Abstract
Background
Emotional expression among people with dementia (PWD) may inform person-centered approaches to care and improvements in dementia-related quality of life.
Objectives
To examine frequency and variability of positive emotional expression and negative emotional expression, personal factors influencing positive and negative emotional expression, and trajectories of emotional expression among PWD during daytime hours.
Methods
We conducted a secondary analysis of daytime positive and negative emotional expressions of 30 PWD living in residential long term care who completed twelve 20-minute observation periods occurring hourly as part of a multi-site study of wandering behavior. Hierarchical linear modeling was used to examine relationships between influencing factors and frequency of emotional expressions; group-based trajectory analysis was applied to identify clusters of individuals with similar daytime patterns of emotional expression.
Results
Time of day (rate ratio [RR] = 1.05) and impaired mobility (RR = 1.37) significantly influenced positive emotional expression; gender (RR = 1.85), age (RR = 1.03), and education (RR = 0.54) were significantly related to negative emotional expression. Three distinct trajectory groups were identified for positive emotional expression: a low stable group, a fluctuating group displaying afternoon peaking, and a fluctuating group displaying morning peaking. Two trajectory groups were identified for negative emotional expression: a consistent pattern and an inconsistent pattern.
Discussion
PWD showed a broad range of emotional expression and significant within-person variation in daytime positive and negative emotional expressions. Observed emotional display is a promising measure of psychological well-being among PWD that, if more fully understood, could guide care approaches to improve quality of life.
Keywords: affect, dementia, group-based trajectory model, psychological well-being
Recent emphasis on person-centered care approaches has raised awareness of emotional well-being as an important care outcome, both because it influences other health outcomes and because it is a core component of quality of life (QoL) (Centers for Medicare & Medicaid Services [CMS], 2011; Finnema, Dröes, Ribbe, & Van Tilburg, 2000; Finnema et al., 2005). Even when measurement of emotion poses challenges, such as among the rapidly growing population of people with dementia (PWD), attention to psychological well-being is important (Jonker, Gerritsen, Bosboom, & Van Der Steen, 2004). Emotion-oriented care, defined as care adjusted to the feelings and emotional needs of PWD (Finnema et al., 2000), has been shown to improve outcomes such as behavioral disturbance and QoL (Finnema et al., 2000; Finnema et al., 2005). With the number of persons with dementia projected to grow to 9 million people in North America by 2050 (Plassman et al., 2011), the need to understand the emotional responses of PWD to illness and care is urgent.
In an integrative review of theoretical and measurement issues in studying QoL in dementia, Jonker et al. (2004) argued that psychological well-being indicated by an individual’s emotional responses should serve as the core dimension of QoL among PWD. A conceptual model of QoL was proposed, in which psychological well-being is influenced not only by objective personal and environmental circumstances, but by the PWD’s subjective evaluation of those circumstances indicated by their emotional responses (positive and negative affect). Better understanding of the relationships among factors influencing emotional responses, and thus psychological well-being among PWD across the spectrum of disease severity, is needed to advance the scientific basis for improving care that optimizes QoL.
PWD experience disease-related language, comprehension and memory deficits that preclude self-report of emotions. Nevertheless, PWD retain the ability to express emotions via the face, voice tone, and body posture (Lawton, Van Haitsma, & Klapper, 1996; Vogelpohl & Beck, 1997).
The issue of whether PWD lose the ability to form emotions has been debated (Lawton, 1994); however, a growing body of empirical work demonstrates that PWD preserve the ability to reliably express emotion even in the later stages of disease (Kolanowski, Hoffman, & Hofer, 2007; Lawton, Van Haitsma, & Klapper, 1996; Magai, Cohen, Gomberg, Malatesta, & Culver, 1996; Vogelpohl & Beck, 1997). Moreover, studies have shown that PWD respond to treatment for emotional disorders such as anxiety and depression (Ferretti, McCurry, Logsdon, Gibbons, & Teri, 2001; Lopez et al., 2003).
Historically, research on emotion in PWD has been focused on mood disorders such anxiety and depression. Although it is important to identify and treat mood disorders because they are a modifiable determinant of emotional distress, psychological well-being is more than the absence of a psychiatric disorder (Keyes, 2007). Daily emotional expression, an indicator of positive and negative effect, may be a more salient indicator of psychological well-being among PWD than absence or presence of psychiatric symptoms. In contrast to mood disorders, which last days, weeks or months, emotional expressions last seconds and change more rapidly (Kolanowski, Litaker, & Catalano, 2002). The degeneration of frontal lobe function and memory deficits associated with progressive dementia result in behavior that becomes more stimulus-bound (Mesulam, 2008), and thus, the lives of PWD are become increasingly carried out in the “here and now” (Kolanowski et al., 2002; Mesulam, 2008). Observed emotional expression is therefore a particularly promising approach to measuring psychological well-being of PWD in moderate to advanced stages of illness because it focuses on immediate responses to daily events.
Emotional expression in adults is characterized by positive and negative dimensions that represent distinct components of emotion rather than opposite poles on a continuum (Brod, Stewart, Sands, & Walton, 1999; Lawton, 1994; Meeks, Van Haitsma, Kostiwa, & Murrell, 2012). Among normal adults, positive emotional expression fluctuates systematically throughout the day, with maximum positive affect occurring at midday in young adults (Clark & Watson, 1988; Clark, Watson, & Leeka, 1989; Egloff, Tausch, Kohlmann, & Krohne, 1995; Thayer, Takahashi, & Pauli, 1988). In contrast, negative affect has shown no systematic diurnal variation (Clark et al., 1989; Thayer, 1987; Thayer et al., 1988).
Empirical studies of older adults have suggested a decline in intensity of emotional expression with increasing age (Gross et al., 1997; Isaacowitz, Charles, & Carstensen, 2000). Despite this age-associated blunting of emotional expression, psychological well-being remains stable with aging and is generally positive (Carstensen et al., 2011; Löckenhoff & Carstensen, 2007; May, Rahhal, Berry, & Leighton, 2005; Scheibe & Carstensen, 2010).
Although the majority of research on emotion has focused on normal adults rather than PWD, studies suggest that the capacity for experiencing and expressing emotions is preserved in PWD, even with advanced disease. Consistent with the literature on age-related effects, several studies of PWD confirm that cognitively impaired people show impoverished emotional expression (Asplund, Norberg, Adolfsson, & Waxman, 1991; Daffner, Scinto, Weintraub, Guinessey, & Mesulam, 1992). However, PWD preserve the ability to express emotions facially (Kolanowski, Hoffman, & Hofer, 2007; Magai et al., 1996), and at least three studies confirm that mid- to late-stage dementia patients retain the ability to express a wide range of basic emotions (Kolanowski et al., 2007; Kolanowski et al., 2002; Magai et al., 1996). This body of literature, viewed from the perspective of Jonker et al.’s (2004) model of QoL in PWD highlights the potential for using emotional expression as an indicator of psychological well-being among PWD. Further studies are needed to better understand how emotional expression varies within individuals and what factors are associated with individual differences in intraindividual change in emotional expression of PWD. The purpose of this study was therefore to explore daytime patterns of emotional expression in PWD with moderate to severe dementia living in residential long-term care. Three specific questions were addressed:
What is the frequency and variability of positive emotional expression (PEE) and negative emotional expression (NEE) of PWD during daytime hours?
Does observable emotional expression in PWD vary by personal characteristics, severity of cognitive impairment, and time of day?
What are the trajectories of PEE and NEE among PWD during daytime hours?
Methods
Data Sources and Procedure
A secondary analysis was conducted of repeated measures data obtained from a subset of participants enrolled in a multi-site descriptive study of factors influencing wandering behavior in PWD who lived in residential long-term care. Institutional review board approval was obtained at each participating institution. Written consent was obtained from legal proxies and assent was also obtained from participants prior to every observation.
In the parent study, participants (N = 185) were PWD recruited from 17 nursing homes and six assisted living facilities in Michigan and Pennsylvania selected by a random cluster sampling approach. Inclusion criteria for the parent study were: age 65 years or older, English-speaking, Mini-Mental Status Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) score < 24/30, DSM-IV criteria for dementia met, and ambulatory. Participants who met inclusion criteria were randomly assigned to twelve 20-minute observation periods once per hour on two non-consecutive days. All observation periods were videotaped and occurred between 8:00 a.m. and 8:00 p.m. A full description of the procedures used in the parent study has been published elsewhere (Algase, Antonakos, Beattie, & Beel-Bates, 2009).
A subset of measures collected in the parent study were extracted for those participants who had completed all twelve scheduled emotional expression observations; these measures included emotional expression ratings, time of day, and personal factors hypothesized to be related to emotional expression. These encounters ensured a sufficient number of observations to capture each participant’s emotional variation. Thus, 30 PWD with 360 observations were included in these analyses.
Measures
Emotional expression of PWD was measured by the Observable Displays of Affect Scale (ODAS) (Vogelpohl & Beck, 1997). The ODAS was specifically developed for coding videotaped emotional expressions; it consists of 34 behaviors including six subscales (e.g., facial displays, vocalizations, and body movement/posture categorized by positive and negative quality) (Vogelpohl & Beck, 1997). Trained research assistants coded the presence or absence of each behavior from a 20 minute videotape using the Noldus Observer® 5.0 software. Inter-rater reliability and test-retest reliability for the ODAS ranges from .68 to 1.00 (Vogelpohl & Beck, 1997). In the parent study, an inter- and intra-rater agreement among coders was established at greater than 95% using training videotapes before coding for the ODAS measures began. Reliability was assessed throughout the study by sampling 10% of the videotapes and retraining coders if needed. Reliability of the ODAS in this study was measured by Cronbach’s alpha, and ranged from .70 to .80.
The MMSE (Folstein, Folstein, & McHugh, 1975) was used to assess cognitive impairment. Participants who were too impaired to complete testing were assigned a score of -1. Personal characteristics included age, gender, ethnicity, education, and mobility. Ethnicity was dichotomized as Caucasian or other. Mobility was dichotomized as independently ambulatory or ambulatory with assistance. Education was categorized as 1 = junior high school or less, 2 = high school; and 3 = college or higher. Time of day was the military time at which an observation was made. Chart review data were used to derive measures of nondementia-related personal characteristics.
Statistical Analysis
Descriptive statistics were examined to address the first research question. Two-level hierarchical linear modeling (HLM) was employed to address the second question. Repeated observations of emotional expression (level-1) data are nested within each person (level-2). For such data, HLM is a one of the best options because it estimates the errors for each participant separately (Bryk, Raudenbush, & Congdon, 1996). Because emotional displays were expressed as frequency (count) data, assumptions of traditional linear regressions were violated. Poisson regression with over-dispersion was chosen as an analytical approach because it is consistent with the counts used to index PEE and NEE during each observation. The level-1 model examined within-person variability of emotional expressions throughout the day. The level-2 model examined interindividual differences in emotional expression based on cognition and other personal characteristics. The HLM 6.0 software package (Scientific Software International, Inc.) was utilized to estimate these statistical models.
For the third research question, a semi-parametric, group-based trajectory analysis (GBTA) (Nagin, 2005) was used to identify clusters of individuals following similar patterns of emotional expression displays over time. Parameters of the GBTA model were estimated in SAS PROC TRAJ (SAS V9); the GBTA is more restrictive than the general growth mixed model of which it may be considered a sub-model (Li, Duncan, Duncan & Acock, 2001). The SAS PROC TRAJ analysis was used to group people with similar PEE and NEE trajectories, respectively. PROC TRAJ was specially designed for use to identify cases with similar trajectories, or patterns of change over time; more specifically, the number of distinct patterns of change (groups of cases) can be identified (Jones, Nagin, & Roeder, 2001). A series of models containing from two to six groups was systematically examined and compared; each group contained linear, quadratic, and cubic change coefficients. Model fit in GBTA is judged using a function of the Bayesian information criterion (BIC) that simultaneously considers model complexity and overall fit, and penalizes highly parameterized models to balance with the interests of parsimony (Nagin, 2005). Values for the function of BIC computed in SAS PROC TRAJ are negative, and the optimal model is indicated by the value closest to 0 (i.e., the maximum value).
Results
Most participants lived in a nursing home (67%) and were female (73%) and Caucasian (80%), with a mean age of 83.97 (SD = 5.77). The majority of participants ambulated independently (70%). Mean MMSE score was 6.93 (range = −1 to 21). Over 85% of participants had moderate to severe cognitive impairment (MMSE ≤ 16).
Description of Emotional Expression
As shown Table 1, PWD showed an average of 13.51 (SD = 12.49) episodes of PEE per observation; only 1.57 (SD = 2.26) episodes of NEE were noted per observation. Positive facial emotional displays such as smile and relaxed facial expression were the most frequently observed PEE indicators (M = 5.89, SD = 5.88). Negative body/posture emotional displays such as repetitive body movements and closed posture were the most frequently observed NEE (M = 1.00, SD = 1.71).
Table 1.
Overall Mean Frequency of Positive and Negative Emotion Expression (per 20 minutes)
| Behavior | M | (SD) | Range |
|---|---|---|---|
| Positive facial emotional expression | 5.89 | (5.88) | 0.00 – 38.50 |
| Positive verbal emotional expression | 1.91 | (3.00) | 0.00 – 22.71 |
| Positive body/posture emotional expression | 5.72 | (5.26) | 0.00 – 28.80 |
| Sum of PEE items | 13.51 | (12.49) | 0.00 – 90.01 |
| Negative facial emotional expression | 0.44 | (0.86) | 0.00 – 9.75 |
| Negative verbal emotional expression | 0.13 | (0.63) | 0.00 – 15.88 |
| Negative body/posture emotional expression | 1.00 | (1.71) | 0.00 – 35.00 |
| Sum of NEE items | 1.57 | (2.26) | 0.00 – 50.88 |
Note. PEE = positive emotional expression; NEE = negative emotional expression.
Poisson Model
Table 2 contains the two-level Poisson HLM results for PEE and NEE. After examining bivariate relationships between predictor variables and emotional expression (p < .05), time of day, gender, and mobility were selected as factors that may influence PEE; gender, age, education were selected as predictors of NEE. With each passing hour of the day, PWD typically showed a 0.05 unit increase in PEE (Confidence Interval (CI) = [1.02 - 1.08]), while other variables were held constant. Participants who needed assistance with ambulation showed a 0.37 unit hourly increase in PEE (CI = [1.00 - 1.89]) compared with those who were independent.
Table 2.
Two-Level Poisson HLM for Positive Emotional Expression and Negative Emotional Expression
| Variable | Positive Emotional Expression
|
Negative Emotional Expression
|
||||||
|---|---|---|---|---|---|---|---|---|
| b | (SE) | RR | 95% CI | b | (SE) | RR | 95% CI | |
| Intercept | 2.16 ** | (0.17) | 8.71 | [6.16 – 12.32] | 0.04 | (0.22) | 1.04 | [0.66 – 1.66] |
| Observation-level predictors | ||||||||
| Time of day | 0.05 ** | (0.01) | 1.05 | [1.02 – 1.08] | ||||
| Person-level predictors | ||||||||
| Mobility (assisted) | 0.32* | (0.15) | 1.37 | [1.00 – 1.89] | ||||
| Gender (female) | 0.28 | (0.16) | 1.33 | [0.94 – 1.87] | 0.62* | (0.23) | 1.85 | [1.15 – 2.99] |
| Age | 0.03* | (0.01) | 1.03 | [1.01 – 1.06] | ||||
| Education (high school) | −0.21 | (0.20) | 0.81 | [0.53 – 1.22] | ||||
| Education (college or higher) | −0.62* | (0.25) | 0.54 | [0.32 – 0.91] | ||||
| Random effects | Variance | χ2 | Variance | χ2 | ||||
|
|
||||||||
| Intercept | 0.02 | 30.80 | 0.14 | 66.32** | ||||
| Time of day | 0.00 | 24.76 | ||||||
| Level-1 | 7.92 | χ2 | 1.34 | |||||
Note. SE = standard error; RR = rate ratio; CI = confidence interval.
p < .05,
p < .01.
Females expressed more NEE than males. Specifically, compared to males, females showed an increase in NEE of 0.85 (CI = [1.15 - 2.99]). As age increased in one unit from the sample mean age (83.97 years), the observed frequency of NEE increased by 0.03 (CI = [1.01 - 1.06]). Participants with college education or higher showed 0.46 (CI = [0.32 - 0.91]) less NEE than participants who had a junior high school education or lower.
Group-Based Trajectory Models
Since PEE and NEE represent different dimensions of emotion, we estimated separate models for each dimension. The parameter estimates for these models are displayed in Table 3. Using BIC, three groups with distinctive trajectories were identified for PEE, and two groups with distinctive trajectories for NEE. The BIC values are listed in Table 4. For PEE, Group 1 was defined by a linear parameter showing a trend toward increased positive emotion over the course of the day that approached significance (p = .068). Group 2 and Group 3 were defined by the cubic parameter suggesting a more complex pattern of daily emotional expression. For NEE, Group 1 was defined by a consistency in emotional expression group and by an inconsistent group. Group 1 showed no diurnal variation in NEE (p =. 199).
Table 3.
Parameter Estimates for Group Trajectories and Group Membership
| Group | Parameter | Positive Emotional Expression
|
Negative Emotional Expression
|
||||
|---|---|---|---|---|---|---|---|
| Estimate | (SE) | p | Estimate | (SE) | p | ||
| 1 | Intercept | 3.80 | (3.39) | .263 | 0.56 | (0.39) | .150 |
| Linear | 0.45 | (0.25) | .068 | 0.04 | (0.03) | .199 | |
| 2 | Intercept | 251.72 | (89.00) | .005 | −69.50 | (27.35) | .012 |
| Linear | −63.87 | (21.38) | .003 | 17.51 | (6.48) | .007 | |
| Quadratic | 5.32 | (1.65) | .001 | −1.33 | (0.49) | .007 | |
| Cubic | −0.14 | (0.04) | .001 | 0.03 | (0.01) | .007 | |
| 3 | Intercept | −162.88 | (95.97) | .091 | |||
| Linear | 45.73 | (23.14) | .048 | ||||
| Quadratic | −3.62 | (1.78) | .043 | ||||
| Cubic | 0.09 | (0.04) | .038 | ||||
Note. SE = standard error; PEE = positive emotional expression; NEE = negative emotional expression.
Table 4.
BIC Values for to Select a Model Having the Optimal Number of Groups
| No. of Groups | PEE | NEE |
|---|---|---|
| 1 | −1397.96 | −695.63 |
| 2 | −1388.43 | −683.84 |
| 3 | −1385.22 | −685.49 |
| 4 | −1392.29 | −700.21 |
Note. PEE = positive emotional expression.
NEE = negative emotional expression. The maximum value of the function of BIC estimated in PROC TRAJ is used to select the optimal model.
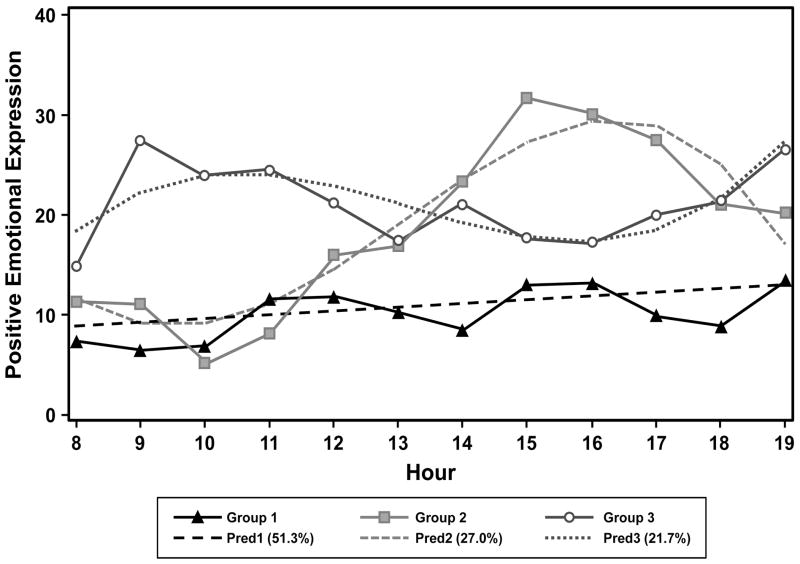
The proportion of participants in each PEE group and the group trajectories are depicted in Figure 1. Solid lines represent observed data, while broken lines represent predicted curves. PEE group 1 with low stable daytime PEE comprises about 51% of the participants (n = 15). The trajectory for this group was basically flat, and residents in this group consistently expressed little PEE. Group 2, with fluctuating PEE and an afternoon peak, comprised about 27% of the participants (n = 9). Specifically, low peak time was around 10:00 a.m.; high peak time was around 6:00 p.m. Group 3 was made up of participants who had high PEE in the morning and low PEE in the afternoon, and accounted for 22% of the residents (n = 6). This group also had higher average PEE than the other two groups. Group 3 was labeled as having fluctuating PEE with a morning peak.
Figure 1.
Predicted and observed positive emotional expression for each trajectory group.
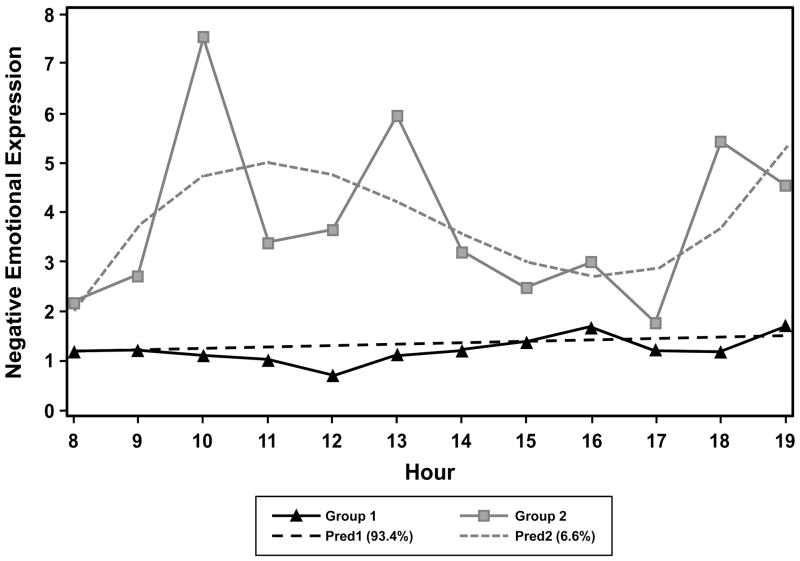
Figure 2 shows both predicted and observed NEE in Group 1 and Group 2. Group 1 displayed low stable levels of NEE, and accounted for 93% of the residents (n = 28). Group 2 displayed higher, fluctuating NEE than Group 1, and constituted only 7% of the residents (n = 2). Specifically, residents in Group 2 showed high NEE in the morning, low NEE in the afternoon, and high NEE again in the early evening.
Figure 2.
Predicted and observed negative emotional expression for each trajectory group.
Discussion
The results of the study provide evidence that PWD display a broad range of emotional expression. Specifically, on average, for each 20 minute observation PWD showed 13.51 (SD = 12.49) episodes of PEE and 1.57 (SD = 2.26) episodes of NEE, even though over 85% of participants had moderate to severe cognitive impairment. This result supports previous studies, which suggest that PWD express positive and negative emotions even in advanced stages of disease (Kolanowski et al., 2007; Magai et al., 1996; Yao & Algase, 2008).
We currently lack normative data on rates of observed emotional expression among healthy older adults, and since there was no control group available in this study, it is uncertain whether PWD had lower rates of emotional expression than people without dementia. In addition, PWD expressed more positive emotion than negative emotion, contrary to the popularly held belief that PWD in residential long-term care were usually anxious, angry, or depressed. This result is consistent with a case study by Kolanowski et al. (2002), who also reported displays of participant’s happiness and found that the mean score of PEE was 13 times that of the NEE mean score.
The model of QoL in PWD proposed by Jonker et al. (2004) posits that environmental and personal factors and the individual’s subjective evaluation of those factors influence psychological well-being. Factors found to be related to emotional expression are consistent with this theory, but predictors differed for PEE and NEE. Time of day and impaired mobility were significant predictors of PEE; gender, age, and education were related to NEE. In bivariate analyses, females showed more of both PEE and NEE than did males (result not shown). Several studies reported that women are more likely to be emotional than men, although their samples were limited to healthy adults (Bagozzi, Wong, & Yi, 1999; Feldman, 1995). Notably, Bagozzi et al. (1999) reported that these gender differences persist regardless of cultural differences. Consistent with other studies, education was negatively correlated with negative affect and is more pronounced in later life (Meeks & Murrell, 2001; Rhodewalt & Zone, 1989). Meeks & Murrell (2001) reported negative affect as a mediator between educational levels and life satisfaction. In other words, higher education levels were associated with lower levels of negative affect, explaining how education influenced life satisfaction. Our finding was consistent with their work, even though the measurement approaches differed.
Another important finding was that severity of cognitive impairment was unrelated to variation in both positive and negative emotional expression. A possible explanation of how PWD manage to preserve emotional expression regardless of cognitive function lies in how the brain changes in dementia. The basal ganglia and amygdala, among the key brain areas with respect to emotional functions, are relatively unaffected by the neural degeneration associated with Alzheimer’s disease (Eldridge, Masterman, & Knowlton, 2002). As dementia progresses, the influence of cognition on emotion may also weaken (Eldridge et al., 2002). This explanation might support the lack of relationship between severity of cognitive impairment and both positive and negative emotional expression.
This study also showed that there were significant daily emotional variations among PWD: three types of PEE and two types of NEE trajectories were found. Our findings suggest that PWD not only preserve the ability to display emotion but also exhibit a variety of emotional expression patterns, consistent with studies of emotion in adults who do not have dementia. Although no prior study was found that examined daytime variation in emotions among PWD, it should be noted that Kolanowski et al. (2007) examined variations of positive and negative emotion in PWD across a 12-day period. They also showed significant within-person variation in positive and negative emotion (approximately 40% to 60%) across days.
Study findings also suggested that environmental influences may play a role in emotional expression, in a manner consistent with research findings in younger adults. Studies of emotional variability among college students showed diurnal PEE variations similar to those found in this study; the authors suggested that a potential reason might be the similar homogeneity of schedules and ages of samples (Clark et al., 1989; Thayer, 1987; Thayer et al., 1988). In this study, the PWDs who belonged to the low stable emotional expression group may not have been scheduled to participate in any activities, leading to limited emotional expression, either positive or negative. On the other hand, PWD who had been scheduled to interact with other residents or staff, such as those with impaired mobility, might display patterns of emotional expression which were subject to fluctuation. The extent to which these environmental influences on patterns of emotional expression are themselves influenced by individual factors such as personality, lifetime personal habits will require prospective study with larger samples. Such studies would greatly enhance our ability to deliver truly person-centered care by helping to isolate the most salient factors for caregivers to accommodate in residential care settings.
The link between emotional expression and social engagement provides another example of environmental influences on emotional expression suggested by Jonker et al.’s (2004) model of QoL in PWD and other research. Magai et al. (1996) speculated that PWD showed lower frequencies of positive and negative emotional expressions because of lack of social stimulation. More recent studies have shown that engagement in activities was associated with better psychological well-being, defined as the balance between positive and negative emotional expression (Chung, 2004). Further studies would allow a more refined examination of how personal factors may influence responses to increased social interaction. Clinical observations made from a person-centered care perspective also suggest that the nature of the relationships between social interaction and psychological well-being are complex; simply increasing social interaction without consideration of the personal factors that influence subjective appraisal and emotional response may have the unintended effect of reducing psychological well-being. We currently lack information on which personal factors to take into consideration when varying social interactions.
This study has two key limitations. First, its small sample size (n = 30) and location (only two states) limit generalizability. However, according to 2004 National Nursing Home Survey, of the 1.5 million nursing home residents, 71.1% were female; 85.5% were Caucasian; and 45.2% were aged 85 years and older (Jones, Dwyer, Bercovitz, & Strahan, 2009), which is similar to characteristics of study participants. Second, this study is a secondary analysis and included only PWD who were ambulatory and assented to observations. Prospective longitudinal studies with broader inclusion criteria are required to justify any causal inference linking contributing factors with emotional expression. Although 30 PWD are not sufficient to draw definitive conclusions from GBTA, these exploratory analyses provide direction for future research.
Future studies should consider clinical applications of the current findings and further examination of the factors that influence psychological well-being in PWD in residential care. Translational of research measures such as the ODAS into tools that staff can readily use to assess emotional responses of PWD to care would fill an important gap. Future studies should continue to examine the relationship between person and environment factors and QoL in PWD. While this study demonstrated relationships between personal factors and psychological well-being in PWD, other factors such as techniques for delivering personal care, or timing and content of social or recreational events and family visits, merit further study.
Personal factors related to dementia such as severity of disability, activity of daily living status and other variables unrelated to dementia that may influence emotional responses such as personality traits or other personal preferences should also be explored in future studies. Studies of the relationships between emotional expression, daily events, and ADL co-occurring at each observation will provide more specific data that may aid in the design of intervention programs to improve psychological well-being of PWD.
Conclusion
Emotions of PWD are often regarded as blunted or diminished, and their emotions are ignored or discounted. In the present study, people with dementia showed not only a broad range of emotional displays but also significant within-person variation in positive and negative emotional expressions during daytime. These findings suggest that strategies such as encouraging nursing home residents to participate in social activities and to interact with other residents and staff in a manner tailored to their personal profile and emotional response may improve the psychological well-being of PWD. In addition, development of clinically useful tools to assess emotional response is needed to implement and evaluate person-centered care approaches to improve QoL.
Acknowledgments
Funding source: Preparation of this manuscript was funded by the Duke University School of Nursing, Trajectories of Chronic Illness and Care Systems postdoctoral fellowship program. Data for this project was obtained with support from the National Institute of Nursing Research, R01 NR04569, PI D. Algase.
Footnotes
There is no conflict of interest.
Contributor Information
Kyung Hee Lee, Duke University, School of Nursing.
Donna L. Algase, University of Toledo, College of Nursing.
Eleanor S. McConnell, Duke University, School of Nursing, Clinical Nurse Researcher, Geriatric Research, Education and Clinical Center (GRECC), Durham Veterans Affairs Medical Center.
References
- Algase DL, Antonakos CL, Beattie E, Beel-Bates CA, Yao L. New parameters for daytime wandering. Research In Gerontological Nursing. 2009;2:58–68. doi: 10.3928/19404921-20090101-02. [DOI] [PubMed] [Google Scholar]
- Asplund K, Norberg A, Adolfsson R, Waxman HM. Facial expressions in severely demented patients—a stimulus–response study of four patients with dementia of the Alzheimer type. International Journal of Geriatric Psychiatry. 1991;6:599–606. [Google Scholar]
- Bagozzi RP, Wong N, Yi Y. The role of culture and gender in the relationship between positive and negative affect. Cognition and Emotion. 1999;13:641–672. [Google Scholar]
- Brod M, Stewart AL, Sands L, Walton P. Conceptualization and measurement of quality of life in dementia: The Dementia Quality of Life Instrument (DQoL) Gerontologist. 1999;39:25–35. doi: 10.1093/geront/39.1.25. [DOI] [PubMed] [Google Scholar]
- Bryk A, Raudenbush S, Congdon R. HLM: Hierarchical linear and nonlinear modeling with the HLM/2L and HLM/3L programs. Chicago: Scientific Software International; 1996. [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, Nesselroade JR. Emotional experience improves with age: Evidence based on over 10 years of experience sampling. Psychology and Aging. 2011;26:21–33. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. State operations manual. 2011 Retrieved from http://cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf.
- Chung JCC. Activity participation and well-being of people with dementia in long-term care settings. OTJR: Occupation Participation and Health. 2004;24:22–31. [Google Scholar]
- Clark LA, Watson D. Mood and the mundane: Relations between daily life events and self-reported mood. Journal of Personality and Social Psychology. 1988;54:296–308. doi: 10.1037//0022-3514.54.2.296. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- Daffner KR, Scinto LF, Weintraub S, Guinessey JE, Mesulam MM. Diminished curiosity in patients with probable Alzheimer’s disease as measured by exploratory eye movements. Neurology. 1992;42:320–328. doi: 10.1212/wnl.42.2.320. [DOI] [PubMed] [Google Scholar]
- Egloff B, Tausch A, Kohlmann CW, Krohne HW. Relationships between time of day, day of the week, and positive mood: Exploring the role of the mood measure. Motivation and Emotion. 1995;19:99–110. [Google Scholar]
- Eldridge LL, Masterman D, Knowlton BJ. Intact implicit habit learning in Alzheimer’s disease. Behavioral Neuroscience. 2002;116:722–726. [PubMed] [Google Scholar]
- Feldman LA. Valence focus and arousal focus: Individual differences in the structure of affective experience. Journal of Personality and Social Psychology. 1995;69:153–166. [Google Scholar]
- Ferretti L, McCurry SM, Logsdon R, Gibbons L, Teri L. Anxiety and Alzheimer’s disease. Journal of Geriatric Psychiatry & Neurology. 2001;14:52–58. doi: 10.1177/089198870101400111. [DOI] [PubMed] [Google Scholar]
- Finnema E, Dröes RM, Ribbe M, Van Tilburg W. The effects of emotion-oriented approaches in the care for persons suffering from dementia: A review of the literature. International Journal of Geriatric Psychiatry. 2000;15:141–161. doi: 10.1002/(sici)1099-1166(200002)15:2<141::aid-gps92>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Finnema E, Droes RM, Ettema T, Ooms M, Ader H, Ribbe M, van Tilburg W. The effect of integrated emotion-oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: A randomized clinical trial. International Journal of Geriatric Psychiatry. 2005;20:330–343. doi: 10.1002/gps.1286. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Götestan CS, Hsu AY. Emotion and aging: Experience, expression, and control. Psychology & Aging. 1997;12:590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Charles ST, Carstensen LL. Emotion and cognition. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, NJ: Erlbaum; 2000. pp. 593–631. [Google Scholar]
- Jones AL, Dwyer LL, Bercovitz AR, Strahan GW. The National Nursing Home Survey: 2004 overview. Vital and Health Statistics. 2009;13(167):1–155. [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29:374–393. [Google Scholar]
- Jonker C, Gerritsen DL, Bosboom PR, Van der Steen JT. A model for quality of life measures in patients with dementia: Lawton’s next step. Dementia and Geriatric Cognitive Disorders. 2004;18:159–164. doi: 10.1159/000079196. [DOI] [PubMed] [Google Scholar]
- Keyes CLM. Promoting and protecting mental health as flourishing: A complementary strategy for improving national mental health. American Psychologist. 2007;62:95–108. doi: 10.1037/0003-066X.62.2.95. [DOI] [PubMed] [Google Scholar]
- Kolanowski AM, Hoffman L, Hofer SM. Concordance of self-report and informant assessment of emotional well-being in nursing home residents with dementia. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:P20–P27. doi: 10.1093/geronb/62.1.p20. [DOI] [PubMed] [Google Scholar]
- Kolanowski AM, Litaker MS, Catalano PA. Emotional well-being in a person with dementia. Western Journal of Nursing Research. 2002;24:28–43. doi: 10.1177/01939450222045699. [DOI] [PubMed] [Google Scholar]
- Lawton MP. Quality of life in Alzheimer disease. Alzheimer Disease & Associated Disorders. 1994;8:138–150. [PubMed] [Google Scholar]
- Lawton MP, Van Haitsma K, Klapper J. Observed affect in nursing home residents with Alzheimer’s disease. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1996;51:3–14. doi: 10.1093/geronb/51b.1.p3. [DOI] [PubMed] [Google Scholar]
- Li F, Duncan TE, Duncan SC, Acock A. Latent growth modeling of longitudinal data: A finite growth mixture modeling approach. Structural Equation Modeling. 2001;8:493–530. [Google Scholar]
- Löckenhoff CE, Carstensen LL. Aging, emotion, and health-related decision strategies: Motivational manipulations can reduce age differences. Psychology and Aging. 2007;22:134–146. doi: 10.1037/0882-7974.22.1.134. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, DeKosky ST. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- Magai C, Cohen C, Gomberg D, Malatesta C, Culver C. Emotional expression during mid- to late-stage dementia. International Psychogeriatrics. 1996;8:383–395. doi: 10.1017/s104161029600275x. [DOI] [PubMed] [Google Scholar]
- May CP, Rahhal T, Berry EM, Leighton EA. Aging, source memory, and emotion. Psychology and Aging. 2005;20:571–578. doi: 10.1037/0882-7974.20.4.571. [DOI] [PubMed] [Google Scholar]
- Meeks S, Murrell SA. Contribution of education to health and life satisfaction in older adults mediated by negative affect. Journal of Aging and Health. 2001;13:92–119. doi: 10.1177/089826430101300105. [DOI] [PubMed] [Google Scholar]
- Meeks S, Van Haitsma K, Kostiwa I, Murrell SA. Positivity and well-being among community-residing elders and nursing home residents: What is the optimal affect balance? Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2012;67:460–467. doi: 10.1093/geronb/gbr135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Annals of Neurology. 2008;64:367–378. doi: 10.1002/ana.21534. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR, Wallace RB. Incidence of dementia and cognitive impairment, not dementia in the United States. Annals of Neurology. 2011;70:418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodewalt F, Zone JB. Appraisal of life change, depression, and illness in hardy and nonhardy women. Journal of Personality and Social Psychology. 1989;56:81–88. doi: 10.1037//0022-3514.56.1.81. [DOI] [PubMed] [Google Scholar]
- Scheibe S, Carstensen LL. Emotional aging: Recent findings and future trends. Journals of Gerontology Series B-Psychological Sciences & Social Sciences. 2010;65:135–144. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer RE. Problem perception, optimism, and related states as a function of time of day (diurnal rhythm) and moderate exercise: Two arousal systems in interaction. Motivation and Emotion. 1987;11:19–36. [Google Scholar]
- Thayer RE, Takahashi PJ, Pauli JA. Multidimensional arousal states, diurnal rhythms, cognitive and social processes, and extraversion. Personality and Individual Differences. 1988;9:15–24. [Google Scholar]
- Vogelpohl TS, Beck CK. Affective responses to behavioral interventions. Seminars in Clinical Neuropsychiatry. 1997;2:102–112. doi: 10.1053/SCNP00200102. [DOI] [PubMed] [Google Scholar]
- Yao L, Algase D. Emotional intervention strategies for dementia-related behavior: a theory synthesis. Journal of Neuroscience Nursing. 2008;40:106–115. doi: 10.1097/01376517-200804000-00010. [DOI] [PubMed] [Google Scholar]