Abstract
Horizontal cells are lateral interneurons that participate in visual processing in the outer retina but the cellular mechanisms underlying transmitter release from these cells are not fully understood. In non-mammalian horizontal cells, GABA release has been shown to occur by a non-vesicular mechanism. However, recent evidence in mammalian horizontal cells favors a vesicular mechanism as they lack plasmalemmal GABA transporters and some soluble NSF attachment protein receptor (SNARE) core proteins have been identified in rodent horizontal cells. Moreover, immunoreactivity for GABA and the molecular machinery to synthesize GABA have been found in guinea pig horizontal cells, suggesting that if components of the SNARE complex are expressed they could contribute to the vesicular release of GABA. In this study we investigated whether these vesicular and synaptic proteins are expressed by guinea pig horizontal cells using immunohistochemistry with well-characterized antibodies to evaluate their cellular distribution. Components of synaptic vesicles including vesicular GABA transporter, synapsin I and synaptic vesicle protein 2A were localized to horizontal cell processes and endings, along with the SNARE core complex proteins, syntaxin-1a, syntaxin-4 and synaptosomal-associated protein 25 (SNAP-25). Complexin I/II, a cytosolic protein that stabilizes the activated SNARE fusion core, strongly immunostained horizontal cell soma and processes. In addition, the vesicular Ca2+-sensor, synaptotagmin-2, which is essential for Ca2+-mediated vesicular release, was also localized to horizontal cell processes and somata. These morphological findings from guinea pig horizontal cells suggest that mammalian horizontal cells have the capacity to utilize a regulated Ca2+-dependent vesicular pathway to release neurotransmitter, and that this mechanism may be shared among many mammalian species.
Keywords: mammalian visual system, retina, synaptic proteins, synaptic vesicle
Introduction
Horizontal cells play an important, although not fully understood, role in visual information processing by interacting with photoreceptors and bipolar cells in the outer plexiform layer (OPL). There are two types of horizontal cells in the mammalian retina that separately serve the cone and rod pathways. The dendrites of B-type horizontal cells contact cones and their axon terminal system contacts rods, whereas A-type horizontal cells contact cones exclusively as they have no axon terminals (for review see Peichl et al., 1998); both types are immunostained by antibodies to calbindin (Uesugi et al., 1992; Peichl & González-Soriano, 1994; Raven & Reese, 2002; Hirano et al., 2007). Physiological evidence demonstrates that horizontal cells contribute to center-surround properties, at least in part, through feedback onto photoreceptors in some species (Baylor et al., 1971; O’Bryan, 1973; Burkhardt, 1977; Verweij et al., 2003; Babai & Thoreson, 2009) and feedforward onto bipolar cells in other species (Dowling & Werblin, 1969; Yang & Wu, 1991; Billups & Attwell, 2002; Zhang & Wu, 2009). Small, clear-core vesicles in horizontal cell tips that invaginate the synaptic triad have been demonstrated at the ultrastructural level (Dowling et al., 1966; Linberg & Fisher, 1988). The localization of the vesicular GABA transporter (VGAT) to horizontal cell endings in mammalian retinas (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Guo et al., 2009b) supports the view that horizontal cells can concentrate GABA into vesicles, as VGAT mediates the uptake and storage of GABA and glycine in neurons (Burger et al., 1991; Liu & Edwards, 1997; McIntire et al., 1997; Chaudhry et al., 1998; Gasnier, 2004). Mammalian horizontal cells have also been found to express L-type (Ueda et al., 1992; Löhrke & Hofmann, 1994; Rivera et al., 2001) and N-type (Schubert et al., 2006; Witkovsky et al., 2006) calcium channels, suggesting the possibility of a Ca2+-dependent vesicular release mechanism. Immunocytochemical studies demonstrate that GABAA or GABAC receptors or both (Vardi et al., 1992, 1994; Greferath et al., 1993, 1995; Grigorenko & Yeh, 1994; Enz et al., 1996; Wässle et al., 1998) are expressed in mammalian photoreceptors, bipolar cells and horizontal cells, suggesting that they may be potential targets of GABA released from horizontal cells.
Although a vesicular mechanism pertaining to the release of GABA from horizontal cells has not been established unequivocally, some protein components of the neuronal exocytotic machinery are expressed in mammalian horizontal cells. In central neurons, GABA release relies on Ca2+-dependent vesicular mechanisms (Olsen & Tobin, 1990; Macdonald & Olsen, 1994; Poncer et al., 1997). Moreover, new observations in guinea pig horizontal cells report the lack of plasmalemmal GABA transporter expression (Guo et al., 2009b) but the presence of GABA and the biosynthetic machinery to synthesize GABA (Guo et al., 2009a). Studies in some mammalian horizontal cells have identified soluble NSF attachment protein receptor (SNARE) proteins that are classically associated with synaptic vesicles and exocytosis, including synaptosomal-associated protein (SNAP-25) (Catsicas et al., 1992; Ullrich & Südhof, 1994; Grabs et al., 1996; von Kriegstein et al., 1999; Greenlee et al., 2001), syntaxin-1 (Nag & Wadhwa, 2001; Hirano et al., 2005), syntaxin-4 (Sherry et al., 2006; Hirano et al., 2007) and complexin I/II (Hirano et al., 2005). However, there have not been any studies evaluating these proteins comprehensively in a single animal model.
The aim of the present study was to address the hypothesis that guinea pig horizontal cells could release GABA through a Ca2+-dependent vesicular mechanism. The guinea pig is an emerging animal model for retina research and has been used to study the cellular organization and function of the retina, including ganglion cells (Demb et al., 1999), amacrine cells (Oh et al., 1999; Fujieda et al., 2000; Kao & Sterling, 2006) and Müller cells (Malgorzata Goczalik et al., 2005; Rillich et al., 2009), and is unique because it exhibits robust expression of proteins related to GABA neurotransmission (Guo et al., 2009a). This is the first study to systematically evaluate vesicular and synaptic-related proteins in this species, which is necessary to validate the guinea pig for future studies evaluating retinal anatomy and synaptic function. Our findings in a mammalian species other than rodents provide a basis for understanding common mechanisms underlying transmitter release from mammalian horizontal cells.
Materials and methods
Animals
Adult Hartley guinea pigs (CRL 051) of either sex were purchased from Charles River Laboratories (Wilmington, MA, USA). All experiments were performed in accordance with the guidelines for the welfare of experimental animals issued by the UCLA Animal Research Committee and the U.S. Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Guinea pigs used for retinal tissue collection were killed by isoflurane inhalation anesthesia (Novaplus, Lake Forest, IL, USA) and decapitated.
Tissue preparation
Guinea pig eyes were enucleated, the cornea, lens and vitreous were removed, and the eyecups were immersion fixed in 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (PB) (pH 7.4) for 15–30 min at 4°C. The fixed eyecups were subsequently transferred to a 30% sucrose solution overnight at 4°C for tissue cryoprotection. The eyecups were then briefly washed in 0.1 M PB, embedded in OCT compound (Sakura Finetek Inc., Torrance, CA, USA) and rapidly frozen with dry ice. Cryostat sections of 10–12 μm were made perpendicular to the vitreal surface and retinal sections were collected onto gelatin-coated slides. Sections were then air dried and stored at −20°C.
Immunohistochemistry
Immunohistochemical labeling was performed using an indirect immunofluorescence method (Hirano et al., 2005, 2007). Retinal frozen sections were thawed for 15 min at 37°C on a tissue warming tray, then rinsed three times with 0.1 M PB (pH 7.4) for 10 min per rinse. Retinal sections were then incubated in a blocking solution of 10% normal goat serum, 1% bovine serum albumin and 0.5% Triton X-100 in 0.1 M PB for 1 h at room temperature (22°C). The blocking solution was removed and the primary antibody solution was immediately added to the sections. The sections were incubated with the primary antibody solution for 12–16 h at 4°C in a humidified chamber. Primary antibody solution contained 3% normal goat serum, 1% bovine serum albumin, 0.05% sodium azide and 0.5% Triton X-100 in 0.1 M PB, pH 7.4. Retinal sections were rinsed three times for 10 min per rinse with 0.1 M PB to remove excess primary antibody and then incubated in secondary antibodies conjugated with Alexa 568 or Alexa 488 (1 : 500; Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature in 0.1 M PB containing 0.5% Triton X-100. For labeling of cone photoreceptors, sections were incubated in fluorescein isothiocyanate-conjugated peanut agglutinin (1 : 500, Vector Labs, Burlingame, CA, USA) for 1 h, after primary antibody labeling. To remove the secondary antibody solution, sections were washed three times in 0.1 M PB for 10 min per rinse, air-dried and mounted using Aqua Poly/Mount (Polysciences, Inc., Warrington, PA, USA).
Antibodies
The optimal working dilution for each antibody was determined experimentally. Mouse monoclonal antibody against calbindin (1 : 2500; Sigma-Aldrich, St Louis, MO, USA; C9848 clone CB-955) and rabbit polyclonal antibody against calbindin (1 : 10 000; Swant, Bellinzona, Switzerland; CB38) were used as markers of type A and B horizontal cells in mammalian retina (Uesugi et al., 1992; Peichl & González-Soriano, 1994; Raven & Reese, 2002; Hirano et al., 2007). Antibodies used to identify synaptic vesicles were as follows: mouse monoclonal antibody against VGAT cytoplasmic domain (1 : 200; Synaptic Systems, Göttingen, Germany; 131 011 clone 117G4) to identify GABA-containing vesicles; mouse monoclonal antibody to synapsin I (1 : 100; Millipore, Billerica, MA, USA; MAB10137 clone 3C5) to identify conventional synapses; mouse monoclonal antibody to adult zebrafish hindbrain protein (Trevarrow et al., 1990), which recognizes synaptotagmin-2 in mouse [1 : 200; Zebrafish International Resource Center, Eugene, OR, USA; Znp-1 (Fox & Sanes, 2007)]; and rabbit polyclonal antibody to synaptic vesicle protein 2 (SV2)A (1 : 500; Synaptic Systems, 119 002) to identify synaptic vesicles. SNARE complex and SNARE-related proteins were identified with the following antibodies: rabbit polyclonal antibodies against complexin I/II [1 : 15 000; Synaptic Systems; 122 102, which recognizes both complexin I and II (Reim et al., 2001)]; rabbit monoclonal antibody to SNAP-25 (1 : 60 000; Sigma-Aldrich; S9684); mouse monoclonal antibody to syntaxin-1a (HPC-1) (1 : 1000; Sigma-Aldrich; S0664) and rabbit polyclonal antibody to syntaxin-4 (1 : 1000; Millipore; AB5330). Bipolar cells were identified with a rabbit polyclonal antibody to protein kinase C α (1 : 30 000; Sigma-Aldrich; P4334). Protein kinase C α is a widely used marker of retinal bipolar cells (Negishi et al., 1988; Young et al., 1988; Greferath et al., 1990; Haverkamp et al., 2000; Ghosh et al., 2001). The characterization and evidence for the appropriate use of antibodies are summarized in Table 1; additional information about the antibodies used can be found in the Appendix S1 of the Supporting information.
Table 1.
Primary antibodies
| Antibody | Host | Immunogen | Source | Dilution | Reference |
|---|---|---|---|---|---|
| CaBP | Rabbit | Recombinant rat calbindin D-28k (CB) | Swant, Bellinzona, Switzerland CB38 | 1 : 10 000 | Haverkamp & Wässle (2000), Strettoi et al. (2002), Loeliger & Rees (2005), Damiani et al. (2008), Gaillard et al. (2008) |
| Mouse | Bovine kidney calbindin-D | Sigma-Aldrich, St Louis, MO, USA, C9858, clone CB-955 | 1 : 2500 | Renteria et al. (2005), Deng et al. (2006), Lee et al. (2006), Gargini et al. (2007), Hirano et al. (2007), Damiani et al. (2008), Ettaiche et al. (2009), Kyhn et al. (2009) | |
| Complexin I/II | Rabbit | Synthetic peptide EEERKAKHARMEAEREKVRQQIRDKYGLKKKEEKEAE (aa 45–81 in complexin II) coupled to key-hole limpet hemocyanin via an added N-terminal cysteine residue | Synaptic Systems, Göttingen, Germany, 122 102 | 1 : 15 000 | Reim et al. (2001), Hirano et al. (2005), Xue et al. (2008) |
| PKCα | Rabbit | Synthetic peptide corresponding to aa 659–672 from the C-terminal variable (V5) region of rat PKCα | Sigma-Aldrich, P4334 | 1 : 30 000 | Wang et al. (2001), Elshatory et al. (2007) |
| SNAP-25 | Rabbit | Synthetic peptide corresponding to the N-terminal of human SNAP-25 (synaptosome-associated protein-25) aa 9–29 with C-terminally added lysine conjugated to KLH | Sigma-Aldrich, S9684 | 1 : 60 000 | Frassoni et al. (2005), Szklarczyk et al. (2007) |
| SV2A | Rabbit | Synthetic peptide EEGFRDRAAFIRGAKD (aa 2–17 in human) coupled to key-hole limpet hemocyanin via an added N-terminal cysteine residue | Synaptic Systems, 119 002 | 1 : 500 | Janz et al. (1999), Janz & Südhof (1999), von Kriegstein & Schmitz (2003), Wang et al. (2003), Witkovsky et al. (2008) |
| Synapsin I | Mouse | Recombinant human synapsin 1 | Millipore, Billerica, MA, USA, MAB10137 clone 3C5 | 1 : 100 | De Camilli et al. (1983), Mandell et al. (1990, 1992), Smith et al. (1993), Hirano et al. (2005) |
| Syntaxin-1a | Mouse | Synaptosomal plasma-membrane fraction from adult rat hippocampus | Sigma-Aldrich, S0664 | 1 : 1000 | Barnstable et al. (1985), Inoue et al. (1992), Morgans et al. (1996), Hirano et al. (2005) |
| Syntaxin-4 | Rabbit | Highly purified corresponding to residues 2–23 of rat or mouse syntaxin-4 (accession Q08850) | Millipore, AB5330 | 1 : 1000 | Gouraud et al. (2002), Spurlin et al. (2004), Sherry et al. (2006), Spurlin & Thurmond (2006), Hirano et al. (2007) |
| VGAT | Mouse | Synthetic peptide AEPPVEGDIHYQR (aa 75–87 in rat) coupled to key-hole limpet hemocyanin via an added N-terminal cysteine | Synaptic Systems, 131 011, clone 117G4 | 1 : 200 | McIntire et al. (1997), Sagné et al. (1997), Jellali et al. (2002), Johnson et al. (2003), Guo et al. (2009) |
| Znp-1 (synaptotagmin-2) | Mouse | 1–5-day zebrafish embryo | Zebrafish International Resource Center, Eugene, OR, USA | 1 : 200 | Fox & Sanes (2007), Wässle et al. (2009) |
aa, amino acids; KLH, keyhole limpet hemocyanin; PKCα, protein kinase C α.
Confocal microscopy
Retinal sections were examined and analyzed with an LSM 510 META laser scanning microscope (Zeiss, Thornwood, NY, USA) equipped with an argon laser for 488 nm excitation and two helium/neon lasers for 543 and 633 nm excitation, respectively, using a C-Apochromat 40 × 1.2 n.a. water objective. During acquisition of signals from double-labeled specimens, scans with each laser were performed sequentially to prevent spectral bleed-through. Specific band-pass filters were used to achieve proper separation of signals (single labeling, 488/505LP; double labeling, 488/505–530 and 543/560LP). To increase the signal-to-noise ratio, images were averaged online (e.g. n = 4) and the scan speed and photomultiplier detector gain were decreased. Digital images were acquired at a magnification zoom of 1.5 × and a resolution of 2048 × 2048 pixels. Confocal images were acquired at an optical thickness between 0.5 and 0.7 μm and approximately 1.0 Airy Units. The tortuous coursing of horizontal cell processes and spray of horizontal cell endings necessitated stacks through the OPL for clearer, more complete images of the localization of signals; however, images of individual scans of a single optical slice are available in supporting Figs S1–S8. For projections, 6–10 optical sections were acquired with a total thickness ranging from 2.5 to 6.3 μm and compressed for viewing. Digital confocal images were saved as Zeiss .LSM files and final publication quality images were exported in the .TIFF format at 300 dpi using LSM 510 META software version 4.2 (Zeiss). Images were adjusted for contrast and brightness, labeled and formatted using Photoshop CS3 (Adobe Systems, Inc., San Jose, CA, USA), and saved at 300 dpi at their final magnification.
Results
Vesicular GABA transporter expression in the outer retina
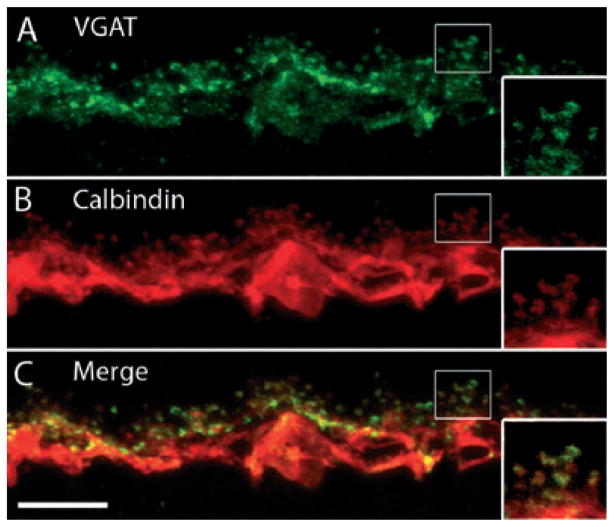
The VGAT immunoreactivity has been localized to horizontal cell processes and terminals in mouse, rat, monkey and human retinas (Haverkamp et al., 2000; Cueva et al., 2002; Jellali et al., 2002; Guo et al., 2009b). To determine if VGAT was also present in guinea pig horizontal cells, double labeling of guinea pig retinal sections with VGAT and calbindin D-28kD (CaBP) antibodies was performed. A- and B-type horizontal cells were identified by immunoreactivity for CaBP, which is an immunohistochemical marker of horizontal cell bodies, dendrites and axons in guinea pig retinas (Peichl & González-Soriano, 1994). CaBP densely labeled horizontal cell somata and processes but horizontal cell endings were generally labeled with less intensity. However, VGAT prominently labeled multiple, laterally running horizontal cell processes, as well as the horizontal cell endings in the OPL (Fig. 1). Individual VGAT-labeled puncta were in clusters near the proximal ONL, indicative of horizontal cell endings. These findings confirmed the presence of VGAT in horizontal cell processes and endings and also established VGAT as a useful marker of horizontal cell endings in guinea pig retina.
Fig. 1.
VGAT immunoreactivity is localized to horizontal cell processes and endings in the OPL. A vertical section of guinea pig retina was double labeled with antibodies to CaBP and VGAT. (A) VGAT immunostaining revealed horizontal cell processes and terminals and a punctate-like pattern of labeling in the OPL. Horizontal cell somata were also faintly labeled. (B) CaBP immunoreactivity was strong throughout the horizontal cell somata, processes and terminals. (C) Merged images reveal the co-localization of VGAT and CaBP to horizontal cells, especially in the processes and endings. Insets reveal a digital magnification of the boxed region, showing the bulb-like endings emerging from laterally coursing horizontal cell processes. Confocal images were scanned at 0.5 μm intervals and a total of 10 optical images were obtained and compressed for viewing. Scale bar: 10 μm.
Synaptic vesicle proteins are expressed in mammalian horizontal cells
Synaptic vesicle protein 2A co-localizes with vesicular GABA transporter
The SV2 is a ubiquitous integral membrane protein of synaptic vesicles that participates in Ca2+-stimulated exocytosis and is present on all synaptic and secretory vesicles (Buckley & Kelly, 1985). There are three known isoforms of this protein, SV2A, SV2B and SV2C (Bajjalieh et al., 1994; Janz & Südhof, 1999), each of which may have synapse-specific functions. A previous study has shown differential expression of these isoforms in the mouse retina, with SV2A broadly expressed at conventional synapses and prevalent in cone terminals and developing horizontal cells in the OPL (Wang et al., 2003). To assess whether SV2A is present in guinea pig horizontal cell processes or putative horizontal cell release sites, double-labeling experiments with VGAT and SV2A antibodies were performed. These experiments revealed co-localized immunoreactivity in horizontal cell endings in the OPL producing a punctate pattern (Fig. 2, arrow), with numerous labeled puncta identifying horizontal cell endings. SV2A labeling of photoreceptor terminals was also seen, which showed no co-localization with VGAT.
Fig. 2.
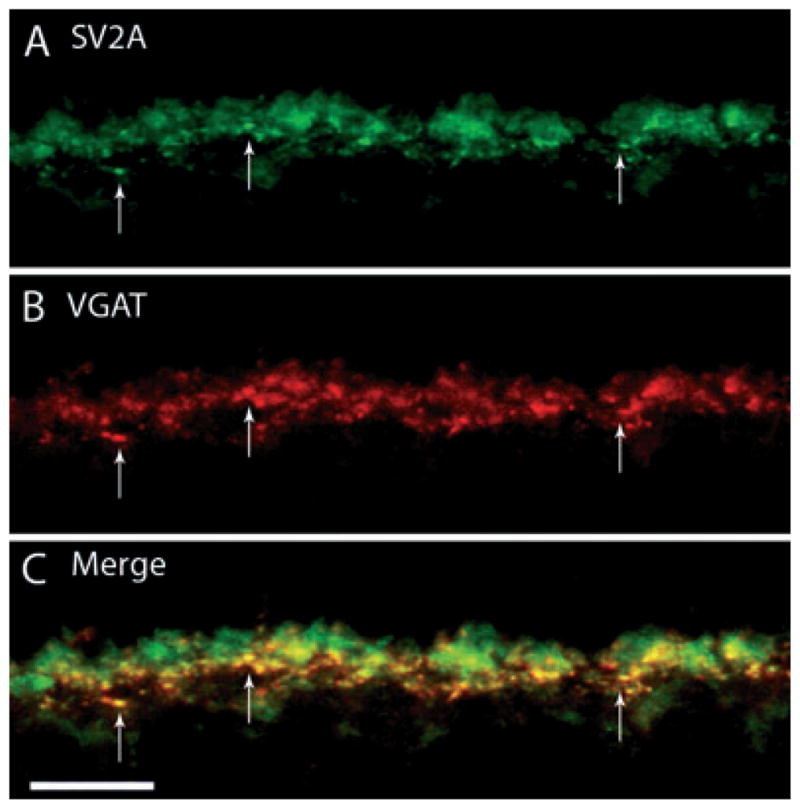
SV2A, a synaptic vesicle protein, labels horizontal cell terminals. A vertical section of guinea pig retina was double labeled with antibodies to SV2A and VGAT. (A) SV2A immunolabeling was localized to horizontal cell endings in the OPL, as well as photoreceptor terminals. (B) VGAT antibodies labeled horizontal cell terminals in the outer retina. (C) Merged images reveal the co-localization of SV2A labeling with that of VGAT. Arrows point to co-localized puncta along the OPL. Confocal images were scanned at 0.5 μm intervals and a total of eight optical images were obtained and compressed for viewing. Scale bar: 10 μm.
Synaptotagmin-2 localizes to horizontal cell processes and endings
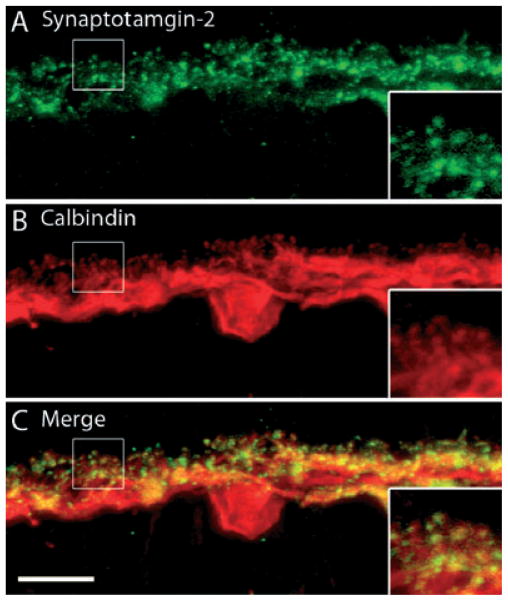
Synaptotagmin-2 is an important synaptic protein that has been shown to function as a trigger for fast, Ca2+-mediated vesicular exocytosis in central and neuromuscular synapses (Pang et al., 2006) and is required for synaptic exocytosis. Because synaptotagmin has been shown to be required for tightly regulated and synchronous synaptic exocytosis, which is characteristic of neurotransmission, we examined the expression of this protein in guinea pig horizontal cells. We used the Znp-1 monoclonal mouse antibody, which has been shown to recognize synaptotamin-2 through western blot and immunoprecipitation analysis (Fox & Sanes, 2007). In guinea pig retina, synaptotagmin-2 and CaBP immunostaining were co-localized to horizontal cell processes and the endings emerging from these processes (Fig. 3). These findings parallel the results of a previous report demonstrating a differential distribution of synaptotagmin-1 and synaptotagmin-2 in mouse retinas, with synaptotagmin-2 as the prevailing isoform present in mouse horizontal cells (Fox & Sanes, 2007).
Fig. 3.
Synaptotagmin-2, a sensor for Ca2+-triggered vesicular release, localizes to horizontal cell processes and their terminals. A vertical section of guinea pig retina was double labeled with antibodies to synaptotagmin-2 and CaBP. (A) Synaptotagmin-2 antibodies labeled the processes in the OPL, with more intensely labeled dots throughout OPL. (B) CaBP immunostaining is in horizontal cell somata, processes and horizontal cell endings. (C) Merged images show co-localization of synaptotagmin-2 and CaBP immunostaining in horizontal cell processes and endings. The inset reveals a digital magnification of the boxed region indicating horizontal cell processes extending from the OPL. Confocal images were scanned at 0.7 μm intervals and a total of 10 optical images were obtained and compressed for viewing. Scale bar: 10 μm.
Synapsin I labels horizontal cells in a punctate pattern
Synapsin I is a synaptic vesicle-associated membrane protein (VAMP) that has been shown to be absent in ribbon synapses but is a marker of conventional synapses (Mandell et al., 1990). The expression of synapsin I in horizontal cells was examined because they are thought to mediate trafficking of synaptic vesicles to their target membrane through interactions with the cell cytoskeleton (Hirokawa et al., 1989; Bennett et al., 1991). Double-labeling experiments with synapsin I and CaBP antibodies showed co-localization at the horizontal cell processes and endings (Fig. 4A–C). Synapsin I antibodies also labeled several cell bodies in the inner nuclear layer. Double-labeling experiments with synapsin I and protein kinase C α (a rod bipolar cell marker) antibodies (Fig. 4D–F) revealed that synapsin I also labels rod dendrites, as has been reported in the rabbit retina (Hirano et al., 2005).
Fig. 4.
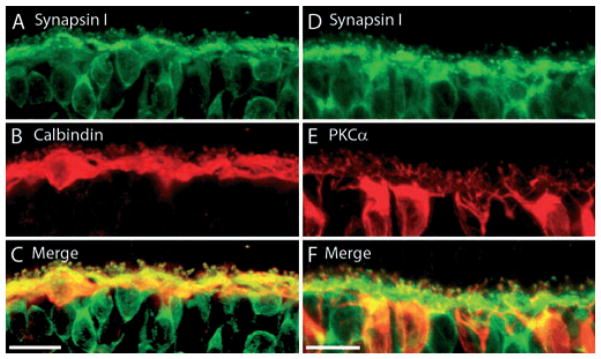
(A–C) Synapsin I, a marker of conventional synapses, is present in horizontal cells. A vertical section of guinea pig retina was double labeled with antibodies to synapsin I and CaBP. (A) Synapsin I immunolabeling was present throughout the OPL, with labeled puncta just above the OPL. Synapsin I also labels bipolar cell bodies just below the OPL. (B) CaBP immunoreactivity is strongest in the cell bodies, but also along the horizontal cell processes and their endings. (C) Merged images reveal the co-localization of synapsin I and CaBP immunoreactivity in the horizontal cell fine processes and endings emerging from the main processes. Confocal images were scanned at 0.5 μm, and a total of 11 optical images were obtained and compressed for viewing. Scale bar: 20 μm. (D–F) Synapsin I is also localized to rod bipolar cell dendrites. A vertical section of guinea pig retina was double labeled with antibodies to synapsin I and PKCα. (D) Synapsin I immunolabeling is present throughout the OPL with labeling of the bipolar cell somata as well. There are also punctate-like areas of more intense immunostaining throughout the OPL. (E) Protein kinase Cα (PKCα), a marker of rod-bipolar cells labels bipolar cell soma and the dendritic tree. (F) Merged images reveal co-localization between synapsin I labeling and PKCα at the rod bipolar dendrites. Confocal images were scanned at 0.8 μm intervals, and a total of 10 optical images were obtained and compressed for viewing. Scale bar: 10 μm.
SNARE proteins, the mediators of vesicular fusion, in mammalian horizontal cells
Central to the regulation of vesicular fusion are the SNARE proteins as they not only provide the energy to drive bilayer fusion but they also confer a degree of specificity to the fusion process (Jahn & Scheller, 2006). The synaptic proteins syntaxin, SNAP-25 and synaptobrevin or VAMP form the essential protein core complex for catalyzing synaptic vesicle fusion in the conventional model of exocytosis (Weber et al., 1998) and are probably necessary to carry out vesicular transmitter release in horizontal cells. Our immunohistochemical studies have evaluated the expression of SNARE proteins in the guinea pig retina by focusing on syntaxin-1a, syntaxin-4, SNAP-25 and complexin I/II.
Syntaxin-1a labels horizontal cell endings in the outer plexiform layer
There are multiple syntaxin isoforms, at least four to date (isoforms 1–4), which participate in vesicle fusion by targeting the plasma membrane (Chen & Scheller, 2001; Brandie et al., 2008; Aran et al., 2009). Syntaxin-1a is an integral membrane protein classically involved in Ca2+-regulated secretion in neurons and neuroendocrine cells (for reviews see Jahn & Südhof, 1999; Jahn & Scheller, 2006; Lang & Jahn, 2008). In the retina, syntaxin-1a is restricted to conventional synapses and is notably absent in photoreceptor and ribbon synapses (Brandstätter et al., 1996a; Hirano et al., 2005; Sherry et al., 2006; Curtis et al., 2008). To examine the distribution of this protein in the guinea pig retina, we performed double-labeling experiments with syntaxin-1a and CaBP antibodies, which showed immunolabeling of amacrine cells as well as within horizontal cell processes and endings (Fig. 5). These results agree with previous reports in rat (Barnstable et al., 1985; Inoue et al., 1992; Morgans et al., 1996) and rabbit (Hirano et al., 2005) retina.
Fig. 5.
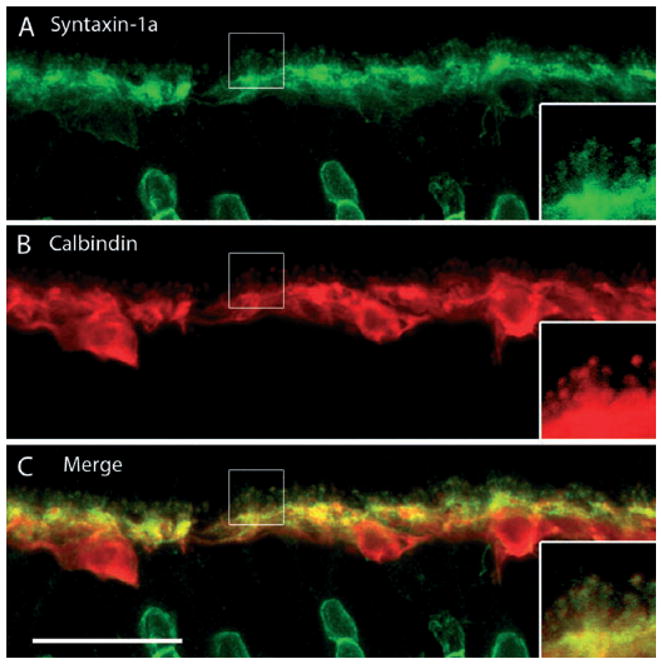
Syntaxin-1a, a SNARE core protein, localizes to amacrine cells and horizontal cell processes and endings. (A) Syntaxin-1a immunolabeling labels horizontal cell processes and endings in the OPL, as well as amacrine cells in the inner nuclear layer (INL). The horizontal cell somata are more faintly labeled. Labeled amacrine cells can be seen in the INL at the bottom of the image. (B) CaBP immunoreactivity is present in horizontal cell somata, their processes and terminal endings. (C) Merged images reveal the co-localization of syntaxin-1a and CaBP immunoreactivity in the OPL to the laterally running horizontal cell processes, as well as the finer endings that emerge from them. Confocal images were scanned at 0.6 μm intervals and a total of nine optical images were obtained and compressed for viewing. Insets reveal digital magnification of the boxed region, highlighting the horizontal cell process and endings. Scale bar: 20 μm.
Syntaxin-4 co-localizes with vesicular GABA transporter in horizontal cells
A recent study has demonstrated robust syntaxin-4 immunoreactivity in horizontal cell processes and tips in mouse, rat and rabbit retinas (Hirano et al., 2007). To examine the distribution of syntaxin-4 in guinea pig retina, double-labeling experiments with syntaxin-4 and VGAT antibodies were performed, which confirmed a similar staining pattern between these antibodies (Fig. 6A–C). Co-localization of VGAT and syntaxin-4 occurred along horizontal cell processes and endings, whereas the cell bodies were only faintly labeled. Similar to what has been observed in other mammalian species (Hirano et al., 2007), syntaxin-4 densities were seen at regular intervals along the OPL, which correspond to clusters of horizontal cell endings abutting the cone pedicle, which were labeled with peanut agglutinin (Fig. 6D–F). The arrow in Fig. 6D points to a horizontal cell terminating at a rod spherule, which is more distal to horizontal cells than cone pedicles, demarcated in Fig. 6E by an asterisk.
Fig. 6.
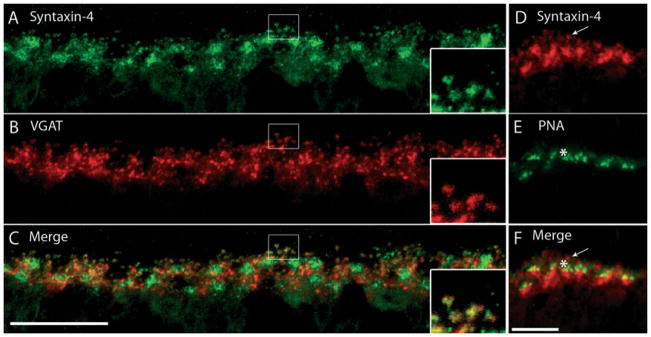
Syntaxin-4, a SNARE complex protein, localizes to horizontal cell processes and endings. (A–C) A vertical section of guinea pig retina was double labeled with antibodies to syntaxin-4 and VGAT. (A) Syntaxin-4 immunolabeling was robust along the OPL with a punctate pattern. There were also regions of more intense staining in the OPL that were located along horizontal cell processes and near the soma. (B) VGAT immunolabeling was localized to the laterally running processes of horizontal cells, as well as the finer processes and endings emerging from them. (C) Merged images reveal the co-localization of syntaxin-4-labeled puncta and VGAT-labeled endings of horizontal cells. The insets are a digital magnification of the boxed region, which demonstrates staining of the delicate processes and endings of horizontal cells by both syntaxin-4 and VGAT antibodies. Confocal images were scanned at 0.5 μm intervals and a total of 10 optical images were obtained and compressed for viewing. Scale bar: 20 μm. (D–F) Syntaxin-4 does not label cone photoreceptor terminals. (D) Dense spots of syntaxin-4 immunoreactivity occur at regular intervals along the OPL. (E) Cone pedicles, labeled with peanut-agglutinin (PNA), produce a similar punctate pattern of densities along the OPL. (F) Merged images reveal that the syntaxin-4-immunoreactive clusters correspond to horizontal cell endings grouped together just underneath cone pedicles. The arrow points to a horizontal cell ending that continues up past the cone pedicle (indicated by the asterisk) to terminate at a rod spherule, which is more distal to horizontal cells than cone pedicles. Confocal images were scanned at 0.5 μm intervals and a total of eight optical images were obtained and compressed for viewing. Scale bar: 10 μm.
SNAP-25 strongly labels horizontal cell processes and endings
SNAP-25, a critical component of the neural SNARE complex that facilitates membrane fusion between synaptic vesicles and the presynaptic plasma membrane, has been reported in multiple retinal cell types, including horizontal cells (Catsicas et al., 1992). Recently, it was shown that SNAP-25 not only subserves cholinergic and glutamatergic neurotransmission but is also critical for evoked GABA release and is expressed by mature GABAergic neurons (Tafoya et al., 2006). We tested whether horizontal cells in guinea pig retina express SNAP-25 with double-labeling experiments with both CaBP and SNAP-25 antibodies. In guinea pig, immunoreactivity using the SNAP-25 anti-rabbit antibody was robust in horizontal cell endings, identified by co-labeling with CaBP antibody (Fig. 7). Immunostaining was very weak or absent in horizontal cell somata (arrow) but the horizontal cell terminals and lateral processes along the OPL beneath photoreceptor terminals were intensely labeled. SNAP-25 also labeled the bipolar cell bodies (asterisk), dendrites and axons, and has been reported to occur in other retinal cell types (Galli et al., 1995; Brandstätter et al., 1996a; von Kriegstein et al., 1999; Morgans & Brandstätter, 2000; von Kriegstein & Schmitz, 2003).
Fig. 7.
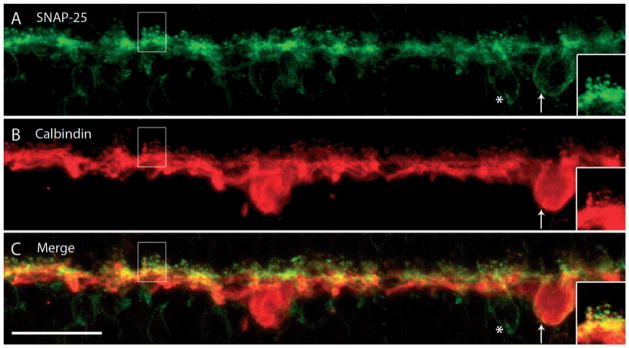
SNAP-25, a SNARE complex protein, is expressed in horizontal cell processes and endings. A vertical section of guinea pig retina was double labeled with anti-SNAP-25 and anti-CaBP antibodies. (A) SNAP-25 is expressed in the OPL at the fine processes and endings emerging from the laterally distributed processes throughout. There was also faint labeling of cell soma within the inner nuclear layer underneath the OPL. (B) CaBP is expressed in horizontal cell somata, processes and endings. (C) Merged images show the co-localization of SNAP-25 and CaBP to horizontal cell terminals. Although SNAP-25 faintly labeled a horizontal cell body, as indicated by co-localization with CaBP (arrow), it also weakly labeled nearby bipolar cell bodies (asterisk). The insets are a digital magnification of the boxed region. Confocal images were scanned at 0.7 μm intervals and a total of 10 optical images were obtained and compressed for viewing. Scale bar: 20 μm.
Complexin I/II labels horizontal cell soma, processes and endings
Fast Ca2+-triggered fusion requires a host of proteins, including complexins. These are soluble SNARE complex-binding proteins that have been shown to have an essential role in synaptic fusion by regulating a late step in Ca2+-dependent neurotransmitter release (Reim et al., 2001; Tang et al., 2006). They control the force transfer from SNARE complexes to membranes in fusion (Maximov et al., 2009) by serving as ‘grappling proteins’ to hold the SNARE complex into an activated but frozen state (Rizo & Rosenmund, 2008; Südhof & Rothman, 2009). Given that these are essential proteins involved in Ca2+-dependent vesicular fusion, we tested whether they are expressed in guinea pig horizontal cells. Double-labeling experiments with complexin I/II and CaBP showed that complexin I/II immunoreactivity was localized to all parts of the guinea pig horizontal cells, including the soma, processes and endings (Fig. 8). These results agree with earlier findings in mouse and rabbit retina, which revealed complexin I/II immunoreactivity in the entire horizontal cell, including the endings, at both the light and electron microscopy level (Hirano et al., 2005; Reim et al., 2005). Amacrine cells are also labeled with the complexin I/II antibody (Fig. 8, asterisk).
Fig. 8.
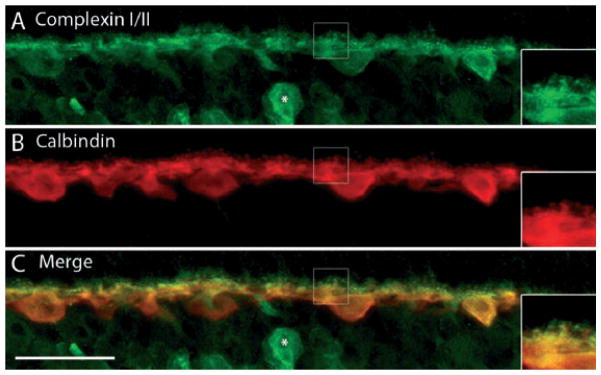
Complexin I/II, a cytosolic SNARE-associated protein, labels horizontal cell soma, processes and endings. A vertical section of guinea pig retina was double labeled with complexin I/II and CaBP antibodies. (A) Complexin I/II immunolabeling is very robust in horizontal cell somata and the larger laterally running processes, as well as the finer processes and endings emerging from them. A labeled amacrine cell is indicated by the asterisk. (B) CaBP expression is also robust in horizontal cell bodies, processes and terminals. (C) Merged images reveal the co-localization of complexin I/II to all parts of the horizontal cells, including the distal processes and endings. Insets are digital magnification images of the boxed region, highlighting several horizontal cell terminals and the localization of complexin I/II to the endings. Confocal images were scanned at 0.7 μm intervals and a total of 10 optical images were obtained and compressed for viewing. Scale bar: 20 μm.
Discussion
This study provides novel insights and morphological evidence for the mechanism of transmitter release from horizontal cells. It has been shown previously that both GABA and the biosynthetic machinery to synthesize GABA are present in guinea pig horizontal cells (Guo et al., 2009a). This study extends these findings to reveal key protein components involved in Ca2+-dependent and SNARE protein-dependent exocytosis. Most notably, the expression of VGAT, synaptotagmin-2, SNAP-25 and syntaxin-1a and syntaxin-4 at horizontal cell tips and processes argues strongly in favor of a regulated exocytotic vesicular pathway for GABA release from guinea pig horizontal cells. However, the nature and trafficking course of the vesicles storing and releasing GABA in guinea pig horizontal cells are currently unknown. Furthermore, the existence of Ca2+-regulated vesicular exocytosis in mammalian horizontal cells is still debated. Based on our findings, GABA probably utilizes a vesicular-regulated secretory pathway in mammalian horizontal cells.
Synaptic vesicle proteins in mammalian horizontal cells
Generally, synaptic vesicle fusion with the plasma membrane involves the SNARE proteins syntaxin-1a, SNAP-25 and VAMP-1 for transmitter release (Südhof & Jahn, 1991; Südhof, 2004; Takamori et al., 2006) but there are many examples of heterogeneity in SNARE core complex combinations within the central nervous system. For instance, astroglial precursor cells, oligodendrocytes and microglia, which undergo vesicular release of glutamate and aspartate, express SNAP-23, an analog of SNAP-25, VAMP-3 and syntaxin-1a (Parpura et al., 1995; Hepp et al., 1999; Maienschein et al., 1999; Montana et al., 2004). In this instance, the interaction of SNAP-23, VAMP-3 and syntaxin-1a forms the core SNARE complex, mediating general membrane insertion mechanisms including secretion, phagocytosis and myelinogenesis (Hepp et al., 1999; Ni et al., 2007). Within the retina, ribbon synapses in photoreceptors express syntaxin-3 (Ullrich & Südhof, 1994; Morgans et al., 1996; Sherry et al., 2006; Curtis et al., 2008), VAMP-1 and VAMP-2 (Sherry et al., 2003; Morgans et al., 2009), and SNAP-25 (Ullrich & Südhof, 1994; Brandstätter et al., 1996b; Greenlee et al., 2001; Mazelova et al., 2009). The syntaxins also show heterogeneity in the retina, with syntaxin-1 and syntaxin-2 in conventional amacrine cell synapses in a non-overlapping fashion, syntaxin-3b in glutamatergic ribbon synapses of photoreceptors and bipolar cells, and syntaxin-4 in horizontal cells (Sherry et al., 2006; Hirano et al., 2007; Curtis et al., 2008). Thus, although the paradigm of SNARE-mediated fusion may be universal, the substrates mediating this action may involve greater isoform variability, which may be the case for proteins involved in SNARE-mediated vesicular release from mammalian horizontal cells.
Mechanism of transmitter release in mammalian horizontal cells
Studies in non-mammalian retinas have argued for GABA uptake and release occurring via a Na+-dependent and Ca2+-independent transport process in these horizontal cells due to the presence of a plasmalemmal GABA transporter (Schwartz, 1982, 1987; Yazulla et al., 1985; Ayoub & Lam, 1987; O’Malley & Masland, 1989; Connaughton et al., 2008; Nelson et al., 2008). In non-mammalian retinas GABA is synthesized and accumulates in cone-driven horizontal cells (Yazulla & Brecha, 1981; Zucker et al., 1984; Yazulla et al., 1989; Marc, 1992; Connaughton et al., 1999), and is released when horizontal cells are depolarized (Ayoub & Lam, 1984; Yang & Wu, 1989, 1993; Kamermans & Werblin, 1992). Evidence for mammalian plasmalemmal transmitter release is absent in mammals, which prompted the hypothesis that mammalian horizontal cells might utilize an alternative mechanism of transmitter release. High-affinity transport of GABA or GABA analogs has not been reported in any adult mammalian horizontal cells (Goodchild & Neal, 1973; Ehinger, 1977; Agardh & Ehinger, 1982, 1983; Agardh et al., 1986; Brecha & Weigmann, 1994) and studies in the developing and adult mouse retina found that GABA transporter (GAT-1 and GAT-3) transcripts and proteins were limited to Müller cell processes (Brecha & Weigmann, 1994; Durkin et al., 1995; Honda et al., 1995; Johnson et al., 1996; Hu et al., 1999; Casini et al., 2006; Guo et al., 2009b). However, L-glutamic acid decarboxylase, the synthesizing enzyme for GABA, is known to be present in horizontal cells particularly in early development (Schnitzer & Rusoff, 1984). Glutamate, the synthetic precursor of GABA, and the glutamate transporter, excitatory amino acid carrier 1, have been localized to the somata of rat and cat horizontal cells (Rauen et al., 1996; Fyk-Kolodziej et al., 2004). The non-synaptic location of this transporter has been implicated in the synthesis and release of GABA in the hippocampus (Coco et al., 1997; Sepkuty et al., 2002), supporting the notion that horizontal cells may also utilize excitatory amino acid carrier 1 to accumulate glutamate for the subsequent synthesis of GABA.
Ultrastructural studies demonstrate the presence of small, spherical, clear-core agranular vesicles classically associated with synaptic vesicles containing fast neurotransmitters such as glutamate and GABA (for review see De Camilli & Jahn, 1990; Torrealba & Carrasco, 2004) within rabbit, mouse, rat, cat, monkey and human horizontal cells (Dowling & Boycott, 1966; Linberg & Fisher, 1988; Spiwoks-Becker et al., 2001). Reports of vesicles clustered at membrane specializations in horizontal cell processes (Dowling et al., 1966; Raviola & Gilula, 1975; Linberg & Fisher, 1988) adjacent to photoreceptor terminals are small in number and the vesicles do not aggregate preferentially at the cell membrane (Dowling & Boycott, 1966; Spiwoks-Becker et al., 2001). Although a previous study by Loeliger & Rees (2005) speculated that only one type of horizontal cell contains GABA in the adult guinea pig retina, GABA release probably occurs from both A- and B-type horizontal cells in the guinea pig, as all calbindin-immunoreactive horizontal cell bodies were shown to contain GABA as well as glutamic acid decarboxylase 65 (GAD65) immunoreactivity (Guo et al., 2009a).
Although these findings support the hypothesis that horizontal cells release GABA in the outer retina, they do not exclude the possibility that there may be other sources of GABA. Interplexiform cells, which have been reported in all vertebrate retinas (Boycott et al., 1975; Savy et al., 1991; Nguyen-Legros et al., 1997), are a subtype of amacrine cells whose processes ramify in both plexiform layers and to the inner nuclear layer and contain dopamine (Dowling & Ehinger, 1975), GABA (Nakamura et al., 1980; Witkovsky et al., 2008), L-glutamic acid decarboxylase and VGAT (Witkovsky et al., 2008) in varicosities along the interplexiform cell processes. GABAergic interplexiform cells co-localized with SV2A, indicating that GABA may also be released from these cells at a conventional synapse (Witkovsky et al., 2008). A second possible source of GABA in the outer retina is the indoleamine-accumulating type 3 cells (Sandell & Masland, 1989) located at the outer edge of the inner nuclear layer (Sandell & Masland, 1989; Massey et al., 1992). These cells arborize widely in the OPL and specifically take up 3H-GABA (Sandell & Masland, 1989) or the GABA analog 3H-muscimol (Massey et al., 1992). However, these cells are likely to have a minor influence on GABA levels in the OPL overall as they are concentrated in the ventral retina and they only ramify in some retinal regions, making it more probable that these cells represent developmental anomalies (Sandell & Masland, 1989). In guinea pigs, horizontal cells are probably the predominant cellular source of GABA in the outer retina (Guo et al., 2009a) as tyrosine hydroxylase-immunoreactive interplexiform cell processes are not present in the inner nuclear layer or OPL of guinea pig retina (Oh et al., 1999; Fujieda et al., 2000; Loeliger & Rees, 2005). Furthermore, there was no evidence of tyrosine hydroxylase-immunolabeled amacrine cell processes in the guinea pig ramifying in the OPL (Oh et al., 1999).
Several studies support the notion that all cells in the photoreceptor triad are end targets of GABA released from horizontal cells. Mammalian horizontal cells exhibit GABA-induced currents (Feigenspan & Weiler, 2004), consistent with GABAA receptor expression on horizontal cells (Greferath et al., 1994, 1995; Blanco et al., 1996). These findings suggest that GABA acts as an auto-receptor. GABAA,C receptors have also been detected on bipolar cell dendrites (Greferath et al., 1994; Enz et al., 1996; Vardi et al., 1998; Pattnaik et al., 2000; Delgado et al., 2009), which provides evidence in support of horizontal cells mediating feedforward action onto bipolar cells. ON-bipolar cells require that GABAergic input be depolarizing to provide them with the corrective horizontal cell input analogous to that received by OFF-bipolar cells. Indeed, Duebel et al. (2006) reports that ON-bipolar cells employ a somatodendritic [Cl-](i) gradient to invert GABAergic horizontal cell input, thereby depolarizing ON-bipolar cells with a high dendritic [Cl-](i). There are several studies showing the presence of GABAA,C receptor immunoreactivity at photoreceptor terminals (Greferath et al., 1995; Picaud et al., 1998; Haverkamp & Wässle, 2000; Pattnaik et al., 2000). However, GABA’s action at photoreceptor terminals remains controversial. GABA-evoked currents are reported for mouse and pig cones (Picaud et al., 1998; Pattnaik et al., 2000) but the predominant finding is a lack of GABA-evoked currents in mammalian cones (Verweij et al., 2003).
There are still many questions that must be answered regarding the mechanism underlying transmitter release from horizontal cells. The results of this study argue for regulated vesicular-mediated exocytosis as the underlying mechanism of release and suggest that the guinea pig retina is uniquely suited for functional studies of mammalian horizontal cells. Understanding the mechanism of release will contribute to understanding how these cells function and communicate within the OPL.
Supplementary Material
Appendix S1. Antibody characterization.
Fig. S1. Single confocal image of VGAT and CaBP immunoreactivity in guinea pig vertical section.
Fig. S2. Single confocal image of SV2A and VGAT immunoreactivity in guinea pig vertical section.
Fig. S3. Single confocal image of synaptotagmin-2 and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S4. Single confocal image of synapsin I and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S5. Single confocal image of syntaxin-1a and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S6. Single confocal image of syntaxin-4 and VGAT immuno-reactivity in guinea pig vertical section.
Fig. S7. Single confocal image of SNAP-25 and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S8. Single confocal image of complexin I/II and CaBP immunoreactivity in guinea pig vertical section.
Acknowledgments
We thank Drs Salvatore Stella Jr, Arlene Hirano, Chenying Guo, Iona Raymond and Steve Barnes for their insightful comments on this manuscript. Supported by NIH EY 15573 and the Jules Stein Eye Institute EyeSTAR program. N.C.B. is a VA Senior Career Research Scientist.
Abbreviations
- CaBP
calbindin D-28kD
- OPL
outer plexiform layer
- PB
phosphate buffer
- SNAP-25
synaptosomal-associated protein
- SNARE
soluble NSF attachment protein receptor
- SV2
synaptic vesicle protein 2
- VAMP
vesicle-associated membrane protein
- VGAT
vesicular GABA transporter
References
- Agardh E, Ehinger B. (3H)-muscimol, (3H)-nipecotic acid and (3H)-isoguvacine as autoradiographic markers for GABA neurotransmission. J Neural Transm. 1982;54:1–18. doi: 10.1007/BF01249274. [DOI] [PubMed] [Google Scholar]
- Agardh E, Ehinger B. Retinal GABA neuron labelling with [3H]isoguvacine in different species. Exp Eye Res. 1983;36:215–229. doi: 10.1016/0014-4835(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Agardh E, Bruun A, Ehinger B, Storm-Mathisen J. GABA immunoreactivity in the retina. Invest Ophthalmol Vis Sci. 1986;27:674–678. [PubMed] [Google Scholar]
- Aran V, Brandie FM, Boyd AR, Kantidakis T, Rideout EJ, Kelly SM, Gould GW, Bryant NJ. Characterization of two distinct binding modes between syntaxin 4 and Munc18c. Biochem J. 2009;419:655–660. doi: 10.1042/BJ20082293. [DOI] [PubMed] [Google Scholar]
- Ayoub GS, Lam DM. The release of gamma-aminobutyric acid from horizontal cells of the goldfish (Carassius auratus) retina. J Physiol. 1984;355:191–214. doi: 10.1113/jphysiol.1984.sp015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub GS, Lam DM. Accumulation of gamma-aminobutyric acid by horizontal cells isolated from the goldfish retina. Vision Res. 1987;27:2027–2034. doi: 10.1016/0042-6989(87)90117-9. [DOI] [PubMed] [Google Scholar]
- Babai N, Thoreson WB. Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J Physiol. 2009;587:2353–2364. doi: 10.1113/jphysiol.2009.169656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG, O’Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol. 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AF, Hayes NV, Baines AJ. Site specificity in the interactions of synapsin 1 with tubulin. Biochem J. 1991;276(Pt 3):793–799. doi: 10.1042/bj2760793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J Physiol. 2002;545:183–198. doi: 10.1113/jphysiol.2002.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco R, Vaquero CF, de la Villa P. The effects of GABA and glycine on horizontal cells of the rabbit retina. Vision Res. 1996;36:3987–3995. doi: 10.1016/s0042-6989(96)00145-9. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Dowling JE, Fisher SK, Kolb H, Laties AM. Interplexiform cells of the mammalian retina and their comparison with catecholamine-containing retinal cells. Proc R Soc Lond B Biol Sci. 1975;191:353–368. doi: 10.1098/rspb.1975.0133. [DOI] [PubMed] [Google Scholar]
- Brandie FM, Aran V, Verma A, McNew JA, Bryant NJ, Gould GW. Negative regulation of syntaxin4/SNAP-23/VAMP2-mediated membrane fusion by Munc18c in vitro. PLoS ONE. 2008;3:e4074. doi: 10.1371/journal.pone.0004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstätter JH, Lohrke S, Morgans CW, Wässle H. Distributions of two homologous synaptic vesicle proteins, synaptoporin and synaptophysin, in the mammalian retina. J Comp Neurol. 1996a;370:1–10. doi: 10.1002/(SICI)1096-9861(19960617)370:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Wässle H, Betz H, Morgans CW. The plasma membrane protein SNAP-25, but not syntaxin, is present at photoreceptor and bipolar cell synapses in the rat retina. Eur J Neurosci. 1996b;8:823–828. doi: 10.1111/j.1460-9568.1996.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Brecha NC, Weigmann C. Expression of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter in the rat retina. J Comp Neurol. 1994;345:602–611. doi: 10.1002/cne.903450410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PM, Hell J, Mehl E, Krasel C, Lottspeich F, Jahn R. GABA and glycine in synaptic vesicles: storage and transport characteristics. Neuron. 1991;7:287–293. doi: 10.1016/0896-6273(91)90267-4. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977;40:53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Casini G, Rickman DW, Brecha NC. Expression of the gamma-aminobutyric acid (GABA) plasma membrane transporter-1 in monkey and human retina. Invest Ophthalmol Vis Sci. 2006;47:1682–1690. doi: 10.1167/iovs.05-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas S, Catsicas M, Keyser KT, Karten HJ, Wilson MC, Milner RJ. Differential expression of the presynaptic protein SNAP-25 in mammalian retina. J Neurosci Res. 1992;33:1–9. doi: 10.1002/jnr.490330102. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Coco S, Verderio C, Trotti D, Rothstein JD, Volterra A, Matteoli M. Non-synaptic localization of the glutamate transporter EAAC1 in cultured hippocampal neurons. Eur J Neurosci. 1997;9:1902–1910. doi: 10.1111/j.1460-9568.1997.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Behar TN, Liu WL, Massey SC. Immunocytochemical localization of excitatory and inhibitory neurotransmitters in the zebrafish retina. Vis Neurosci. 1999;16:483–490. doi: 10.1017/s0952523899163090. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R, Bender AM. Electrophysiological evidence of GABAA and GABAC receptors on zebrafish retinal bipolar cells. Vis Neurosci. 2008;25:139–153. doi: 10.1017/S0952523808080322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva JG, Haverkamp S, Reimer RJ, Edwards R, Wässle H, Brecha NC. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol. 2002;445:227–237. doi: 10.1002/cne.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LB, Doneske B, Liu X, Thaller C, McNew JA, Janz R. Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. J Comp Neurol. 2008;510:550–559. doi: 10.1002/cne.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani D, Alexander JJ, O’Rourke JR, McManus M, Jadhav AP, Cepko CL, Hauswirth WW, Harfe BD, Strettoi E. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–4887. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Harris SM, Jr, Huttner WB, Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983;96:1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado LM, Vielma AH, Kähne T, Palacios AG, Schmachtenberg O. The GABAergic system in the retina of neonate and adult Octodon degus, studied by immunohistochemistry and electroretinography. J Comp Neurol. 2009;514:459–472. doi: 10.1002/cne.22023. [DOI] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell’s nonlinear receptive field. J Neurosci. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Wang L, Dong W, He S. Lateral components in the cone terminals of the rabbit retina: horizontal cell origin and glutamate receptor expression. J Comp Neurol. 2006;496:698–705. doi: 10.1002/cne.20959. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188:270–273. doi: 10.1126/science.804181. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Werblin FS. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969;32:315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Brown JE, Major D. Synapses of horizontal cells in rabbit and cat retinas. Science. 1966;153:1639–1641. doi: 10.1126/science.153.3744.1639. [DOI] [PubMed] [Google Scholar]
- Duebel J, Haverkamp S, Schleich W, Feng G, Augustine GJ, Kuner T, Euler T. Two-photon imaging reveals somatodentritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron. 2006;49:81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Brain Res Mol Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Glial and neuronal uptake of GABA, glutamic acid, glutamine and glutathione in the rabbit retina. Exp Eye Res. 1977;25:221–234. doi: 10.1016/0014-4835(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci. 2007;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Brandstätter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest Ophthalmol Vis Sci. 2009;50:2417–2426. doi: 10.1167/iovs.08-3028. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Weiler R. Electrophysiological properties of mouse horizontal cell GABAA receptors. J Neurophysiol. 2004;92:2789–2801. doi: 10.1152/jn.00284.2004. [DOI] [PubMed] [Google Scholar]
- Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503:280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Frassoni C, Inverardi F, Coco S, Ortino B, Grumelli C, Pozzi D, Verderio C, Matteoli M. Analysis of SNAP-25 immunoreactivity in hippocampal inhibitory neurons during development in culture and in situ. Neuroscience. 2005;131:813–823. doi: 10.1016/j.neuroscience.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci. 2000;17:63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- Fyk-Kolodziej B, Qin P, Dzhagaryan A, Pourcho RG. Differential cellular and subcellular distribution of glutamate transporters in the cat retina. Vis Neurosci. 2004;21:551–565. doi: 10.1017/S0952523804214067. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Bonfield S, Gilmour GS, Kuny S, Mema SC, Martin BT, Smale L, Crowder N, Stell WK, Sauve Y. Retinal anatomy and visual performance in a diurnal cone-rich laboratory rodent, the Nile grass rat (Arvicanthis niloticus) J Comp Neurol. 2008;510:525–538. doi: 10.1002/cne.21798. [DOI] [PubMed] [Google Scholar]
- Galli T, Garcia EP, Mundigl O, Chilcote TJ, De Camilli P. v- and t-SNAREs in neuronal exocytosis: a need for additional components to define sites of release. Neuropharmacology. 1995;34:1351–1360. doi: 10.1016/0028-3908(95)00113-k. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Haverkamp S, Wässle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647. doi: 10.1523/JNEUROSCI.21-21-08636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild M, Neal MJ. The uptake of 3Hγ -aminobutyric acid by the retina. Br J Pharmacol. 1973;47:529–542. doi: 10.1111/j.1476-5381.1973.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouraud S, Laera A, Calamita G, Carmosino M, Procino G, Rossetto O, Mannucci R, Rosenthal W, Svelto M, Valenti G. Functional involvement of VAMP/synaptobrevin-2 in cAMP-stimulated aquaporin 2 translocation in renal collecting duct cells. J Cell Sci. 2002;115:3667–3674. doi: 10.1242/jcs.00053. [DOI] [PubMed] [Google Scholar]
- Grabs D, Bergmann M, Urban M, Post A, Gratzl M. Rab3 proteins and SNAP-25, essential components of the exocytosis machinery in conventional synapses, are absent from ribbon synapses of the mouse retina. Eur J Neurosci. 1996;8:162–168. doi: 10.1111/j.1460-9568.1996.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Greenlee MH, Roosevelt CB, Sakaguchi DS. Differential localization of SNARE complex proteins SNAP-25, syntaxin, and VAMP during development of the mammalian retina. J Comp Neurol. 2001;430:306–320. doi: 10.1002/1096-9861(20010212)430:3<306::aid-cne1032>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Greferath U, Müller F, Wässle H, Shivers B, Seeburg P. Localization of GABAA receptors in the rat retina. Vis Neurosci. 1993;10:551–561. doi: 10.1017/s0952523800004764. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Müller F, Wässle H. Localization of GABAA receptors in the rabbit retina. Cell Tissue Res. 1994;276:295–307. doi: 10.1007/BF00306115. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Fritschy JM, Stephenson A, Möhler H, Wässle H. GABAA receptor subunits have differential distributions in the rat retina: in situ hybridization and immunohistochemistry. J Comp Neurol. 1995;353:553–571. doi: 10.1002/cne.903530407. [DOI] [PubMed] [Google Scholar]
- Grigorenko EV, Yeh HH. Expression profiling of GABAA receptor beta-subunits in the rat retina. Vis Neurosci. 1994;11:379–387. doi: 10.1017/s0952523800001723. [DOI] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL, Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA synthesizing enzyme, GAD65 and the GABA vesicular transporter. J Comp Neurol. 2009a;518:1647– 1669. doi: 10.1002/cne.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Stella SL, Jr, Hirano AA, Brecha NC. Plasmalemmal and vesicular gamma-aminobutyric acid transporter expression in the developing mouse retina. J Comp Neurol. 2009b;512:6–26. doi: 10.1002/cne.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Hepp R, Perraut M, Chasserot-Golaz S, Galli T, Aunis D, Langley K, Grant NJ. Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia. 1999;27:181–187. doi: 10.1002/(sici)1098-1136(199908)27:2<181::aid-glia8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Brecha NC. Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. J Comp Neurol. 2005;488:70–81. doi: 10.1002/cne.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Vila A, Brecha NC. Robust syntaxin-4 immunoreactivity in mammalian horizontal cell processes. Vis Neurosci. 2007;24:489–502. doi: 10.1017/S0952523807070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989;108:111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Yamamoto M, Saito N. Immunocytochemical localization of three subtypes of GABA transporter in rat retina. Brain Res Mol Brain Res. 1995;33:319–325. doi: 10.1016/0169-328x(95)00150-q. [DOI] [PubMed] [Google Scholar]
- Hu M, Bruun A, Ehinger B. Expression of GABA transporter subtypes (GAT1, GAT3) in the developing rabbit retina. Acta Ophthalmol Scand. 1999;77:261–265. doi: 10.1034/j.1600-0420.1999.770303.x. [DOI] [PubMed] [Google Scholar]
- Inoue A, Obata K, Akagawa K. Cloning and sequence analysis of cDNA for a neuronal cell membrane antigen, HPC-1. J Biol Chem. 1992;267:10613–10619. [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Janz R, Südhof TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience. 1999;94:1279–1290. doi: 10.1016/s0306-4522(99)00370-x. [DOI] [PubMed] [Google Scholar]
- Janz R, Goda Y, Geppert M, Missler M, Südhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Jellali A, Stussi-Garaud C, Gasnier B, Rendon A, Sahel JA, Dreyfus H, Picaud S. Cellular localization of the vesicular inhibitory amino acid transporter in the mouse and human retina. J Comp Neurol. 2002;449:76–87. doi: 10.1002/cne.10272. [DOI] [PubMed] [Google Scholar]
- Johnson J, Chen TK, Rickman DW, Evans C, Brecha NC. Multiple gamma-aminobutyric acid plasma membrane transporters (GAT-1, GAT-2, GAT-3) in the rat retina. J Comp Neurol. 1996;375:212–224. doi: 10.1002/(SICI)1096-9861(19961111)375:2<212::AID-CNE3>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Werblin F. GABA-mediated positive autofeedback loop controls horizontal cell kinetics in tiger salamander retina. J Neurosci. 1992;12:2451–2463. doi: 10.1523/JNEUROSCI.12-07-02451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YH, Sterling P. Displaced GAD65 amacrine cells of the guinea pig retina are morphologically diverse. Vis Neurosci. 2006;23:931–939. doi: 10.1017/S0952523806230293. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Schmitz F. The expression pattern and assembly profile of synaptic membrane proteins in ribbon synapses of the developing mouse retina. Cell Tissue Res. 2003;311:159–173. doi: 10.1007/s00441-002-0674-0. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Schmitz F, Link E, Südhof TC. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur J Neurosci. 1999;11:1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Kyhn MV, Warfvinge K, Scherfig E, Kiilgaard JF, Prause JU, Klassen H, Young M, la Cour M. Acute retinal ischemia caused by controlled low ocular perfusion pressure in a porcine model. Electrophysiological and histological characterisation. Exp Eye Res. 2009;88:1100–1106. doi: 10.1016/j.exer.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Lang T, Jahn R. Core proteins of the secretory machinery. Handb Exp Pharmacol. 2008;184:107–127. doi: 10.1007/978-3-540-74805-2_5. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Mann LB, Rickman DW, Lim EJ, Chun MH, Grzywacz NM. AII amacrine cells in the distal inner nuclear layer of the mouse retina. J Comp Neurol. 2006;494:651–662. doi: 10.1002/cne.20838. [DOI] [PubMed] [Google Scholar]
- Linberg KA, Fisher SK. Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. J Comp Neurol. 1988;268:281–297. doi: 10.1002/cne.902680211. [DOI] [PubMed] [Google Scholar]
- Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu Rev Neurosci. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- Loeliger M, Rees S. Immunocytochemical development of the guinea pig retina. Exp Eye Res. 2005;80:9–21. doi: 10.1016/j.exer.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Löhrke S, Hofmann HD. Voltage-gated currents of rabbit A- and B-type horizontal cells in retinal monolayer cultures. Vis Neurosci. 1994;11:369–378. doi: 10.1017/s0952523800001711. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Maienschein V, Marxen M, Volknandt W, Zimmermann H. A plethora of presynaptic proteins associated with ATP-storing organelles in cultured astrocytes. Glia. 1999;26:233–244. doi: 10.1002/(sici)1098-1136(199905)26:3<233::aid-glia5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Malgorzata Goczalik I, Raap M, Weick M, Milenkovic I, Heidmann J, Enzmann V, Wiedemann P, Reichenbach A, Francke M. The activation of IL-8 receptors in cultured guinea pig Muller glial cells is modified by signals from retinal pigment epithelium. J Neuroimmunol. 2005;161:49–60. doi: 10.1016/j.jneuroim.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990;5:19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Czernik AJ, De Camilli P, Greengard P, Townes-Anderson E. Differential expression of synapsins I and II among rat retinal synapses. J Neurosci. 1992;12:1736–1749. doi: 10.1523/JNEUROSCI.12-05-01736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE. Structural organization of GABAergic circuitry in ectotherm retinas. Prog Brain Res. 1992;90:61–92. doi: 10.1016/s0079-6123(08)63609-2. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL, Marc RE. All indoleamine-accumulating cells in the rabbit retina contain GABA. J Comp Neurol. 1992;322:275–291. doi: 10.1002/cne.903220213. [DOI] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009;122:2003–2013. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans C, Brandstätter JH. SNAP-25 is present on the Golgi apparatus of retinal neurons. Neuroreport. 2000;11:85–88. doi: 10.1097/00001756-200001170-00017. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brandstätter JH, Kellerman J, Betz H, Wässle H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J Neurosci. 1996;16:6713–6721. doi: 10.1523/JNEUROSCI.16-21-06713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Kensel-Hammes P, Hurley JB, Burton K, Idzerda R, McKnight GS, Bajjalieh SM. Loss of the Synaptic Vesicle Protein SV2B results in reduced neurotransmission and altered synaptic vesicle protein expression in the retina. PLoS ONE. 2009;4:e5230. doi: 10.1371/journal.pone.0005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag TC, Wadhwa S. Differential expression of syntaxin-1 and synaptophysin in the developing and adult human retina. J Biosci. 2001;26:179–191. doi: 10.1007/BF02703642. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, McGuire BA, Sterling P. Interplexiform cell in cat retina: identification by uptake of gamma-[3H]aminobutyric acid and serial reconstruction. Proc Natl Acad Sci USA. 1980;77:658–661. doi: 10.1073/pnas.77.1.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Nelson R, Bender AM, Connaughton VP. Transporter-mediated GABA responses in horizontal and bipolar cells of zebrafish retina. Vis Neurosci. 2008;25:155–165. doi: 10.1017/S0952523808080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Savy C. Dopaminergic and GABAergic retinal cell populations in mammals. Microsc Res Tech. 1997;36:26–42. doi: 10.1002/(SICI)1097-0029(19970101)36:1<26::AID-JEMT3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- O’Bryan PM. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973;235:207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Kim IB, Lee EJ, Kim KY, Kim HI, Chun MH. Immunocytological localization of dopamine in the guinea pig retina. Cell Tissue Res. 1999;298:561–565. doi: 10.1007/s004419900122. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB J. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- O’Malley DM, Masland RH. Co-release of acetylcholine and gamma-aminobutyric acid by a retinal neuron. Proc Natl Acad Sci USA. 1989;86:3414–3418. doi: 10.1073/pnas.86.9.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Südhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- Pattnaik B, Jellali A, Sahel J, Dreyfus H, Picaud S. GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. J Neurosci. 2000;20:6789–6796. doi: 10.1523/JNEUROSCI.20-18-06789.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, González-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Peichl L, Sandmann D, Boycott BB. Comparative anatomy and function of mammalian horizontal cells. In: Chalupa L, Finlay BL, editors. Development and Organization of the Retina. Plenum Press; New York: 1998. pp. 147–172. [Google Scholar]
- Picaud S, Pattnaik B, Hicks D, Forster V, Fontaine V, Sahel J, Dreyfus H. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J Physiol. 1998;513 (Pt 1):33–42. doi: 10.1111/j.1469-7793.1998.033by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gahwiler BH, Thompson SM. Either N- or P-type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron. 1997;18:463–472. doi: 10.1016/s0896-6273(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Rauen T, Rothstein JD, Wässle H. Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res. 1996;286:325–336. doi: 10.1007/s004410050702. [DOI] [PubMed] [Google Scholar]
- Raven MA, Reese BE. Horizontal cell density and mosaic regularity in pigmented and albino mouse retina. J Comp Neurol. 2002;454:168–176. doi: 10.1002/cne.10444. [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Südhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Reim K, Wegmeyer H, Brandstätter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K, Brose N. Structurally and functionally unique complexins at retinal ribbon synapses. J Cell Biol. 2005;169:669–680. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria RC, Strehler EE, Copenhagen DR, Križaj D. Ontogeny of plasma membrane Ca2+ ATPase isoforms in the neural retina of the postnatal rat. Vis Neurosci. 2005;22:263–274. doi: 10.1017/S0952523805223027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillich K, Gentsch J, Reichenbach A, Bringmann A, Weick M. Light stimulation evokes two different calcium responses in Muller glial cells of the guinea pig retina. Eur J Neurosci. 2009;29:1165–1176. doi: 10.1111/j.1460-9568.2009.06682.x. [DOI] [PubMed] [Google Scholar]
- Rivera L, Blanco R, de la Villa P. Calcium-permeable glutamate receptors in horizontal cells of the mammalian retina. Vis Neurosci. 2001;18:995–1002. [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagné C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH. Shape and distribution of an unusual retinal neuron. J Comp Neurol. 1989;280:489–497. doi: 10.1002/cne.902800312. [DOI] [PubMed] [Google Scholar]
- Savy C, Simon A, Nguyen-Legros J. Spatial geometry of the dopamine innervation in the avascular area of the human fovea. Vis Neurosci. 1991;7:487–498. doi: 10.1017/s0952523800009779. [DOI] [PubMed] [Google Scholar]
- Schnitzer J, Rusoff AC. Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J Neurosci. 1984;4:2948–2955. doi: 10.1523/JNEUROSCI.04-12-02948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Weiler R, Feigenspan A. Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J Neurophysiol. 2006;96:1278–1292. doi: 10.1152/jn.00191.2006. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22:6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Frishman LJ. Differential distribution of vesicle associated membrane protein isoforms in the mouse retina. Mol Vis. 2003;9:673–688. [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Nikulasson S, De Girolami U, De Gennaro LJ. Immunohistochemistry of synapsin I and synaptophysin in human nervous system and neuroendocrine tumors. Applications in diagnostic neuro-oncology. Clin Neuropathol. 1993;12:335–342. [PubMed] [Google Scholar]
- Spiwoks-Becker I, Vollrath L, Seeliger MW, Jaissle G, Eshkind LG, Leube RE. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic beta-cells. Mol Endocrinol. 2006;20:183–193. doi: 10.1210/me.2005-0157. [DOI] [PubMed] [Google Scholar]
- Spurlin BA, Park SY, Nevins AK, Kim JK, Thurmond DC. Syntaxin 4 transgenic mice exhibit enhanced insulin-mediated glucose uptake in skeletal muscle. Diabetes. 2004;53:2223–2231. doi: 10.2337/diabetes.53.9.2223. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Porciatti V, Falsini B, Pignatelli V, Rossi C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J Neurosci. 2002;22:5492–5504. doi: 10.1523/JNEUROSCI.22-13-05492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991;6:665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A, Oyler G, McKay R, Gerfen C, Conant K. Cleavage of neuronal synaptosomal-associated protein of 25 kDa by exogenous matrix metalloproteinase-7. J Neurochem. 2007;102:1256–1263. doi: 10.1111/j.1471-4159.2007.04625.x. [DOI] [PubMed] [Google Scholar]
- Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci. 2006;26:7826–7838. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Südhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Carrasco MA. A review on electron microscopy and neurotransmitter systems. Brain Res Brain Res Rev. 2004;47:5–17. doi: 10.1016/j.brainresrev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Kaneko A, Kaneda M. Voltage-dependent ionic currents in solitary horizontal cells isolated from cat retina. J Neurophysiol. 1992;68:1143–1150. doi: 10.1152/jn.1992.68.4.1143. [DOI] [PubMed] [Google Scholar]
- Uesugi R, Yamada M, Mizuguchi M, Baimbridge KG, Kim SU. Calbindin D-28k and parvalbumin immunohistochemistry in developing rat retina. Exp Eye Res. 1992;54:491–499. doi: 10.1016/0014-4835(92)90127-e. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Südhof TC. Distribution of synaptic markers in the retina: implications for synaptic vesicle traffic in ribbon synapses. J Physiol Paris. 1994;88:249–257. doi: 10.1016/0928-4257(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Vardi N, Masarachia P, Sterling P. Immunoreactivity to GABAA receptor in the outer plexiform layer of the cat retina. J Comp Neurol. 1992;320:394–397. doi: 10.1002/cne.903200310. [DOI] [PubMed] [Google Scholar]
- Vardi N, Kaufman DL, Sterling P. Horizontal cells in cat and monkey retina express different isoforms of glutamic acid decarboxylase. Vis Neurosci. 1994;11:135–142. doi: 10.1017/s0952523800011172. [DOI] [PubMed] [Google Scholar]
- Vardi N, Morigiwa K, Wang TL, Shi YJ, Sterling P. Neurochemistry of the mammalian cone ‘synaptic complex’. Vision Res. 1998;38:1359–1369. doi: 10.1016/s0042-6989(98)00007-8. [DOI] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MM, Janz R, Belizaire R, Frishman LJ, Sherry DM. Differential distribution and developmental expression of synaptic vesicle protein 2 isoforms in the mouse retina. J Comp Neurol. 2003;460:106–122. doi: 10.1002/cne.10636. [DOI] [PubMed] [Google Scholar]
- Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Shen C, McRory J. Differential distribution of voltage-gated calcium channels in dopaminergic neurons of the rat retina. J Comp Neurol. 2006;497:384–396. doi: 10.1002/cne.20995. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Gábriel R, Križaj D. Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas. J Comp Neurol. 2008;510:158–174. doi: 10.1002/cne.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc Natl Acad Sci USA. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Effects of prolonged light exposure, GABA, and glycine on horizontal cell responses in tiger salamander retina. J Neurophysiol. 1989;61:1025–1035. doi: 10.1152/jn.1989.61.5.1025. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Feedforward lateral inhibition in retinal bipolar cells: input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc Natl Acad Sci USA. 1991;88:3310–3313. doi: 10.1073/pnas.88.8.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Effects of GABA on horizontal cells in the tiger salamander retina. Vision Res. 1993;33:1339–1344. doi: 10.1016/0042-6989(93)90041-t. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Brecha N. Localized binding of [3H]muscimol to synapses in chicken retina. Proc Natl Acad Sci USA. 1981;78:643–647. doi: 10.1073/pnas.78.1.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S, Cunningham J, Neal M. Stimulated release of endogenous GABA and glycine from the goldfish retina. Brain Res. 1985;345:384–388. doi: 10.1016/0006-8993(85)91022-4. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, Vitorica J, de Blas AL. Immunocytochemical localization of GABAA receptors in goldfish and chicken retinas. J Comp Neurol. 1989;280:15–26. doi: 10.1002/cne.902800103. [DOI] [PubMed] [Google Scholar]
- Young S, Rothbard J, Parker PJ. A monoclonal antibody recognising the site of limited proteolysis of protein kinase C. Inhibition of down-regulation in vivo. Eur J Biochem. 1988;173:247–252. doi: 10.1111/j.1432-1033.1988.tb13991.x. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci. 2009;29:789–797. doi: 10.1523/JNEUROSCI.4984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker C, Yazulla S, Wu JY. Non-correspondence of [3H]GABA uptake and GAD localization in goldfish amacrine cells. Brain Res. 1984;298:154–158. doi: 10.1016/0006-8993(84)91160-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Antibody characterization.
Fig. S1. Single confocal image of VGAT and CaBP immunoreactivity in guinea pig vertical section.
Fig. S2. Single confocal image of SV2A and VGAT immunoreactivity in guinea pig vertical section.
Fig. S3. Single confocal image of synaptotagmin-2 and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S4. Single confocal image of synapsin I and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S5. Single confocal image of syntaxin-1a and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S6. Single confocal image of syntaxin-4 and VGAT immuno-reactivity in guinea pig vertical section.
Fig. S7. Single confocal image of SNAP-25 and CaBP immuno-reactivity in guinea pig vertical section.
Fig. S8. Single confocal image of complexin I/II and CaBP immunoreactivity in guinea pig vertical section.