Abstract
Objective
To provide an overview of the diagnosis and management of phenylketonuria (PKU) in childhood with an emphasis on aspects relevant to family physicians providing ongoing care.
Sources of information
The author’s experience as the clinic physician in a regional pediatric PKU clinic is supplemented with references providing evidence for key points.
Main message
While metabolic clinics typically provide guidance regarding the specific management of PKU, the family doctor has an essential role in providing ongoing medical care.
Conclusion
Children and families have much to gain from strong relationships with family doctors, and family doctors can confidently provide care with awareness of the very few potential special needs of patients with PKU.
Résumé
Objectif
Donner un aperçu du diagnostic et de la prise en charge de la phénylcétonurie (PCU) en mettant l’accent sur les aspects pertinents pour les médecins de famille qui dispensent des soins continus.
Sources des données
L’expérience de l’auteure en tant que médecin clinicienne dans une clinique pédiatrique régionale de la PCU est complétée par des références fournissant des données probantes à l’appui des points saillants.
Message principal
Même si les cliniques du métabolisme offrent habituellement des conseils concernant la prise en charge spécifique de la PCU, les médecins de famille jouent un rôle essentiel dans la prestation des soins médicaux continus.
Conclusion
Les enfants et les familles ont beaucoup à gagner de relations étroites avec les médecins de famille et ces derniers peuvent en toute confiance offrir des soins en étant conscients des besoins spéciaux potentiels très restreints des patients atteints de la PCU.
Case
During a busy Friday afternoon clinic, you receive a telephone call from your provincial newborn screening laboratory, indicating that a baby you delivered 6 days earlier has a positive screening result for phenylketonuria (PKU). Now what? What is your role in diagnosing and treating PKU, and in providing future health care for this child?
For most family doctors, the management of PKU will never be an issue, as they might never have affected children in their practices. Those who do might understandably be uncertain about how best to work with such children and their families. Although PKU is an uncommon condition that requires some specialized care, the role of the family doctor is essential in providing complete health care for the child and his or her family. This brief overview of the disease and the family doctor’s role in its management will set the stage for a relationship that is satisfying and valuable to the doctor, the patient, and the family.
Sources of information
The information provided is based on the author’s experience as a clinic physician in a regional PKU clinic, and on the experiences of many families attending the clinic. Specific points are supported by key references.
Main message
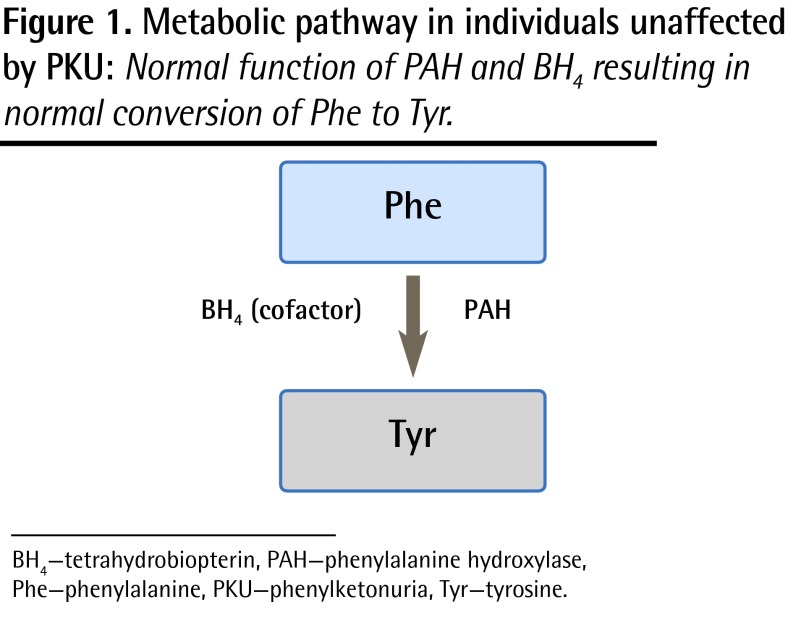
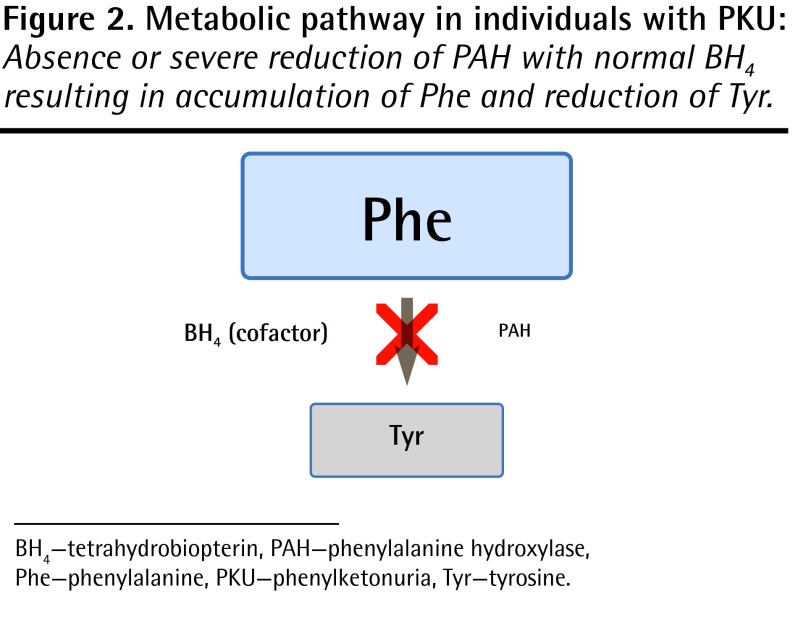
Phenylketonuria is an inherited metabolic disease of protein metabolism affecting approximately 1 in 14 000 children born in Canada.1 Given the number of births in each province, this means that there might be anywhere from 0 to 12 new cases of PKU diagnosed per province per year. The autosomal recessive inheritance pattern predicts a 25% recurrence risk with each pregnancy, and it is therefore not unusual to have more than 1 affected child in a family. The gene for classic PKU is found on chromosome 12, and mutations affect the production of the enzyme phenylalanine hydroxylase. The normal function of phenylalanine hydroxylase is to catalyze the conversion of phenylalanine (Phe) to tyrosine (Tyr) (Figure 1). The mutations associated with PKU result in reduced or absent enzyme production, or production of an enzyme with reduced or absent function. The consequences of this are the accumulation of abnormal amounts of Phe and reduction in Tyr in the blood (Figure 2). In untreated individuals, the clinical presentation of PKU consists of mental retardation, behaviour problems, fair skin and hair, eczema, and a mousy odour. The cause of the mental retardation is not completely understood, but it might be a consequence of abnormally high levels of Phe in the brain, reduced levels of neurotransmitters (for which Tyr is a precursor), or other unknown mechanisms.
Figure 1.
Metabolic pathway in individuals unaffected by PKU: Normal function of PAH and BH4 resulting in normal conversion of Phe to Tyr.
BH4 —tetrahydrobiopterin, PAH—phenylalanine hydroxylase, Phe—phenylalanine, PKU—phenylketonuria, Tyr—tyrosine.
Figure 2.
Metabolic pathway in individuals with PKU: Absence or severe reduction of PAH with normal BH4 resulting in accumulation of Phe and reduction of Tyr.
BH4 —tetrahydrobiopterin, PAH—phenylalanine hydroxylase, Phe—phenylalanine, PKU—phenylketonuria, Tyr—tyrosine.
Diagnosis
All provinces and territories in Canada conduct newborn screening for PKU, which allows for early detection and treatment, and obviates the devastating consequences of the condition. Most cases are identified within the first week of life. When newborn screening identifies a high Phe level, the child will generally be referred to a centre familiar with the diagnosis and management of metabolic disease. As is the case with all screening tests, the diagnosis must be confirmed by repeating measurement of Phe levels, as well as conducting additional tests to rule out other very rare causes of elevated Phe levels.
Early management
Treatment of PKU involves dramatic reduction of dietary whole protein sources in order to reduce the intake of Phe. However, as Phe is an essential amino acid, growth cannot occur if it is completely eliminated from the diet. Each child’s individual “tolerance” of Phe will be different based on the level of enzyme activity present. Therefore, the amount of Phe that can be consumed in the diet must be tailored to the child’s specific needs. This is achieved by consuming measured amounts of PKU formula, which contains all amino acids required for growth except Phe, and consumption of a diet with small amounts of whole protein (which contains Phe).
Typically, breastfeeding or feeding of regular formula will initially be discontinued for an infant with PKU for a period of time (usually less than 72 hours) to allow for rapid reduction of Phe levels. During this time the infant will consume only specialized PKU formula without Phe. As levels begin to decrease, feeding of breast milk or standard infant formula will be reintroduced in combination with PKU formula until Phe levels stabilize in the target range. Because PKU formula is completely Phe-free, PKU formula can never be used for extended periods (ie, longer than 24 to 48 hours) as a sole source of nutrition. The Phe requirements will change as the child grows, and feeding recommendations will generally be made by metabolic physicians and dietitians experienced in PKU management.
Monitoring
Blood levels are measured periodically by obtaining a blood sample by heel or finger poke collected onto a newborn screening card. Although this process is somewhat analogous to blood glucose testing in a patient with diabetes, there is an important difference: Phe levels must be measured in a clinical laboratory by tandem mass spectrometry, a process that requires a full day to complete and that is typically available only in specialized regional laboratories. As such, while it is possible to obtain results on the same day, in stable patients there might be a delay of several days before results are available. A list of Canadian Newborn Screening Laboratories can be found on the Canadian Agency for Drugs and Technologies in Health website.2
There is some controversy regarding appropriate target levels for Phe, and little evidence with which to defend specific recommendations. However, during childhood, many centres in North America aim for levels of approximately 120 to 360 μmol/L until 12 years of age, as per the National Institutes of Health Consensus Statement, with some liberalization to approximately 600 μmol/L thereafter.3
Diet
In most centres, metabolic dietitians will have a pivotal role in helping families manage the PKU diet. The dietitian will provide ongoing guidance on issues such as the introduction of complementary foods (ie, “solids”), changes in formula, and the need for nutrient supplementation. Infant PKU formula is used for about the first year of life, with complementary foods introduced at about 6 months. After 1 year of age, children are generally transitioned to an alternative formula suitable for use throughout childhood and into adulthood. While infant PKU formulas contain all the necessary macronutrients and micronutrients (except Phe), formulas for older children and adults are much more variable in their composition. The dietitian will provide guidance regarding different formula options and their advantages for individual children. Specialized PKU formula is provided free of charge to infants and children with PKU in all provinces, and to adults with PKU in many provinces. In addition to formula, children will consume a variety of foods. As the PKU diet emphasizes fruit and vegetable intake, it might be assumed that children with PKU have a healthier diet than unaffected children. While that might be true, many less healthy choices such as candy contain low or no Phe, and might be included in the diet of the child with PKU. As for any child, excessive consumption of these low-nutrient, high-calorie foods can lead to poor diet quality and excessive weight gain. In addition to fruits and vegetables, children can also consume measured amounts of regular grain products such as breads, rice, or pasta. However, the relatively high Phe content of these foods means that the quantities that can be included in the diet might be quite limited. An alternative option to increase dietary variety is to substitute special low-protein medical foods. These are food products designed for use in medically prescribed diets such as the PKU diet. The products are intended to look and taste like foods that would ordinarily be severely restricted or eliminated from the diet and are available through such manufacturers as Cambrooke Foods (www.cambrookefoods.com) and Applied Nutrition Corp (www.dietforlife.com). Over the past decade the variety and palatability of such foods has increased enormously and this has improved quality of life for many individuals with PKU. However, most of these specialized products are considerably more expensive than their higher-protein counterparts, and funding for such products might be limited or nonexistent, is highly variable between provinces, and changes frequently. Low-protein cookbooks offer another option for increasing the variety of foods available for inclusion in the PKU diet. Information regarding access to low-protein foods, recipes, and other diet-related resources specific to the Canadian context can be found at www.canpku.org.
Other treatments
The mainstay of PKU management is dietary protein restriction. However, other options to help with management are possible in specific circumstances. The drug sapropterin is a pharmacologic preparation of biopterin, which is a cofactor in the conversion of Phe to Tyr. In a limited number of PKU patients, the addition of sapropterin to dietary therapy will result in a reduction of Phe levels and allow for some degree of dietary liberalization. This is not related to biopterin deficiency, as biopterin levels and function are assessed during the confirmation of the diagnosis of PKU. The effect is not observed in all patients and it is variable in magnitude. Clinical trials to further elucidate the role of this drug are ongoing. Other therapies provide some further therapeutic options for adult patients, but they are not routinely used in children.4
Outcomes
Children with PKU who receive early treatment have excellent outcomes, with cognitive development consistently in the normal range.5,6 While previous recommendations suggested that dietary control was only necessary during the period of brain development, there is good evidence that benefits continue throughout the lifespan. It is now clear that maintenance of lower Phe levels by lifelong dietary therapy has a positive effect on concentration, mood, and attention, resulting in improved cognitive function.7,8 Despite these improvements, challenges remain. Measurable differences in executive functioning (eg, planning, organization) exist between affected and unaffected individuals, even when diagnosis and treatment occur early in infancy. School and learning problems are more common among children with PKU, and neuropsychological assessment might be helpful in early identification and program planning.5,9
Patients with PKU in general practice
In general, individuals with PKU require little in the way of specialized care. However, several issues might require special consideration and management.
Medications and supplements:
Although most medications are not contraindicated in PKU, some are sweetened with the artificial sweetener aspartame, which contains Phe. This information should be readily available from a pharmacist and should be checked each time a course of medication is started, as formulations change frequently. If a medication does contain aspartame, consultation with a pharmacist should be helpful in identifying an alternative preparation. In addition, some multivitamin or mineral supplements might also contain amino acids or skim milk powder and thus might be sources of Phe. Changes in Phe levels coinciding with the addition of new medications or supplements should prompt a review with a pharmacist to determine whether the new preparation could be the source of additional dietary Phe intake.
Poor fluid or energy intake:
Children with PKU acquire the usual assortment of childhood illnesses, which might be accompanied by decreased intake of food and fluids. While this is often mild and self-limited, more severe or long-lasting illnesses might require special management. When children with PKU decrease their energy and protein intake, they will experience some muscle catabolism to meet their immediate needs for glucose and protein synthesis. Because muscle contains substantial amounts of Phe, this will result in elevated Phe levels during illness. Ideally, this is managed by encouraging continued intake of low-Phe, higher energy–containing fluids, generally in the form of PKU formula. However, if this is unsuccessful, inpatient management for administration of intravenous dextrose and lipids to rehydrate and increase energy intake might be warranted. If proximity to a metabolic centre or the resources available locally are limiting factors, this could be done at a local hospital with guidance from the metabolic team, or the child could be transferred for care. As the family physician is likely the first point of contact for such mild to moderate illnesses, awareness of the need for monitoring, communication with the metabolic team, and intervention if necessary are key to the family physician’s role. Specific protocols and recommendations for their implementation can be obtained from local metabolic clinics.
School and behavioural issues:
Although many children with PKU have no obvious school or behavioural issues, some experience challenges, and the emergence of a picture resembling attention deficit disorder (ADD) is not uncommon. While children with PKU and ADD are often treated for ADD using the same approaches that are used in other children, it can be extremely valuable to obtain detailed neuropsychological testing to inform school program planning and optimize therapy. Consultation with the metabolic centre might be helpful in ensuring access to appropriate testing and follow-up.
Pregnancy and PKU:
As girls with PKU move into adolescence, it is critically important that they understand the effects of PKU on future pregnancies. As PKU is inherited in an autosomal recessive fashion, all children of a mother with PKU will inherit 1 affected gene. However, as the likelihood of having a partner who is a carrier for PKU is relatively low (approximately 2% in North America), any offspring are unlikely to inherit a second affected gene from the father, and would mostly likely be PKU carriers. However, Phe is actively transported across the placenta during pregnancy, and fetal Phe levels exceed those of the mother. The consequences of high fetal Phe levels during pregnancy are devastating for the fetus, with frequent occurrence of microcephaly, developmental delay, congenital heart disease, and various other abnormalities. Many of these effects are related to high Phe levels in the earliest days of gestation, often before recognition of pregnancy. Therefore, meticulous Phe control before pregnancy, or as early as possible during pregnancy, provides the best chance for an optimal outcome. Ensuring that the young woman with PKU is aware of the importance of PKU management before and during pregnancy, is familiar with contraceptive options, and is aware of the need for early diagnosis of pregnancy are all critically important roles for family doctors caring for adolescent girls with PKU. An excellent summary of maternal PKU provides more detailed information and specific recommendations.10
Conclusion
Although PKU is an uncommon condition, it is one in which the family physician can have a vital role in the provision of regular medical care, monitoring, coordination of care, advocacy, and family support. Most children with PKU require little in the way of specialized medical support. Awareness of the few situations that might require specialized management specific to PKU will enable the family physician to confidently provide ongoing care. For children with PKU and their families, having someone they know and trust available to help is the most valuable resource that they can have. For the family doctor, it is an opportunity to watch, and sometimes help, families overcome considerable life challenges to help their children grow into healthy, happy, and successful adults.
EDITOR’S KEY POINTS
Although phenylketonuria (PKU) is an uncommon condition that requires some specialized care, family physicians can have a vital role in the provision of regular medical care, monitoring, coordination of care, advocacy, and family support.
Treatment of PKU involves dramatic reduction of dietary whole protein sources in order to reduce the intake of the essential amino acid phenylalanine (Phe). In most centres, metabolic dietitians will have a pivotal role in helping families manage the PKU diet. Blood levels are measured periodically by obtaining a blood sample by heel or finger poke.
Family physicians should be aware that some medications contain aspartame, which contains Phe, and some supplements might also contain amino acids or skim milk powder and thus might also be sources of Phe. During severe or prolonged illness, muscle catabolism due to poor energy intake might also raise Phe levels, and special management might be required. Further, as female patients with PKU approach adolescence, special consideration around pregnancy and contraception is needed.
POINTS DE REPÈRE DU RÉDACTEUR
Quoique la phénylcétonurie (PCU) soit un problème rare qui exige certains soins spécialisés, les médecins de famille jouent un rôle essentiel dans la prestation des soins médicaux habituels, la surveillance, la coordination des soins, la promotion de la santé et le soutien à la famille.
Le traitement de la PCU comporte une réduction dramatique des sources alimentaires de protéines entières afin de réduire l’apport en acide aminé essentiel phénylalanine (Phe). Dans la plupart des centres, des diététiciennes spécialisées en métabolisme exercent un rôle central pour aider les familles à suivre la diète requise pour la PCU. Les concentrations sanguines sont mesurées périodiquement en obtenant un spécimen de sang par piqûre au talon ou au doigt.
Les médecins de famille devraient savoir que certains médicaments contenant de l’aspartame, qui renferme du Phe, et certains suppléments qui pourraient aussi contenir des acides aminés ou du lait écrémé en poudre, pourraient conséquemment être des sources de Phe. Durant une maladie grave ou prolongée, le catabolisme musculaire dû à un faible apport énergétique peut aussi faire augmenter les taux de Phe et une prise en charge spéciale pourrait être nécessaire. De plus, avec les patientes atteintes de PCU qui approchent de l’adolescence, il est nécessaire d’accorder une attention particulière aux questions entourant la grossesse et la contraception.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Competing interests
None declared
References
- 1.Alberta Health and Wellness . Newborn metabolic screening in Alberta 2002–2005. Edmonton, AB: Provincial Health Office; 2006. Available from: www.health.alberta.ca/documents/Newborn-Metabolic-Report-2002-05.pdf. Accessed 2012 Aug 25. [Google Scholar]
- 2.Canadian Agency for Drugs and Technologies in Health [website] Newborn screening for disorders and abnormalities in Canada. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2013. Available from: www.cadth.ca/products/environmental-scanning/environmental-scans/newborn-screening. Accessed 2013 Jun 26. [Google Scholar]
- 3.Phenylketonuria: screening and management. NIH consensus statement. Bethesda, MD: National Institutes of Health; 2000. Available from: www.nichd.nih.gov/publications/pubs/pku/sub3.cfm. Accessed 2012 Aug 24. [Google Scholar]
- 4.Van Spronsen FJ, de Groot MJ, Hoeksma M, Reijngoud DJ, van Rijn M. Large neutral amino acids in the treatment of PKU: from theory to practice. J Inherit Metab Dis. 2010;33(6):671–6. doi: 10.1007/s10545-010-9216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gassió R, Fuste E, Lopez-Sala A, Artuch R, Vilaseca MA, Campistol J. School performance in early and continuously treated phenylketonuria. Pediatr Neurol. 2005;33(4):267–71. doi: 10.1016/j.pediatrneurol.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Brumm VL, Grant ML. The role of intelligence in phenylketonuria: a review of research and management. Mol Genet Metab. 2010;99(Suppl 1):S18–21. doi: 10.1016/j.ymgme.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Brumm VL, Azen C, Moats RA, Stern AM, Broomand C, Nelson MD, et al. Neuropsychological outcome of subjects participating in the PKU adult collaborative study: a preliminary review. J Inherit Metab Dis. 2004;27(5):549–66. doi: 10.1023/b:boli.0000042985.02049.ff. [DOI] [PubMed] [Google Scholar]
- 8.Gassió R, Campistol J, Vilaseca MA, Lambruschini N, Cambra FJ, Fuste E. Do adult patients with phenylketonuria improve their quality of life after introduction/resumption of a phenylalanine-restricted diet? Acta Paediatr. 2003;92(12):1474–8. [PubMed] [Google Scholar]
- 9.Antshel KM. ADHD, learning, and academic performance in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S52–8. doi: 10.1016/j.ymgme.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Genetics Maternal phenylketonuria. Pediatrics. 2008;122(2):445–9. [Google Scholar]