Abstract
Variations in fat preference and intake across humans are poorly understood in part because of difficulties in studying this behavior. The objective of this study was to develop a simple procedure to assess fat discrimination, the ability to accurately perceive differences in the fat content of foods, and assess the associations between this phenotype and fat ingestive behaviors and adiposity. African-American adults (n=317) were tested for fat discrimination using 7 forced choice same/different tests with Italian salad dressings that ranged in fat-by-weight content from 5–55%. Performance on this procedure was determined by tallying the number of trials in which a participant correctly identified the pair of samples as “same” or “different” across all test pairs (ranging from 1–7). Individuals who received the lowest scores on this task (≤3 out of 7 correct) were classified as fat non-discriminators (n=33) and those who received the highest scores (7 out of 7 correct) were classified as fat discriminators (n=59). These 2 groups were compared for the primary outcome variables: reported food intake, preferences, and adiposity. After adjusting for BMI, sex, age, and dietary restraint and disinhibition, fat non-discriminators reported greater consumption of both added fats and reduced fat foods (p<0.05 for both). Fat non-discriminators also had greater abdominal adiposity compared to fat discriminators (p<0.05). Test-retest scores performed in a subset of participants (n=40) showed moderate reliability of the fat discrimination test (rho=0.53;p<0.01). If these results are replicated, fat discrimination may serve as clinical research tool to identify participants who are at risk for obesity and other chronic diseases due to increased fat intake.
Keywords: oral fat perception, fat intake, obesity
1. Introduction
Obesity continues to be a critical public health issue due to its increasing prevalence (1) and association with various forms of morbidity and mortality (2;3). Both genetic (4) and environmental (5) factors contribute to the development of obesity. One environmental factor that has repeatedly been shown to correlate strongly with obesity is a high- fat diet (5–7). Dietary fat has over twice the energy density of carbohydrates and proteins, but it tends to be less satiating (8). Furthermore, within the context of foods, fat is a highly palatable and reinforcing nutrient (9–13). Despite this, not all humans over-consume fat when it is available, and the considerable variation in human fat preferences and consumption patterns may have genetic underpinnings (14;15).
In comparison to basic tastes, like bitter and sweet, fat perception is more difficult to study because it is a complex stimulus imparting gustatory, textural and olfactory cues, and it is found in a wide range of physical forms (e.g. liquids, solids, semi-solids). A variety of approaches have been taken to better understand human fat perception, but there is presently no universally accepted approach. With respect to fat test stimuli, studies have used either sweet-fat combinations, such as dairy products (9;10;16–20) or cake frostings (21) or non-sweet added fats that can be easily manipulated, such as salad dressings (22;23). In addition, other studies have tested differences in the ability to perceive fat by using emulsions of free fatty acids (24–26). All of these methods have limitations. Although the use of sugar-fat mixtures may emulate real-life palatable foods such as cakes and ice cream, there may be difficulties in assessing fat content in these mixtures because sweetness tends to mask the ability to accurately perceive fat content (17;21). In addition, dairy samples require refrigeration, making them impractical for studies done outside the laboratory. In work by Tepper and Nurse (22;23) using salad dressings, only high (40% fat) and low (10% fat) samples were used, so interpreting participant responses is limited from a psychophysical perspective. While the testing of free fatty acids offers more experimental control over the stimuli, the ecological validity for predicting experiences with real foods is uncertain. Further, since FFAs are highly vulnerable to lipid oxidation, their use outside the laboratory would not be feasible. Developing a simple screening procedure to assess oral fat perception ability would advance the field.
The purpose of this study was to develop and pilot test a simple, screening procedure for assessing the ability to discriminate fat content of test stimuli. A second focus of this study was to determine if individuals with different fat discrimination abilities differ by reported fat intake, preference for high-fat foods, and weight status. The present report was part of a larger study conducted with African-Americans to determine the association between fat ingestive behaviors and variation in the CD36 gene, a fatty acid translocase and putative oral fat sensor (Keller et al., under review). This population is highly vulnerable to obesity (1;27) and its comorbidities (28), so testing these associations in this cohort may have additional clinical relevance. We hypothesized that decreased oral fat discrimination would be associated with increased fat preference, fat intake, and BMI. While previous studies have found evidence to support this hypothesis using sweetened dairy (18;19) or free fatty acids (26), other studies that have tested this notion using unflavored milks have been unsupportive (29;30).
2. Methods
2.1. Study Design
A cross-sectional design was used. Participants completed a 1-hour testing session during which fat discrimination was assessed, food preference and intake questionnaires were administered, anthropometrics were measured, and saliva samples were collected for DNA processing (data presented elsewhere). All testing was done in the Taste and Eating Laboratory at St. Luke’s Roosevelt Hospital, New York, NY.
2.2. Participants
Three hundred (n = 317) African-American men (n = 137) and women (n = 180), ages 18 to 65 (mean ± SD = 35.3 ± 11. 3), participated in the study. Participants were recruited by advertisements posted on popular internet websites and flyers posted in and around the study site. Eligible participants were healthy and not currently on a diet or taking medications that could affect taste, food intake, and/or body weight. In addition, individuals who had food allergies/restrictions, disliked Italian salad dressings, or smoked more than one pack of cigarettes per week were excluded. Participants who were enrolled in the study were instructed to fast for two hours prior to their scheduled visit, and light smokers were instructed to abstain from smoking the day of their participation in the study. These instructions were also confirmed in writing via letters mailed to participants.
2.3. Ethics
Research protocols were approved by the St. Luke’s Roosevelt Hospital Institutional Review Board. Participants gave written informed consent prior to participation and were offered modest compensation for time and travel. Research Authorization forms were used in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
2.4. Questionnaires
Participants completed food frequency and preference questionnaires that both contained the same 83 food items, selected because they have predominate fat orosensory and/or nutritional properties. The food frequency questionnaire was a semi-quantitative instrument developed specifically for use in this study in which participants reported intake of standard servings per day, week, or month. On the food preference questionnaire, participants reported liking for each food on a 170-mm horizontal visual analogue scale (VAS) anchored with “dislike extremely” and “like extremely”, with higher numerical ratings signifying greater reported liking.
For analysis, foods from these questionnaires were grouped into the following categories based on sensory and/or nutritional properties: fast foods; red meats (beefs, sausages, pork, etc.); white meats (chicken, fish, and seafood); low- and reduced-fat foods; added fats and spreads; sweet-fats; and nuts and nut butters. In addition, a composite group of high-fat foods, excluding nuts and nut butters, was also analyzed. Some of the foods were included in more than one category if they met the definitions for inclusion in both. For example, bacon was considered both a “red meat” and a “high-fat food”. For the food frequency questionnaire, reported data were converted to monthly servings for standardization across the categories. The foods listed within each category are listed in Table 1.
Table 1.
Foods Contained in Each Category for the Liking and Intake Questionnairesa
| Group | Foods |
|---|---|
|
| |
| Fast Foods | Burger King®, KFC®, McDonald’s®, Popeye’s®, Taco Bell®, Wendy’s® |
| Red Meats | bacon, ground beef, lean ground beef, steak, hot dogs, pork (fattier cuts), salami, sausage |
| White Meat & Eggs | chicken (baked & fried), eggs, fish (all kinds), seafood (all kinds) |
| Low-Fat and Reduced-fat Foods | baked chicken, lean ground beef, reduced-fat ice cream, skim milk, low-fat yogurt, fat-free and reduced fat salad dressings, baked potato chips, reduce fat cottage cheese, low-fat frozen yogurts, reduced-fat spreads |
| Added Fats & Spreads | butter, half-and-half, sour cream, canola oil, lard, mayo, margarine, olive oil, salad dressings, vegetable oils |
| Sweet-Fats | ice cream, cake, dark chocolate, milk chocolate, cookies, croissants, doughnuts, Danish pastry, muffins |
| Nuts and Nut Butters | almonds, cashews, coconut, macadamia nuts, peanuts and peanut butters, pecans, pistachios, sunflower seeds, walnuts |
|
| |
| High-fat Foods (Excluding nuts and nut butters)b | bacon, beef, steak, fried chicken, hot dogs, pork (fattier cuts), eggs, salami, sausage, canola oil, olive oil, margarine, mayo, butter, salad dressing (full fat), cheeses, lard, ice cream, whole milk, cake, chips, corn chips, cookies, croissants, Danish, doughnuts, French fries |
Not all 83 food items from the questionnaires are represented in the above groups.
High-fat Foods is a composite group of foods with high overall fat content (excluding nuts and nut butters)
In addition, the Three Factor Eating Questionnaire (TFEQ) was administered to participants to measure levels of dietary restraint and disinhibition (31). The restraint subscale measures individuals’ tendency to intentionally restrict food intake in order to control their weight. The disinhibition subscale measures individuals’ tendency to lose control over eating due to emotional states or social cues. These cognitive variables may impact food intake and body weight, so they were treated as covariates in the final analyses.
2.5. Anthropometrics
Subjects were weighed in light clothing and stocking feet by trained researchers. Height and weight were measured and recorded to the nearest 0.25 inches and 0.5 pounds using a stadiometer and balance beam scale. To calculate body mass index (BMI), height and weight were converted to meters and kilograms, respectively, and the formula BMI=kg/m2 was applied. Waist circumference was measured in the standing position immediately above the iliac crest and recorded to the nearest 0.25 inch.
2.6. Fat Discrimination Test
Italian salad dressings that varied in fat content were used to assess oral fat discrimination. Salad dressing was used because it is familiar to most individuals and is available in a variety of fat contents. In addition, this food can be served at room temperature and is easy to transport, so applying this test in large scale population studies is feasible.
2.7. Taste Test-Stimuli
Seven samples of Italian salad dressing ranging in fat-by-weight content from 5% to 55% were prepared with Good Seasons Italian Dressing Mix (Kraft Foods Global, Inc., Northfield, IL), Heinz apple cider vinegar (H.J. Heinz Co., Pittsburg, PA), Mazola canola oil (ACH Food Companies, Inc., Memphis, TN), and distilled water (see Table 1). Recipes were based on the work of Tepper and Nurse (22;23), but modified to include a broader range of fat levels. All dressings were mixed in a standard kitchen blender (Black and Decker, Model No. BL10450HB) on high power for 45 s per batch to ensure uniform consistency. For the dressings with 30% fat content or less, carrageenan (Viscarin SD 389, FMC Corp., Philadelphia, PA) was added as a thickener. The dressings were not noticeably different in viscosity, mouthfeel, and texture, which was confirmed by informal pilot testing with research staff.
2.8. Procedures for Assessing Fat Discrimination
Salad dressings were prepared on the day of the test session. Prior to the study, a randomized order of presentation for the 7 pairs of salad dressing was selected and this order was maintained for all participants. A standardized order was used because of the difficulty in counter-balancing 7 pairs across 350 participants (the targeted enrollment). For each trial, participants were presented with a pair of salad dressings that were either the same in fat concentration, or different. All dressings were assigned three-digit random numbers and served in 60 mL black soufflé cups. The first dressing in the pair was always the 55% fat content dressing. Participants were instructed to taste a small amount (approximately 10 mL) of the 55% fat content dressing, followed by an unsalted cracker and distilled water to cleanse the palate, and then taste the second dressing and mark whether they were the “same” or “different”. A 2-minute interval was provided between each pair of salad dressings to prevent sensory fatigue. All comparison tests were performed under red light to mask visual cues. Nose clips were not worn during this procedure.
2.9. Scoring of Fat Discrimination Test
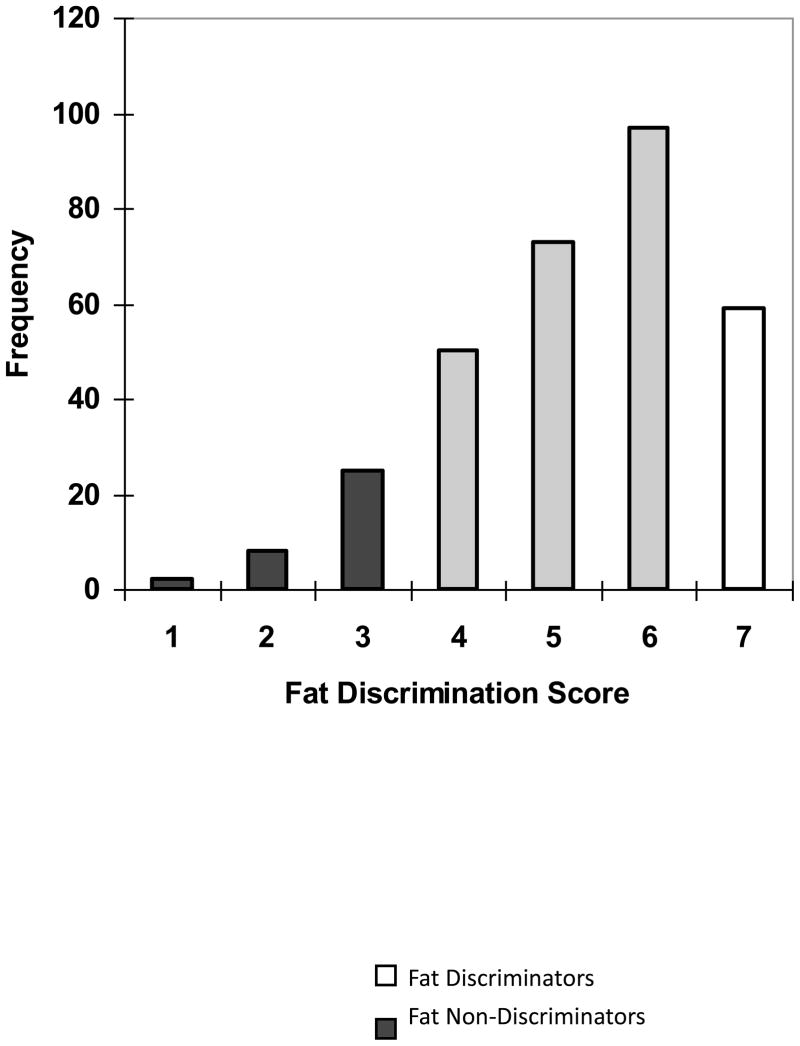
Participants received one point for each pair that they correctly identified as “same” or “different”. Scores ranged from 1–7, with higher scores depicting greater fat discrimination. For the entire cohort (n=317), mean (SD) fat discrimination scores were mean 5.2 (1.4) [Figure 1]. For primary analyses, individuals who received 7 out of 7 on this test were classified as fat discriminators (n=59) and those who received scores of 3 or less were classified as fat non-discriminators (n=33). An additional 3 participants scored only 1 out of 7 correct, and would have been included as non-discriminators, but they were dropped from the final analysis because examination of the other responses on the test suggested that they either did not taste the samples or did not understand the procedures. For example, before the discrimination test, we asked participants to rate perceived creaminess, oiliness, and fat content of the lowest (5%), medium (35%) and highest (55%) fat samples (Keller et al., under review). The participants that were ultimately dropped from the analysis gave identical ratings to each salad dressing, or in one case, gave no responses, regardless of fat content, while for other subjects, responses varied. Reported fat intake, preference, and adiposity were compared between these groups. Group cut-offs were defined a priori and were based on a power analysis done for the genetics portion of the study. Based on the allele frequencies of the 5 polymorphisms studied and assuming a co-dominant relationship between fat discrimination and CD36 genotype, we needed approximately 35 participants per fat discrimination group to detect differences at an alpha of 0.05 and 80% power. Total participant enrollment was capped at 350 due constraints of the funding mechanism, so 35 participants corresponded to the top and bottom 10% of fat discrimination scores, or based on the distribution of scores (Figure 1), 7 out of 7 correct and ≤3 out of 7 correct.
Figure 1.
Frequency distribution of fat discrimination scores for all participants (n=317). Individuals who scored ≥ 3 out of 7 were classified as fat non-discriminators (black bars). Individuals who scored 7 out of 7 were classified as fat discriminators (white bars). Individuals who scored between 3–7 (gray bars) were not used in main study analyses.
2.10. Test-Retest Reliability of Fat Discrimination
Reliability of fat discrimination was tested in a sub-set (n=40) of the cohort who were randomly selected and were available to come back within one week of the original test. Fat discrimination status was classified at the first and second visit as either “fat discriminator”, “fat non-discrimator” or “neither” (falling in the gray area on Figure 1). Fat discrimination status was compared at the two visits by Spearman’s correlation coefficients (rho=0.53; p<0.01).
2.11. Statistical Analyses
Data were analyzed using SPSS for Windows (Version 16.0; SPSS Inc, Chicago, IL). All statistical tests were computed at a critical value of p ≤ 0.05 and all hypotheses were two-tailed. Data are displayed as mean (SD), unless otherwise labeled.
Descriptive statistics (means, SDs, SEs) were generated to characterize both the cohort as a whole, and participants classified as fat discriminators and non-discriminators. Independent t-tests were used to compare values for age, waist circumference, BMI, and cognitive dieting variables between discriminators and non-discriminators. Pearson’s Chi-Square tests were used to compare the frequency breakdown of dichotomous variables between fat discrimination groups. The primary objectives of the study were tested with General Linear Model (GLM) Analysis of Covariance (ANCOVA), where the independent variable was fat discrimination group and the dependent variables were mean reported intake from each of the food groups, mean reported preferences from each of the food groups, waist circumference, and BMI. Covariates included in these models were age, sex, dietary restraint and disinhibition, and BMI (included only when the dependent variable was food intake or preference).
3. Results
3.1. Participant Characteristics
For the entire cohort (n=317), 43.2% (n = 137) were male and 56.8% (n = 180) were female. Mean (SD) BMI was 29.2 (6.9) kg/m2 and age was 35.3 (11.3) years. Mean (SD) waist circumference was 94.5 (16.3) cm and differed by sex (t=2.4; p<0.05), with mean waist circumferences of 97.0 (16.6) and 92.5 (16.5) cm for males and females, respectively. Approximately 70% of the participants were either overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥ 30.0 kg/m2). For the remainder of the results, only individuals classified as fat non-discriminators (n=33) and fat discriminators (n=59) will be described.
Descriptive characteristics of the two fat discriminator groups are displayed in Table 2. The percentage breakdown of males and females in each group did not differ, and discriminators and non-discriminators were the same for age, BMI, and dietary restraint and disinhibition. When disinhibition was explored separately by sex, females discriminators reported higher disinhibition than male discriminators (t=−2.2; p<0.05).
Table 2.
Characteristics of Fat Discriminators and Fat Non-discriminators
| Fat Non-Discriminators (n = 33) | Fat Discriminators (n = 59) | |
|---|---|---|
| Sex | n (%) | n (%) |
| Male | 16 (48.5 %) | 24 (40.7 %) |
| Female | 17 (51.5 %) | 35 (59.3 %) |
|
| ||
| Mean ± SD | Mean ± SD | |
| Age (y) | 37.3 ± 11.1 | 36.7 ± 12.8 |
| BMI (kg/m2) | 29.9 ± 7.0 | 29.8 ± 7.1 |
| Dietary restraint (All) | 7.3 ± 4.2 | 8.5 ± 4.3 |
| Male | 6.9 ± 4.0 | 7.0 ± 3.4 |
| Female | 7.6 ± 4.6 | 9.6 ± 4.6 |
| Dietary disinhibition (All) | 5.8 ± 3.8 | 5.2 ± 3.5 |
| Male | 5.1 ± 3.9 | 4.0 ± 2.9a |
| Female | 6.5 ± 3.7 | 6.0 ± 3.7b |
Significantly different from one another at p< 0.05.
3.2. Fat Discrimination Status and Reported Food Intake
Reported intake of monthly servings from each food group as a function of fat discrimination status is displayed in Table 3. In all food groups, monthly intake values reported for non-discriminators were higher than for discriminators, even after adjusting for covariates that may impact these relationships (age, sex, BMI, dietary restraint and disinhibition). However, only differences in reduced fat foods (F(1,91) = 5.2; p<0.05) and added fats (F(1,91) = 4.4; p<0.05) were significant. Non-discriminators reported a greater number of monthly servings of these foods than discriminators, with reported amounts of over 40 additional servings per month from both added fats and reduced fat foods. There was trend for non-discriminators to also report greater intake from all high-fat foods combined (F(1,91) = 2.7; p = 0.10). For reported intake of red meats (p=0.26), white meats (p=0.50), sweet-fats (p=0.14), and nuts and nut butters (p=0.25), non-discriminators and discriminators were not different.
Table 3.
Reported Monthly Intake (in standard servings) from Food Groups in Fat Non-Discriminators and Discriminators*
| Monthly Servings | Fat Non-Discriminators (n = 33) | Fat Discriminators (n = 59) |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Red Meats (beef, pork, & sausages) | 65.8 ± 14.1 | 45.6 ± 10.6 |
| White Meats (chicken, fish, and seafood) | 75.3 ± 14.6 | 62.9 ± 10.9 |
| Added Fats & Spreads¥ | 138.3 ± 16.3 | 95.2 ± 12.1 |
| Sweet Fats | 78.5 ± 13.1 | 54.0 ± 9.8 |
| Nuts and Nut Butters | 67.0 ± 20.0 | 37.8 ± 14.9 |
| Reduced Fat Foods¥ | 102.0 ± 13.4 | 63.3 ± 9.9 |
| High-fat Foods§ | 309.7 ± 40.0 | 227.1 ± 29.7 |
The general linear models above contain the following covariates: BMI, age, sex, dietary restraint and disinhibition
p ≤0.10 (N.S. Trend)
p <0.05
3.3. Fat Discrimination Status and Reported Food Preferences
Mean reported food preferences for each food group as a function of fat discrimination status are displayed in Table 4. There was a trend for non-discriminators to report higher mean preferences for added fats than discriminators (F(1,91)=2.6; p=0.10). Mean food preferences between discriminators and non-discriminators were not different for any of the other food groups: red meats (p=0.17), white meats (p=0.39), sweet-fats (p=0.63), nuts and nut butters (p=0.74), reduced fat foods (p=0.82), and high-fat foods (p=0.14).
Table 4.
Reported Food Preference Ratings in Fat Non-Discriminators and Discriminators*
| Average Reported Preference (0–170 mm) | Fat Non-Discriminators (n = 33) | Fat Discriminators (n = 59) |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Red Meats (beef, pork, & sausages) | 118.0 ± 6.4 | 106.7 ± 4.9 |
| White Meats (chicken, fish, and seafood) | 127.4 ± 6.0 | 121.0 ± 4.6 |
| Added Fats & Spreads§ | 101.2 ± 4.4 | 92.3 ± 3.3 |
| Sweet Fats | 126.7 ± 5.4 | 123.5 ± 4.0 |
| Nuts and Nut Butters | 94.6 ± 7.3 | 97.7 ± 5.4 |
| Reduced Fat Foods | 97.5 ± 3.4 | 96.9 ± 2.5 |
| High-fat Foods | 120.2 ± 4.6 | 111.5 ± 3.4 |
The general linear models above contain the following covariates: BMI, age, sex, dietary restraint and disinhbition
p ≤0.10 (N.S. Trend)
3.4. Fat Discrimination Status and Adiposity
Fat non-discriminators had higher waist circumferences than discriminators (t=2.1; p=0.05), with mean (SD) equal to 38.6 (7.6) and 35.7 (5.2), respectively. However, after including sex and age in the model, the difference in waist circumference between discriminators and non-discriminators was only a trend (F(df 1,72) = 3.50; p=0.07). Body mass indexes between the groups did not differ (p=0.17), means equal to 30.1 (7.1) and 28.1 (5.6), for non-discriminators and discriminators, respectively.
4. Discussion
The purpose of this study was to test the association between a simple procedure to assess the ability to discriminate differences in fat content and reported fat intake behaviors and obesity. While it is well-known that high-fat foods are pleasurable, the relationship between differences in oral perception of this nutrient and more complex behaviors like fat preference and selection are unclear. Our findings reveal that African-American adults with poor fat discrimination ability reported higher monthly intake of both added fats, like butters, fat spreads, and oils, and reduced and low-fat foods, like fat-free and low-fat dairy products and snacks. Interestingly, greater reported dietary experience with added fats actually corresponded to a poorer ability to discriminate the fat content of similar foods (salad dressings) in the laboratory, although the causal relationship between these variables cannot be determined. In addition, there was also a suggestion, albeit non-significant, for non-discriminators to report greater monthly intake of all high-fat foods combined. These differences in reported intake remained after adjusting for age, sex, BMI, and dietary restraint and disinhibition. These results suggest that fat discrimination may be a useful measure for assessing an individual’s likelihood of consuming excess amounts of some high-fat and fat-modified foods, however additional studies to confirm this are needed.
The hypothesis that decreases in oral fat perception may be associated with increased fat preference and intake was first proposed by Hayes and Duffy (19) and in a more recent study by Stewart and colleagues (26). Hayes and Duffy (19) used a series of sweet-fat dairy samples to show that individuals who perceived 6-n-propylthiouracil (PROP) as less bitter a marker for reduced sensitivity to a range of tastes and textures, including fat (23;32) liked samples with greater fattiness and sweetness. In two recent reports from Stewart and colleagues, individuals who were classified as hyposensitive to orally administered fatty acids were heavier (26;33), and reported greater fat consumption in the diet (26), although the latter effect was only a trend. The present study extends this work by demonstrating that this phenomenon can also be observed by using a simple discrimination task to assess oral response to triglycerides in a common food medium. This increases the number of participants that can be tested with this procedure, making this method more practical for large-scale community or genetic-association studies. In addition, the present study reports relationships between oral fat discrimination and intake patterns, along with suggested trends in reported fat preferences that warrant follow-up investigation to determine clinical relevance.
The present observation that African-American adults who were poor at discriminating fat content in the laboratory reported greater intake of added fats warrants additional speculation. Foods included in this category were salad dressings, butters and margarines, cooking oils and half-and-half. The foods in this group are high in fat content, ranging from 50–100% fat. Furthermore, these foods are not eaten alone, but rather are added to other foods to enhance flavor (e.g., adding butter to bread) and/or to mask potentially bitter flavors (e.g., adding cream to coffee). Increased reported intake of these foods among fat non-discriminators suggests that they either add more on any given occasion or they use added fats more frequently. Increased consumption of added fats is a dietary pattern that has been associated with cardiovascular risk factors (34)and poor overall nutrient intake (35). Many sources of added fats also tend to be high in saturated and/or trans-fats, both of which have been associated with increased risk for cardiovascular diseases (36;37). While the mechanism behind the fat discrimination phenotype requires additional study, previous researchers have speculated that poor ability to discriminate a particular dietary component might lead to excess intake due to an impaired ability to detect the nutrient at lower levels (19). Further elucidation of potential gustatory mechanisms of fat transduction, such as CD36 and the GPR family of proteins (38), may also help to clarify the mechanism behind fat discrimination. Both CD36, a fatty acid translocase, and GPR40 and GPR120, G-coupled protein receptors, are expressed in the mouth and in the gastrointestinal tract [for review see (38). Studies in animals suggest that CD36 is necessary for oral fat detection and preference (39;40), while both GPR40 and GPR120 may be involved with preferences for some fatty acids. Determining the genetic underpinnings of oral fat discrimination may increase the understanding of this phenotype and help determine its biological relevance to fat ingestive behaviors.
In addition to differences in reported intake of added fats, fat non-discriminators also reported greater intake of reduced-fat foods, even after adjusting for differences in body weight and dieting behavior. The reason for this is unclear, although it is possible that non-discriminators are more accepting of the flavor of fat-modified foods because they are less able to detect differences between these foods and higher fat versions. This finding is intriguing, as it suggests that certain individuals may be more willing to use fat modified foods, and this has implications for weight reduction efforts. Additional studies are needed to confirm this.
This study had several limitations. First, it was conducted in a single ethnic group, African-Americans, in order to maintain similar allele frequencies for the genetic portion of the study. It is not known if these results will translate to other ethnic groups. The procedure used to measure fat discrimination involved forced choice same/different tests in which each sample was compared to the highest fat salad dressing (55% fat-by-weight). While we gave participants a short rest period and had them eliminate residual salad dressing from the mouth with a cracker and water, it is possible that some of the oil remained and made it difficult to distinguish one sample from another. We are presently modifying this procedure to use lower fat salad dressings as the reference sample and in our pilot studies (Donovan JD, unpublished data), the results have been similar. In addition, the fat discrimination procedure used did not allow us to differentiate whether participants made decisions based on basic taste (e.g. fatty acid, sweetness, or sourness) or textural properties of the salad dressings. We opted to use Italian salad dressings made with canola oil because this oil is high in long-chain polyunsaturated fatty acids (linoleic and oleic) and these fat sources have been reported to serve as ligands for putative fat sensors, such as CD36 (41). It is possible that using oils of varying fatty acid composition may produce different results than those reported here. Previous research suggests that preferences for fats are context specific(42); a preference for high-fat salad dressings may not translate to a preference for high-fat milks. However, the present results agree with those reported by Stewart and colleagues who assessed oral sensitivity to fats using solutions of long-chain free fatty acids (26). This suggests that the phenomenon of poor oral fat discrimination may generalize to broader food acceptance and dietary behaviors. A further limitation is that we did not measure the amount of free fatty acids or the viscosity of our salad dressings and doing so would allow us to make additional speculation about the biological mechanisms underlying this phenotype. Finally, although food frequency questionnaires have well-known biases (43), similar methods were used to assess intake in recent reports from other laboratories (26). The fact that similar relationships were observed between the present findings and those from other laboratories strengthens the validity of these reports.
Conclusions
The results of this and other studies (18;19;26) support the need to develop a valid and reliable method of measuring oral fat perception that can be applied in basic research and translational studies. The present findings suggest that measuring fat discrimination may be useful to identify individuals who are at risk for increased consumption of added fats like butters, spreads and oils. Further, assessing fat discrimination may also help identify individuals who are likely to consume reduced fat products, as well. This information could improve the ability to prescribe appropriate weight loss diets in a clinical setting. In addition, the fat discrimination phenotype may be a useful tool for the food production industry to improve the design of fat modified foods.
Highlights.
Fat is a nutrient sensed by oral, olfactory, and texture cues that adds palatability to many foods.
There are no standardized procedures for studying oral fat perception.
We developed a simple tool to measure the ability to discriminate fat content.
Poor fat discrimination ability was linked to increased intake of added fats.
This tool may be used to identify risk for obesity and excess fat intake.
Acknowledgments
Funding for this study came from NIH grant K01DK068008 and an NIH/NIDDK Pilot and Feasibility Award (KLK). Additional support came from the Obesity Research Center Grant (NIH grant 5P30DK026687-27) and from DK52431, DK63608, and DK26687 (WKC).
Supported by: NIH Pilot and Feasibility Study through the New York Obesity Research Center.
Appendix A.
Recipes for Italian Salad Dressings used for Measuring Fat Discrimination
| Fat-by-Weight | Distilled Content (%)Water (mL) | Apple Cider Vinegar (mL) | Canola Oil (mL) | Seasoning Packeta | Carrageenanb (g) |
|---|---|---|---|---|---|
| 5% | 157.0 | 39.0 | 12.0 | 1 | 4.0 |
| 10% | 143.0 | 41.0 | 23.0 | 1 | 3.2 |
| 20% | 114.0 | 47.0 | 46.0 | 1 | 1.8 |
| 30% | 85.0 | 53.0 | 69.0 | 1 | 0.9 |
| 40% | 56.0 | 59.0 | 92.0 | 1 | 0.0 |
| 50% | 28.0 | 65.0 | 114.0 | 1 | 0.0 |
| 55% | 8.0 | 65.0 | 127.0 | 1 | 0.0 |
Good Seasons Mild Italian Dressing Packet®
Viscarin® Carrageenan
Footnotes
Disclosure
There are no conflicts of interest on behalf of any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.McHugh MD. Fit or fat? A review of the debate on deaths attributable to obesity. Public Health Nurs. 2006;23:264–70. doi: 10.1111/j.1525-1446.2006.230309.x. [DOI] [PubMed] [Google Scholar]
- 3.Pi-Sunyer FX. Health implications of obesity. Am J Clin Nutr. 1991;53:1595S–1603S. doi: 10.1093/ajcn/53.6.1595S. [DOI] [PubMed] [Google Scholar]
- 4.Ahituv N, Kavaslar N, Schackwitz W, et al. Medical sequencing at the extremes of human body mass. Am J Hum Genet. 2007;80:779–91. doi: 10.1086/513471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vamosi M, Heitmann BL, Kyvik KO. The relation between an adverse psychological and social environment in childhood and the development of adult obesity: a systematic literature review. Obesity Rev. 2010;11:177–84. doi: 10.1111/j.1467-789X.2009.00645.x. [DOI] [PubMed] [Google Scholar]
- 6.Astrup A. The role of dietary fat in obesity. Sem Vasc Med. 2005;5:40–7. doi: 10.1055/s-2005-871740. [DOI] [PubMed] [Google Scholar]
- 7.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr. 1995;49:79–90. [PubMed] [Google Scholar]
- 8.Cotton JR, Burley VJ, Weststrate JA, Blundell JE. Dietary fat and appetite: similarities and differences in the satiating effect of meals supplemented with either fat or carbohydrate. J Hum Nutr Diet. 2007;20:186–99. doi: 10.1111/j.1365-277X.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- 9.Drewnowski A, Brunzell JB, Sande K, Iverius PH, Greenwood MR. Cream or sugar: obese subjects’ preferences for sweetened high-fat foods. Proc IVth Int Congr Obes; New York. 1983. [Google Scholar]
- 10.Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet Tooth Reconsidered: Taste Responsiveness in Human Obesity. Physiol & Behav. 1985;35:617–22. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 11.Drewnowski A, Shrager EE, Lipsky C, Stellar E, Greenwood MR. Sugar and fat: sensory and hedonic evaluation of liquid and solid foods. Physiol & Behav. 1989;45:177–83. doi: 10.1016/0031-9384(89)90182-0. [DOI] [PubMed] [Google Scholar]
- 12.Drewnowski A. Human preferences for sugar and fat. In: Fernstrom JD, Miller GD, editors. Appetite and body Weight Regulation: Sugar, Fat, and Macronutrient Substitutes. London: CRC Press, Inc; 1994. [Google Scholar]
- 13.Schiffman SS. Orosensory perception of dietary fat. Curr Dir Psychol Sci. 1998;7:137–43. [Google Scholar]
- 14.Bauer F, Elbers CC, Adan RAH, et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90:951–9. doi: 10.3945/ajcn.2009.27781. [DOI] [PubMed] [Google Scholar]
- 15.Cai G, Cole SA, Bastarrachea-Sosa RA, MacCluer JW, Blangero J, Comuzzie AG. Quantitative trait locus determining dietary macronutrient intakes is located on human chromosome 2p22. Am J Clin Nutr. 2004;80:1410–4. doi: 10.1093/ajcn/80.5.1410. [DOI] [PubMed] [Google Scholar]
- 16.Drewnowski A, Greenwood MR. Cream and sugar: Human preferences for high-fat foods. Physiol Behav. 1983;30:629–33. doi: 10.1016/0031-9384(83)90232-9. [DOI] [PubMed] [Google Scholar]
- 17.Drewnowski A. Individual differences in sensory preferences for fat in model sweet dairy products. Acta Psychologica. 1993;84:103–10. doi: 10.1016/0001-6918(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 18.Hayes JE, Duffy VB. Revisiting sugar-fat mixtures: sweetness and creaminess vary with phenotypic markers of oral sensation. Chem Senses. 2007;32:225–36. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- 19.Hayes JE, Duffy VB. Oral sensory phenotype identifies level of fat and sugar required for maximal liking. Physiol & Behav. 2008;95:77–87. doi: 10.1016/j.physbeh.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni AP. Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutr. 2004;79:372–8. doi: 10.1093/ajcn/79.3.372. [DOI] [PubMed] [Google Scholar]
- 21.Drewnowski A, Schwartz M. Invisible Fats: Sensory Assessment of Sugar/Fat Mixtures. Appetite. 1990;14:203–17. doi: 10.1016/0195-6663(90)90088-p. [DOI] [PubMed] [Google Scholar]
- 22.Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiol & Behav. 1997;61:949–54. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 23.Tepper BJ, Nurse RJ. PROP taster status is related to fat perception and preference. Ann NY Acad Sci. 1998;30:802–04. doi: 10.1111/j.1749-6632.1998.tb10662.x. [DOI] [PubMed] [Google Scholar]
- 24.Mattes RD. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem Senses. 2009;34:145–50. doi: 10.1093/chemse/bjn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattes RD. Oral thresholds and suprathresold intensity ratings for free fatty acids on 3 tongue sites in humans: implications for trandsuctions mechanisms. Chem Senses. 2009;34:415–23. doi: 10.1093/chemse/bjp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RSJ. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104:145–52. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL. Disparities in obesity prevalence in the United States: black women at risk. Am J Clin Nutr. 2009;89:1001–2. doi: 10.3945/ajcn.2009.27592. [DOI] [PubMed] [Google Scholar]
- 28.Cossrow N, Falkner B. Race/ethnic issues in obesity and related co-morbidities. J Clin Endocrin Metab. 2004;89:2590–4. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 29.Pangborn RM, Dunkley WL. Difference-preference evaluation of milk by trained judges. J Dairy Sci. 1964;47:1414–6. [Google Scholar]
- 30.Pangborn RM, Bos KE, Stern JS. Dietary fat intake and taste response to fat in milk by under-, normal, and overweight women. Appetite. 1985;6:25–40. doi: 10.1016/s0195-6663(85)80048-9. [DOI] [PubMed] [Google Scholar]
- 31.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 32.Duffy VB. Perceived creaminess of high-fat milk products varies wiht genetic taste status. Chemical Senses. 1996;21:598 (abstr). [Google Scholar]
- 33.Stewart JE, Seimon RV, Otto B, Keast RSJ, Clifton PM, Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr. 2011;93:703–11. doi: 10.3945/ajcn.110.007583. [DOI] [PubMed] [Google Scholar]
- 34.Nettleton JA, Polak JF, Russell T, Burke GL, Jacobs DR. Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:647–54. doi: 10.3945/ajcn.2009.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie LD, Spector P, Stevens MJ, et al. Dietary patterns in adolescence are related to adiposity in young adulthood in black and white females. J Nutr. 2007;137:399–406. doi: 10.1093/jn/137.2.399. [DOI] [PubMed] [Google Scholar]
- 36.Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–32. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC. Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Diet Assoc. 2010;110:585–92. doi: 10.1016/j.jada.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Mattes RD. Is there a fatty acid taste? Annu Rev Nutr. 2009;29:1–23. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol- Reg Int & Comp Physiol. 2007;293:R1823–32. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 41.Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Memb Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- 42.Mela DJ, Sacchetti DA. Sensory preferences for fats: relationships with diet and body composition. Am J Clin Nutr. 1991;53:908–15. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- 43.Carithers TC, Talegawkar SA, Rowser ML, et al. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109:1184–93. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]