Abstract
Background
A decrease in the prevalence of hepatitis C virus antibody (anti-HCV) has been reported among voluntary blood donors in some regions of China. However, the prevalence of HCV among volunteer blood donors in other regions of China has not been reported. The aim of this study was to investigate the seroprevalence of HCV among 559,890 first-time volunteer blood donors recruited during 2004 through 2007 at the Guangzhou Blood Center, China.
Study Design and Methods
Anti-HCV was detected using two different third-generation enzyme immunoassay kits. HCV RNA was detected using reverse transcription–polymerase chain reaction (RT-PCR) targeting the 5′-untranslated region of HCV.
Results
Among 559,890 donors, 1877 (0.335%) were positive for anti-HCV. The anti-HCV+ rate was significantly higher in males than females (0.37% vs. 0.28%; p < 0.001) and significantly lower among donors living in Guangdong Province than donors who had migrated from other locations (0.30% vs. 0.40%; p < 0.001). Among the 1877 anti-HCV+ donors, 450 were randomly selected for HCV nucleic acid amplification by RT-PCR. Of these, 270 (60%) were HCV RNA+ and 180 (40%) were HCV RNA–.
Conclusions
Many donors from outside Guangdong Province were migrant laborers from other areas in China, suggesting that there is regional heterogenicity in HCV prevalence within China. The overall anti-HCV+ rate reported here is among the lowest reported among blood donors in China reflecting the effect of the current recruitment of exclusively volunteer donors.
Chronic infection with hepatitis C virus (HCV) is a major and growing public health problem. Globally, approximately 170 million people are infected with HCV; however, the prevalence varies greatly among countries, from 0.2% to 26%.1-6 The rapid global spread of HCV is believed to have occurred primarily because of efficient transmission through blood transfusion and parenteral exposures with contaminated equipment.7 In countries where HCV antibody screening and nucleic acid amplification testing (NAT) are mandatory for all blood donors, new HCV-infected donors often have a history of injecting illicit drugs, contaminated medical procedures, or other parenteral exposures. Risk factors also include tattooing, ear and body piercing, household or sexual contact, and unknown sources of infection.8 One of the most consistent characteristics of HCV is its ability to cause chronic infection in a high percentage of individuals. Chronic HCV infection often leads to liver cirrhosis and hepatocellular carcinoma.9,10
As blood-borne pathogens, both HCV and the human immunodeficiency virus (HIV) were frequently detected among paid blood donors in China during the early 1990s.11-14 To improve the safety of blood supply and reduce the risk of transfusion-transmitted diseases, the Chinese government has, since 1998, outlawed the use of paid blood donors. As a result, Chinese blood banks now rely on various other methods to recruit blood donors; these include employer-organized donor recruitment and the use of student donors, replacement donors, and in the recent past, only volunteer donors. This transition in the blood donor recruitment methods in China has been associated with a gradual decrease in the seroprevalence of anti-HCV among donors.13 The HCV seroprevalence among the general population of China has been estimated to be about 3.2%, while among paid blood donors the prevalence has been 5.7% or higher.15-17 However, among employer-organized donors and volunteer donors, the prevalence has ranged between 1.1, 2.3, and 0.46%, respectively13,18,19 Although a progressive decrease in the overall prevalence of anti-HCV has been observed among donors in some regions, the HCV prevalence among first-time volunteer blood donors in China has not been reported. From January 2004 to December 2007, a total of 559,890 first-time volunteer blood donors were evaluated at the Guangzhou Blood Center, in Guangdong Province. In this study, we report the prevalence of anti-HCV in this cohort of donor population.
Materials and Methods
Subjects and samples
From January 2004 to December 2007, a total of 559,890 first-time volunteer blood donors were recruited at the Guangzhou Blood Center. Before donation each volunteer was asked to answer a standardized risk factor questionnaire created by the Chinese Ministry of Public Health. The questionnaire identifies donors for exclusion who had a history of blood product transfusion, injection drug use, receipt of a tattoo, ear or body piercing, surgery, or other invasive medical procedures. Before blood collection, all donors underwent rapid testing for hepatitis B surface antigen (HBsAg; colloidal gold strip method). After a negative result was obtained, donors free of reported risk on the questionnaire were accepted to donate blood. Donated blood was stored at 4°C for less than 24 hours before serum was tested using routine screening assays for alanine aminotransferase (ALT; ≥40 IU/L by the speed rate method or ≥25 IU/L by the Reitman method), anti-HIV-1, anti-HIV-2, HBsAg, and anti-HCV and a serologic test for syphilis. These assays were performed in two rounds with different reagents from two different manufacturers. All reagents used in the screening assays are licensed by the Chinese National Food and Drug Administration and have passed the lot release process of the Chinese National Institute for the Control of Pharmaceutical and Biological Products with the Chinese National Reference Panels. If a donor gave blood more than once, only the first-time donation was included in this analysis.
Anti-HCV assays
Anti-HCV was assayed using third-generation enzyme immunoassay (EIA) reagents from two different manufacturers, following the manufacturers' protocols. The first assay was the enzyme-linked immunosorbent assay diagnostic kit for anti-HCV, from Kehua Biotech Co. Ltd (Shanghai, China), and the second was the Ortho HCV 3.0 enzyme-linked immunosorbent assay kit from Ortho-Clinical Diagnostic, Inc. (Raritan, NJ). Samples reactive in either or both tests were considered as anti-HCV+ and were not used for transfusion.
Reverse transcription–polymerase chain reaction to detect HCV RNA
From each 140 μL of serum, total RNA was extracted using a viral RNA mini kit following the manufacturer's protocol (QIAamp, Qiagen, Chatsworth, CA). After extraction, RNA was converted into cDNA in 20 μL of volume containing random hexamers (Promega, Madison, WI) and 15 units of AMV reverse transcriptase (Promega) and the reaction was incubated at 37°C for 60 minutes. With the converted cDNA as a template, the 5′-untranslated region of the HCV genome was amplified. This was done using the Primer STAR HS (Premix) kit (Takara, Dalian, China) and the primers previously described.20 Each polymerase chain reaction (PCR) volume was 25 μL. The first-round PCR contained 2 μL of cDNA and 10 pmol of each outer primer; the second-round PCR contained 1 μL of the first-round PCR product and 10 pmol of each inner primer. The amplification was performed for one cycle at 94°C for 2 minutes, followed by 40 cycles, each consisting of 94°C for 40 seconds, 50°C for 40 seconds, and 72°C for 40 seconds, with the last cycle of extension at 72°C for 10 minutes. After amplification, PCR products were resolved in 2% agarose gel stained with ethidium bromide.
Statistical analysis
Participants in this study were classified into groups before being subjected to statistical analyses: 1877 of 559,890 volunteer blood donors were identified as being anti-HCV+. Of the 1877 anti-HCV+ donors, 450 were randomly selected for reverse transcription (RT)-PCR assays, which subsequently classified the 450 donors into those that were HCV RNA+ and those that were HCV RNA–. Donors were further classified by sex (male/female) and location of origin (Guangdong/outside Guangdong).
Chi-square tests and analysis of variance tests were performed, as appropriate, to test the associations between HCV prevalence and donor age, sex, and location of origin. In the comparison of HCV prevalence rates of donors originating from Guangdong and migrants from outside Guangdong, the rates were standardized according to the overall sex and age distribution of all the 559,890 donors. Statistical analyses were performed using computer software (SPSS, Version 14.0 for Windows, SPSS, Inc., Chicago, IL). Comparisons at the p < 0.05 level were judged to be significant.
Results
Characteristics of the donor population and anti-HCV seroprevalence
Of the 559,890 first-time volunteer blood donors, 357,673 (63.88%) were male and 202,217 (36.12%) were female; 345,385 (61.69%) were from Guangdong Province, and 214,505 (38.31%) were from areas other than Guangdong. The latter included donors from all provinces, municipalities, and special administrative regions of China, most of them representing off-season migrant laborers traveling from rural areas to work in factories around the Pearl River Delta (see Table 1). The 559,890 donors were between 18 and 55 years of age and had a mean age of 29.7 years (standard deviation [SD], 7.79 years). Overall, 1877 of the 599,890 donors were positive for anti-HCV: of these, 1308 (69.69%) were male and 569 (30.31%) were female; 1028 (54.77%) were from Guangdong Province and 849 (45.23%) were from elsewhere. The anti-HCV+ donors were between 20 and 55 years of age and had a mean age of 33.1 years (SD, ±8.02 years). Therefore, the anti-HCV+ donors were on average 3.4 years older (p < 0.001) than anti-HCV− donors and were significantly more likely to be male (p < 0.001) and have origins from locations other than Guangdong (p < 0.001; Table 1). The anti-HCV+ prevalence was 0.335% (95% confidence interval [CI], 0.334%-0.336%) among all 559,890 donors, 0.366% (95% CI = 0.364%-0.368%) among the 357,673 male donors, and 0.281% (95% CI = 0.279%-0.283%) among the 202,217 female donors (p < 0.001), 0.298% (95%CI = 0.297%-0.300%) among the 345,385 donors from Guangdong Province, and 0.396% (95%CI = 0.394%-0.398%) among the 214,505 donors from elsewhere (p < 0.001).
Table 1. HCV seroprevalence among 559,890 first-time voluntary donors at the Guangzhou Blood Center by age, sex, and location of origin, 2004 through 2007.
| Guangdong Province | Other locations | Total | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Donor group | Number of donors | Anti-HCV+(%) | Number of donors | Anti-HCV+(%) | Number of donors | Anti-HCV+(%) |
| Males | 212,780 | 734 (0.345)* | 144,893* | 574 (0.396) | 357,673 | 1,308 (0.366)* |
| Females | 132,605 | 294 (0.222) | 69,612 | 275 (0.395) | 202,217 | 569 (0.281) |
| Ages (years), mean ± SD | 29.7 (±8.52) | 33.1 (±8.68)* | 29.6 (±6.44)†‡ | 33.3 (±7.10) | 29.7 (±7.79) | 33.1 (±8.02)* |
| Total | 345,385 | 1,028 (0.298) | 214,505 | 849 (0.396)*§ | 559,890 | 1,877 (0.335) |
p < 0.001.
Comparing with the total number of donors from Guangdong Province.
p < 0.05.
Comparing with the anti-HCV+ rate among donors from Guangdong Province.
After stratification by both geographic origin and sex, 212,780 (61.6%) male and 132,605 (38.4%) female donors were from Guangdong Province, and 144,893 (67.5%) male and 69,612 (32.5%) female donors were from other areas. The sex ratios and mean ages were both significantly different (p < 0.05) between these two geographic groups. When the 1877 anti-HCV+ donors were similarly stratified, 734 males and 294 females were from Guangdong Province, and 574 males and 275 females were from elsewhere; the sex ratios and mean ages were not significantly different between the two geographic groups. The anti-HCV+ prevalence among donors of Guangdong origin was significantly higher (p < 0.001) in males (0.345%) than females (0.222%). However, the anti-HCV+ prevalence was similar (p > 0.05) in males (0.396%) and females (0.395%) from areas outside Guangdong Province (Table 1).
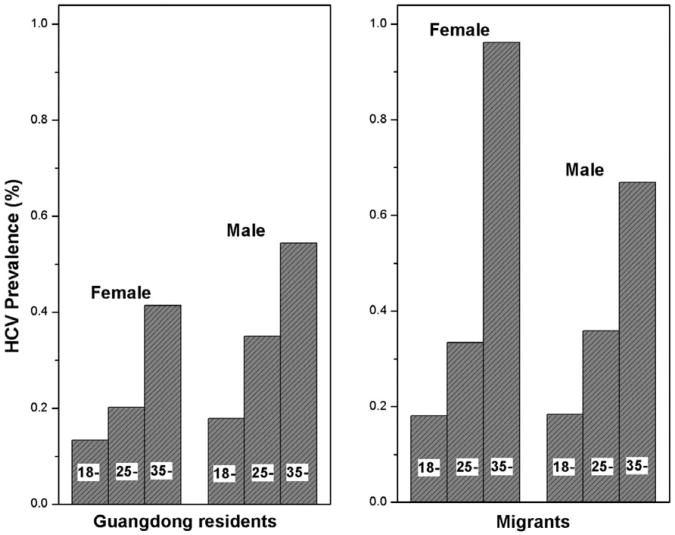
According to the age distribution of all donors, the age-standardized anti-HCV+ rates were calculated for different groups. Although a birth cohort effect was observed, the age-standardized anti-HCV+ rates did not significantly deviate from the crude rates (Table 2 and Fig. 1).
Table 2. Age-standardized HCV rates for donors from Guangdong Province and from other locations.
| Guangdong residents | Migrants | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Sex and age (years) | Donors | HCV+ | Rate (%) | Age-adjusted rate* (%) | Donors | HCV+ | Rate (%) | Age-adjusted rate* (%) |
| Female | 0.220 | 0.407 | ||||||
| 18-24 | 53,949 | 72 | 0.133 | 21,576 | 39 | 0.181 | ||
| 25-34 | 48,731 | 98 | 0.201 | 35,959 | 120 | 0.334 | ||
| ≥35 | 29,926 | 124 | 0.414 | 12,075 | 116 | 0.961 | ||
| Male | 0.352 | 0.388 | ||||||
| 18-24 | 67,054 | 120 | 0.179 | 27,171 | 50 | 0.184 | ||
| 25-34 | 92,212 | 323 | 0.350 | 85,142 | 306 | 0.359 | ||
| ≥35 | 53,513 | 291 | 0.544 | 32,582 | 218 | 0.669 | ||
Age-standardized rate according to the age distribution of all donors.
Fig. 1.
Sex- and age-specific HCV prevalence for local Guangdong residents and migrants.
Of the 1877 anti-HCV+ donors, 450 were selected for NAT assay. Among the 450 donors, 307 (68.2%) were male and 143 (31.9%) were female; 265 (58.9%) were from Guangdong Province and 185 (41.1%) were from elsewhere. The ages of these 450 donors ranged between 21 and 54 years, with a mean age of 33.3 years (SD, 6.93 years). Comparing with the 1877 anti-HCV+ donors, the 450 selected donors showed no significant differences in sex, age, and location of origin. Using an in-house RT-PCR amplification assay targeting the 5′-untranslated region of the HCV genome, HCV RNA was detected among 270 (60%) of the 450 donors and was undetectable in 180 (40%). There was no difference in the age and location of origin of these two groups (Table 3). However, females were significantly more common (p < 0.001) in the HCV RNA− group than in the HCV RNA+ group.
Table 3. HCV RNA prevalence by NAT assay among 450 selected donors who were reactive on EIA tests by age, sex, and location of origin, Guangzhou Blood Center, 2004 through 2007*.
| Donor characteristics | Positive (270) | Negative (180) | Total (450) |
|---|---|---|---|
| Sex | |||
| Male | 204 (66.4)† | 103 (33.5) | 307 |
| Female | 66 (46.1) | 77 (53.9) | 143 |
| Residence | |||
| Guangdong Province | 166 (62.6) | 99 (37.4) | 265 |
| Other areas | 104 (56.2) | 81 (43.8) | 185 |
| Age (years) | 34.4 ± 6.79 | 31.7 ± 6.81 |
Data are reported as number (%) or mean ± SD.
p < 0.001.
Commonly, an elevated ALT level is considered to be a biochemical marker for active hepatitis. To differentiate biochemical hepatitis from past HCV infection, we examined the ALT levels of the 180 anti-HCV+ but HCV RNA− donors: 13 had elevated ALT levels (10 male, three female; 11 from Guangdong, two from elsewhere).
Discussion
Blood transfusion was a major risk factor for HCV infection before the virus was identified in 1989. This transmission route remained common in China during 1992 through 1994, when more than 500,000 donors were found to be infected with HCV due to contaminated blood collection, mostly plasma collection in for-profit facilities.21,22 To eliminate this risk, the Chinese government has outlawed the use of paid blood donors since 1998.13 Blood donor recruitment models in China have therefore changed from the use of paid donors to selecting donors from employer-organized recruitment drives, donors among students, and volunteer donors among low-risk populations.19 In recent years, the proportion of volunteer donors have rapidly increased, from 5% in 1998 to 71.5% at the end of 2004. They now constitute the main source of the blood supply in all major blood banks in China.13,23 Although data on anti-HCV prevalence have not been reported among donors recruited by different methods from a single geographic region, temporal trends of decreasing HCV seroprevalence have been observed as the methods of donor recruitment changed. In 1993 through 1994, the HCV prevalence among blood donors was 6.5%. From 1997 to 2000, it decreased to 4.6%. The prevalence was 2.3% in 2000 through 2001 and it decreased further to 0.41% to 0.46% in 2003 and 0.335% in the current study during 2004 through 2007.18,19,21,22 These trends indicate that the changes in blood donor populations resulting from different methods of recruitment have resulted in a decreasing seroprevalence of HCV among donors. These trends also likely reflect the reduced proportion of donors who are in the seronegative window period or who have a false-negative serologic test for other reasons. The residual risk of transmission of HCV from a seronegative donor was recently estimated to be approximately 1 in 40,000 to 60,000 donations in China.24,25 A decrease in anti-HCV+ donations has been reported among donors in the United States (from 0.63% to 0.40% during 1992-1996) and Canada (from 0.34% to 0.08% during 1993-2006).26,27 A stable low prevalence of anti-HCV (0.04%-0.10%) has also been maintained among donors in Hong Kong since 1991 (http://www.info.gov.hk/hepatitis). The geographic proximity and social composition of Hong Kong makes it an ideal model for the optimization of donor recruitment models in Guangzhou.
In this study, an anti-HCV+ frequency of 0.335% was found among 559,890 first-time volunteer blood donors, who had origins throughout China and who were recruited and screened during 2004 through 2007 at the Guangzhou Blood Center, one of the largest blood centers in the country. The seroprevalence of HCV appears to be substantially lower among blood donors than in the general population in China, because of the currently successful screening and selection of donors who are at a lower risk for infection. The seroprevalence in the donor population in the Guangzhou Blood Center is approximately 10-fold lower than the estimate of a 3.2% seroprevalence of anti-HCV in the general population of China.15 Although with slightly different testing algorithm, this seroprevalence estimate in the early 1990s has been found to be stable in the late 1990s in a subsequent study.16 Reported lower anti-HCV+ seroprevalence among volunteer blood donors than among the general population has been attributed to the implementation of effective donor selection criteria that are designed to exclude high-risk individuals from donating.26,27 There are also other factors possibly contributing to the decrease, such as the birth cohort effect and regional variations in HCV prevalence. The birth cohort effect refers to higher anti-HCV frequencies observed among older people, from exposure to unscreened blood transfusions and other contaminated injections in the past that have been reduced among younger cohorts.27,28
A nationwide anti-HCV rate was estimated from a study of 66,975 individuals screened in 1992 who were a representative sample of the general population of China.15 These subjects were aged 1 to 59 years and originated from all provinces of China. The overall anti-HCV+ rate was estimated to be 3.2% and a strong birth cohort effect was observed. The anti-HCV+ prevalence was lowest (2.08%) among infants, children, and young adults and highest (3.96%) in the 50- to 59-years age group, and the anti-HCV+ seroprevalence increased significantly with age. When the study population was stratified according to geographic origin, the anti-HCV+ prevalence was higher (3.5%) in the north than the south (2.9%).15 During the 12 years between that earlier report and the present study, in 2004 through 2007, many older individuals with higher anti-HCV+ prevalence have been replaced with younger individuals with lower anti-HCV+ frequencies. Persons over the age of 55 are not accepted for blood donation in China. In addition, the majority of the donors in the current study were from the south of China and all were between 18 and 55 years of age when they volunteered to donate blood. Taken together, these temporal and spatial variations could have contributed significantly to the lower anti-HCV seroprevalence found in this study. In the United States and Canada, the anti-HCV prevalence among first-time volunteer blood donors has been estimated to be approximately one-third to one-fifth of the HCV infection among the general population.6,26-28 If this ratio of HCV prevalence among blood donors to that in the general population also exists in China, approximately 1.0% to 1.68% of the Chinese population might be currently infected with HCV, representing approximately 13 million to 22 million people. These estimates are lower than those previously reported in 1992.15
Several other factors could affect the anti-HCV+ prevalence among this cohort of donors. A proportion of the volunteer blood donors were among migrants from rural areas, who were temporarily employed in Guangzhou city. During the “farming-busy” seasons and holidays, these individuals typically travel back and forth from the city to remote rural areas. Nationally, the total number of such internal migrants is estimated to be approximately 100 million; they have among the highest rates of HIV and HCV transmission in the country, among the general population.11 These individuals can be identified when they donate blood because their permanent rural addresses are shown on their identification documents that must be presented at the time they register for donation. More than 30 million migrants are employed around the Pearl River Delta; they may be motivated to donate blood because, if they have a record of volunteer blood donation in Guangzhou, they or their family members will receive free blood transfusions anywhere in the country in the future, if this is needed. Migrant laborers may be at higher risk for HCV infection than permanent city residents because of their lower socioeconomic status and more frequent parenteral exposures in higher-risk locations.
Third-generation EIAs to detect anti-HCV are now routinely used for screening potential donors in nearly all blood banks in China. These screening assays are quite sensitive to detect infections with HCV after the early infection-seronegative window period has passed.25 A positive screening HCV EIA test is used as a criteria for exclusion of a blood donor in China. Nevertheless, not all donors who have reactive EIA tests have active HCV infections.29,30 To increase the sensitivity of the HCV EIA screening assays and to improve the positive predictive value of the testing results, the Guangzhou blood bank routinely screens prospective donors using EIA assays from two different manufacturers. Donors who are reactive with either screening assay are excluded and their blood is not transfused. However, some donors who are reactive on only one assay may more commonly have false-positive tests. Other persons with a reactive EIA may have cleared a previous HCV infection spontaneously and be noninfectious.31-33 The overall frequency of spontaneous clearance of an HCV infection has been reported to be approximately 20% to 30% in areas where Genotype 1 infection predominates.34,35 The proportion is higher after infection with HCV Genotypes 2 and 3.32,36,37 The HCV genotype distribution in China and other areas in Asia differs from that in the United States and Western Europe. Genotypes 3 and 6 are more common in Asia, contributing approximately 25% to 30% or more to all HCV infections, whereas 65% to 80% of infected persons in Western countries are infected with Genotype 1.34,35 Variations in the natural history of infection by different HCV genotypes in our population could account for the somewhat greater proportion of RNA-negative, EIA repeatedly reactive donors (40%) than has been reported among blood donors in the United States and Europe.17,18,22-24
Females also have higher rates of spontaneous clearance of HCV infections than males.32-34 Spontaneous virus clearance of an HCV infection could account for a large proportion of the anti-HCV+, RNA− donors found in this study. This is supported by the finding that HCV RNA–, EIA+ donors were statistically more common among women in our study.
Although confirmatory testing with recombinant immunoblot assay or a NAT assay is recommended and routinely used by blood banks in the United States and Europe to confirm a repeat-reactive EIA HCV screening result, the availability of these tests is very limited in China due to the high cost and the requirement for qualified personnel and specialized equipment. Therefore, blood banks in China report HCV infection rates among donors based only on an anti-HCV+ screening assay. The substantial reduction in the prevalence of HCV infection among donors at the Guangzhou blood collection center in the years after the regulation requiring the collection of blood only from voluntary donors, suggests that an important reduction in the risk of the transmission of HCV by blood transfusion has occurred in China in the past decade.
Acknowledgments
The project described was supported by a grant from the National Institute of Allergy and Infectious Diseases (1R01AI080734-01A109, LL); a grant from the Bureau of Health, Guangzhou Municipality, China (No. 2008-ZDI-10, YF); and a grant from the National Natural Science Foundation of China (No. 30872162, YF).
References
- 1.World Health Organization. Hepatitis C global prevalence. Wkly Epidemiol Rec. 1999;74:425–7. [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis C. Wkly Epidemiol Rec. 1997;72:65–9. [Google Scholar]
- 3.Aymard JP, Botte C, Contal P, Janot C, Treiff F. Seroprevalence of hepatitis C-antibodies among blood donors: study of second generation ELISA & RIBA tests and surrogate markers. Pathol Biol (Paris) 1993;41:149–53. [PubMed] [Google Scholar]
- 4.Shakil AO, Conry-Cantilena C, Alter HJ, Hayashi P, Kleiner DE, Tedeschi V, Krawczynski K, Conjeevaram HS, Sallie R, Di Bisceglie AM. Volunteer blood donors with antibody to hepatitis C virus: clinical, biochemical, virologic, and histologic features. The Hepatitis C Study Group. Ann Intern Med. 1995;123:330–7. doi: 10.7326/0003-4819-123-5-199509010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Panigrahi AK, Panda SK, Dixit RK, Rao KV, Acharya SK, Dasarathy S, Nanu A. Magnitude of hepatitis C virus infection in India: prevalence in healthy blood donors, acute and chronic liver disease. J Med Virol. 1997;51:167–4. [PubMed] [Google Scholar]
- 6.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 7.Prati D. Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review. J Hepatol. 2006;45:607–16. doi: 10.1016/j.jhep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Alter MJ. Transmission of hepatitis C virus—route, dose, and titer. N Engl J Med. 1994;330:784–6. doi: 10.1056/NEJM199403173301111. [DOI] [PubMed] [Google Scholar]
- 9.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–9. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 10.Zoulim F, Chevallier M, Maynard M, Trepo C. Clinical consequences of hepatitis C virus infection. Rev Med Virol. 2003;13:57–68. doi: 10.1002/rmv.371. [DOI] [PubMed] [Google Scholar]
- 11.Beach MV. “Blood heads” and AIDS haunt China's countryside. Lancet. 2001;357:49. doi: 10.1016/s0140-6736(05)71551-8. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J, Jing J. AIDS: China and AIDS—the time to act is now. Science. 2002;296:2339–40. doi: 10.1126/science.1074479. [DOI] [PubMed] [Google Scholar]
- 13.Shan H, Wang JX, Ren FR, Zhang YZ, Zhao HY, Gao GJ, Ji Y, Ness PM. Blood banking in China. Lancet. 2002;360:1770–5. doi: 10.1016/S0140-6736(02)11669-2. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Liu Z, Detels R. HIV-1 infection in commercial plasma donors in China. Lancet. 1995;346:61–2. doi: 10.1016/s0140-6736(95)92698-4. [DOI] [PubMed] [Google Scholar]
- 15.Xia GL, Liu CB, Cao HL, Li S, Zhan MY, Sub CA, Nan JH, Qi XQ. Prevalence of hepatitis B and C virus infections in the general Chinese population: results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Int Hepatol Commun. 1996;5:62–73. [Google Scholar]
- 16.Lei XZ, Shigeko N, Deng XW, Wang S, Qin S, Liu L, Tang H, Zhao L, Lei B, Yoshihiro A. Prevalence of hepatitis C virus infection in the general population and patients with liver disease in China. Hepatol Res. 1999;14:135–43. [Google Scholar]
- 17.Wang Y, Tao QM, Zhao HY, Tsuda F, Nagayama R, Yamamoto K, Tanaka T, Tokita H, Okamoto H, Miyakawa Y. Hepatitis C virus RNA and antibodies among blood donors in Beijing. J Hepatol. 1994;21:634–40. doi: 10.1016/s0168-8278(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 18.Ding X, Gu H, Zhong ZH, Zilong X, Tran HT, Iwaki Y, Li TC, Sata T, Abe K. Molecular epidemiology of hepatitis viruses and genotypic distribution of hepatitis B and C viruses in Harbin, China. Jpn J Infect Dis. 2003;56:19–22. [PubMed] [Google Scholar]
- 19.Zhao SM, Jiang TL, Gao FX, Lu L, Zheng HQ, Hu J, Fan YH, Li B, Xiao RR, Yury K. Analysis of true voluntary blood donors with anti-HCV prevalence and implications for donor management in Chongqing, China. Transfus Med. 2007;17:210–1. doi: 10.1111/j.1365-3148.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 20.Nainan OV, Lu L, Gao FX, Meeks E, Robertson BH, Margolis HS. Selective transmission of hepatitis C virus genotypes and quasispecies in humans and experimentally infected chimpanzees. J Gen Virol. 2006;87:83–91. doi: 10.1099/vir.0.81268-0. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Ren QH, Zhu ZY, Qu DM, Qiu ZK, Li JF, Mei H, Yan HY. Investigation and analysis of HCV infection in Chinese blood donors. Acta Acad Med Sinicae. 1998;203:240–1. [Google Scholar]
- 22.Shi XL, Ren QH, Zhu ZY, Qu DM, Ji Y, Peng DH, Ni SQ. Hepatitis C virus infection in blood donors in the People's Republic of China. Transfusion. 1999;39:913. doi: 10.1046/j.1537-2995.1999.39080913.x. [DOI] [PubMed] [Google Scholar]
- 23.Ren FR, Lv QS, Zhuang H, Li JJ, Gong XY, Gao GJ, Liu CL, Wang JX, Yao FZ, Zheng YR, Zhu FM, Tiemuer MH, Bai XH, Shan H. Significance of the signal-to-cutoff ratios of anti-hepatitis C virus enzyme immunoassays in screening of Chinese blood donors. Transfusion. 2005;45:1816–22. doi: 10.1111/j.1537-2995.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 24.Shan H, Ren FR, Zhao HY, Zhang YZ, Wen GX, Yao FZ, Gao GJ, Yan LX, Jiang CF, Bai XH, Tiemuer MH, Tu YQ, Zhu FM, Zheng YR, Cui L, Liu CL, Gong XY, Lv QS, Zheng P, Ziermann R, Ness P, Wang JX. A multi-Chinese blood center study testing serologic-negative donor samples for hepatitis C virus and human immunodeficiency virus with nucleic acid testing. Transfusion. 2007;47:2011–6. doi: 10.1111/j.1537-2995.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 25.Shang G, Seed CR, Wang F, Nie D, Farrugia A. Residual risk of transfusion-transmitted viral infections in Shenzhen, China, 2001 through 2004. Transfusion. 2007;47:529–39. doi: 10.1111/j.1537-2995.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- 26.Glynn SA, Kleinman SH, Schreiber GB, Busch MP, Wright DJ, Smith JW, Nass CC, Williams AE. Trends in incidence and prevalence of major transfusion-transmissible viral infections in US blood donors, 1991-1996. Retrovirus Epidemiology Donor Study (REDS) JAMA. 2000;284:229–35. doi: 10.1001/jama.284.2.229. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien SF, Fan W, Xi G, Yi QL, Goldman M, Fearon MA, Infante-Rivard C, Chiavetta JA, Willems B, Pi D, Fast M, Delage G. Declining hepatitis C rates in first-time blood donors: insight from surveillance and case-control risk factor studies. Transfusion. 2008;48:902–9. doi: 10.1111/j.1537-2995.2007.01618.x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy EL, Brazman SM, Glynn SA, Ameti DY, Thomson RA, Williams AE, Nass CC. Risk factors for hepatitis C virus infection in United States blood donors. NHLBI Retrovirus Epidemiology study (REDS) Hepatology. 2000;31:756–62. doi: 10.1002/hep.510310329. [DOI] [PubMed] [Google Scholar]
- 29.Alter MJ, Kuhnert WL, Finelli L Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–13. [PubMed] [Google Scholar]
- 30.Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, Ishak KG, Iber FL, Toro D, Samanta A, Koretz RL, Perrillo RP, Goodman ZD, Knodell RG, Gitnick G, Morgan TR, Schiff ER, Lasky S, Stevens C, Vlahcevic RZ, Weinshel E, Tanwandee T, Lin HJ, Barbosa L. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: a National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–63. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 31.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 32.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M, Pape GR. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–8. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 33.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, Shaw-Stiffel T, Weston SJ, Thiede H, Wald A, Rosen HR. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474–82. doi: 10.1086/522608. [DOI] [PubMed] [Google Scholar]
- 35.Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, Vlahov D, Chien D, Shyamala V, Ray SC, Thomas DL. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–8. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 36.Santantonio T, Medda E, Ferrari C, Fabris P, Cariti G, Massari M, Babudieri S, Toti M, Francavilla R, Ancarani F, Antonucci G, Scotto G, Di Marco V, Pastore G, Stroffolini T. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154–9. doi: 10.1086/507640. [DOI] [PubMed] [Google Scholar]
- 37.Soriano V, Mocroft A, Rockstroh J, Ledergerber B, Knysz B, Chaplinskas S, Peters L, Karlsson A, Katlama C, Toro C, Kupfer B, Vogel M, Lundgren J EuroSIDA Study Group. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008;198:1337–44. doi: 10.1086/592171. [DOI] [PubMed] [Google Scholar]