujours convenable à toutes les visites médicales, d’autres méthodes plus conviviales peuvent aider à combler les lacunes. Alors, cette étude examine la relation entre la prise de pouls manuelle et la variabilité de la fréquence cardiaque. Les rythmes manuels ont aussi été comparés au rythme cardiaque dérivé de la VFC.
Méthodologie :
Quarante-huit étudiants en chiropratique ont été examinés par la mesure de la variabilité de la fréquence cardiaque (SDNN et fréquence cardiaque moyenne) et par deux mesures manuelles du pouls radial. Les critères d’admissibilité étaient le fait d’être des étudiants en chiropratique. Un participant n’était pas admissible s’il avait un indice de masse corporelle supérieur à 30, s’il était âgé de plus de 35 ans, et s’il avait des antécédents : a) d’étourdissements en position debout, b) de traitement pour des troubles psychiatriques, et c) de diabète. Aucun critère d’inadmissibilité n’a été retenu contre les deux participants restants qui étaient aussi des bénévoles servant d’échantillon de commodité. Les rapports linéaires entre les méthodes de mesure de pouls manuelle et les deux mesures de variabilité de la fréquence cardiaque (SDNN et fréquence moyenne) ont été testés à l’aide d’une analyse de corrélation de Pearson et de régression linéaire simple.
Résultats :
Des corrélations inverses de niveau modéré (prévues) ont été observées entre les deux méthodes de mesure de pouls manuelle et la SDNN (r = −0,640, 95 % CI −0,781, −0,435; r = −0,632, 95 % CI −0,776, −0,425). Des relations directes de niveau élevé (prévues) ont été observées entre les méthodes de mesure de pouls manuelle et le rythme cardiaque dérivé de la technique de VFC (r = 0,934, 95 % CI 0,885, 0,962; r = 0,941, 95 % CI 0,897, 0,966).
Conclusion :
La prise de pouls manuelle peut se présenter comme une option pratique dans l’évaluation de la variabilité autonome. De plus, cette étude démontre une relation importante entre le pouls manuel et le rythme cardiaque dérivé de la technique de VFC.
Keywords: fréquence cardiaque, rythme cardiaque, chiropratique, pouls, ajustement, manipulation
Introduction
One approach in chiropractic care of patients pertains to the analysis and adjustment of vertebral subluxation, a condition with various theoretical underpinnings. Others may prefer to call the target of chiropractic intervention a “functional articular lesion,” where the purpose of the intervention is to “produce (a) beneficial neurologic effect.”1 In either case, a measurable neurological outcome of some type is presupposed. For purposes of this study, the “adjustable lesion” is referred to as vertebral subluxation since the author considers this to be a more familiar term within the profession. Briefly, vertebral subluxation is theorized to consist of some type of minor biomechanical aberrancy between two vertebrae, resulting in some type of (and yet still-to-be defined) neurological disturbance. The present study focuses on a potentially useful neurological predictor, if not also a useful outcome variable that may be related to putative subluxation.
One aspect of subluxation theory involves the potential effect of subluxation on the autonomic nervous system (ANS), the health of which can be assessed in terms of “autonomic variability” measures.2
R.W. Stephenson advanced the idea that subluxation interferes with the body’s ability to adapt.3 In current day terminology, neurological adaptability, particularly in regard to the ANS is described by the complexity model as it is known in medicine.4 In chiropractic, neuro-adaptabilty is typically analyzed with pattern analysis.5 Briefly, the concept is that variation in certain autonomic functions, such as heart rate, is considered to represent a healthy nervous system. A higher amount of heart rate variability is neurologically healthier than lower heart rate variability in terms of various cardiological and noncardiological diseases.2 There are exceptions to this concept. For example, higher variation in blood pressure has been correlated with atherosclerosis and diabetic nephropathy in patients with Type 2 Diabetes.6
Many chiropractors who focus on vertebral subluxation may wish to choose from a variety of options for assessing ANS adaptability/variability. The number of these options is currently limited. Thus, additional evidence-based options would seem helpful to increase feasibility in chiropractic practice for assessing ANS adaptability.
One way to test a potentially useful option for assessing autonomic variability is to compare it to a gold standard for autonomic variability such as heart rate variability (HRV). One of the main measures in HRV is the standard deviation of normal-to-normal beats (SDNN),7 having a unit in milliseconds (ms), representing the amount of variability of the heart rate. A higher SDNN value is considered healthier than a lower SDNN value.2 Another main measure in HRV is mean heart rate. Both of these measures (SDNN and mean heart rate) are considered as ANS markers.
Sessions for HRV testing are typically either 5 minutes or 24 hours. The shorter time frame is an approach commonly used in chiropractic research, in regard to: a) before and after care findings for HRV and pain,8 b) correlation with health perception,9 and c) correlation with area of the spine that was adjusted.10
One medical study that used the 5 minute approach for HRV found a moderate strength, statistically significant inverse correlation between SDNN and heart rate that was derived from a 10 second electrocardiogram (ECG) recording.11 While that study used a technology-based method to obtain the resting heart rate (10 second ECG recording), the authors commented on the practical appeal of employing manual methods of heart (pulse) rate for autonomic assessment in routine clinical practice.11 Other studies using heart rate variability have also shown the inverse relationship between resting heart rate and heart rate variability,12–15 again using technology-based methods for the derivation of the heart rate. The inverse relationship between SDNN and heart rate means that as heart variability increases (considered a neurologically healthy occurrence), pulse rate decreases (also considered a neurologically healthy occurrence).
Manual pulse rate as obtained with, say, radial artery palpation, is used for a variety of purposes, including the assessment of “autonomic nervous system tone.”16 A lower pulse rate is considered healthier than a higher pulse rate.17 One previous study compared the average of four 15 second pulse readings taken manually to HRV (SDNN) and found a moderate strength, statistically significant inverse (expected) correlation between SDNN and the manual pulse rates.18 The present study further tests this correlation with: a) a different sample of participants, and b) different methods for obtaining the manual pulse readings (two 15 second times instead of four).
The present study further builds on the aforementioned study18 by comparing: a) SDNN to the mean heart rate derived from the HRV session itself and b) mean heart rate derived in HRV to the manual pulse rates. The manual pulse rate has been shown to be strongly correlated with heart rate derived from technology.19–20
The manual pulse rate times used in the present study were 15 seconds. Although 30 seconds is a more common time frame for manual pulse measurement in a health care setting, the differences between pulse rates taken with 15-, 30-, or 60-second time frames have not been found to be statistically significant.21
The aim of the present study is to determine what, if any, relationship exists between manual pulse rate and HRV. In particular, pulse rate is compared to the HRV values of SDNN and heart rate (derived from the HRV recording).
Research hypotheses
An inverse relationship was expected between SDNN and pulse rate since lower heart rate is considered neurologically healthier than a higher pulse rate, and a higher SDNN value is considered neurologically healthier than a lower SDNN value. A direct relationship was expected in the secondary analysis comparing the different methods of heart rate measurement.
Methods
Sample characteristics
The study was approved by the Institutional Review Board at Sherman College of Chiropractic. The recruitment of participants at the College consisted of a combination of global emails to all students, along with invitations in the classrooms from the author. Most of the participants within the sample (n = 46) ended up being part of another study on subclinical orthostatic hypotension, the exclusion criteria for which consisted of: a) body mass index greater than 30, b) dizziness upon standing, c) past treatment of psychiatric disorders, d) history of diabetes, and e) age greater than 35 years. No formal exclusion criteria were applied to the two additional participants. All participants were chiropractic students who participated on strictly a voluntary basis.
Examination
The two examination procedures consisted of: 1) A 5 minute HRV exam using a Biopac Heart Rhythm Scanner (Version 1, Clinical Edition, Biocom Technologies, Poulsbo, WA); and 2) Two manually-palpated radial pulse measurements, each taken over a 15-second interval, 15 seconds apart. The readings were timed with a digital timer with the first pulse count beginning on the first target second number on the timer (i.e., starting with beat #1 on the zero second mark). The 15 second results were multiplied by 4 to obtain a beats per minute (BPM) measurement.
After a minimum of 5 minutes rest in the seated position, the two tests (HRV and manual pulse) were performed with the participant continuing to be seated. For pulse rate, the first pulse rate (Pulse 1), as well as the mean of Pulse 1 and the second pulse rate (“mean of Pulse1 and Pulse2”) were used in the analysis. From the HRV data, SDNN and mean heart rate (“mean heart rate in HRV”) were used.
Data analysis
Pearson’s r was used to test for a linear association between SDNN and each of the following heart rate methods:
Mean heart rate, derived from the 5 minute HRV session (the gold standard measurement of resting heart rate in the present study);
Pulse 1;
Mean of Pulse1 and Pulse2.
Patient characteristic were also measured. Spearman’s correlation coefficient was used to assess for nonlinear, but still monotonically trending, associations between body mass index (BMI) and age. An association between SDNN and sex was examined using a t-test for independent samples. BMI was calculated using the formula cited by the Centers for Disease Control and Prevention based on height, weight, and a conversion factor. 22 In addition, simple linear regression (rather than multiple linear regression, which showed problems with collinearity) was used to test the linear relationship between dependent variable heart rate derived from HRV and the two manual pulse rate methods and to examine the magnitude of the difference in HRV-derived heart rate for every one-unit change in manually assessed pulse rate. Since HRV and pulse rates typically are different for male and female,23 correlations were also performed by sex.
Analyses were performed in Stata IC 12.1 (StataCorp, College Station, TX). Confidence intervals for correlation coefficients were obtained, and comparisons of correlation coefficients between sexes were performed using an online calculator.24 Two tailed p-values less than or equal to the traditional alpha level 0.05 were considered statistically significant.
Results
Data were collected from a total of 48 chiropractic student volunteers (19 female, 29 male; 39.6% and 60.4% respectively), each of whom underwent both HRV and manual radial pulse rate assessments during a single visit. The mean age of the participants was 26.4 years (SD 4.3), with a mean BMI of 24.7 (SD 3.0; Table 1).
Table 1.
Summary statistics, including patient characteristics. BMI = body mass index. SDNN = standard deviation of normal-to-normal beats in HRV. Pulse 1 and mean Pulse1 Pulse2 are manual methods of pulse measurement.
| Variable | n | mean | SD | Min | Max |
|---|---|---|---|---|---|
| Age | 48 | 26.4 | 4.3 | 20.0 | 34.0 |
| BMI | 48 | 24.7 | 3.0 | 18.8 | 31.4 |
| SDNN (ms) | 48 | 62.2 | 31.8 | 11.9 | 155.0 |
| Mean HR in HRV | 48 | 71.5 | 12.3 | 50.2 | 106.2 |
| Pulse 1 | 48 | 71.9 | 12.6 | 48.0 | 112.0 |
| Mean Pulse1 Pulse2 | 48 | 71.7 | 12.2 | 50.0 | 112.0 |
Correlation of SDNN and patient characteristics
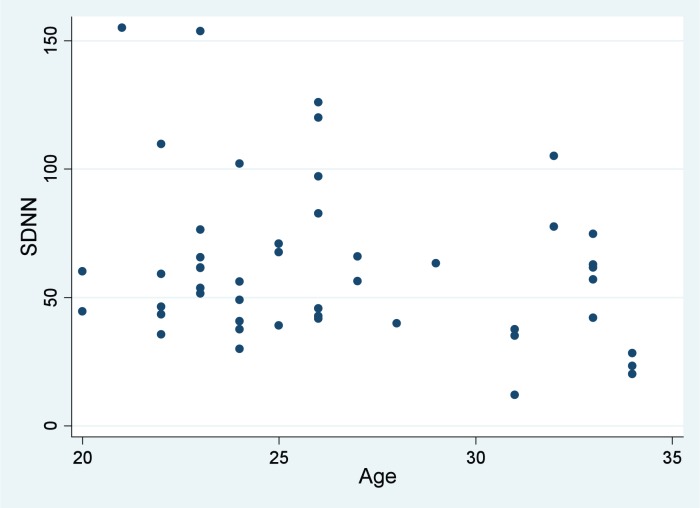
BMI and age exhibited nonlinear relationships with SDNN according to scatter plot inspection (Figures 1 and 2). Correlation coefficients are provided in Table 2. The correlations of age and BMI with SDNN were not statistically significant (p > 0.05; Table 2). Mean SDNN for females was 52.8 (95% CI 42.1, 63.4) compared to 68.4 (95% CI 54.8, 82.0) for males, a difference that was not statistically significant (p = 0.0678) but potentially clinically important given that the mean difference was 15.6.
Figure 1.
Scatter plot for SDNN and age.
Figure 2.
Scatter plot for SDNN and BMI.
Table 2.
Testing SDNN against three pulse predictors and three patient characteristic variables. Pearson correlation is used for continuous variables exhibiting a linear relationship in their scatter plots (#s 1–3 in list) while Spearman is used for correlations where nonlinear relationships were observed (variables 4–5). CI = confidence interval
| Variable | n | r | 95% CI for r | p |
|---|---|---|---|---|
| 1) Mean HR in HRV | 48 | −0.661 | −0.795, −0.465 | < 0.0001 |
| 2) Pulse 1 | 48 | −0.640 | −0.781, −0.435 | < 0.0001 |
| 3) Mean Pulse1Pulse2 | 48 | −0.632 | −0.776, −0.425 | < 0.0001 |
| 4) Age | 48 | −0.199 | −0.457, 0.090 | 0.1761 |
| 5) BMI | 48 | 0.103 | −0.186, 0.376 | 0.4845 |
Correlation of SDNN and pulse rate
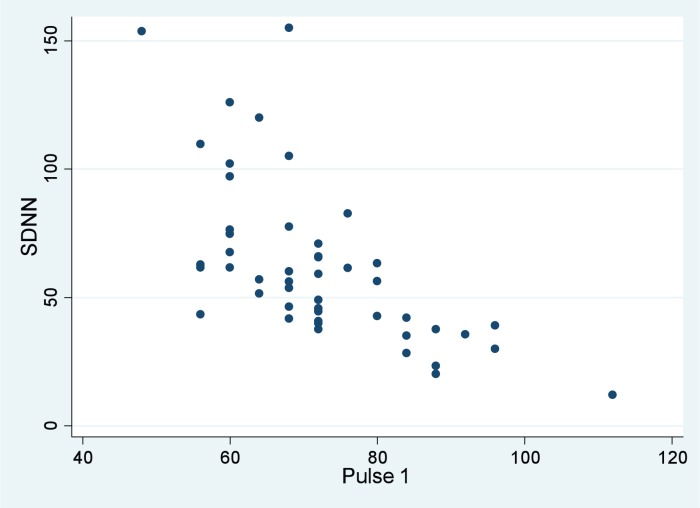
Mean SDNN was 62.2 milliseconds (SD 31.8; Table 1). The different pulse rate measurements showed essentially the same correlations with SDNN. These associations were statistically significant and reflected moderate-strength inverse (expected) relationships between SDNN and the following variables: Mean heart rate in HRV (r = −0.661, p < 0.0001); Pulse 1 (r = −0.640, p < 0.0001); Mean Pulse1 and Pulse2 (r = −0.632, p < 0.0001). The scatter plot in Figure 3 shows, graphically, the relationship between SDNN and Pulse 1.
Figure 3.
SDNN and Pulse 1. As manual pulse rate increases (horizontal axis), SDNN decreases (vertical axis), as expected.
Since the correlations of the manual methods were so similar, Pulse 1 was arbitrarily selected as the manual pulse method to be correlated with SDNN, stratified by sex. Here, correlation with SDNN revealed similar correlations: r = −0.676, p = 0.0015 for females; and r = −0.630, p = 0.0003 for males. The difference between these two correlation coefficients was not statistically significant (p = 0.8026).
Relationships between mean heart rate in HRV and manual pulse rate
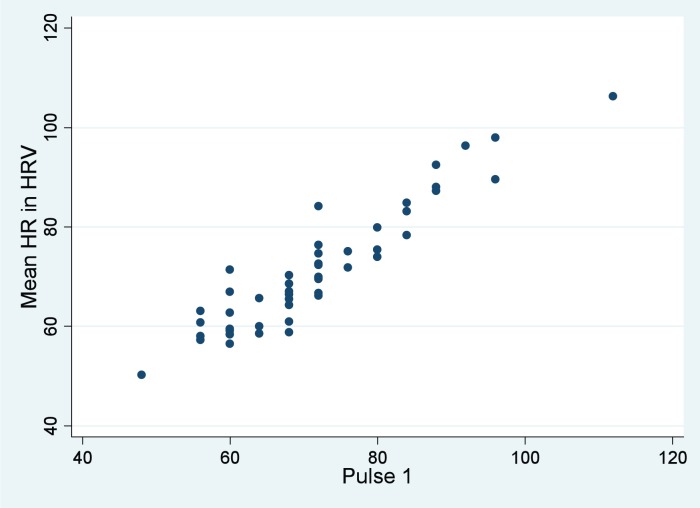
Mean heart rates for the three methods studied were as follows: a) mean heart rate in HRV: 71.5 BPM (SD 12.3); b) Pulse 1: 71.9 BPM (SD 12.6); and c) mean of Pulse1 and Pulse2: 71.7 BPM (SD 12.2). Very strong and direct correlations were observed between mean heart rate in HRV and both Pulse 1 (r = 0.934, p < 0.0001; Figure 4) and the mean of Pulse1 and Pulse2 (r = 0.941, p < 0.0001; Table 3). Since the correlation coefficients were similar for both manual methods, Pulse 1 was again used for correlations by sex with mean heart rate in HRV. Here, similar correlations were found between sexes: r = 0.950, p < 0.0001 for females; and r = 0.919, p < 0.0001 for males. The difference between these two correlations was not statistically significant (p = 0.4354).
Figure 4.
Mean heart rate (HR) in HRV versus Pulse1 manual pulse rate. As manual pulse increases (horizontal axis), so too does mean heart rate derived from technology in HRV vertical axis), as expected.
Table 3.
Testing mean heart rate in HRV against the two manual pulse methods using Pearson correlations (r, p for r) and linear regression (coefficient, p for regression coefficient). MP1P2 = mean pulse 1 pulse 2. P1 = Pulse 1. CI = confidence interval.
| Pearson | Linear regression | ||||||
|---|---|---|---|---|---|---|---|
| Covariable | n | r | 95% CI for Pearson r | p | Coefficient | 95% CI for regression coefficient | p |
| MP1P2 | 48 | 0.941 | 0.897, 0.966 | < 0.0001 | 0.95 | 0.86, 1.0 | < 0.001 |
| P1 | 48 | 0.934 | 0.885, 0.962 | < 0.0001 | 0.91 | 0.82, 1.0 | < 0.001 |
In linear regression analyses using mean heart rate in HRV as the dependent variable, the R-squared value was 0.877 for Pulse 1 and 0.887 for the mean of Pulse1 and Pulse 2. The regression coefficient was 0.91 for Pulse 1 (p < 0.005; 95% CI 0.8, 1.0) and 0.95 for the mean of Pulse1 and Pulse2 (p < 0.005; 95% CI 0.86, 1.05). This means that for every 1 BPM change in manual pulse rate, the mean heart rate in HRV would also expected to change in the same direction by approximately 1 BPM.
Discussion
In regard to SDNN, the heart rates (mean heart rate in HRV, Pulse 1, and mean Pulse1 and Pulse2) revealed the expected (inverse) relationships with SDNN. That is, a lower pulse (considered neurologically healthier than a higher pulse) is related to higher heart rate variability (considered neurologically healthier than lower HRV). Age, sex, and BMI did not have associations with SDNN that were statistically significant, although there was a nearly statistically significant difference in SDNN between males and females. This nearly significant finding is consistent with findings in another study that used a 24 hour monitoring protocol.23 There, a significant mean difference of 35 milliseconds SDNN was observed between males and females aged 10–29 years, and a smaller mean difference of 17 milliseconds SDNN was observed between males and females aged 30–49 years.23 In the present study, the analysis was not stratified by age group, however a difference of 15.6 milliseconds SDNN was observed between sexes. Interestingly, other research using the same HRV technology used in the present study did not find a statistically significant mean difference in SDNN between sexes.25 In any event, the present study did not show that sex had an effect on the strength or significance of the correlations between manual pulse rate with the HRV findings (SDNN and mean heart rate in HRV).
The present study revealed statistically significant correlations between manual and technology based pulse rate measurements, which may in turn be useful proxy measures of autonomic variability, and potential changes in autonomic variability after vertebral adjustment. Even aside from its correlation with heart variability, manually assessed pulse rate stands on its own as a marker for autonomic health in other studies. Correlations between the manual pulse rate methods and mean heart rate in HRV were very strong (and statistically significant) as expected.
One of the strengths of the current study is that the count method for the manual pulse reading began with “1” instead of “zero” on the zero second mark. In this regard, pulse rate measurement using the former method (starting with “1” count on the zero second mark) has been shown to be more strongly associated with heart rate derived from ECG.21
Admittedly, a formal sample size calculation was not conducted in advance of the study. However, a posteriorly, it was determined that in order to detect a statistically significant, moderate-strength correlation (e.g., absolute value of r between 0.400 and 0.700), a sample size of 24 would be needed.26 Consequently, for at least a moderate strength correlation, the sample size in the present study appeared to be adequate.
In linear regression, the average change in pulse rates was essentially a 1:1 ratio between mean heart rate in HRV and either of the manual pulse rate methods. However, mean Pulse1 Pulse2 showed a slightly stronger association with the presumed gold standard for heart rate in this study (i.e., heart rate derived from HRV), which suggests that the average of two pulse rate measurements may be the preferred method over any single determination in future studies.
Limitations to the study are that the participants comprised a convenience sample and were relatively healthy, making the generalizability of these findings to other patient populations limited. Additionally, p-values were not adjusted for multiple hypothesis tests. However even if multiple testing had been adjusted for, these findings would remain statistically significant due to the already-existing very low p-values in correlation and regression results.
Conclusion
In this study of relatively healthy chiropractic students, manual pulse rates showed: a) a moderate inverse correlation with the SDNN value in heart rate variability, and b) a strong direct correlation with heart rate derived from HRV technology. Manual pulse rate determinations may be a useful proxy measure for chiropractors and chiropractic researchers seeking to assess the global neurological effect of vertebral adjustment on putatively diagnosed vertebral subluxation. Additional research involving more representative patient populations are needed to verify the findings derived from the current study. Further studies to assess the association between manual pulse rate and both health status and clinically significant changes in health status following vertebral adjustment are also needed.
References
- 1.Winterstein J. Semantics. Outreach. National university of Health Sciences. 2003 Oct-Nov;:1, 3. [Google Scholar]
- 2.Lauer MS. Autonomic function and prognosis. Clev Clin J Med. 2009:S18–S22. doi: 10.3949/ccjm.76.s2.04. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson RW. The Chiropractic Text Book. Davenport, IA: Palmer School of Chiropractic; 1927. p. 203. [Google Scholar]
- 4.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;268(8):984. [PubMed] [Google Scholar]
- 5.Hart JF, Boone WR. Pattern analysis of paraspinal temperatures: a descriptive report. J Vert Sublux Res. 1999–2000;3(4):1–8. [Google Scholar]
- 6.Okada H, et al. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2011;220:155–159. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Szajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Dean D, Nosco D, Strathopulos D, Floros M. Effect of chiropractic care on heart rate variability and pain in a multisite clinical study. J Manip Physiol Ther. 2006;29:267–274. doi: 10.1016/j.jmpt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Hawk C, Zhang J. The relationship of heart rate variability to health status and health behavior. Top Integr Health Care. 2011;2(2) ID: 2.2004. [Google Scholar]
- 10.Welch A, Boone R. Sympathetic and parasympathetic responses to specific diversified adjustments to chiropractic vertebral subluxations of the cervical and thoracic spine. J Chiropr Med. 2008;7(3):86–93. doi: 10.1016/j.jcm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nussinovitch U, et al. The efficiency of 10-second resting heart rate for the evaluation of short-term heart rate variability indices. Pace. 2011;34:1498–1502. doi: 10.1111/j.1540-8159.2011.03178.x. [DOI] [PubMed] [Google Scholar]
- 12.Carney RM, Rich MW, teVelde A, Saini J, Clark K, Freedland KR. The relationship between heart rate, heart rate variability and depression in patients with coronary artery disease. J Psychosom Res. 1988;32(2):159–164. doi: 10.1016/0022-3999(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 13.Van Hoogenhuyze D, et al. Reproducibility and relation to mean heart rate of heart rate variability in normal subjects and in patients with congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1991;68(17):1668–1576. doi: 10.1016/0002-9149(91)90327-h. [DOI] [PubMed] [Google Scholar]
- 14.Ramaekers D, Ector H, Aubert AE, Rubens A, Van de Werf F. Heart rate variability and heart rate in healthy volunteers. Eur Heart J. 1998;19:1334–1341. doi: 10.1053/euhj.1998.1084. [DOI] [PubMed] [Google Scholar]
- 15.Soliman EZ, Elsala MA, Li Y. The relationship between high resting heart rate and ventricular arrhythmogenesis in patients referred to ambulatory 24 h electrocardiographic recording. Europace. 2010;12:261–265. doi: 10.1093/europace/eup344. [DOI] [PubMed] [Google Scholar]
- 16.Hsia J, et al. Resting heart rate as a low tech predictor of coronary events in women: prospective cohort study. BMJ. 2009;338:b219. doi: 10.1136/bmj.b219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verrier RL, Tan A. Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm. 2009;6(11 Suppl):S68–S75. doi: 10.1016/j.hrthm.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart J. Association between heart rate variability and novel pulse rate variability methods. Ann Vert Sublux Res. 2012 Jul 9;:65–71. [Google Scholar]
- 19.Erikssen J, Rodahl K. Resting heart rate in apparently healthy middle-aged men. Eur J Appl Physiol Occup Physiol. 1979;42(1):61–69. doi: 10.1007/BF00421105. [DOI] [PubMed] [Google Scholar]
- 20.Runcie CJ, Reeve W, Reidy J, Dougall JR. A comparison of measurements of blood pressure, heart rate and oxygenation during inter-hospital transport of the critically ill. Intensive Care Med. 1990;16(5):317–322. doi: 10.1007/BF01706357. [DOI] [PubMed] [Google Scholar]
- 21.Hwu YJ, Coates VE, Lin FY. A study of the effectiveness of different measuring times and counting methods of human radial pulse rates. J Clin Nurs. 2000;9:146–152. doi: 10.1046/j.1365-2702.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 22.Healthy weight – it’s not a diet, it’s a lifestyle. Centers for Disease Control and Prevention; [Cited 2013 Mar 28]. Available from: http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#Interpreted. [Google Scholar]
- 23.Umetani K, Singer DH, McCRaty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 24.Lowry R. VassarStats: Website for statistical computation. Vassar College; [Cited 2013 May 15–16]. Available from: http://vassarstats.net/ [Google Scholar]
- 25.Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manip Physiol Ther. 2007;30(5):374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Baldi B, Moore DS. The practice of statistics in the life sciences. New York: Freeman; 2009. p. 721. [Google Scholar]