Abstract
Therapeutic trials in Duchenne Muscular dystrophy (DMD) exclude young boys because traditional outcome measures rely on cooperation. The Bayley-III Scales of Infant and Toddler Development (Bayley-III) have been validated in developing children and those with developmental disorders but have not been studied in DMD. Expanded Hammersmith Functional Motor Scale (HFMSE) and North Star Ambulatory Assessment (NSAA) may also be useful in this young DMD population. Clinical evaluators from the MDA-DMD Clinical Research Network were trained in these assessment tools. Infants and boys with DMD (n=24; 1.9±0.7 years) were assessed. The mean Bayley-III motor composite score was low (82.8 ± 8; p=<.0001)(normal=100 ± 15). Mean gross motor and fine motor function scaled scores were low (both p=<.0001). The mean cognitive comprehensive (p=.0002), receptive language (p=<.0001), and expressive language (p=.0001) were also low compared to normal children. Age was negatively associated with Bayley-III gross motor (r=−0.44 p=.02) but not with fine motor, cognitive, or language scores. HFMSE (n=23) showed a mean score of 31 ± 13. NSAA (n =18 boys; 2.2 ± 0.4years) showed a mean score of 12 ± 5. Outcome assessments of young boys with DMD are feasible and in this multicenter study were best demonstrated using the Bayley-III.
1. Introduction
1.1. Early Motor Development in DMD infants and boys
There are many excellent multicenter studies that demonstrate progression of weakness in boys with Duchenne Muscular Dystrophy (DMD). Most use measures that require active cooperation. Early careful DMD natural history studies relied on manual muscle testing using Medical Research Council (MRC) testing and functional outcomes [1–4]. These outcomes have been extended to include quantitative measurements, which allow better inter-rater reliability for ambulatory boys and are currently used in multicenter trials [5–13]. Additional functional measures including timed functional tests [4, 10, 14–17] activity monitoring [18, 19] and the six-minute walk test [10, 19, 20] are also very reliable for ambulatory boys. The North Star Ambulatory Assessment (NSAA) tests 17 items ranging from standing to running 10 meters has recently been shown to have excellent inter-rater reliability in a multicenter trial of boys with DMD as young as 5 years [21–23].
Few clinical trials have included younger boys or infants because traditional MRC or quantitative testing may not be reliable or even possible. Both manual muscle testing and quantitative strength testing have been shown to be less reliable in children younger than age six years [4, 24]. Furthermore, motor skills do improve in pre-school boys with DMD, albeit at a slower rate than normal boys. Prior studies of untreated younger boys demonstrate effectively this “honeymoon” period in DMD when function and strength may improve but does not equal the improvement present in normal children[1, 10, 19, 25, 26]. This can complicate interpretation of functional gains during clinical trials in younger boys with DMD. Reliable, age-normed measures are needed that can be performed in infants and younger boys with DMD. A pilot study of baseline motor function in 33 young boys with DMD (mean age 3.4 years) shows markedly different gross motor skills compared to age-matched controls using the Hammersmith Motor Ability Score[27]. However, there was a “ceiling effect” using this early form of the Hammersmith. In the same study, the locomotor quotient of the Griffiths’ scales demonstrated deterioration in young boys with DMD [27]. This latter measurement showed a highly significant negative correlation with age.
An expanded version of the Hammersmith Functional Motor Scales (HFMSE) has recently been validated in both SMA-II and SMA-III and includes additional gross motor skills including jumping and climbing up and down stairs [28, 29]. Finally, the Bayley III infant motor scale, while not yet tested in children with neuromuscular disorders, has been validated in many studies of children with motor delay in the first years of life[30–38].
Novel therapeutic interventions for boys with DMD may be most effective in the youngest age group, but these boys present significant difficulties for clinical trials because of the lack of validated outcomes. In order to have this challenging population of infants and young boys “trial ready” we studied outcomes in a multicenter cohort after careful clinical evaluator training. We wanted to capture data from the youngest boys and therefore chose the Bayley III and the HFMSE which have both been validated in infants from birth. We also studied the NSAA to test its ability to assess the youngest ambulatory boys with DMD.
1.2. Early Mental Development in DMD infants and boys
Cyrulnik et al demonstrated that cognitive delay in some boys with DMD is clearly recognized by parents[39]. Hinton et al have also characterized verbal and memory skills in older boys with DMD [40]. The earliest study of mental development in young boys with DMD (6 boys who were a subset of Brooke et al’s original study) was done using the Denver developmental assessment [1]. This diagnostic tool has since been revised to improve both social and speech/language assessments [41]. However, as it does not provide a quotient, it is not possible to quantify and would also be difficult to standardize between sites. The most detailed work in mental development of young boys with DMD using the Griffith’s Mental Development Scales (GMDS) shows that early delays can be detected using careful standardized assessments [42].
The most validated scales of language and cognition in all infants and young children through age six years are the Bayley III scales of infant development which are validated through 42 months of age[43] and the Stanford Binet Intelligence Scale 5th edition (ages 3 years and older)[44]. Both provide measurable quotients. While the Bayley III has not been used in boys with DMD, it has been used in hundreds of studies of children at risk for both motor and mental delays including premature infants, children with hypoxic ischemic injuries, children with Down syndrome and children with immune deficiency [30–38].
We had two objectives in determining if we could reliably assess motor, cognitive, language, and social emotional development in young boys with DMD. First, if a reliable scale can measure development in infants and young boys with DMD, it could be used as and outcome measure in clinical trials. Some treatments may work best in infants and young boys before severe fibrosis develop and this would allow inclusion of this important group. Second, information about early cognitive or intellectual deficits may lead to specific early cognitive or social intervention for infants and boys with DMD.
2. Methods
2.1 Participants
We included boys with DMD who were at least one month but less than three years old. All six DMD Clinical Research sites received human studies approval and informed consent was obtained from a parent prior to enrollment in the study. Every site enrolled at least three boys for this study which involved a single visit; Washington University (N=5), Nationwide Children’s (N=5) University of California-Davis (N=4), University of Minnesota (N=4), Boston Children’s (N=3), Newcastle (N=3). Mutations in the DMD gene were defined for all boys (Table 1). Thirteen had a primary relative (brother or uncle) with DMD; three of these boys were enrolled based on an elevated serum CK consistent with DMD, and the results of mutation analysis in their brothers were accepted. Three boys were identified by newborn screening[45]. None were taking corticosteroids.
Table 1. Age and DMD mutations.
Asterisks mark subjects in whom mutation analysis from an affected brother was accepted. Implications of reading frame are discussed in the text. The last two columns describe predicted effect of the mutation on expression of other relevant DMD isoforms by deletion of either the appropriate promoter/exon 1 or by downstream domains.
| Subject | Age (years) | Family History | Mutation | Exon(s) | Frame | Disruption of Dp140 isoform | Disruption of Dp71 isoform |
|---|---|---|---|---|---|---|---|
| 1 | 0.37 | Yes | Deletion | 3–32 | In | no | no |
| 2 | 0.41 | No | Deletion | 3–41 | In | no | no |
| 3 | 0.63 | Yes | Deletion | 45 | Out | yes | no |
| 4 | 0.69 | Yes | Nonsense* (c.615T>A; p.TyrX) | 7 | Out | no | no |
| 5 | 1.22 | Yes | Duplication | 2 | Out | no | no |
| 6 | 1.23 | Yes | Deletion | 45–50 | Out | yes | no |
| 7 | 1.42 | Yes | Deletion | 46–50 | Out | yes | no |
| 8 | 1.7 | No | Deletion | 12 | Out | no | no |
| 9 | 1.72 | Yes | Deletion* | 46 | Out | yes | no |
| 10 | 1.81 | Yes | Deletion | 45–50 | Out | yes | no |
| 11 | 2.02 | No | Deletion | 58 | Out | yes | no |
| 12 | 2.07 | Yes | Deletion* | 8–9 | Out | no | no |
| 13 | 2.21 | Yes | Nonsense (c.2353C>T; p.Gln785X) | 19 | Out | no | no |
| 14 | 2.24 | No | Deletion | 51–57 | Out | yes | no |
| 15 | 2.41 | No | Deletion | 53–55 | Out | yes | no |
| 16 | 2.41 | No | Deletion | 45 | Out | yes | no |
| 17 | 2.43 | No | Deletion | 49–52 | Out | yes | no |
| 18 | 2.44 | No | Deletion | 58–64 | Out | yes | yes |
| 19 | 2.61 | Yes | Deletion | 18–25 | In | no | no |
| 20 | 2.71 | No | Deletion | 46–52 | Out | yes | no |
| 21 | 2.8 | No | Nonsense (c.2791G>T; p.Glu931X) | 21 | Out | no | no |
| 22 | 2.82 | No | Deletion | 45 | Out | yes | no |
| 23 | 2.85 | Yes | Deletion | 12–44 | Out | unknown+ | no |
| 24 | 2.99 | No | Deletion | 17 | Out | no | no |
2.2 Measures
The clinical assessments included are summarized in Table 2. Total testing time was generally 90–150 minutes. The tests administered included the following:
Table 2.
Clinical assessments used in infants and young boys with DMD.
| Test | Age range | Domains | Test Administration | Time to administer |
|---|---|---|---|---|
| Bayley III Scales of Infant development | 0–42 months |
|
Trained clinical evaluator | 90–120 minutes |
| Adaptive Behavior Subtest of Bayley-III | 0–42 months |
|
Parent self-report | 15–30 minutes |
| HMFSE | 0–6 years* |
|
Trained clinical evaluator | 20–30 minutes |
| North Star ** | Ambulatory |
|
Trained clinical evaluator | 10–15 minutes |
The age range of the HMFSE is approximate as these skills are gained over time but typically developing children would have achieved all by six years.
The 17 activities tested with the North Star include standing (1), walking (2), standing up from a chair(3), standing on each leg (4, 5), climbing on a box step with each leg (6, 7), descending from box step with each leg (8, 9), getting to sitting(10), rising from the floor (11), lifting head(12), standing on heels (13), jumping (14), hopping on each leg (15, 16) and running 10 meters(17).
a) Bayley III Scales of Infant Development
The Bayley III Scales of infant development includes assessment of cognition, language (receptive and expressive) and motor function (gross and fine). The Bayley III provides a measureable and validated cognitive quotient[43]. Bayley III language assessment is divided into two subtests consisting of receptive and expressive assessments. When these two language subtests are combined a composite score is determined. Bayley III Motor assessment includes scaled scores for fine motor and gross motor as well as a composite score. In addition, the Bayley III social emotional scale includes items which test both social emotional competence and sensory processing and is based on earlier work by Greenspan [46].
b) Adaptive Behavioral Subtest of Bayley
(ABS) is a detailed, validated parental questionnaire which allows calculation of social emotional and adaptive behavioral assessment scores [43]. Subscales include Communication, Community Use, Functional Pre-Academic, Home Living, Health and Safety, Leisure, Self-Care, Self-Direction, Social, and Motor. Subscale scores in normal children are standardized to a mean of 10 and a standard deviation of 3. Based on these subscales, four composite scores are calculated which include the general adaptive composite (GAC), conceptual adaptive domain (CON), social adaptive domain (SO) and practical adaptive domain (PR). The general adaptive composite (GAC) reflects all subscales. The conceptual adaptive domain (CON) is calculated by adding the communication, functional pre-academics, and self-direction subscale scores. The social adaptive (SO) composite score considers the leisure and the social subscale scores. Finally, the practical adaptive domain reflects the community use, health and safety, home living, and self-care subscales. The norms for these composite scores in normal children are mean = 100, sd =15.
c) Expanded Hammersmith Functional Motor Scales (HFMSE)(Table 3)
Table 3. Expanded Hammersmith Functional Motor Scale.
Each activity is scored on a three point scale 0–2 (0= unable to complete, 1 completes with modification, 2 completes fully).
| Activity Tested | Estimated age range when normal children should complete this activity |
|---|---|
| 1. Sitting on chair | 6–10 mos |
| 2. Long sitting, legs extended | 7–11 mos |
| 3. Hand To Head while sitting | 10–12 mos |
| 4. Two hands to Head while sitting | 10–12 mos |
| 5. Rolling to each side | 4–5 mos |
| 6/7. Rolling prone to supine Right and left | 5–9 mos |
| 8. Rolling Supine to prone to right | 6–9 mos |
| 9. Rolling Supine to prone to left | 6–9 mos |
| 10. Sitting to lying | 6–8 mos |
| 11. Props on Forearms | 4–6 mos |
| 12. Lifts head from Prone | 1–2 mos |
| 13. Props on extended arms | 8–11 mos |
| 14. Lying to sitting | 7–11 mos |
| 15. Four-point kneeling | 7–10 mos |
| 16. Crawling | 7–12 mos |
| 17. Lifts head from supine | 8–11 mos |
| 18. Supported standing | 8–10 mos |
| 19. Stand unsupported | 9–13 mos |
| 20. Stepping | 12–15 mos |
| 21/22. Right and left hip flexion in supine | 8–15 mos |
| 23/24. High kneeling to right/left half Kneel | 18–36 mos |
| 25/26. High kneeling to standing, leading with right/left leg | 18–36 mos |
| 27. Stand to sitting on floor | 9–13 mos |
| 28. Squat | 10–15mos |
| 29. Jumps 12 inches forward | 24–38 mos |
| 30. Ascends 4 stairs with railing | 18–24 mos |
| 31. Descends 4 stairs with railing | 24–48 mos |
| 32. Ascends 4 stairs without arm support | 36–48 mos |
| 33. Descends 4 stairs without arm support. | 36–56 mos |
The HFMSE was administered to all participants with DMD. While this scale and prior forms of it were originally developed for children with SMA type II and III, we thought recent work suggested the HFMSE might be useful in young DMD boys [47, 48]. Over the last decade, several versions of the Hammersmith Functional Motor Scales have been developed[29, 47, 49–51]. The first version, by Main et[50] showed good reliability in children over age 30 months in both SMA and normal children but poor reliability in younger children. Krosshell et al then showed that a modified version Hammersmith functional motor scale (MHFMS) showed excellent reliability (ICC 0.96) in very young, non ambulatory children with SMA type 2 or 3 (range 9–30 months) [29]. Healthy participants had MHFMS scores ranging from 36 to 40 and achieved maximum test scores at 12 months of age[29]. The Expanded Hammersmith Functional Motor Scale the (HFMSE) was developed using some skills from the Hammersmith Functional Motor Scale developed by Main et al [50] and skills from the Gross Motor Function Measure [52] including standing, squatting, jumping, and stair climbing and showed good reliability in a large cohort of ambulatory subjects with SMA 2 and 3, extending to as young as 20 months [47, 48]. We chose to use the HFMSE because of this reliability and because it would capture early ambulation milestones.
d) North Star Ambulatory Assessment (NSAA)(Table4)
Table 4. North Star Ambulatory Assessment.
Each activity is scored on a three point scale 0–2 (0= unable to complete, 1 completes with modification, 2 completes fully).
| Activity Tested | Estimated age range when normal children should complete this activity |
|---|---|
| 1. Stand | 9–13 mos |
| 2. Walk | 9–15 mos |
| 3. Stand up from a chair | 9–14 mos |
| 4/5. Stand on right/left leg for three seconds | 2.5–3.5 years |
| 6/7. Climb on box step (15 cm high) with right/left leg leading | 14–22 mos |
| 8/9. Descend from box step with right /left leg leading | 16–26 mos |
| 10. Gets from lying to a sitting position | 6–11 mos |
| 11. Rises from supine to standing | 10–14 mos |
| 12. Lifts head from supine position | 8–11 mos |
| 13. Stands on heels | 3–4 years |
| 14. Jump up | 20–38 mos |
| 15/16. Hop on right/left leg clearing floor | 3–5 years |
| 17. Run 10 meters. | 15–26 mos |
The NSAA tests functional activities including standing, getting up from the floor, negotiating steps, hopping, and running[22, 23, 53, 54]. It was designed to be used in boys with DMD who are able to stand and has been validated in boys over the age of 3 years. The assessment is based on a 3 point rating scale of 2= ability to perform the test normally, 1= modified method or assistance to perform test, 0=unable to perform the test. Thus, total score can range from 0 (completely non-ambulant) to 34 (able to complete all tasks fully). The 17 activities tested become progressively difficult (Table 4). Item 1 requires a boy be able to stand. We therefore used this assessment only in those who were at least able to stand using a modified method, thus achieving at least 1 point. We realized that the NSAA is limited because there is not yet data in typically developing children and we could only score those who were already ambulating.
2.2 Training
Training required all clinical evaluators (CE) to attend a three-day session at Washington University in Saint Louis. All CE’s completed didactic and interactive training for the HFMSE (by SOR and ES, Boston Children’s Hospital) and the NSAA (by ME, Newcastle). For the Bayley III, CE’s received didactic and healthy infant training (by MMC, Washington University). Then each site was required to recruit at least two additional well infants (under and over age 18 months) and perform a videotaped practice Bayley III. This was reviewed and scored by MMC and if needed, additional well infants were evaluated. Upon completion, CEs at each site were certified (prior to recruitment of boys with DMD). Training for the HFMSE (by SOR and ES) and NSAA (by ME) was also performed during this meeting. Retraining for all outcomes was performed 12 months into the study.
2.3 Statistical analyses
Descriptive statistics were performed with SAS 9.3 (SAS Institute, Cary, NC) and GraphPad Prism (GraphPad Software, La Jolla, CA). P-values are all 2-tailed.
3. Results
3.1. Bayley III Scales of Infant Development and Adaptive Behavioral Assessment Score (ABAS)
All 24 infants and young boys with DMD were able to complete the gross motor component of the Bayley III (mean age 1.9 ± 0.7 years; range=0.37–2.99) and 23 were able to complete fine motor component. One child (age 2.4 years) became irritable and did not complete the fine motor component. All 24 completed the language and cognitive assessment of the Bayley III.
3.1.a Bayley III Cognitive
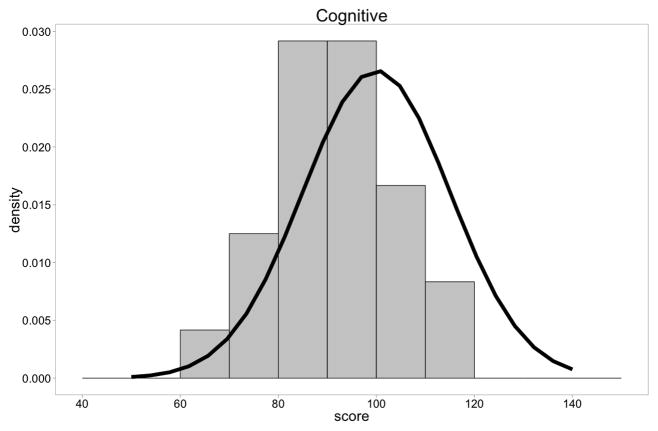
On the Bayley III cognitive scale, the mean cognitive comprehensive score for the group was 89.6 with a range of 65–115. The plot of the individual children demonstrated a clear shift to the left very similar to what has been described for standard IQ testing in older boys with DMD (t = −4.43, p = .0002). (Figure 1A)
Figure 1.
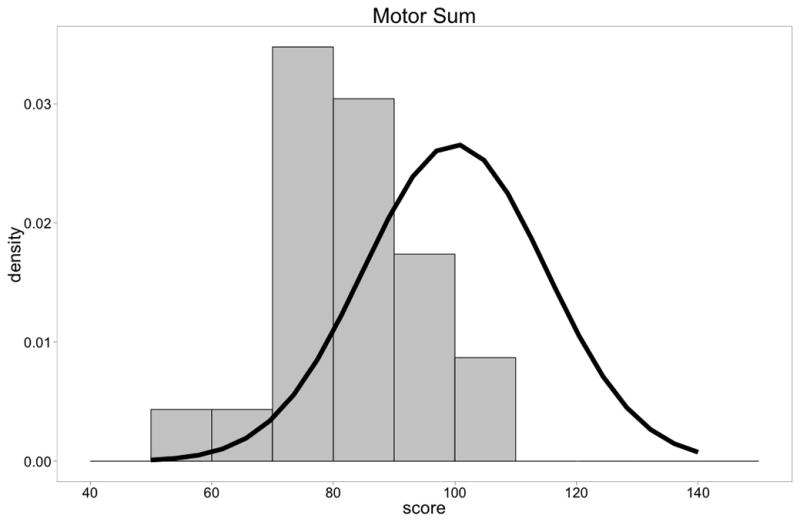
Figure 1A. Distribution of Bayley-III Cognitive Composite scores of DMD boys compared to normal children. There was a shift of the distribution to the left (t = −4.36, p=.0001).
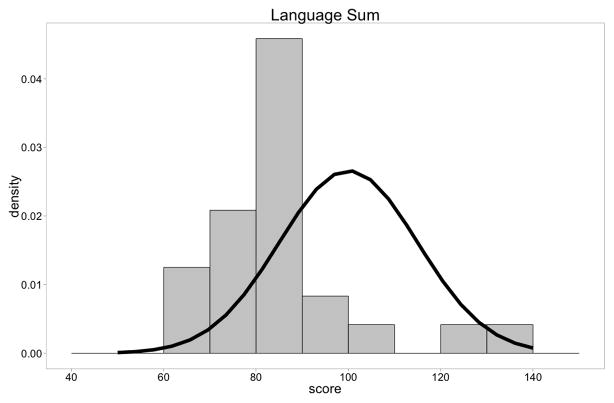
Figure 1B. Distribution of Bayley-III Language Composite scores of DMD boys compared to normal children(n=24). There was a shift of the distribution to the left (t=−4.42, p<.0001).
Figure 1C. Distribution of Bayley-III Motor Composite scores of DMD boys compared to normal children(n=23). There was a shift of the distribution to the left (t =−7.60, p=<.0001).
3.1.b Bayley III language (Table 5)
Table 5. Bayley-III Language and Cognitive assessment of infants and young boys with DMD (n=24).
Both receptive and expressive language means were significantly lower than normal children. The language composite score combines these two scaled scores.
| Subtest | Normal | DMD | t test and p value |
|---|---|---|---|
| Language Receptive Scaled Score | 10±3 | 7.0±1.6 (2–14) | t=−5.98 (p=<.0001) |
| Language Expressive Scaled Score | 10±3 | 8.0± 2.3 (3–18) | t=−3.01 (p=.005) |
| Language Composite | 100±15 | 85.8±9.9 (5–135) | t=−4.42 (p=.0001) |
| Cognitive Composite | 100±15 | 89.5±8.7 (65–115) | t=−4.36 (p=.0001) |
On the Bayley III receptive and expressive language assessments and the composite language assessment, infants and young boys with DMD again demonstrated mean performances that were lower than average (Table 5) with a shift of data to the left (t = −4.61, p= .0001)(Figure 1B).
3.1.c Bayley III Motor Assessment
On the Bayley III Motor assessment, motor composite assessment showed average performance that was lower than normal children (t = −7.99, p = <.0001) (Figure 1C). Gross motor, as expected appeared more affected being more affected than fine motor (Table 6). The data showed that most boys performed more than a standard deviation less than the mean.
Table 6. Bayley-III Motor assessment of infants and young boys with DMD.
One infant was not able to complete the fine motor testing and therefore the Motor composite score (which combines both Fine and Gross motor Scaled scores) is also calculated with n=24.
| Subtest | Normal | DMD | t test and p value |
|---|---|---|---|
| Fine Motor Scaled Score (n=23) | 10±3 | 7.9 ±1.9 (3–11) | t=−4.4 (p=.0001) |
| Gross Motor Scaled Score (n=24) | 10±3 | 6.1 ± 1.5 (3–10) | t=−9.8 (p=<.0001) |
| Motor Composite Score (n=23) | 100±15 | 82.2 ±8.4 (58–103) | t=−7.60 (p=<.0001) |
3.2 Comparison of DMD boys’ performance of Bayley III with age
3.2.1 Bayley III Gross and fine motor scaled scores versus age
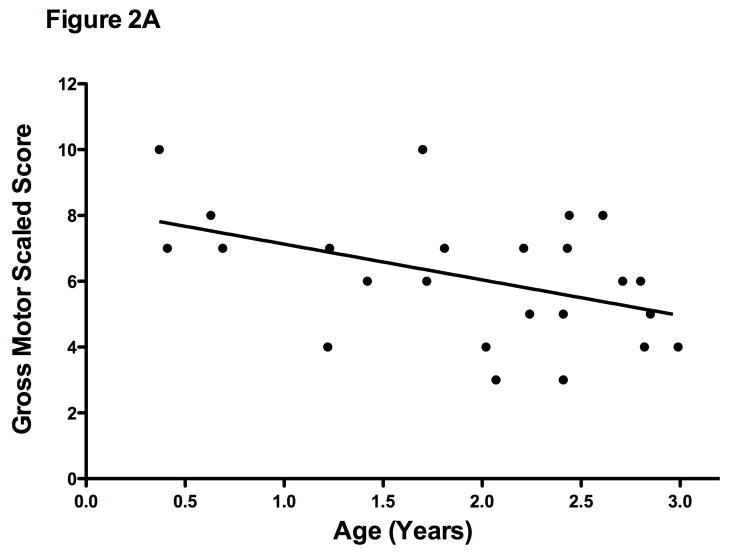
Lower gross motor-scaled scores were associated with increasing age (r= −0.44; p= .02) (Figure 2A). This may be a function of the test better identifying deficits as boys get slightly older or may suggest that these children are losing ground compared to their peers even in this early age group. Fine motor-scaled scores, while low, did not vary significantly with age (r=−0.12; p= 0.5).
Figure 2.
Figure 2A. Bayley-III Gross Motor Scaled Scores versus Age (n=24). There was a negative correlation with age (r = −0.45; p=.03) demonstrating motor function relative to typically developing children is low.
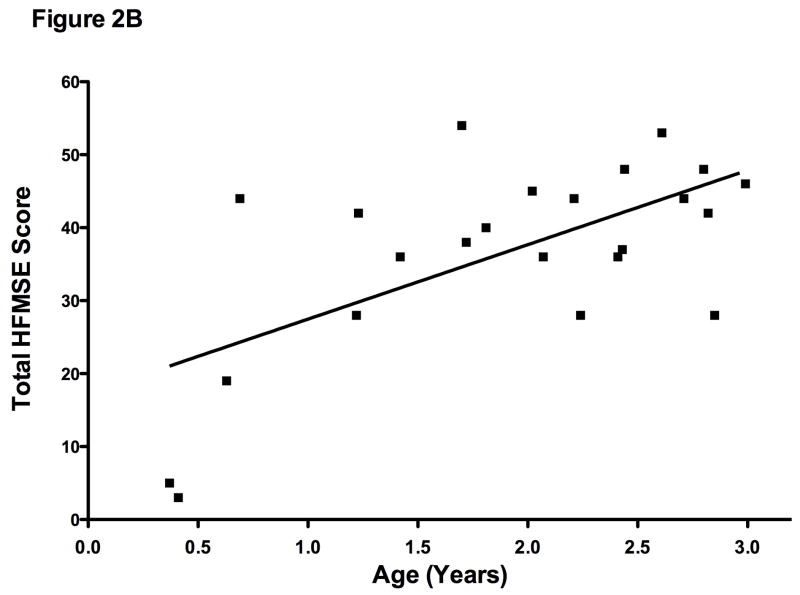
Figure 2B. Hammersmith Functional Motor Extended versus Age (n=23). There was a positive correlation with age (r = 0.63 p=.001) showing absolute motor gains are demonstrated across time.
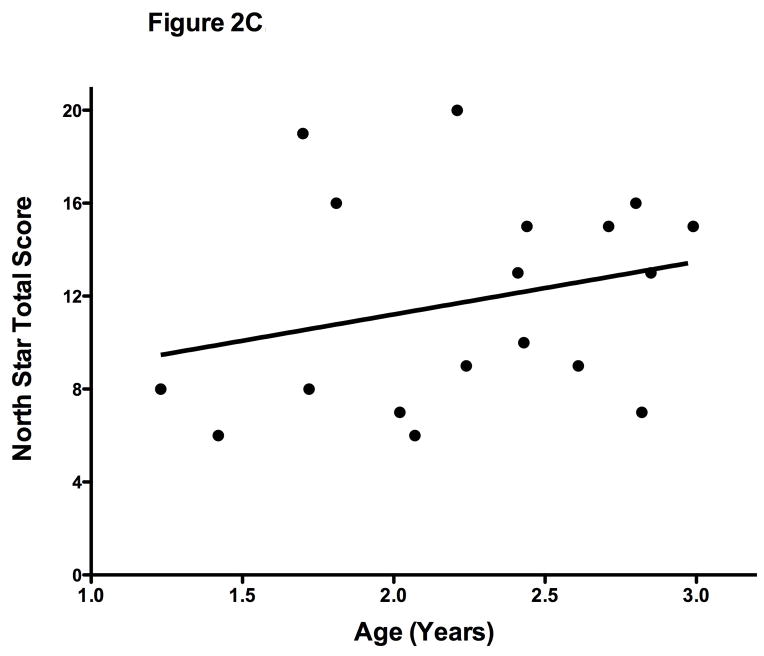
Figure 2C. North Star Ambulatory Assessment (n=18). There was not a significant correlation with age (r = 0.26; p=.3).
3.2.2. Bayley III Cognitive and Language scores versus age
The Bayley III Cognitive and Language composite scores did not vary significantly with age (r=−0.13 and r=0.10; p=0.5 and 0.6). However, cognitive and language composite scores were very tightly correlated with each other (r= 0.80; p= <.0001). This positive correlation between cognitive and language is commonly seen in other populations including typically developing children[43].
3.3 Adaptive Behavior Subtest (ABS)
The results for each ABS are shown in Table 7. The lowest ABS subscale scores were Functional Pre-academic (7.8 ± 2.8, Self-care (6.2 ± 2.9) and Motor (7.4 ± 4.0). The general adaptive composite (GAC) reflects all subscales and showed a mean composite score of 84 ± 15. The conceptual adaptive domain (CON) composite score for our population was 86 ± 15. The mean social adaptive (SO) composite score was 86 ±10. Finally, the mean practical adaptive (PR) was 85 ±13. Despite the fact that the motor subscale (7.4 ± 4.0) is not considered in the CON, SO or PR composite scores, on average the infants and young boys are one standard deviation lower than normally developing boys. The ABS questionnaire reflects similar pattern of performance demonstrated by direct examination (Bayley III) and provides good evidence that these deficits are apparent to their primary caregiver.
Table 7. Adaptive Behavior Subtest of Bayley-III in infants and young boys with DMD.
Incomplete reports and age of infants account for the variable numbers. Community use, Functional use, and Home living are only scored for infants over one year of age.
| Sub-scale | Communication | Community Use | Functional Pre-Academic | Home Living | Health and Safety | Leisure | Self Care | Self Direction | Social | Motor |
|---|---|---|---|---|---|---|---|---|---|---|
| n= | 22 | 18 | 18 | 18 | 22 | 22 | 22 | 22 | 22 | 22 |
| nl mean =10 | 8.4 | 8.8 | 7.8 | 8.7 | 7.9 | 8.4 | 6.2 | 8.7 | 7.8 | 7.4 |
| s.d. ±3 | ± 2.9 | ± 2.9 | ± 2.8 | ± 3.3 | ± 2.6 | ± 3.1 | ± 2.9 | ± 3.2 | ± 2.9 | ±4.0 |
| t value p value |
t=−1.6 p=.01 |
t=−1.7 p=.04 |
t=−3.2 p=.002 |
t=−1.7 p=.05 |
t=−3.8 p=.0006 |
t=−2.3 p=.03 |
t=−6.1 p<.0001 |
t=−1.8 p=.04 |
t=−3.4 p=.001 |
t=−3.1 p=.002 |
3.4. Social Emotional Scale of the Bayley III
Twenty-two completed the Social Emotional Scale of the Bayley III. The social emotional composite scores ranged from 55–135 (mean 92 ± 17). Six boys had social emotional scores less than 75 which correspond to the 5th percentile or less. The social emotional scores did not vary with age (r=0.1; p=0.47).
3.5. Expanded Hammersmith Functional Motor Scale (HFMSE)
The HFMSE was performed on 23 boys. The mean total score was 36.7 ± 13. As expected, this score increased with age (r = 0.63; p = .001) (Figure 2B). HFMSE scores for population based typically developing children do not yet exist. However, we demonstrate that is feasible to perform this assessment and in time may allow one to detect early motor plateau or decline in individual children.
3.6. Performance on the North Star Ambulatory Assessment
We tested only the 18 boys who were able to stand independently for at least 3 seconds (thus achieving 2 points). As expected the mean age was older (2.2 ± 0.5 years) compared to those who could not (0.9 ± 0.6 years). As with the HFMSE, the NSAA has also not yet been studied in typically developing children. The scores in this group of boys with DMD boys did not correlate with age (r= 0.24; p =0.3). (Figure 2C). We also noted striking variability in the performance of different boys of similar ages. This observation corresponds to the clinical fact that some DMD boys meet some early milestones and are therefore are not recognized as delayed in these first years. However, we do demonstrate it is feasible to begin to use the early skills in this scale (standing, walking) in the boys over age two years. Following this scale across time may be useful if data in typically developing children is obtained.
4. Discussion
There is limited evidence of the motor, cognitive and language function in boys with DMD who are less than 5 years old. Previous work using the Griffiths’ scale supports this approach where normative controls allowed Smith et al to compare development of boys with DMD to that of typically developing children at a single site[27, 42]. The Bayley-III is the most widely used standardized measure in the clinical research and early intervention setting for children with developmental delay. Earlier versions of the Bayley have been favorably compared to the Griffiths’ scale in children with other severe developmental disabilities[55]. We chose to study infants and young boys with DMD using the Bayley III for two reasons. First, we had the ability to train clinical evaluators in its use whereas training in the Griffiths’ scale is not widely available. Second, we wanted to determine if, with careful training, a multicenter outcome trial would allow comparison of this challenging group across ages and against normative populations. In this cross sectional population of boys with DMD, we demonstrate that these infants and young boys have measurable differences in motor, cognitive, language, and social development compared to typically developing children.
The normative sample for the Bayley III which includes the Adaptive Behavior Questionnaire included 1700 children from age 1 through 42 months and was stratified using by gender, region, race/ethnicity, and parent education. The Bayley III is not designed to be predictive but descriptive and may underreport deficits. However, in this multi-center study we were able to reliably identify group differences in infants and young boys with DMD compared to typically developing children.
With regard to gross motor development, we demonstrated that Bayley III scaled gross motor scores correlated negatively with age of the infants and boys. It has been known for many years that boys with DMD gain motor skills and function over the first 6–7 years (the “honeymoon” period). Our work suggests that while there are gains in motor skills (demonstrated on the HFMSE), relative to their peers, even infants and young boys with DMD may be losing ground and show less maturational improvement with age. Follow-up of this cohort of boys will further clarify this question and determine if these tools are sensitive to change across time. Given the gain in skills with age on the HFMSE, it is likely that a therapeutic agent would need to produce greater improvement over time in skills on this instrument or a greater percentage of patients achieving defined levels of performance or milestones. This fact makes scales such as the HFMSE and the NSAA much less useful as they have not been validated in typically developing children.
Early motor deficits in young boys with DMD under age three have also not been reported using the HFMSE or the NSAA. While we demonstrate that the HFMSE can be tested in young boys with DMD, and that the absolute scores increase with age as expected, this measure did not have any advantage over the Bayley III which has the strong advantage of being well standardized in normal children. Once ambulatory, we were also able to score all boys with DMD using the North Star Ambulatory assessment. The lack of relationship with age of the NSAA may imply that it might not be a useful measure of disease progression in the under age three population. Longitudinal assessments will be required to determine this. Furthermore, both the HFMSE and the NSAA suffer from lack of validation in typically developing children.
Many boys with DMD have social deficits which have generally been appreciated at or just before school age [40, 56, 57]. Here, we also demonstrate lower average performance on measures of cognitive and social emotional development based both on objective clinical evaluator assessments (via the SE Scale of the Bayley III) and by parent observation (via the Adaptive Behavior Subscale) in children younger than three years. These two assessments correlated well with each other. In the study of other populations of children, this correlation has also been recognized. Early recognition of these cognitive and social deficits may have implications regarding therapy and early intervention services. Social and cognitive deficits are not typically included as outcome in neuromuscular treatment trials. Our data shows that both objective raters and caregivers may detect these deficits and that these deficits are present early in the disease course.
Our subjects were genetically well characterized. Only three had mutations predicted to preserve an open reading frame, more commonly associated with the milder Becker muscular dystrophy (BMD) phenotype. However, two of these in-frame mutations (deletion of exons 3–41, and deletion of exons 18–25) have been previously reported to be associated with DMD. The third (deletion of exons 3–32) has been reported only once to the Leiden database (www.dmd.nl) without a clearly defined phenotype, but may be expected to be associated with a DMD-like phenotype by analogy to many other large deletions affecting the N-terminus of the dystrophin protein [58].
It has been previously reported that deletions affecting expression of either the Dp140 or Dp71 isoforms of dystrophin may be related to cognitive impairment in DMD patients [59] [60] As seen in Table 1, the vast majority (23 out of 24) of mutations represented in our cohort are predicted not to affect Dp71 expression. Effects on Dp140 expression could be predicted in 23 subjects, among whom expression would be expected to be disrupted in 13. In boys whose mutations did not disrupt the Dp140 brain dystrophin, there were trends for higher cognitive comprehensive (mean 93.5 versus 86.9; p=.10) and language comprehensive scores (mean 91.4 versus 81.7; p=.10) but no difference in motor scores (83.5 versus 81.5; p=.35).
Clinical trials are underway which may fundamentally change the natural history of DMD. Some therapeutic options may work best early, prior to the secondary sequella of fibrosis of muscle. Traditionally, these clinical trials have excluded all young boys and infants as traditional outcome measures have not been possible in this age group. While the very young, less than six month, age group will remain challenging, developmental scales such as the Bayley III allow them to be studied. In this study, we demonstrate that is possible to characterize and quantitate cognitive, language, and motor deficits at baseline evaluation. The most consistent deficits in the young population were in the gross motor domain. Since future treatments are likely to preserve skeletal muscle fibers and improve muscle function, it is likely that the motor scales of the Bayley III, and other functional scales which focus on gross motor function (such as the HFMSE and North Star) will play an important role in these clinical trials. Over time, we hope to demonstrate sensitivity to change for each of these assessment tools in this challenging population.
Acknowledgments
This work was funded by the Muscular Dystrophy Association DMD-center grants to AMC, JDM, CMM, JWD, and BTD. This publication was also made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Authors disclosure statement. The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brooke M, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. determination of the “power” of theapeutic trials based on the natural history. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 2.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation of Duchenne muscular dystrophy; interesting results in a trial of prednisone. 1987;44:812–817. doi: 10.1001/archneur.1987.00520200016010. [DOI] [PubMed] [Google Scholar]
- 3.Mendell JR, Province MA, Moxley RT, 3rd, et al. Clinical investigation of Duchenne muscular dystrophy. A methodology for therapeutic trials based on natural history controls. Archives of neurology. 1987;44:808–11. doi: 10.1001/archneur.1987.00520200012009. [DOI] [PubMed] [Google Scholar]
- 4.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 1995;74:S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Florence JM, Pandya S, King WM, et al. Clinical trials in Duchenne dystrophy; Standardization and reliability of evaluation procedures. 1984;64:41–45. doi: 10.1093/ptj/64.1.41. [DOI] [PubMed] [Google Scholar]
- 6.Connolly AM, Schierbecker J, Renna R, Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. 2002;12:917–25. doi: 10.1016/s0960-8966(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 7.Escolar DM, Henricson EK, Mayhew J, et al. Clinical evaluator reliability for quantitative and manual muscle testing measures of strength in children. 2001;24:787–93. doi: 10.1002/mus.1070. [DOI] [PubMed] [Google Scholar]
- 8.Pandya S, Florence JM, King WM, Robison JD, Oxman M, Province MA. Reliability of Goniometric measurements in patients with Duchenne muscular dystrophy. 1985;65:1339–1342. doi: 10.1093/ptj/65.9.1339. [DOI] [PubMed] [Google Scholar]
- 9.Buyse GM, Goemans N, Henricson E, et al. CINRG pilot trial of oxatomide in steroid-naive Duchenne muscular dystrophy. 2007. [DOI] [PubMed] [Google Scholar]
- 10.Mayhew JE, Florence JM, Mayhew TP, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle & nerve. 2007;35:36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 11.Escolar DM, Hache LP, Clemens PR, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–52. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spurney CF, Rocha CT, Henricson E, et al. CINRG pilot trial of coenzyme Q10 in steroid-treated Duchenne muscular dystrophy. Muscle & nerve. 2011;44:174–8. doi: 10.1002/mus.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman A, Clemens PR, Tesi-Rocha C, et al. Liquid formulation of pentoxifylline is a poorly tolerated treatment for duchenne dystrophy. Muscle & nerve. 2011;44:170–3. doi: 10.1002/mus.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle & nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 15.Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB. The natural history of Duchenne muscular dystrophy: a caveat for therapeutic trials. Transactions of the American Neurological Association. 1981;106:195–9. [PubMed] [Google Scholar]
- 16.Fowler WM, Jr, Abresch RT, Aitkens S, et al. Profiles of neuromuscular diseases. Design of the protocol American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 1995;74:S62–9. doi: 10.1097/00002060-199509001-00002. [DOI] [PubMed] [Google Scholar]
- 17.Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. 2007;17:290–6. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald CM, Widman LM, Walsh DD, Walsh SA, Abresch RT. Use of step activity monitoring for continuous physical activity assessment in boys with Duchenne muscular dystrophy. Archives of physical medicine and rehabilitation. 2005;86:802–8. doi: 10.1016/j.apmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.McDonald CM, Henricson EK, Han JJ, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. 2010;42:966–74. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 20.McDonald CM, Henricson EK, Han JJ, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle & nerve. 2010;41:500–10. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 21.Mazzone ES, Messina S, Vasco G, et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. 2009;19:458–61. doi: 10.1016/j.nmd.2009.06.368. [DOI] [PubMed] [Google Scholar]
- 22.Mazzone E, Martinelli D, Berardinelli A, et al. North Star Ambulatory Assessment, 6- minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. 2010;20:712–6. doi: 10.1016/j.nmd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Mayhew A, Cano S, Scott E, Eagle M, Bushby K, Muntoni F. Moving towards meaningful measurement: Rasch analysis of the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Developmental medicine and child neurology. 2011;53:535–42. doi: 10.1111/j.1469-8749.2011.03939.x. [DOI] [PubMed] [Google Scholar]
- 24.Merlini L, Dell’Accio D, Granata C. Reliability of dynamic strength knee muscle testing in children. The Journal of orthopaedic and sports physical therapy. 1995;22:73–6. doi: 10.2519/jospt.1995.22.2.73. [DOI] [PubMed] [Google Scholar]
- 25.Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: Patterns of clinical progression and effects of supportive therapy. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 26.Mazzone E, Vasco G, Sormani MP, et al. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology. 2011;77:250–6. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- 27.Smith RA, Newcombe RG, Sibert JR, Harper PS. Assessment of locomotor function in young boys with Duchenne muscular dystrophy. Muscle & nerve. 1991;14:462–9. doi: 10.1002/mus.880140513. [DOI] [PubMed] [Google Scholar]
- 28.O’Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. 2007;17:693–7. doi: 10.1016/j.nmd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Krosschell KJ, Scott CB, Maczulski JA, Lewelt AJ, Reyna SP, Swoboda KJ. Reliability of the Modified Hammersmith Functional Motor Scale in young children with spinal muscular atrophy. Muscle & nerve. 2011;44:246–51. doi: 10.1002/mus.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants’ developmental test performance. 2004;93:1391–6. [PubMed] [Google Scholar]
- 31.Almeida KM, Dutra MV, de Mello RR, Reis AB, Martins PS. Concurrent validity and reliability of the Alberta Infant Motor Scale in premature infants. Jornal de pediatria. 2008;84:442–8. doi: 10.2223/JPED.1836. [DOI] [PubMed] [Google Scholar]
- 32.Baillieu N, Potterton J. The extent of delay of language, motor, and cognitive development in HIV-positive infants. 2008;32:118–21. doi: 10.1097/NPT.0b013e3181846232. [DOI] [PubMed] [Google Scholar]
- 33.Glascoe FP, Byrne KE. The usefulness of the Developmental Profile-II in developmental screening. 1993;32:203–8. doi: 10.1177/000992289303200402. [DOI] [PubMed] [Google Scholar]
- 34.Lichtenberger EO. General measures of cognition for the preschool child. Mental retardation and developmental disabilities research reviews. 2005;11:197–208. doi: 10.1002/mrdd.20076. [DOI] [PubMed] [Google Scholar]
- 35.Moore DG, Goodwin JE, Oates JM. A modified version of the Bayley Scales of Infant Development-II for cognitive matching of infants with and without Down syndrome. 2008;52:554–61. doi: 10.1111/j.1365-2788.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- 36.Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Archives of pediatrics & adolescent medicine. 2005;159:1032–5. doi: 10.1001/archpedi.159.11.1032. [DOI] [PubMed] [Google Scholar]
- 37.van Schie PE, Becher JG, Dallmeijer AJ, Barkhof F, Weissenbruch MM, Vermeulen RJ. Motor outcome at the age of one after perinatal hypoxic-ischemic encephalopathy. Neuropediatrics. 2007;38:71–7. doi: 10.1055/s-2007-984449. [DOI] [PubMed] [Google Scholar]
- 38.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. 2000;343:378–84. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 39.Cyrulnik SE, Fee RJ, De Vivo DC, Goldstein E, Hinton VJ. Delayed developmental language milestones in children with Duchenne’s muscular dystrophy. The Journal of pediatrics. 2007;150:474–8. doi: 10.1016/j.jpeds.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinton VJ, Fee RJ, Goldstein EM, De Vivo DC. Verbal and memory skills in males with Duchenne muscular dystrophy. Developmental medicine and child neurology. 2007;49:123–8. doi: 10.1111/j.1469-8749.2007.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. 1992;89:91–7. [PubMed] [Google Scholar]
- 42.Smith RA, Sibert JR, Harper PS. Early development of boys with Duchenne muscular dystrophy. Developmental medicine and child neurology. 1990;32:519–27. doi: 10.1111/j.1469-8749.1990.tb16978.x. [DOI] [PubMed] [Google Scholar]
- 43.Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio: Harcourt Assessment; 2005. [Google Scholar]
- 44.Roid G. Stanford Binet Intelligence Scales. 5. Riverside Publishing; 2006. [Google Scholar]
- 45.Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of neurology. 2012;71:304–13. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 46.Greenspan SI. Clinical assessment of emotional milestones in infancy and early childhood. Pediatric clinics of North America. 1991;38:1371–85. doi: 10.1016/s0031-3955(16)38225-6. [DOI] [PubMed] [Google Scholar]
- 47.Glanzman AM, O’Hagen JM, McDermott MP, et al. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. Journal of child neurology. 2011;26:1499–507. doi: 10.1177/0883073811420294. [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann P, McDermott MP, Darras BT, et al. Observational study of spinal muscular atrophy type 2 and 3: functional outcomes over 1 year. 2011;68:779–86. doi: 10.1001/archneurol.2010.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krosschell KJ, Maczulski JA, Crawford TO, Scott C, Swoboda KJ. A modified Hammersmith functional motor scale for use in multi-center research on spinal muscular atrophy. 2006;16:417–26. doi: 10.1016/j.nmd.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Main M, Kairon H, Mercuri E, Muntoni F. The Hammersmith functional motor scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. 2003;7:155–9. doi: 10.1016/s1090-3798(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 51.Mercuri E, Messina S, Battini R, et al. Reliability of the Hammersmith functional motor scale for spinal muscular atrophy in a multicentric study. 2006;16:93–8. doi: 10.1016/j.nmd.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Boyce WF, Gowland C, Hardy S, et al. Development of a quality-of-movement measure for children with cerebral palsy. Physical therapy. 1991;71:820–8. doi: 10.1093/ptj/71.11.820. discussion 828–32. [DOI] [PubMed] [Google Scholar]
- 53.Scott E, Mawson SJ. Measurement in Duchenne muscular dystrophy: considerations in the development of a neuromuscular assessment tool. 2006;48:540–4. doi: 10.1017/S0012162206001137. [DOI] [PubMed] [Google Scholar]
- 54.Scott E, Eagle M, Mayhew A, et al. Development of a functional assessment scale for ambulatory boys with Duchenne muscular dystrophy. 2012;17:101–9. doi: 10.1002/pri.520. [DOI] [PubMed] [Google Scholar]
- 55.Beail N. A comparative study of profoundly multiply handicapped children’s scores on the Bayley and the Griffiths developmental scales. 1985;11:31–6. doi: 10.1111/j.1365-2214.1985.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 56.Hinton VJ, De Vivo DC, Fee R, Goldstein E, Stern Y. Investigation of Poor Academic Achievement in Children with Duchenne Muscular Dystrophy. 2004;19:146–154. doi: 10.1111/j.1540-5826.2004.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hinton VJ, Fee RJ, De Vivo DC, Goldstein E. Poor facial affect recognition among boys with duchenne muscular dystrophy. Journal of autism and developmental disorders. 2007;37:1925–33. doi: 10.1007/s10803-006-0325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flanigan KM, Dunn DM, von Niederhausern A, et al. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Human mutation. 2009;30:1657–66. doi: 10.1002/humu.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felisari G, Martinelli Boneschi F, Bardoni A, et al. Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology. 2000;55:559–64. doi: 10.1212/wnl.55.4.559. [DOI] [PubMed] [Google Scholar]
- 60.Daoud F, Angeard N, Demerre B, et al. Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Human molecular genetics. 2009;18:3779–94. doi: 10.1093/hmg/ddp320. [DOI] [PubMed] [Google Scholar]