Abstract
Animal studies show that CD36, a fatty acid translocase, is involved in fat detection and preference, but these findings have not been reported in humans. The objective of this study was to determine whether human genetic variation in 5 common CD36 polymorphisms is associated with oral fat perception of Italian salad dressings, self-reported acceptance of high-fat foods and obesity in African-American adults (n = 317). Ratings of perceived oiliness, fat content, and creaminess were assessed on a 170-mm visual analogue scale (VAS) in response to salad dressings that were 5%, 35%, and 55% fat-by-weight content. Acceptance of added fats and oils and high-fat foods was self-reported and anthropometric measures were taken in the laboratory. DNA was isolated from saliva and genotyped at 5 CD36 polymorphisms. Three polymorphisms, rs1761667, rs3840546, and rs1527483 were associated with the outcomes. Participants with the A/A genotype at rs1761667 reported greater perceived creaminess, regardless of the fat concentration of the salad dressings (P < 0.01) and higher mean acceptance of added fats and oils (P = 0.02) compared to those with other genotypes at this site. Individuals who had C/T or T/T genotypes at rs1527483 also perceived greater fat content in the salad dressings, independent of fat concentration (P = 0.03). BMI and waist circumference were higher in participants who were homozygous for a deletion (D/D) at rs3840546, compared to I/D or D/D individuals (P < 0.001), but only 2 D/D individuals were tested, so this finding needs replication. This is the first study to demonstrate an association between common variants in CD36 and fat ingestive behaviors in humans.
Introduction
The consumption of high-fat diets has been consistently linked to obesity (1), and lifestyle modifications that include increased fruit and vegetable intake and decreased total fat intake have been associated with reduced health risks (2). The factors contributing to increased fat preference and intake, however, are not well understood. Although fat is considered universally palatable to humans (3,4), both fat preference and consumption vary considerably across individuals. Several studies have suggested that genes may contribute to part of this variation (5–7).
Evidence has emerged from animals (8–10) and humans (11,12) that dietary fats, or more specifically fatty acids (FAs), can be perceived by the gustatory system and influence postingestive metabolism. Historically, the perception of dietary fat was thought to be a function of olfactory (13) and textural cues (e.g., oiliness and viscosity) (4,14,15). However, when these cues are masked, animals are still able to distinguish between fatty acid and control solutions (8). Similarly, Mattes has demonstrated that humans can detect a range of free fatty acids that vary in saturation and chain length when visual, olfactory, and textural differences in the samples are controlled (16). Taken together, these studies suggest that humans may have a gustatory mechanism for fat detection, as well.
One candidate oral fat sensor that has received considerable attention is the fatty acid translocase CD36 (homologous to fatty acid transporter in animals). CD36 is an 88 kDa membrane bound protein that is expressed in multiple cell types (17) and has a broad range of functions in immunity, inflammation, and lipoprotein metabolism (18). It is also involved with the transport of long-chain FAs across cell membranes, a first step in fat metabolism (19). Because of its integral role in FA uptake, CD36 has been associated with disruptions in lipoprotein metabolism in animals (20) and humans, including increased risk factors for cardiovascular disease (21) and the metabolic syndrome (22). CD36 is also expressed on taste cells in animals (23,24) and humans (25) where it is likely involved in the detection of FA in the oral cavity, though the mechanism of how this occurs has not been established.
In addition to its role in postoral fat metabolism, in animals, there is strong evidence to suggest that CD36 is involved with preference for this nutrient (24,26,27). CD36-null animals do not show preferences for linoleic acid over water whereas wild-type animals show spontaneous preferences for this FA (24,27). In addition, Sclafani et al. (27) showed that CD36 was required for naive rats to show preferences for triglycerides as well as FA but was not necessary for postoral conditioning of this nutrient. This suggests that CD36 is not only involved in FA detection, but also preference for triglyceride. At present, no studies have reported an association between CD36 and fat preferences in humans.
The primary objective of this study was to determine if there is an association between sequence at 5 common CD36 single-nucleotide polymorphisms (SNPs) and oral fat perception and reported liking of high-fat foods in an ethnic group at high risk for metabolic disease, African Americans. A secondary objective tested the associations between the same CD36 SNPs and adiposity. These five polymorphisms, described in detail in Table 1, were selected because they are common in African Americans and they have previously been associated with fat metabolism in humans (21).
Table 1.
C haracteristics of 5 common haplotype tagging CD 36 polymorphisms
| RfSNPID | Variation | Frequencya | Positionb | Location |
|---|---|---|---|---|
| rs1984112 | A>G | 0.32 | −33137 | 5′ flanking exon 1A |
| rs1761667 | G>A | 0.45 | −31118 | 5′ flanking exon 1A |
| rs1527483 | C>T | 0.12 | 25444 | Intron 11 |
| rs1049673 | G>C | 0.48 | 30294 | Exon 15 (3′-UTR) |
| rs3840546 | 16 bp del. | 0.13 | 27645 | Exon 14 (3′-UTR) |
Frequency of the allele substitution;
Relative to the translation start site on (hg37).
Methods and Procedures
Participants
Three hundred and seventeen (n = 317) African-American males (n = 137) and females (n = 180), ages 18–65 (mean ± s.d. = 35.5 ± 11.3), participated. African Americans were selected because this ethnic group is highly vulnerable to obesity and its related comorbidities and characterizing robust markers of fat preference in this population would improve the ability to identify those at greatest risk for disease (28,29).
Participants were recruited by placing advertisements on popular internet websites and through flyers posted around the hospital study site. Screening for potential participants was conducted on the phone. Exclusion criteria were: food allergies; major medical conditions such as diabetes, hypertension, or diagnosed metabolic syndrome; recent weight loss or dieting; or use of any medications known to affect taste, body weight, or appetite. Participants were also excluded if they were not African-American, as defined by self-report of two African-American biological parents. In addition, moderate to heavy smokers (defined as those smoking more than one pack per week) were excluded. At the end of the phone screening, eligible participants were instructed to fast for 2 h prior to their test session, and light smokers were instructed to abstain from smoking the day of their participation in the study. These instructions were also provided in writing via letters mailed to participants.
Subjects gave written informed consent prior to participation. Protocols were approved by the St. Luke’s Roosevelt Hospital Institutional Review Board. Research Authorization forms were used in compliance with the Health Insurance Portability and Accountability Act.
Study design
This was an exploratory study using a cross-sectional design. Participants (n = 317) attended a 1-h test session at the New York Obesity Research Center. This session included sensory tests to assess oral perception, anthropometric measures, food acceptability questionnaires, and saliva collection for processing of DNA.
Anthropometrics
Height and weight were measured and recorded to the nearest 0.25 inch and 0.5 pound, respectively, by trained researchers using a stadiometer and balance beam scale. Waist circumference was measured in the standing position immediately above the iliac crest and recorded to the nearest 0.25 inch. All anthropometric measures were taken without shoes and in light clothing. BMI was calculated for each subject by converting height and weight measures to m and kg, respectively, and applying the formula BMI = kg/m2.
Oral fat perception
Oral fat perception was assessed using Italian salad dressings prepared with varying amounts of canola oil (rich in long-chain fatty acids). Salad dressing was used because most adults are familiar with this food. Furthermore, commercially available salad dressings can vary widely in fat content, so consumers have experience with this product at a range of fat levels.
Taste test-stimuli
Salad dressings were prepared with Good Season’s Italian Dressing Mix (Kraft Foods Global, Northfield, IL), apple cider vinegar (H.J. Heinz, Pittsburg, PA), canola oil (Mazola, ACH Food Companies, Memphis, TN), and distilled water. Recipes were based on the work of Tepper and Nurse (15), and modified to create a low-fat sample (5% fat-by-weight), a medium-fat sample (35% fat-by-weight) and a high-fat sample (55% fat-by-weight). All dressings were mixed in a standard kitchen blender (Black and Decker, Model No. BL10450HB) on high power for 45 s per batch to ensure uniform consistency. For the 5 and 35% fat salad dressings, carrageenan (Viscarin SD 389, FMC, Philadelphia, PA) was added as a thickener. The dressings were not noticeably different in viscosity, mouthfeel, and texture, which was confirmed by informal pilot testing with research staff. Recipes are available from the authors upon request.
Oral fat perception
Participants were presented with the 5, 35, and 55% fat salad dressings, served in a randomized order in black, 2-oz souffle cups on a plastic serving tray. They were instructed to taste the salad dressings in the order given by taking a small spoonful and keeping the dressing in their mouths for 5 s before expectorating. After sampling the dressing, participants rated perceived oiliness, perceived fat content, and perceived creaminess on a 170-mm visual analogue scale (VAS) anchored on the ends with “extremely low” and “extremely high.” Between samples, participants consumed an unsalted saltine cracker and distilled water to cleanse the palate and were given a 2-min rest period to prevent fatigue. All testing was done under red light to mask visual differences between the salad dressings, but participants did not wear nose clips because we wanted the procedure to have ecological validity to a real eating experience. Following the procedure, a ruler was used to measure the distance between the left anchor and the participant’s response.
Questionnaires
Participants self-reported acceptability of 83 fat containing foods using a questionnaire developed by our laboratory. In developing this questionnaire, we included foods with a range of physical forms (e.g., fluid, solid, semisolid), sensory characteristics (e.g., sweet fats, savory fats) and nutritional properties (e.g., saturated fats, polyunsaturated fats, full fat, and fat-reduced). Participants rated degree of liking of each food on a 170-mm VAS anchored with “dislike extremely” and “like extremely,” with higher numerical ratings denoting greater liking for a food.
For analysis in the present study, two food groups were created to capture preferences for the highest fat food choices: added fats and oils and high-fat foods. Added fats and oils consisted of: butter, half-and-half, sour cream, canola oil, lard, mayonnaise, margarine, olive oil, full-fat salad dressings, and vegetable oil. High-fat foods consisted of: bacon, ground beef, steak, fried chicken, hot dogs, pork (fattier cuts), salami, sausage, cheeses, cake, chips, cookies, muffins, doughnuts, potato chips, corn chips, Danish, and French fries. The rationale for creating the former group was to capture intake of foods that contribute large amounts of fat to the diet. In addition, added fats and oils are frequently used in cooking and meal preparation to enhance the flavor of foods, but increased intake of these food sources has been linked to obesity and other chronic diseases (30). The group of high-fat foods was created to capture foods not included in the added fats and oils group that may also contribute to an unhealthy eating pattern. We included all foods that were high in fat content (>15 g fat/100 g serving), but we excluded nuts, nut butters, avocado, and fatty fishes because of the health benefits associated with these foods. In short, we created two distinct high-fat food groups that have been associated with poor dietary intake and health outcomes. Identifying genetic markers for these specific types of foods may be useful for designing more precise dietary guidance for future intervention studies. In addition, because foods are complex and contain more than one major sensory quality, we focused our analyses on the highest fat choices to better capture acceptability of foods where fat is a major sensory property.
Participants also completed the Three Factor Eating Questionnaire to assess dietary restraint and disinhibition (31). The restraint subscale measures the tendency to intentionally restrict food intake in order to control weight. The disinhibition subscale measures the tendency to lose control over eating due to emotional states or social cues. These cognitive variables are known to impact food intake (32) and body weight (33), so they were treated as covariates in the final analyses.
DNA collection and genotyping
Saliva samples of ~4 ml total volume were collected and DNA was extracted according to manufacturer’s instructions with Oragene DNA Self Collection Kits (DNA Genotek, Ontario, Canada).
PCR was used to amplify DNA fragments in 20 μl reaction volumes with 100 ng genomic DNA, 1× reaction buffer (Boehringer Mannheim) containing [MgCl2] 1.5 mmol/l, 0.25 mmol/l each dNTP, 100 ng of each PCR primer (available upon request), and 1 U Taq polymerase. All thermocycling was performed with 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C, for 30 s, and extension at 72 °C for 30 s. Amplicons of ~200 bp were amplified from genomic DNA using a biotin labeled primer (available upon request) and subsequently purified using streptavidin beads (Amersham Biosciences, Uppsala, Sweden).
Genotyping of 4 polymorphisms at CD36 (rs1984112, rs1761667, rs1527483, and rs1049673) was performed by pyrosequencing according to the manufacturer’s recommended protocol (PSQ96 Biotage, Westborough, MA). Genotyping of rs3840546 was performed by electrophoresis through a 3% agarose gel to score the 16 bp deletion.
Observed allele frequencies at each of the 5 CD36 polymorphisms are found in Table 2 and linkage disequilibrium between the variants in Table 3. The observed frequencies did not differ from Hardy–Weinberg equilibrium at any of five sites.
Table 2.
O bserved allele frequencies for CD36 polymorphisms
| CD36 SNPa | Number of subjects (n) | Percentage (%) |
|---|---|---|
| rs1984112 | ||
| A/A | 165 | 53.6 |
| G/A | 124 | 40.3 |
| G/G | 19 | 6.2 |
| rs1761667 | ||
| A/A | 59 | 19.2 |
| G/A | 152 | 49.4 |
| G/G | 97 | 31.5 |
| rs1527483 | ||
| C/C | 288 | 93.5 |
| C/T | 18 | 5.8 |
| T/T | 2 | 0.6 |
| rs1049673 | ||
| C/C | 201 | 65.5 |
| C/G | 88 | 28.7 |
| G/G | 18 | 5.9 |
| rs3840546 | ||
| I/I | 279 | 91.0 |
| I/D | 25 | 8.2 |
| D/D | 2 | 0.7 |
Missing data due to failed genotyping across 1 or more sites. For rs1984112, rs1761667, and rs1527483, n = 308. For rs1049673, n = 307. For rs3840546, n = 306.
Table 3.
Linkage disequilibrium between CD36 variants
| Variant 1 | Variant 2 | D′ | r2 |
|---|---|---|---|
| rs1984112 | rs1761667 | 0.84 | 0.20 |
| rs1984112 | rs1527483 | 0.58 | 0.01 |
| rs1984112 | rs3840546 | 1.00 | 0.02 |
| rs1984112 | rs1049673 | 0.15 | 0.02 |
| rs1761667 | rs1527483 | 0.08 | 0 |
| rs1761667 | rs3840546 | 0.69 | 0.03 |
| rs1761667 | rs1049673 | 0.33 | 0.03 |
| rs1527483 | rs3840546 | 1.00 | 0.002 |
| rs1527483 | rs1049673 | 1.00 | 0.11 |
| rs3840546 | rs1049673 | 1.00 | 0.02 |
Statistics
Descriptive statistics (means, SDs and SEs) were calculated on all continuous variables (e.g., BMI, age) and frequencies were calculated on categorical variables (e.g., sex). For each SNP, data were analyzed by treating the genotype independently (e.g., A/A vs. G/A vs. G/G). In the case of rs1527483, ratings of oral fat perception did not differ significantly for perceived oiliness (P = 0.50), perceived fat content (P = 0.98) and perceived creaminess (P = 0.54) between C/T and T/T genotypes, so these groups were combined to maximize the number of subjects. General linear model (GLM) repeated measures ANOVA and analysis of covariance were used to test for differences in perceived oiliness, fat content, and creaminess as a function of CD36 genotype. In addition, two-way ANOVA and analysis of covariance were used to test for interaction effects between fat concentration of the salad dressing and CD36 genotype. General linear model ANOVA and analysis of covariance were used to test for differences in reported liking of added fats and oils, high-fat foods, and adiposity (BMI and waist circumference). For each model, a cutoff of P ≤ 0.10 was used for determining the covariates to include. For oral fat perception, covariates included age, sex, and reported intake of Italian salad dressing assessed from a food frequency questionnaire. For liking of added fats and high-fat foods, covariates included dietary restraint and disinhibition. For BMI and waist circumference, covariates included age, dietary restraint, disinhibition, and sex. All main effects are presented as adjusted values. In addition, reported fat liking was also included in models where BMI or waist circumference was the dependent variable to determine if this variable mediated the relationship between the CD36 variant and adiposity.
A P value <0.05 was the cutoff for significance in the ANOVA models. All hypotheses were two-tailed. When appropriate, Scheffé post hoc tests were used to correct for multiple comparisons. All data were analyzed with SPSS, version 18.0 (SPSS, Chicago, IL) for Windows XP.
Results
Participant characteristics
Characteristics of study males (n = 137) and females (n = 180) are reported in Table 3. Only waist circumference differed between these two groups (P < 0.05). For all subjects, mean age was 35.3 ± 11.3 years and BMI was 29.2 ± 6.9 kg/m2. Over 40% of males and 35% of females had BMIs that were in the obese range (Table 4).
Table 4.
Participant characteristics
| Variable | Males (n = 137) | Females (n = 180) |
|---|---|---|
| BMI | n (%) | n (%) |
| BMI < 25.0 | 38 (27.7) | 60 (33.3) |
| BMI 25.0–29.9 | 42 (30.7) | 54 (30.0) |
| BMI ≥30.0 | 57 (41.6) | 66 (36.7) |
| Variable | Mean ± s.d. | Mean ± s.d. |
| Age (year) | 36.2 ± 10.6 | 34.6 ± 11.8 |
| BMI (kg/m2) | 29.8 ± 6.2 | 28.8 ± 7.4 |
| Waist circumference—cma | 97.0 ± 15.6 | 92.5 ± 16.5 |
Significantly different from one another at P < 0.05.
Relationship between CD36 polymorphisms at rs1761667 and oral fat perception
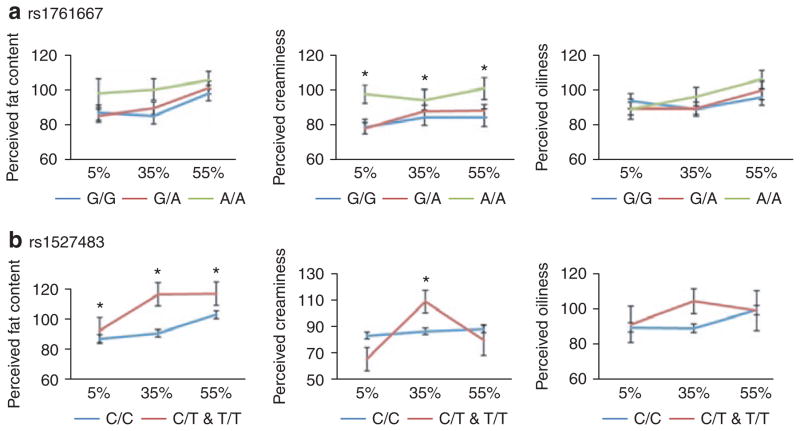
Variation at rs1761667 was associated with perceived creaminess ratings, independent of the fat concentration of the salad dressing (F(2,305) = 5.5; P < 0.01; main effect). According to Scheffé post hoc test, A/A individuals perceived more creaminess in the salad dressing samples than G/A (P = 0.03) and G/G individuals (P = 0.02), regardless of fat concentration of the dressing. There was also a nonsignificant trend for A/A individuals to perceive greater fat content in the salad dressings, compared to G/A and G/G individuals (F(2,305) = 1.8; P = 0.15; main effect). For both perceived creaminess and fat content, the interactions between rs1761667 genotype and fat concentration of the salad dressing were not significant. There were no differences in perceived oiliness as a function of rs1761667 genotype (P = 0.65) (Figure 1a).
Figure 1.
Oral fat perception ratings for 5, 35, and 55% fat-by-weight Italian salad dressings as a function of CD36 genotype at (a) rs1761667 and (b) rs1527483. (a) Ratings for perceived fat content, creaminess, and oiliness as a function of rs1761667 genotype and fat concentration (5, 35, and 55% fat-by-weight). There was a main effect of rs1761667 genotype on ratings of perceived creaminess (F(2,305) = 5.5; P < 0.01). A/A individuals perceived greater creaminess than G/A (P = 0.03) and G/A (P = 0.02) individuals (post hoc, Scheffé). (b) Ratings for perceived fat content, creaminess, and oiliness as a function rs1527483 genotype. There was a main effect of rs1527483 genotype on ratings of perceived fat content (F(1,306) = 4.5; P = 0.02). C/T and T/T individuals (n = 20) tended to perceive greater fat content than C/C individuals (n = 288). There was a significant interaction between rs1527483 genotype and fat concentration on ratings of perceived creaminess (F(2,306) = 3.3; P = 0.04; interaction effect). C/T and T/T individuals rated the 35% fat salad dressing as creamier than C/C individuals (P < 0.05; post hoc, Scheffé).
Other CD36 SNPs
There were no differences in oral fat perception as a function of CD36 genotype at rs1049673, rs1984112, or rs3840546. Results for oral fat perception ratings at rs1527483 are presented in Figure 1b. At rs1527483, there was an overall effect of genotype on ratings of fat content across all samples (F(1,306) = 4.5; P = 0.02; main effect). Individuals who had the C/T or T/T genotypes tended to give higher ratings of fat content than those who had the C/C genotype, regardless of the fat concentration of the salad dressing. For ratings of perceived creaminess, there was a significant interaction between genotype at rs1527483 and fat concentration of the salad dressing (F(2,306) = 3.3; P = 0.04; interaction effect). Individuals who had C/T or T/T genotypes tended to perceive greater creaminess than C/C individuals, but this was only significant at the 35% fat salad dressing (Scheffé post hoc test; P < 0.05).
Relationship between CD36 polymorphisms and reported fat acceptance
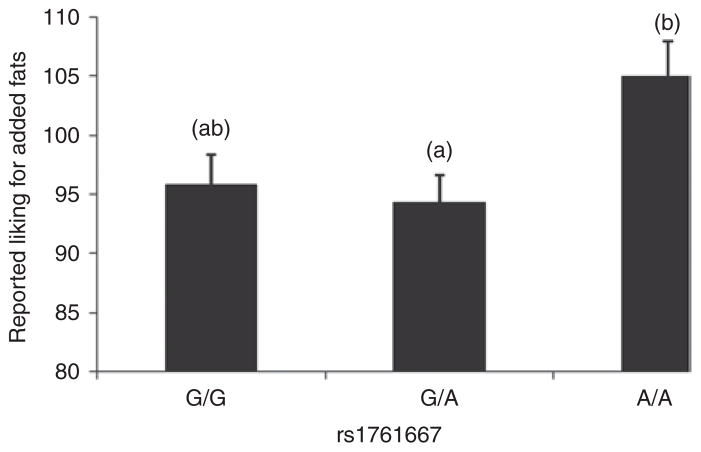
Variation at rs1761667 was associated with mean reported liking for added fats and oils (F(2,307) = 3.73; P = 0.02). According to a Scheffé post hoc analysis, A/A individuals reported greater liking of added fats than G/A individuals, with means ± s.d. of 104.8 ± 23.3 and 93.9 ± 27.8 mm, respectively. The difference in reported acceptability for added fats between A/A and G/G individuals was not significant, according to Scheffé adjustment (P = 0.12) (Figure 2).
Figure 2.
Reported preferences for added fats and oils differed as a function of genotype at rs1761667 (F(2,307) = 3.73; P < 0.05). Individuals who have the A/A genotype (n = 59) reported higher preferences for added fats and oils compared to individuals who had the G/A genotype (n = 152), according to Sheffé post hoc analysis (P = 0.02). Models are adjusted for BMI, dietary restraint and disinhibition. Superscripts above error bars depict significant differences.
Mean liking for high-fat foods showed a similar pattern, with mean ± s.d. for A/A, G/A, and G/G equal to 123.4 ± 25.1, 116 ± 26.9, and 113.5 ± 28.8 mm, respectively. However, the P value for the latter relationship did not reach significance (P = 0.18).
Relationship between CD36 polymorphisms and obesity
Variation at rs3840546 was associated with body weight. After adjusting for covariates, individuals with two (D/D) deletions at rs3840546 had higher BMIs than I/I homozygotes and I/D heterozygotes (F(1,304) = 13.1; P < 0.001). This effect was driven by the 2 D/D individuals who had a mean BMI of 52.4 ± 7.8 kg/m2, as compared to 31.1 ± 6.4 kg/m2 found in I/D individuals and 28.9 ± 6.7 kg/m2. However, due to the small sample size of the D/D group (n = 2), this finding should be interpreted with caution and replication in a larger group is needed.
Waist circumference also differed at rs3840546 (F(1,304) = 10.5; P < 0.001). After adjusting for age, sex, and cognitive attempts to control diet, mean ± s.d. waist circumferences for D/D individuals was 143.5 ± 10.8 cm and this was significantly greater than in I/Ds (98.5 ± 14.9; P = 0.001) and I/Is (93.9 ± 16.0; P < 0.001).
Relationships between BMI and waist circumference and rs3840546 genotype were also examined by adjusting the models for reported liking of high-fat foods. This was done to determine if the relationship between rs3840546 genotype and obesity was mediated by liking of high-fat foods. Separate models were calculated for liking of added fats and oils and high-fat foods. For both measures, inclusion of reported fat liking did not change the P value for the effect due to rs3840546 genotype.
There was an overall effect of rs1527483 genotype on BMI (F(1,304) = 3.1; P = 0.05), but the relationship was not strong enough to withstand post hoc analysis. The presence of one (C/T) or two (T/T) alleles at this site appeared to be protective, as mean BMIs in C/C, C/T, and T/T individuals was 29.5 ± 6.9, 26.7 ± 6.3, and 19.5 ± 1.8 kg/m2, respectively.
Discussion
This was an exploratory study reporting two novel findings. First, genotypes at two SNPs in the CD36 gene, rs1761667 and rs1527483, were associated with oral fat perception. At rs1761667, African Americans who had the A/A genotype perceived Italian salad dressings to be creamier than did G/A and G/G individuals, regardless of how much fat was actually in the salad dressing. This relationship was independent of age, sex, and reported intake of Italian salad dressing in the diet. Second, rs1761667 genotype was also associated with reported acceptance of added fats and oils, a group of foods which includes cooking oils, spreads, and full-fat salad dressings. African Americans who carried the A/A genotype at this site reported greater liking of added fats and oils when compared to G/A, but not G/G individuals, according to Sheffé post hoc analysis. However, average liking ratings by G/A and G/G individuals were not significantly different from one another, so it is possible that in a larger cohort, an effect might have been seen with G/G individuals as well. This relationship was independent of not only BMI, but also cognitive attempts to control food intake. The fact that CD36 showed a stronger relationship to liking of added fats than to high-fat foods in general is interesting because the former group consists of foods that are predominantly composed of triglycerides, such as butter, margarine, and cooking oils. Although fatty acid composition, processing, and source of the oil (34), can influence the overall flavor of these fats, their predominant sensory property is “fatty.” If CD36 is involved with development of fatty acid and triglyceride preferences as shown in animals (24,27), we might expect a stronger association between variation in the gene and reported liking of fats and oils than for more complex high-fat foods that provide other sensory qualities like sweetness (e.g., cookies) or saltiness (e.g., potato chips). Alternatively, most added fats and oils are fluids, and studies have demonstrated that it is easier to perceive fat in this physical form than in solid foods (35). The possibility that CD36 may be a gene target associated with fat intake, through a mechanism of oral fat perception and preference, warrants additional investigation.
Although it is not possible to draw firm conclusions, some speculation as to why A/A individuals like added fats and oils more than G/A and G/G individuals can be made from close examination of the sensory data collected in this study. Although A/A individuals do not appear to discriminate that accurately between salad dressings that range in fat content from 5 to 55% as evidenced by nearly identical perceived fat and creaminess ratings given across the three samples, they perceive the samples to be creamier than do individuals who have other genotypes at this allele. Creaminess is a complex sensory characteristic that consists of both flavor and textural components (14), but overall, it is perceived as a positive attribute of foods that contain fat. In studies that have collected data on both perceived creaminess and liking, there is generally a positive relationship between the two. Most of these studies have examined relationships in ice creams (36,37), or other fluid dairy products (38), however, it is not known if the same relationship exists for oil-based salad dressings. Further complicating this relationship, “creaminess” can be used by consumers to describe both flavor and textural aspects of foods, so identifying the source of variation in this attribute can be difficult. The notion that A/A individuals may like added fats and oils because they perceive them to be creamier is a question that warrants further study. This is especially important because, as in the present study, creaminess can be achieved not only with fat, but also by increasing emulsifiers such as carrageenan. Given that fatty acids are ligands for CD36, it is possible that differences in perceived creaminess ratings between different genotype groups are due to differences in the ability to detect small amounts of free fatty acids in the samples, and as a result, textural attributes like creaminess become more pronounced. Additional studies are needed to confirm this.
The rs1761667 polymorphism that was associated with both oral fat perception and reported fat acceptance in the current study is in the promoter region of the CD36 gene. In whites, G is the minor allele, whereas in African Americans, A is the minor allele. In the present study, the CD36 variant that best predicted oral fat perception and acceptance (rs1761667) occurred at high frequency, with the homozygous “at risk” genotype (A/A) present in about 20% of individuals. These findings suggest that variation in CD36 may represent an important marker for fat perception and acceptance in African Americans, and the impact this may have on metabolic health risks should be further investigated. Ma et al. (21) analyzed identical SNPs to those studied here and found that Italian males with the G/G genotype had higher free fatty acids and triglyceride levels. Similarly, Madden et al. (39) published findings in a small, nonhomogeneous cohort from the UK and demonstrated that G/G individuals were also less likely to show improvements in plasma triacylglycerol levels after a fish oil rich dietary intervention when compared to G/A and A/A individuals. The totality of available evidence suggests that presence of the A/A genotype at rs1761667 may reduce CD36 protein expression and, because of this, confer postingestive benefits on lipid metabolism. The fact that we demonstrated that African Americans with the A/A genotype showed higher preferences for added fats and oils, a dietary behavior that can be associated with increased, not decreased health risk, may suggest that our findings are due to linkage disequilibrium between this and another SNP that was not genotyped. The linkage disequilibrium may differ between whites and African Americans and thus account for the difference in allelic association. Alternatively, because fat preferences are affected by both oral and postoral responses to this nutrient, it is possible that differential postoral responses to fat between A/A vs. G/A & G/G individuals may be mediating this effect. It should be noted, however, that the lipid profiles of the individuals in this study were not tested, and future studies are needed to clarify the relationship between variation at rs1761667 and cardiovascular health in African Americans.
A secondary aim of this study tested the association between 5 CD36 SNPs and adiposity. Presence of the minor allele at rs1527483 was associated with both increased perceived ratings of fat content in Italian salad dressings and decreased BMI, although the latter relationship was only a trend. Stewart et al. (40) recently published data that showed an inverse relationship between oral fatty acid sensitivity and BMI. Participants who were classified as “hypersensitive” to oleic acid had lower BMIs than those classified as “hyposensitive.” Previously, we reported a similar relationship from this cohort; African Americans who were poor at discriminating differences in the fat content of salad dressings had higher BMIs than those who were able to discriminate fat content more accurately (41). In the present study, ratings of perceived fat content in the salad dressing were included in the model as a covariate and this reduced the P value of the relationship between rs1527483 genotype and BMI from P = 0.05 to P = 0.11. In multiple linear regression models that were run as exploratory analyses, perceived fat content ratings of the 35% and 55% fat salad dressings explained ~15% of the total variance in BMI, whereas rs1527483 genotype explained only 11%. However, BMI did not change the significance of the relationship between rs1527483 genotype and perceived fat content when it was included in the model. Taken together with findings from Stewart et al. (40), we speculate that the rs1527483 genotype may impact body weight in part by impacting oral fat perception. An increased oral sensitivity to the fat content of the diet may help consumers detect small changes in fat intake, and as a result, they may be less likely to consume excess amounts of this nutrient. These findings are preliminary and need to be confirmed in larger cohorts.
We also identified another SNP associated with obesity in this cohort that has not been identified previously, rs3840546. Several recent reports have shown relationships between CD36 polymorphisms and BMI in European adolescents (42) European adults, (43) and Korean adults (44), but not all reports have been consistent (45). This is the first study to report an association between rs3840546 and obesity in an African-American population. The mechanisms that mediate the effects of CD36 on body weight are not known, but differences in the metabolic availability of fatty acids for storage vs. oxidation in “at risk” individuals is one possibility (46). In our study, individuals who had a deletion at rs3840546 had mean BMIs that were over 30 kg/m2, which is associated with significant health risks (47). In addition, carrying a deletion at rs3840546 was also associated with increased waist circumference, of significance because the presence of increased abdominal adiposity is associated with greater morbidity than elevated BMI alone (48). One important caveat to mention is that our cohort was small, consisting of only 25 heterozygous (I/Ds) and 2 homozygous (D/D) for the “at risk” genotype. Testing the impact of this SNP on body weight in larger cohorts is necessary to confirm these findings.
The present study had several limitations. First, it was conducted in African Americans, a single ethnicity group. It is unclear if these results will generalize to other ethnic groups. Second, fat preferences were self-reported, and these measures have well-known biases (49). In addition, we did not collect fat liking measures in response to the Italian salad dressings used for sensory testing and doing so could have shed additional light on the relationship between oral fat perception and acceptance. Moreover, for a genetic association study, our sample was small and results are not corrected for multiple testing. There is a debate about the extent to which correction for multiple testing should be applied in exploratory studies (50). Still, results should be interpreted with caution and confirmation studies are needed. Further, an association study is not able to verify the functional significance of these polymorphisms, but is only able to identify regions of the gene that might warrant more rigorous follow-up investigations.
Conclusion
The fatty acid translocase, CD36, has a range of functions in multiple tissues, including recent studies in animals that report an important role in fat preferences. This study provides preliminary evidence that CD36 is involved with oral fat perception and liking of added fats and oils in humans. In addition, we found associations between variation at the CD36 gene and adiposity. At present, these findings suggest that perception and preference for some dietary fats may be in part explained by common heritable variation in African Americans. If these findings are supported by future investigations, the CD36 genotype may be a useful genetic marker of excess fat consumption in an environment in which these foods are plentiful.
Acknowledgments
Funding for this study came from NIH grant K01DK068008 and an NIH/NIDDK Pilot and Feasibility Award (K.L.K.). Additional support came from the Obesity Research Center Grant (NIH grant 5P30DK026687-27) and from DK52431, DK63608, and DK26687 (W.K.C.).
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav. 2004;83:549–555. doi: 10.1016/j.physbeh.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296:695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 3.Drewnowski A, Greenwood MR. Cream and sugar: human preferences for high-fat foods. Physiol Behav. 1983;30:629–633. doi: 10.1016/0031-9384(83)90232-9. [DOI] [PubMed] [Google Scholar]
- 4.Drewnowski A. Sensory properties of fats and fat replacements. Nutr Rev. 1992;50:17–20. doi: 10.1111/j.1753-4887.1992.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 5.Bauer F, Elbers CC, Adan RA, et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90:951–959. doi: 10.3945/ajcn.2009.27781. [DOI] [PubMed] [Google Scholar]
- 6.Collaku A, Rankinen T, Rice T, et al. A genome-wide linkage scan for dietary energy and nutrient intakes: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) Family Study. Am J Clin Nutr. 2004;79:881–886. doi: 10.1093/ajcn/79.5.881. [DOI] [PubMed] [Google Scholar]
- 7.Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA. Heritable variation in food preferences and their contribution to obesity. Behav Genet. 1997;27:373–387. doi: 10.1023/a:1025692031673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuwatari T, Shibata K, Iguchi K, et al. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol Behav. 2003;78:579–583. doi: 10.1016/s0031-9384(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 9.Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- 10.Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann N Y Acad Sci. 1998;855:165–168. doi: 10.1111/j.1749-6632.1998.tb10560.x. [DOI] [PubMed] [Google Scholar]
- 11.Mattes RD. Oral fat exposure alters postprandial lipid metabolism in humans. Am J Clin Nutr. 1996;63:911–917. doi: 10.1093/ajcn/63.6.911. [DOI] [PubMed] [Google Scholar]
- 12.Mattes RD. The taste of fat elevates postprandial triacylglycerol. Physiol Behav. 2001;74:343–348. doi: 10.1016/s0031-9384(01)00578-9. [DOI] [PubMed] [Google Scholar]
- 13.Kinney NE, Antill RW. Role of olfaction in the formation of preference for high-fat foods in mice. Physiol Behav. 1996;59:475–478. doi: 10.1016/0031-9384(95)02086-1. [DOI] [PubMed] [Google Scholar]
- 14.Mela DJ. Sensory assessment of fat content in fluid dairy products. Appetite. 1988;10:37–44. doi: 10.1016/s0195-6663(88)80031-x. [DOI] [PubMed] [Google Scholar]
- 15.Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiol Behav. 1997;61:949–954. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 16.Mattes RD. Oral thresholds and suprathreshold intensity ratings for free fatty acids on 3 tongue sites in humans: implications for transduction mechanisms. Chem Senses. 2009;34:415–423. doi: 10.1093/chemse/bjp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 20.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Bacci S, Mlynarski W, et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum Mol Genet. 2004;13:2197–2205. doi: 10.1093/hmg/ddh233. [DOI] [PubMed] [Google Scholar]
- 22.Love-Gregory L, Sherva R, Sun L, et al. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet. 2008;17:1695–1704. doi: 10.1093/hmg/ddn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuwatari T, Kawada T, Tsuruta M, et al. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–464. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- 24.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons PJ, Kummer JA, Luiken JFP, Boon L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochemica. 2010 doi: 10.1016/j.acthis.2010.08.006.. [DOI] [PubMed] [Google Scholar]
- 26.Gaillard D, Laugerette F, Darcel N, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–R1832. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 28.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89:2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 29.Ogden CL. Disparities in obesity prevalence in the United States: black women at risk. Am J Clin Nutr. 2009;89:1001–1002. doi: 10.3945/ajcn.2009.27592. [DOI] [PubMed] [Google Scholar]
- 30.Halkjaer J, Tjønneland A, Overvad K, Sørensen TI. Dietary predictors of 5-year changes in waist circumference. J Am Diet Assoc. 2009;109:1356–1366. doi: 10.1016/j.jada.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 32.Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obes Rev. 2008;9:409–419. doi: 10.1111/j.1467-789X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 33.Paradis AM, Godin G, Lemieux S, Pérusse L, Vohl MC. Eating behaviours of non-obese individuals with and without familial history of obesity. Br J Nutr. 2009;101:1103–1109. doi: 10.1017/S0007114508055645. [DOI] [PubMed] [Google Scholar]
- 34.Chow C. Fatty Acids in Foods and their Health Implications. New York: Marcel Dekker; 1992. [Google Scholar]
- 35.Drewnowski A, Shrager EE, Lipsky C, Stellar E, Greenwood MR. Sugar and fat: sensory and hedonic evaluation of liquid and solid foods. Physiol Behav. 1989;45:177–183. doi: 10.1016/0031-9384(89)90182-0. [DOI] [PubMed] [Google Scholar]
- 36.Lahteenmaki L, Tuorila H. Liking for ice cream measured with three procedures: side-by-side, after consumption and single samples 1747. J Sensory Stud. 1994;4:455–465. [Google Scholar]
- 37.Prescott J, Bell GA, Gillmore R, et al. Cross-cultural comparisons of Japanese and Australian responses to manipulations of sweetness in foods 1749. Food Qual Pref. 1997;8:45–55. [Google Scholar]
- 38.Richardson-Herman NJ, Stevens R, Walker S, et al. Mapping consumer perceptions of creaminess and liking for liquid dairy products 1748. Food Qual Pref. 2000;11:239–246. [Google Scholar]
- 39.Madden J, Carrero JJ, Brunner A, et al. Polymorphisms in the CD36 gene modulate the ability of fish oil supplements to lower fasting plasma triacyl glycerol and raise HDL cholesterol concentrations in healthy middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78:327–335. doi: 10.1016/j.plefa.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Stewart JE, Feinle-Bisset C, Golding M, et al. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104:145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 41.Breen CL, MacDougall MC, Tepper BJ, McLean J, May D. Decreased ability to discriminate differences in fat content of Italian salad dressings is associated with increased levels of obesity in healthy African-Americans. Chemical Senses. 2008;33:P359. (abstr) [Google Scholar]
- 42.Bokor S, Legry V, Meirhaeghe A, et al. Single-nucleotide polymorphism of CD36 locus and obesity in European adolescents. Obesity. 2009;1038:1–6. doi: 10.1038/oby.2009.412. [DOI] [PubMed] [Google Scholar]
- 43.Heni M, Müssig K, Machicao F, et al. Variants in the CD36 gene locus determine whole-body adiposity, but have no independent effect on insulin sensitivity. Obesity (Silver Spring) 2011;19:1004–1009. doi: 10.1038/oby.2010.251. [DOI] [PubMed] [Google Scholar]
- 44.Yun YM, Song EY, Song SH, Song J, Kim JQ. CD36 polymorphism and its relationship with body mass index and coronary artery disease in a Korean population. Clin Chem Lab Med. 2007;45:1277–1282. doi: 10.1515/CCLM.2007.270. [DOI] [PubMed] [Google Scholar]
- 45.Choquet H, Labrune Y, De Graeve F, et al. Lack of association of CD36 SNPs with early onset obesity: a meta-analysis in 9,973 European subjects. Obesity (Silver Spring) 2011;19:833–839. doi: 10.1038/oby.2010.226. [DOI] [PubMed] [Google Scholar]
- 46.Bonen A, Parolin ML, Steinberg GR, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 47.Pi-Sunyer FX. Health implications of obesity. Am J Clin Nutr. 1991;53:1595S– 1603S. doi: 10.1093/ajcn/53.6.1595S. [DOI] [PubMed] [Google Scholar]
- 48.Shen W, Punyanitya M, Chen J, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity (Silver Spring) 2006;14:727–736. doi: 10.1038/oby.2006.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am J Epidemiol. 2008;167:1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 50.Bendotti C, Garattini S, Samanin R. Eating caused by neuropeptide-Y injection in the paraventricular hypothalamus: response to (+)-fenfluramine and (+)-amphetamine in rats. J Pharm Pharmacol. 1987;39:900–903. doi: 10.1111/j.2042-7158.1987.tb03126.x. [DOI] [PubMed] [Google Scholar]