Abstract
Inhibitory control commonly recruits a number of frontal regions: pre-supplementary motor area (pre-SMA), frontal eye fields (FEFs), and right-lateralized posterior inferior frontal gyrus (IFG), dorsal anterior insula (DAI), dorsolateral prefrontal cortex (DLPFC), and inferior frontal junction (IFJ). These regions may directly implement inhibitory motor control or may be more generally involved in executive control functions. Two go/no-go tasks were used to distinguish regions specifically recruited for inhibition from those that additionally show increased activity with working memory demand. The pre-SMA and IFG were recruited for inhibition in both tasks and did not have greater activation for working memory demand on no-go trials, consistent with a role in inhibitory control. Activation in pre-SMA also responded to response selection demand and was increased with working memory on go trials specifically. The bilateral FEF and right DAI were commonly active for no-go trials. The FEF was also recruited to a greater degree with working memory demand on go trials and may bias top–down information when stimulus–response mappings change. The DAI, additionally responded to increased working memory demand on both go and no-go trials and may be involved in accessing sustained task information, alerting, or autonomic changes when cognitive demands increase. DLPFC activation was consistent with a role in working memory retrieval on both go and no-go trials. The inferior frontal junction, on the other hand, had greater activation with working memory specifically for no-go trials and may detect salient stimuli when the task requires frequent updating of working memory representations.
INTRODUCTION
Response inhibition typically involves withholding a prepotent response when an infrequent stimulus occurs. Inhibitory control recruits the pre-supplementary motor area (pre-SMA), frontal eye fields (FEFs), and a series of right-lateralized prefrontal regions including the inferior frontal gyrus (IFG), dorsal anterior insula (DAI), dorso-lateral pFC (DLPFC), and inferior frontal junction (IFJ; Levy & Wagner, 2011; Swick, Ashley, & Turken, 2011; McNab et al., 2008; Rubia et al., 2001). Although these regions have been implicated in response inhibition, their precise role is unknown. They may be directly involved in the motor control necessary to implement a nonprepotent action plan or may be more generally involved in other aspects common to response inhibition paradigms such as retrieving nonprepotent task goals, response selection under increased demand, or updating attention.
Both the pre-SMA and right posterior IFG (BA 44/BA 45) are structurally and functionally connected to the sub-thalamic nucleus and BG (Swann et al., 2012; Aron, Behrens, Smith, Frank, & Poldrack, 2007; Aron & Poldrack, 2006), forming part of hyperdirect and indirect circuits responsible for motor control (Zandbelt, Bloemendaal, Hoogendam, Kahn, & Vink, 2013; Aron, 2011; Jahfari et al., 2011; Zandbelt & Vink, 2010). However, several studies have suggested that they do not directly implement the motor control necessary to withhold a prepotent response. Instead, they may play related functions necessary for inhibitory control such as updating action plans (Verbruggen, Aron, Stevens, & Chambers, 2010; Mostofsky & Simmonds, 2008; Mars, Piekema, Coles, Hulstijn, & Toni, 2007), context monitoring (Chatham et al., 2012), allocating attention (Sharp et al., 2010), representing expectancy (Zandbelt, Bloemendaal, Neggers, Kahn, & Vink, in press; Shulman et al., 2009), setting response thresholds (Chen, Scangos, & Stuphorn, 2010), or preparing for controlled processing (Swann et al., 2012; Aron, 2011).
During most inhibitory control paradigms, response inhibition occurs infrequently. One study identified an a priori inhibitory control network and found that across all regions activity was significant not only for infrequent inhibit events, but also for infrequent respond and infrequent count events (Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010). Activity was not significantly different between the inhibit and respond events in the IFG and pre-SMA, suggesting that these regions may not reflect inhibitory control but rather other aspects of responding to infrequent events. This is consistent with the notion that the pre-SMA, which plays a central role in inhibitory control (Simmonds, Pekar, & Mostofsky, 2008), is more generally involved in response selection (Mostofsky & Simmonds, 2008; Isoda & Hikosaka, 2007) and setting response thresholds (Chen et al., 2010).
The right-lateralized IFG/DAI and TPJ are commonly recruited during response inhibition but also have been identified as comprising a ventral attention network (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006). The ventral attentional network is active not just when inhibiting a prepotent response but also when infrequent stimuli are responded to such as during the Oddball and Posner Orienting paradigms (Levy & Wagner, 2011). This suggests that the role of the ventral attentional network may not be inhibition per se, but reorienting attention to salient stimulus features and/or changing action plans (Corbetta, Patel, & Shulman, 2008; Corbetta, Kincade, & Shulman, 2002). Studies that have controlled for response type by including both infrequent no-go trials as well as infrequent go trials have concluded that the posterior IFG (BA 44/BA 45), in particular, may implement inhibitory control, while a nearby region, the IFJ may be involved in attention allocation (Cai & Leung, 2011; Levy & Wagner, 2011; Chikazoe, 2010; Chikazoe et al., 2009). Other studies, however, suggest that the right IFG is involved in context monitoring (Chatham et al., 2012) or detection of infrequent stimuli (Sharp et al., 2010) and not response inhibition. The nearby right DAI is also commonly recruited for inhibitory control and a meta-analysis found that this region, rather than the posterior IFG, was the locus of right-lateralized activation for inhibitory control tasks (Swick et al., 2011). The DAI is functionally connected not only to the ventral attentional network but also to the cingulo-opercular network, which has been implicated in sustaining task information over a block of trials (Dosenbach et al., 2007) and also responds to autonomic changes associated with task demand (Critchley, 2005; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Critchley, Melmed, Featherstone, Mathias, & Dolan, 2002; Critchley, Corfield, Chandler, Mathias, & Dolan, 2000). Therefore, this region may play a more general role in executive function, rather than inhibitory control per se.
Both the pre-SMA and right IFG are recruited not only for response inhibition; activity in these regions has also been observed during working memory tasks (Rama, Sala, Gillen, Pekar, & Courtney, 2001; Petit, Courtney, Ungerleider, & Haxby, 1998). A study comparing activation for these two task types has shown that there is overlapping activation in pre-SMA and right IFG, among other regions, for both response inhibition and working memory tasks (McNab et al., 2008). During working memory tasks, these regions may directly contribute to working memory maintenance and retrieval functions (D’Esposito, 2007; Rypma, Berger, & D’Esposito, 2002; D’Esposito, Postle, Ballard, & Lease, 1999), attention (Chun, 2011; Gazzaley, 2011), or updating of working memory contents (Roth, Serences, & Courtney, 2006). Alternatively, activation of these regions may not directly reflect working memory demand, but instead may reflect downstream processing involved in motor response selection and control (Mostofsky & Simmonds, 2008; Petit et al., 1998). While the pre-SMA may be involved in motor aspects of response selection and control, it is directly interconnected with regions representing abstract task information in contrast to the more caudal SMA-proper that is principally connected to primary motor cortex (Mostofsky & Simmonds, 2008; Johansen-Berg et al., 2004). Therefore, it is not clear whether the pre-SMA represents the more abstract aspects of action plans related to task goals or the response representations themselves. The DLPFC, on the other hand, has been traditionally associated with working memory (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). Although it has been implicated in inhibitory control tasks, it plays a clear role in representing and retrieving more abstract task information, which supports inhibitory control under more complex task demands (Simmonds et al., 2008; Mostofsky et al., 2003).
The FEFs are also commonly recruited during inhibitory control (Muggleton, Chen, Tzeng, Hung, & Juan, 2010). This region is classically considered part of the oculomotor circuitry and is more generally recruited when a nonprepotent action plan is implemented. It is intrinsically connected with parietal and higher-order visual regions (Fox et al., 2005), suggesting that it plays a role in biasing stimulus–response mappings when a nonpre-potent task is performed.
The current study examined whether regions that play a role in response inhibition (i.e., pre-SMA, FEF, and right lateralized prefrontal regions: posterior IFG, DAI, DLPFC, and IFJ) are also modulated by working memory demand. This was assessed using two go/no-go tasks: a simple task that had a straightforward stimulus response mapping (green = go, red = no-go) with minimal working memory demand and a repeat task that had an inconsistent stimulus response mapping (color change = go, color repeat = no-go), which required working memory to cue response inhibition. The aim was to distinguish those regions involved in motoric aspects of response selection/inhibitory control from those involved in other executive functions that are required when working memory demand increases.
In line with previous findings (Mostofsky et al., 2003), it was expected that the pre-SMA would be involved in response selection/inhibition for both tasks, whereas the DLPFC would be involved in maintaining goal-relevant task information and would have increased activity associated with greater working memory demand in the repeat task. Additionally, it was expected that response selection would be relatively automatic during simple go trials whereas response selection demand would be increased during repeat go trials, due to the inconsistent stimulus response mappings. In that task, it was expected that activity in the FEF would be increased for repeat go trials associated with biasing task information for response selection. For no-go trials, it was hypothesized that ventral attentional network regions (i.e., right DAI/posterior IFG and TPJ), which are involved in detecting behaviorally relevant stimuli that cue a change from the prepotent action plan (Levy & Wagner, 2011; Corbetta et al., 2002, 2008), would be active in both tasks. Furthermore, given previous findings revealing that the posterior IFG is specifically involved in response inhibition, although the nearby IFJ is more generally modulated by attention demands (Cai & Leung, 2011; Levy & Wagner, 2011; Chikazoe, 2010; Chikazoe et al., 2009), it was expected that activation in the IFJ would be increased associated with updating attention in the repeat task.
METHODS
Participants
Twenty-two healthy, right-handed adults (10 men), aged 20–40 years (mean = 28.97 years, SD = 5.22 years) participated in the study. Participants were recruited through local advertisements and had no history of mental illness or substance abuse. The study was approved by the Johns Hopkins Medicine Institutional Review Board. Informed consent was signed before task participation.
fMRI Behavioral Paradigm
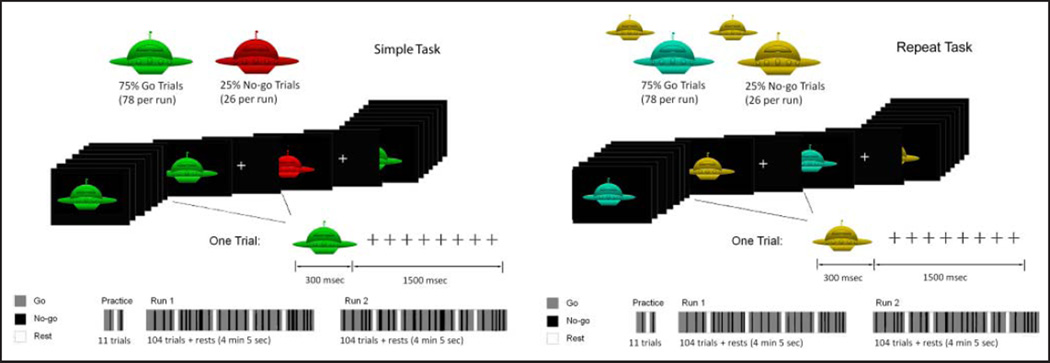
Two go/no-go tasks were performed by all participants (Figure 1). For each trial, spaceship stimuli were presented for 300 msec followed by a 1500-msec ISI. Twelve-second blocks of rest occurred at the beginning, end, and four times throughout each run. The proportion of go/no-go trials was 3:1, with 78 go trials and 26 no-go trials occurring in each run. The first two trials of every block were go trials, and the trials were pseudorandomly ordered so that zero to six go trials preceded a given no-go trial. Participants performed two runs each of the two go/no-go tasks. Each run was preceded by instructions and 20 practice trials. Half of the participants performed the two simple runs first and the other half performed the two repeat runs first.
Figure 1.
Simple and repeat go/no-go tasks. In the simple task, go stimuli were green and no-go stimuli were red. In the repeat task, a change in stimulus color indicated a go, whereas a repetition of stimulus color indicated a no-go.
For the simple go/no-go task, stimulus response associations were well ingrained and easy to remember. Go stimuli were green, whereas no-go stimuli were red. For the repeat task, stimulus response associations were not constant and required working memory to guide response selection. Stimuli were 50% blue and 50% yellow, with neither color consistently cuing a go or no-go event. A change in the stimulus color signaled a go trial, whereas a repetition of the stimulus color signaled a no-go trial. Participants were therefore required to remember the color of the previous stimulus to determine the correct response.
fMRI Acquisition and Preprocessing
Imaging data were acquired on a Philips 3T scanner. This included a high-resolution anatomical scan (MPRAGE, eight-channel head coil, repetition time = 7.99 msec, echo time = 3.76 msec, flip angle = 8°) for image coregistration, segmentation and normalization processing steps. The behavioral task was performed during four fMRI runs (2DSENSE EPI, eight-channel head coil, repetition time = 2500 msec, echo time = 30 msec, flip angle = 70°). Each run was 4 min 5 sec in duration.
Preprocessing of functional data was performed using SPM5 and included slice timing correction; motion correction; coregistration of the first functional image in the run to the MPRAGE image; segmentation of gray matter, white matter, and cerebrospinal fluid using SPM probabilistic tissue priors; normalization to standard Montreal Neurological Institute space; resampling of voxels to 2 mm3; and 8 mm FWHM smoothing.
fMRI Data Analysis
SPM5 was used to create general linear models. For each subject, first-level models included up to five regressors for each trial type for both the simple and repeat tasks: go, no-go, commission errors (i.e., no-go trial errors), omission errors (i.e., go trial errors), and anticipatory responses (i.e., trials on which responses were faster than 200 msec). Each of these regressors was convolved with the canonical hemodynamic response function in SPM5. In addition, six motion parameters and a block parameter were included as regressors for each run.
Second-level, one-sample t tests were then performed across participants to produce group-level activation maps for go and no-go conditions. All activation maps were thresholded at a voxel-wise p value of .001 and multiple comparisons corrected at a cluster-level p value of .05 according to random field theory (Worsley et al., 1996). To examine activation that is common across both tasks, conjunction analyses were performed separately for the go and no-go conditions. These analyses identified voxels that were significantly active for a particular condition during both the simple and repeat tasks. To examine activation that is distinct for the two tasks, one-sample t tests were performed to identify regions that had differential activation for the two tasks (i.e., repeat > simple go, repeat > simple no-go, simple > repeat go, and simple > repeat no-go).
RESULTS
Behavioral Results
Behavioral results are displayed in Table 1. RTs were significantly slower (t(21) = 5.36, p < .001) and intrasubject variability (ISV; standard deviation RT/mean RT) was significantly higher (t(21) = 3.80, p < .001) in the repeat as compared with the simple task. In addition, both commission (t(21) = 6.03, p < .001) and omission error rates (t(21) = 3.52, p = .002) were significantly higher in the repeat task.
Table 1.
Behavioral Results for the Simple and Repeat Tasks
| RT | ISV | Commission Rate | Omission Rate | |
|---|---|---|---|---|
| Simple | 373.12 (107.52) | 0.20 (0.05) | 3.59 (4.72) | 0.36 (1.04) |
| Repeat | 454.01 (128.03) | 0.25 (0.05) | 11.87 (10.37) | 4.97 (10.87) |
fMRI Results
Within-Task Activations
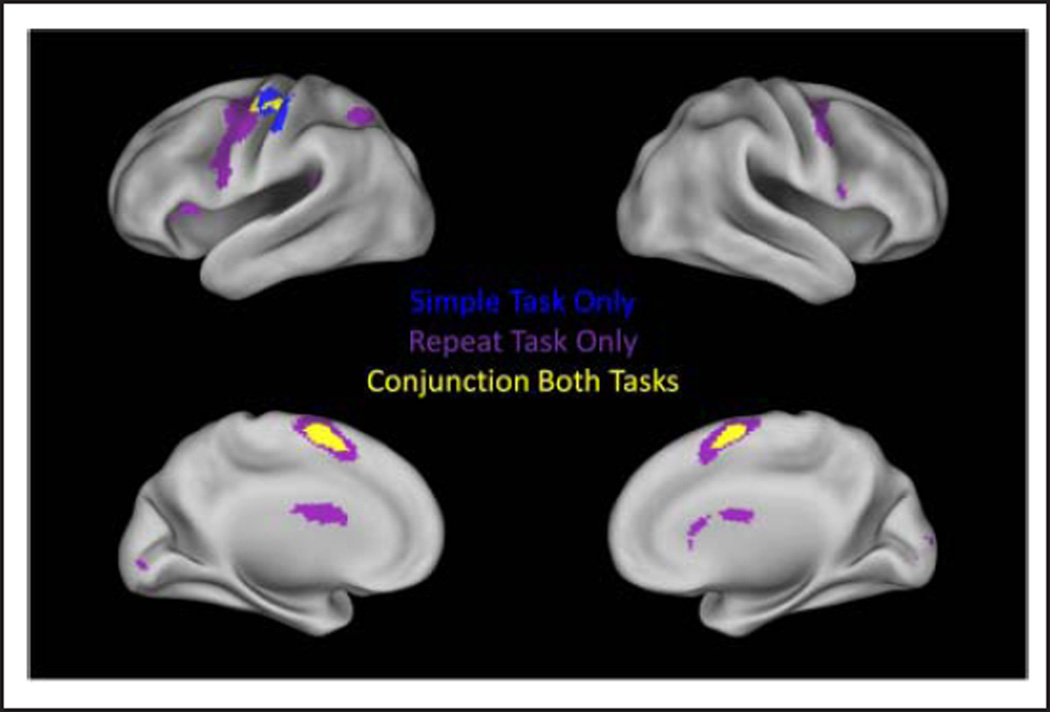
Figure 2 displays those voxels that were significantly active for the go condition in the simple and repeat tasks as well as those voxels that were significantly active for both tasks identified in the conjunction analysis. Go activation that was observed in both tasks (Table 2) included left primary motor/precentral gyrus (M1), the supplementary motor complex (SMC), including both SMA and pre-SMA; left caudate and putamen; left thalamus; and the fourth, fifth, and sixth lobules of the right cerebellum. These regions comprise a well-established left-lateralized motor network (Barber et al., 2012; Mostofsky et al., 2009). The repeat go condition also recruited bilateral dorsal premotor cortex, bilateral middle frontal gyrus (MFG)/IFG, left inferior parietal lobule (IPL), superior parietal lobule (SPL), bilateral caudate and putamen, and a number of cerebellar regions including the fourth, fifth, sixth, and seventh vermal lobules, the Crus 1 lobule of the right cerebellum, and the sixth and Crus 1 lobule of the left cerebellum.
Figure 2.
Go condition activation. Regions with significant activation in the simple task are displayed in blue. Regions with significant activation in the repeat task are displayed in purple. Regions that are significantly active in both tasks are displayed in yellow.
Table 2.
Regions with Significant Activation for the Go Condition in the Simple and Repeat Tasks
| Region | Side | BA | Peak T | p | Size (Voxels) |
x | y | z |
|---|---|---|---|---|---|---|---|---|
| Simple Go | ||||||||
| Superior/medial frontal gyrus (SMA/pre-SMA) |
B | 6/32 | 7.14 | .000 | 448 | −2 | 2 | 62 |
| 10 | 10 | 52 | ||||||
| Precentral gyrus/postcentral gyrus | L | 4/3/6 | 7.44 | .000 | 713 | −38 | −18 | 52 |
| −58 | −18 | 50 | ||||||
| −40 | −22 | 64 | ||||||
| Caudate tail/thalamus | L | 7.4 | .005 | 231 | −16 | −38 | 18 | |
| −12 | −28 | 20 | ||||||
| −6 | −16 | 22 | ||||||
| Caudate/precuneus/hippocampus | R | 30 | 5.45 | .004 | 247 | 22 | −44 | 10 |
| 20 | −42 | 18 | ||||||
| 30 | −46 | 4 | ||||||
| Putamen | L | 6.41 | .001 | 319 | −26 | 0 | −2 | |
| −24 | −10 | 10 | ||||||
| Dentate/cerebellum 4 5 R/cerebellum 6 R | R | 6.15 | .003 | 257 | 18 | −52 | −20 | |
| 8 | −56 | −10 | ||||||
| Repeat Go | ||||||||
| Superior frontal gyrus (pre-SMA) | B | 6/32/8 | 9.23 | .000 | 1450 | 0 | 8 | 62 |
| 8 | 12 | 54 | ||||||
| Precentral gyrus/postcentral gyrus/ MFG/Insula/IFG |
L | 4/6/9/13/44 | 8.18 | .000 | 5397 | −40 | −4 | 46 |
| Caudate | B | −14 | −8 | 24 | ||||
| −18 | 14 | 20 | ||||||
| Putamen | L | −26 | 12 | 4 | ||||
| −24 | 0 | 8 | ||||||
| Precentral gyrus/MFG/IFG | R | 6/9 | 7.260 | .000 | 573 | 36 | −2 | 52 |
| 50 | 2 | 40 | ||||||
| 54 | 8 | 12 | ||||||
| IPL/SPL/precuneus/superior temporal gyrus | L | 7/40 | 7.29 | .000 | 1900 | −40 | −38 | 34 |
| −32 | −42 | 14 | ||||||
| −24 | −50 | 38 | ||||||
| Cerebellum 6 R/vermis 6/cerebellum 4 5 R/ Vermis 7/cerebellum Crus1 R/vermis 4 5/ cerebellum 6 L |
R | 6.23 | .000 | 1284 | 8 | −74 | −20 | |
| 26 | −60 | −24 | ||||||
| 10 | −58 | −18 | ||||||
| Cerebellum 6 L/cerebellum Crus1 L | L | 5.42 | .008 | 225 | −32 | −62 | −26 |
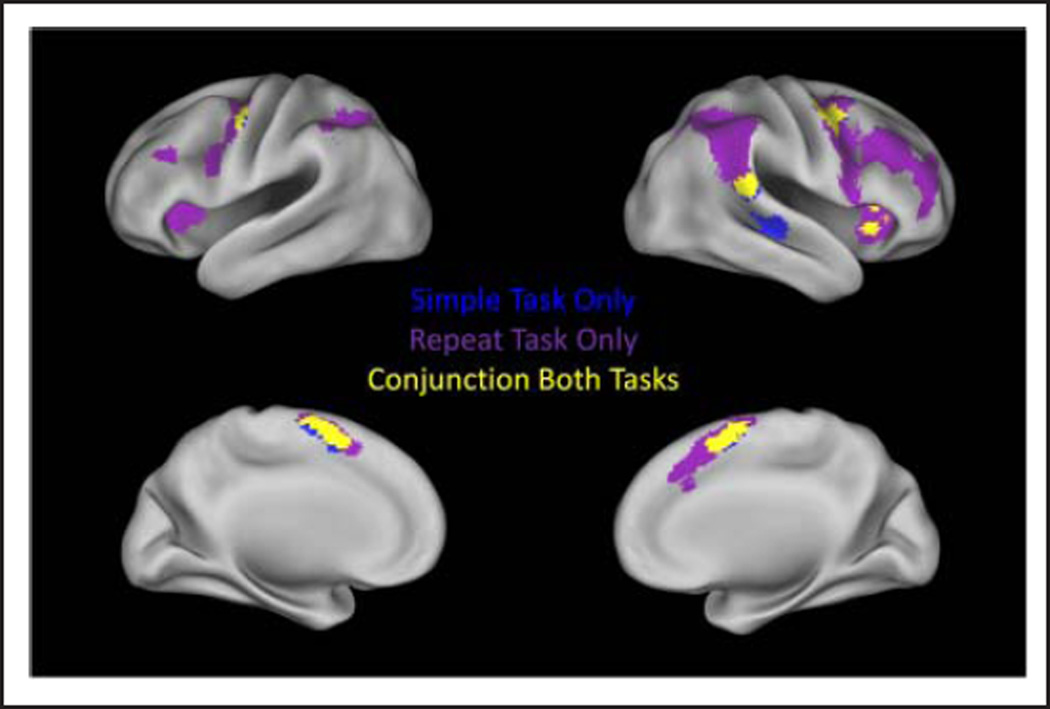
Figure 3 displays those voxels that were significantly active for the no-go condition in both tasks and those voxels identified in the no-go conjunction analysis. No-go activation that was consistent in both included pre-SMA, the right putamen, superior/middle temporal gyrus, DAI/ IFG, TPJ, bilateral dorsal premotor regions, and the sixth and Crus 1 lobules of the left cerebellum (Table 3). The simple no-go condition additionally recruited the left caudate and putamen, the right fusiform, and right middle temporal/superior temporal gyri (BA 22/BA 21), whereas the repeat no-go condition additionally recruited a larger extent of premotor cortex bilaterally, the left DAI, left MFG, and bilateral IPL/SPL. Of note, simple no-go activation occurred in the right IFG at the reported significance threshold but was confined to the frontal operculum/DAI (BA 13/BA 47). This activation did not extend into the right posterior IFG (the area that is commonly associated with response inhibition); however, there was distinct activation that was significant and survived a cluster-level multiple comparisons correction when the voxel-level threshold was reduced to p < .01. This significant but reduced threshold activation was confined within the right posterior IFG (BA 44/BA 45) and did not extend into the right IFJ.
Figure 3.
No-go condition activation. Regions with significant activation in the simple task are displayed in blue. Regions with significant activation in the repeat task are displayed in purple. Regions that are significantly active in both tasks are displayed in yellow.
Table 3.
Regions with Significant Activation for the No-go Condition in the Simple and Repeat Tasks
| Region | Side | BA | Peak T | p | Size (Voxels) | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Simple No-go | ||||||||
| Medial frontal gyrus/superior frontal gyrus (SMA/pre-SMA) |
B | 6/32 | 10.98 | .000 | 729 | −6 | 8 | 54 |
| Precentral gyrus | L | 6 | 8.45 | .005 | 201 | −48 | −4 | 50 |
| MFG/precentral gyrus | R | 6 | 6.48 | .000 | 429 | 40 | 0 | 56 |
| 46 | 6 | 52 | ||||||
| 48 | 6 | 42 | ||||||
| Putamen/insula/IFG | R | 13/47 | 6.31 | .000 | 603 | 24 | 4 | 2 |
| 36 | 20 | 10 | ||||||
| 30 | 12 | 4 | ||||||
| Superior temporal gyrus | R | 22/42 | 6.35 | .002 | 247 | 64 | −36 | 18 |
| Superior temporal/middle temporal gyrus | R | 22/21 | 5.19 | .001 | 271 | 50 | −20 | −6 |
| 54 | −26 | 0 | ||||||
| 60 | −18 | 2 | ||||||
| Putamen/caudate | L | 6.21 | .000 | 411 | −24 | 4 | 0 | |
| −24 | 4 | 14 | ||||||
| −14 | 0 | 20 | ||||||
| Fusiform gyrus | R | 37 | 5.01 | .049 | 121 | 36 | −50 | −16 |
| 40 | −54 | −24 | ||||||
| 36 | −62 | −14 | ||||||
| Cerebelum Crus1 L/cerebelum 6 L/ fusiform gyrus |
L | 37 | 4.79 | .008 | 183 | −34 | −62 | −30 |
| −38 | −64 | −18 | ||||||
| Repeat No-go | ||||||||
| Medial frontal gyrus/superior frontal gyrus (SMA/pre-SMA)/middle cingulate gyrus |
B | 6/32/8 | 10.47 | .000 | 1419 | 8 | 14 | 52 |
| 2 | 10 | 60 | ||||||
| 6 | 30 | 38 | ||||||
| Precentral gyrus/IFG | L | 6/9 | 6.37 | .000 | 662 | −52 | 2 | 42 |
| −46 | −6 | 56 | ||||||
| −34 | −4 | 48 | ||||||
| Precentral gyrus/MFG/superior frontal/IFG | R | 6/9/10/47/8/46 | 11.69 | .000 | 4607 | 46 | 2 | 24 |
| Insula/IFG/putamen | R | 13/44/45 | 34 | 22 | 4 | |||
| 42 | 18 | −2 | ||||||
| Superior temporal gyrus | R | 22 | 13.8 | .000 | 2648 | 62 | −36 | 18 |
| IPL/supramarginal gyrus/angular gyrus/SPL | R | 40/7 | 50 | −40 | 46 | |||
| 38 | −52 | 42 | ||||||
| Insula/IFG | L | 13/47 | 6.94 | .000 | 628 | −32 | 18 | 8 |
| −30 | 24 | 0 | ||||||
| −42 | 8 | 4 | ||||||
| IPL/SPL | 40/7 | 6.19 | 000 | 936 | 40 | 42 | 36 | |
| −28 | −54 | 42 | ||||||
| −36 | −50 | 46 | ||||||
| MFG/superior frontal gyrus | L | 9/46 | 4.74 | .004 | 230 | −46 | 26 | 28 |
| −38 | 42 | 36 | ||||||
| Cerebelum 6 L/cerebelum Crus1 L | L | 5.49 | .003 | 247 | −28 | −66 | −30 | |
| Cerebelum 6 R/cerebelum Crus1 R | R | 5.64 | .013 | 182 | 36 | −62 | −28 |
Conjunction Analysis: Overlapping Activation
The conjunction analyses identified activation that overlapped across both tasks. For the go condition, these regions included the SMC; left M1; left putamen; the tail of the caudate bilaterally; and the fourth, fifth, and sixth lobules of the right cerebellum (Figure 2). For the no-go condition, these regions include the rostral portion of the SMC (i.e., pre-SMA), bilateral dorsal premotor cortex, right IFG/DAI, right TPJ, and the sixth and Crus 1 lobules of the left cerebellum (Figure 3).
Between-Task Activations
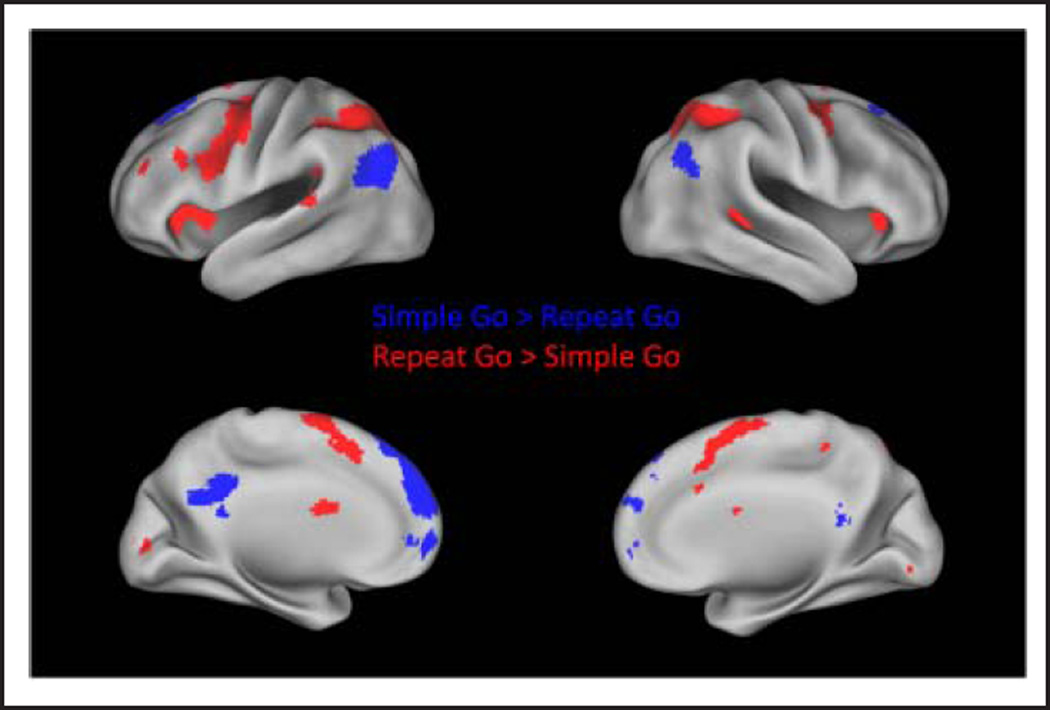
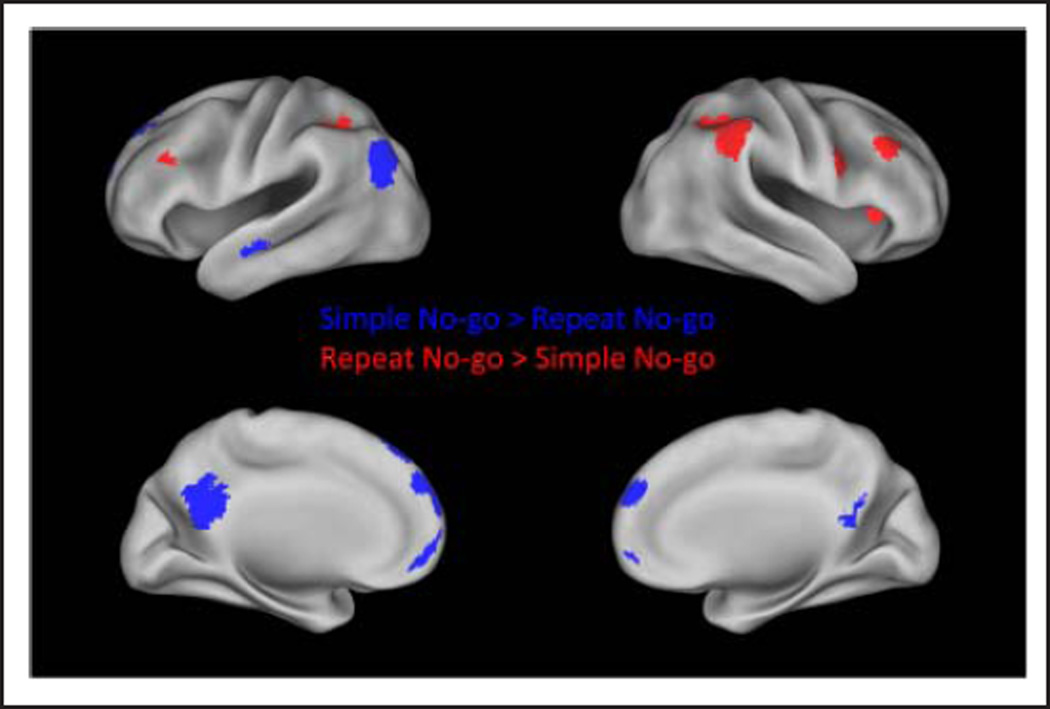
Figure 4 displays regions that were significantly more active for the repeat as compared with the simple task. For the go condition, these regions included the bilateral IPL, bilateral dorsal premotor cortex, SMC, bilateral DAI, right middle temporal gyrus, bilateral caudate, and the left MFG (Figure 4 and Table 4). For the no-go condition, greater activation for the repeat than simple task was observed in bilateral IPL, right DAI, and bilateral MFG (Figure 5 and Table 5). To determine whether subthreshold activation may be present in the posterior IFG, TPJ, FEF, or pre-SMA in the repeat > simple no-go contrast, activation at a reduced voxel-level threshold of p < .01 was examined. There were no significant clusters within those regions at the reduced threshold.
Figure 4.
Regions with significantly greater activation in the simple than repeat task during the go condition are displayed in blue. Regions with significantly greater activation in the repeat than simple task during the go condition are displayed in red.
Table 4.
Regions with Significantly Greater Activation for the Repeat than Simple Task in the Go Condition
| Region | Side | BA | Peak T | p | Size (Voxels) | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Repeat > Simple Go | ||||||||
| IPL/superior parietal/precuneus/middle/ superior occipital/calcarine sulcus/ superior temporal gyrus/supramarginal gyrus |
L | 7/40/17/18 | 10.07 | .000 | 3545 | −42 | −38 | 36 |
| 7.92 | −28 | −50 | 36 | |||||
| 7.62 | −30 | −58 | 50 | |||||
| Precentral gyrus/MFG | R | 6 | 7.87 | .000 | 503 | 40 | −2 | 50 |
| 7.24 | 30 | −6 | 50 | |||||
| 4.72 | 22 | −8 | 46 | |||||
| SPL/IPL/angular gyrus/supramarginal gyrus/ precuneus/superior occipital |
R | 7/40 | 7.75 | .000 | 1873 | 32 | −44 | 40 |
| 6.89 | 20 | −60 | 52 | |||||
| 6.65 | 32 | −48 | 50 | |||||
| Precentral gyrus/IFG/MFG | L | 6/9 | 7.26 | .000 | 1727 | −36 | −6 | 44 |
| 7.01 | −38 | 0 | 30 | |||||
| 6.79 | −44 | −4 | 46 | |||||
| Superior/medial frontal gyrus (SMA/pre-SMA)/ middle cingulate cortex |
B | 6/32/8 | 6.37 | .000 | 1276 | 2 | 12 | 58 |
| 6.33 | −2 | 0 | 66 | |||||
| 5.52 | −16 | 4 | 66 | |||||
| Anterior insula | L | 13 | 6.26 | .000 | 490 | −30 | 28 | −2 |
| 5.26 | −18 | 28 | 10 | |||||
| 4.75 | −30 | 20 | 4 | |||||
| Anterior insula | R | 13 | 5.52 | .006 | 223 | 24 | 28 | 0 |
| 5.16 | 34 | 28 | 2 | |||||
| 3.96 | 38 | 22 | 14 | |||||
| Middle temporal gyrus | R | 5.35 | .013 | 190 | 42 | −34 | 2 | |
| 5.17 | 40 | −28 | −8 | |||||
| Caudate | B | 5.34 | .007 | 214 | 2 | −2 | 20 | |
| 5.26 | −8 | 2 | 18 | |||||
| 4.49 | −16 | −18 | 24 | |||||
| MFG/IFG | L | 10 | 5.24 | .001 | 285 | −34 | 36 | 20 |
| 4.44 | −38 | 24 | 28 | |||||
| 4.36 | −52 | 32 | 24 |
Figure 5.
Regions with significantly greater activation in the simple than repeat task during the no-go condition are displayed in blue. Regions with significantly greater activation in the repeat than simple task during the no-go condition are displayed in red.
Table 5.
Regions with Significantly Greater Activation for the Repeat than Simple Task in the No-go C ondition
| Region | Side | BA | Peak T | p | Size (Voxels) | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Repeat >Simple No go | ||||||||
| IPL/supramarginal gyrus/angular gyrus | R | 40 | 8.86 | .000 | 1320 | 44 | −34 | 40 |
| 8.81 | 38 | −40 | 40 | |||||
| 7.93 | 44 | −42 | 48 | |||||
| Insula | R | 13 | 6.33 | .030 | 139 | 34 | 22 | 6 |
| MFG | R | 9 | 5.46 | .008 | 187 | 40 | 30 | 30 |
| MFG/IFG | L | 9/46 | 5.15 | .036 | 132 | −48 | 28 | 30 |
| IPL | L | 40 | 5.13 | .001 | 281 | −36 | −50 | 36 |
| 5.09 | −44 | −40 | 44 | |||||
| 4.42 | −36 | −50 | 46 | |||||
| IFG/Precentral gyrus | R | 9/44 | 4.97 | .033 | 135 | 54 | 10 | 28 |
Figure 4 displays regions that were significantly more active for the simple go than repeat go condition. These regions included the medial pFC (MPFC), including the superior frontal gyrus and the OFC, the posterior cingulate cortex (PCC), and the middle temporal and angular gyri (Table 6). These regions comprise the default mode network. A similar set of default mode regions were more active for the simple no-go as compared with the repeat no-go condition: the MPFC, including the superior frontal gyrus and the OFC, the PCC/precuneus, the left superior/ middle temporal, and angular gyrus (Figure 5 and Table 7).
Table 6.
Regions with Significantly Greater Activation for the Simple than Repeat Task in the Go Condition
| Region | Side | BA | Peak T | p | Size (Voxels) |
x | y | z |
|---|---|---|---|---|---|---|---|---|
| Simple > Repeat Go | ||||||||
| Medial prefrontal cortex/superior frontal gyrus/ superior medial frontal gyrus |
B | 8/9/10 | 6.78 | .000 | 2108 | −12 | 54 | 24 |
| 6.14 | −8 | 56 | 44 | |||||
| 5.61 | −14 | 34 | 46 | |||||
| Angular gyrus/middle temporal gyrus | L | 39 | 6.06 | .000 | 528 | −46 | −72 | 32 |
| 5.23 | −52 | −70 | 22 | |||||
| Angular gyrus | R | 39 | 5.93 | .006 | 223 | 50 | −68 | 34 |
| PCC/precuneus | B | 31/30 | 4.57 | .000 | 354 | −6 | −42 | 34 |
| 4.32 | 2 | −44 | 18 | |||||
| 4.18 | −4 | −58 | 26 | |||||
| Medial orbital frontal/medial frontal gyrus/ superior medial frontal/anterior cingulate cortex |
B | 10/32 | 4.37 | .002 | 282 | −8 | 48 | 0 |
| 4.27 | 60 | −4 | ||||||
| 3.87 | 48 | −4 |
Table 7.
Regions with Significantly Greater Activation for the Simple than Repeat Task in the No-go Condition
| Region | Side | BA | Peak T | p | Size (Voxels) |
x | y | z |
|---|---|---|---|---|---|---|---|---|
| Simple > Repeat No go | ||||||||
| Angular gyrus/middle occipital gyrus/middle temporal gyrus | L | 39/19 | 7.03 | .000 | 390 | −46 | −72 | 28 |
| 3.78 | −54 | −64 | 16 | |||||
| Superior frontal gyrus/superior medial frontal gyrus | B | 10/9/8 | 6.93 | .000 | 1272 | −2 | 60 | 34 |
| 5.65 | −6 | 42 | 52 | |||||
| 5.49 | 6 | 60 | 28 | |||||
| Middle temporal gyrus | L | 21 | 5.49 | .022 | 150 | −50 | −12 | −18 |
| 4.63 | −40 | −14 | −16 | |||||
| Medial orbital frontal/superior medial frontal gyrus | B | 10/11 | 5.26 | .000 | 308 | 2 | 52 | −12 |
| 5.07 | −8 | 60 | −6 | |||||
| 3.91 | −4 | 66 | 2 | |||||
| PCC/precuneus | B | 31/23/7 | 5.23 | .000 | 628 | −2 | −58 | −28 |
| 4.91 | −14 | −52 | 26 |
DISCUSSION
The current study examined response selection and inhibition during two go/no-go tasks. The simple task used a well-ingrained, consistent stimulus response mapping (green = go, red = no-go) in which working memory demands were minimized. The repeat task had inconsistent stimulus response mappings, which required maintenance of the previous stimulus color (color switch = go, color repeat = no-go) to make the correct response. Regions that were recruited for motor control in both tasks were distinguished from regions that responded to working memory demand and were more active in the repeat task.
Inhibitory Control Circuit
The current study found activation for no-go trials in both tasks in regions that are commonly implicated in inhibitory motor control, including the pre-SMA, right DAI/ posterior IFG, TPJ, striatum, and cerebellum. It has been proposed that these regions work to implement “braking” when it is necessary to stop a prepotent action (Zandbelt, Bloemendaal, Hoogendam, et al., 2013; Aron, 2011; Jahfari et al., 2011; Zandbelt & Vink, 2010). The pre-SMA, right IFG, and striatum are both functionally (Zandbelt & Vink, 2010) and structurally connected (Swann et al., 2012; Aron et al., 2007; Aron & Poldrack, 2006), suggesting that they form cortical BG circuits, which implement motor response inhibition.
A braking function was also proposed by Corbetta and colleagues (2002, 2008) to describe the role of the right TPJ and IFG in visual attention tasks. Consistent with that account, the current study found right-lateralized activity that was common to both tasks within the anterior portion of the TPJ (BA 22/BA 42) and the DAI/IFG (BA 13/ BA 47). When the threshold was reduced for the simple no-go condition, the frontal region extended into the posterior portion of the IFG, the pars opercularis (BA 44) and pars triangularis (BA 45), which have been specifically associated with response inhibition (Cai & Leung, 2011; Levy & Wagner, 2011; Chikazoe, 2010; Chikazoe et al., 2009). This posterior IFG activation was significant and survived cluster-level multiple comparisons correction in the current study.
Together, the TPJ and DAI/IFG are intrinsically connected at rest, forming what has been termed a ventral attentional network (Mars et al., 2012; Fox et al., 2006). They have been traditionally associated with reflexive reorienting tasks (Levy & Wagner, 2011; Corbetta et al., 2002, 2008) and, therefore, are not specifically active for inhibitory control but respond more generally when a non-prepotent action plan is implemented (Hampshire et al., 2010; Chikazoe, Konishi, Asari, Jimura, & Miyashita, 2007).
The pre-SMA is also a well-established region involved in inhibitory control (Simmonds et al., 2008). In the current study, pre-SMA activation was common for both inhibition (during no-go trials) and response selection (during go trials) in the simple and repeat tasks, consistent with previous findings that this region is involved in response selection in addition to inhibition (Mostofsky & Simmonds, 2008). The pre-SMA, therefore, may be directly involved in stopping a planned motor action (Sharp et al., 2010) and may play a general role in setting response thresholds in preparation for response inhibition (Chen et al., 2010). In a study using human intracranial recording, it was found that the pre-SMA was active during a preparatory period and again just before the inhibition was implemented, suggesting that it is involved in both proactive and reactive inhibitory control (Swann et al., 2012). In addition, this region is connected with both the DLPFC and SMA, making it well situated to influence motor commands in line with task goals (Mostofsky & Simmonds, 2008). The pre-SMA was commonly activate for both tasks in the go and no-go conditions. In addition, there was an anterior portion of the pre-SMA, extending into the rostral cingulate zone (RCZ) that was modulated by working memory demand (i.e., showed significant activity for the repeat > simple go contrast). Since response selection was relatively automatic during the simple task, this pre-SMA activity may have reflected increased demands for motor response selection and control in the repeat task, when stimulus response mappings were inconsistent. The RCZ is active during error trials but also plays a role in correct trials that have increased response selection demands (Nee, Kastner, & Brown, 2011). It has been hypothesized that this region responds to action outcome predictions and, in particular, unexpected nonoccurrences (Alexander & Brown, 2011). Whereas activation of this region in the repeat no-go condition is consistent with this interpretation, activation in the repeat–simple go contrast suggests that it may be more generally involved in response selection when stimulus response mappings change and flexible task representations are required. Therefore, the RCZ does not just respond to action outcome frequency, which in the current study is the same for the simple and repeat tasks, but may instead respond to action outcome uncertainty based on the history of previous stimulus associations. This is supported by previous findings that there is overlapping activity in this region for error trials and for novel, uninstructed stimuli (Wessel, Danielmeier, Morton, & Ullsperger, 2012) and also that this region responds to irrelevant stop stimuli (Boehler, Appelbaum, Krebs, Chen, & Woldorff, 2011).
Response Selection and Inhibition under Working Memory Demand
The DLPFC is classically considered to play a role in working memory function. Neurons within the DLPFC maintain task information over delay periods (Goldman-Rakic, 1987, 1996) and are responsible for representing relevant task information even in the face of distractors (Sakai, Rowe, & Passingham, 2002; Miller, Erickson, & Desimone, 1996). The DLPFC and IPL form a frontoparietal network that represents goal-relevant information (Vincent et al., 2008). In the current study, these regions were modulated by working memory demand in both the go and no-go conditions, reflecting the need to maintain the stimulus color from one trial to the next in the repeat task.
The inferior frontal cortex (BA 44/BA 45/BA 47) also plays a role in working memory, which is dissociable from that of the DLPFC (D’Esposito, 2007; Rypma et al., 2002; D’Esposito et al., 1999). This latter region has been implicated in working memory maintenance and may update attention and/or working memory representations (Roth et al., 2006; Brass, Derrfuss, Forstmann, & von Cramon, 2005). The current study found a further dissociation between the DLPFC and distinct parts of the inferior frontal cortex: the right IFG, which was involved with inhibitory control but did not respond to working memory demand, and the right IFJ, which was modulated by working memory demand. This is consistent with the interpretation that the IFJ is involved in attention allocation and updating of task representations (Roth et al., 2006; Brass et al., 2005)
The right IFJ has been commonly implicated in the detection of behaviorally relevant target stimuli (Cai & Leung, 2011; Levy & Wagner, 2011; Chikazoe, 2010; Chikazoe et al., 2009). Although this region is commonly active for infrequent target stimuli that signal a change in action plan, it is also active when target stimuli are equiprobable (Levy & Wagner, 2011) and has been found for presentations of unexpected, task-irrelevant stimuli Asplund, Todd, Snyder, & Marois, 2010). This suggests that the IFJ may be involved in assessing the behavioral relevance of stimuli and updating working memory accordingly. In the current study, the right IFJ was modulated by working memory demand specifically on no-go trials, suggesting that it may be involved in detecting salient stimuli when the task requires frequent updating of working memory representations.
In addition to the frontoparietal network (DLPFC-IPL) and IFJ, the DAI also showed an effect of working memory demand. This region was commonly active for no-go trials in both tasks. In fact, the locus of activation for no-go trials in both tasks was in the right DAI and frontal operculum rather than the right IFG. This is consistent with previous studies that have found this insular region consistently activated by response inhibition tasks (Swick et al., 2011). However, unlike other inhibitory control regions found in the current study (i.e., pre-SMA, right IFG, and right TPJ), activity in this region was also modulated by working memory demand (i.e., significant activity for the repeat > simple no-go contrast). Therefore, the DAI may be more directly involved with accessing relevant maintained task information (Dosenbach et al., 2007) or autonomic changes (Critchley, 2005; Critchley et al., 2000, 2002, 2004), rather than with withholding a prepotent response. The IFG and DAI are commonly active together and the right-side DAI forms part of the ventral attentional network (Fox et al., 2006); however, these two regions seem to serve different functions in inhibitory control. Consistent with this view, a meta-analysis comparing activity in response inhibition and reorienting tasks found that activity in the IFG pars opercularis was specific to response inhibition, whereas activity in the DAI was common to both of these types of task (Levy & Wagner, 2011). Therefore, the IFG may be more directly involved in inhibiting prepotent action plans, whereas the DAI may orchestrate changes in sustained task information (Dosenbach et al., 2007) and/or changes in autonomic state (Critchley, 2005; Critchley et al., 2000, 2002, 2004) that accompany a response inhibition. These changes may be necessary whenever task conditions are challenging, such as during inhibitory control, as well as under working memory demand.
A separate set of regions, bilateral premotor cortex (BA 6), SPL (BA 7/BA 40), and bilateral FEFs, responded to working memory demand for the go trials, but not for the no-go trials. Activation in this set of regions may therefore be associated with guiding response selection when stimulus response mappings are not straightforward. The bilateral premotor cortex (BA 6) and SPL (BA 7/BA 40) form a dorsal attention network, which bias attention to task goals (Corbetta et al., 2008; Fox et al., 2005, 2006). In the current study, response selection was relatively automatic for the simple task because it was consistently mapped to green stimuli, whereas response selection required greater selective attention in the repeat task because the stimulus response mapping changed. Activation of these dorsal attention network regions may therefore reflect greater need for top–down biasing of attention for response selection in the repeat task. The FEF responded to working memory demand for go trials (i.e., in the repeat go > simple go contrast) but was also commonly recruited for inhibitory control in both tasks. This region may therefore reflect additional response control that is necessary whenever the selection of an action plan is not straighforward, both when stimulus response mappings are inconsistent and when the action plan is nonprepotent. The FEF is classically considered part of the circuitry for oculomotor control but is also active for other response modalities (Muggleton et al., 2010; Corbetta et al., 1998). Although it plays a role in inhibitory control (Muggleton et al., 2010), it is more generally implicated in top–down biasing of attention (Corbetta et al., 2008).
All of the regions mentioned so far that were modulated by working memory demand are known to be involved in implementing task control. A separate set of default mode regions were less active for the repeat task as compared with the simple task (i.e., in both the simple > repeat go and simple > repeat no-go contrasts). The default mode is involved in representing internally directed, self-reflective thought (Buckner, Andrews-Hanna, & Schacter, 2008). It is well known that default mode regions show opposing activity with dorsal attention regions, particularly when working memory demand increases (Raichle & Snyder, 2007; Fox et al., 2005; Raichle et al., 2001). In the current study, reduced activation of default mode regions in both of the repeat task conditions reflects suppression of these regions when working memory demand was increased.
Conclusions
The current study examined brain networks involved in motor response selection and inhibition with two tasks involving consistent and inconsistent stimulus response mappings. The effect of working memory demand on brain networks involved in motor control was assessed. Regions that are generally associated with inhibitory control (right IFG, right TPJ, and pre-SMA) were not affected by working memory demand on no-go trials. The DLPFC, IFJ, DAI and FEF, on the other hand, showed unique patterns of activity for the two tasks, which reflect specific roles in guiding goal-directed behavior for motor response selection and inhibition. Activation in default mode regions was suppressed with greater working memory demand during both go and no-go trials.
REFERENCES
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature Neuroscience. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Neuroscience. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. Motor “dexterity”?: Evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cerebral Cortex. 2012;22:51–59. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Chen LC, Woldorff MG. The role of stimulus salience and attentional capture across the neural hierarchy in a stop-signal task. PLoS One. 2011;6:e26386. doi: 10.1371/journal.pone.0026386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cai W, Leung HC. Rule-guided executive control of response inhibition: Functional topography of the inferior frontal cortex. PLoS One. 2011;6:e20840. doi: 10.1371/journal.pone.0020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Claus ED, Kim A, Curran T, Banich MT, Munakata Y. Cognitive control reflects context monitoring, not motoric stopping, in response inhibition. PLoS One. 2012;7:e31546. doi: 10.1371/journal.pone.0031546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. Journal of Neuroscience. 2010;30:14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Current Opinion in Psychiatry. 2010;23:267–272. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cerebral Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of Cognitive Neuroscience. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Chun MM. Visual working memory as visual attention sustained internally over time. Neuropsychologia. 2011;49:1407–1409. doi: 10.1016/j.neuropsychologia.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. Journal of Physiology. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: A functional magnetic resonance study. Neuroimage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, U.S.A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;49:1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the frontal association cortex and its relevance to dementia. Archives of Gerontology and Geriatrics. 1987;6:299–309. doi: 10.1016/0167-4943(87)90029-x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences, U.S.A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nature Neuroscience. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. Journal of Neuroscience. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Piekema C, Coles MG, Hulstijn W, Toni I. On the programming and reprogramming of actions. Cerebral Cortex. 2007;17:2972–2979. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schuffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-based subdivisions of the human right “temporoparietal junction area”: Evidence for different areas participating in different cortical networks. Cerebral Cortex. 2012;22:1894–1903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: An fMRI, within-subjects investigation. Neuropsychologia. 2008;46:2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. Journal of Neuroscience. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Research, Cognitive Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Chen CY, Tzeng OJ, Hung DL, Juan CH. Inhibitory control and the frontal eye fields. Journal of Cognitive Neuroscience. 2010;22:2804–2812. doi: 10.1162/jocn.2010.21416. [DOI] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage. 2011;54:528–540. doi: 10.1016/j.neuroimage.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Courtney SM, Ungerleider LG, Haxby JV. Sustained activity in the medial wall during working memory delays. Journal of Neuroscience. 1998;18:9429–9437. doi: 10.1523/JNEUROSCI.18-22-09429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences, U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Rama P, Sala JB, Gillen JS, Pekar JJ, Courtney SM. Dissociation of the neural systems for working memory maintenance of verbal and nonspatial visual information. Cognitive, Affective & Behavioral Neuroscience. 2001;1:161–171. doi: 10.3758/cabn.1.2.161. [DOI] [PubMed] [Google Scholar]
- Roth JK, Serences JT, Courtney SM. Neural system for controlling the contents of object working memory in humans. Cerebral Cortex. 2006;16:1595–1603. doi: 10.1093/cercor/bhj096. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. Journal of Cognitive Neuroscience. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nature Neuroscience. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences, U.S.A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. Journal of Neuroscience. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage. 2012;59:2860–2870. doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proceedings of the National Academy of Sciences, U.S.A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, Ullsperger M. Surprise and error: Common neuronal architecture for the processing of errors and novelty. Journal of Neuroscience. 2012;32:7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Hoogendam JM, Kahn RS, Vink M. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. Journal of Cognitive Neuroscience. 2013;25:157–174. doi: 10.1162/jocn_a_00309. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SF, Kahn RS, Vink M. Expectations and violations: Delineating the neural network of proactive inhibitory control. Human Brain Mapping. doi: 10.1002/hbm.22047. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PLOS One. 2010;5:e13848. doi: 10.1371/journal.pone.0013848. [DOI] [PMC free article] [PubMed] [Google Scholar]