Abstract
Background
The optimal management of colon injury patients requiring damage control laparotomy (DCL) is controversial. The objective of this study was to assess the safety of colonic resection and anastomosis versus fecal diversion in trauma patients requiring DCL.
Methods
Patients with traumatic colon injuries undergoing DCL between 2000 and 2010 were identified by the database and chart review. Those who died within 48 h were excluded. Patients were divided into two groups: those undergoing one or more colonic anastomoses with or without distal colostomy (group 1) and those undergoing colostomy only or one or more colonic anastomoses with a protecting proximal ostomy (group 2). Variables were compared using Wilcoxon rank sum, χ2, or Fisher exact tests as appropriate.
Results
Sixty-one patients were included (group 1, n = 28 and group 2, n = 33). Fascial closure rates (group 1, 50% versus group 2, 61%; P = 0.45), hospital length of stay (29 versus 23 d; P = 0.89), and in-patient mortality (11% versus 12%; P = 1.0) were similar between groups. There were a total of 11 anastomotic leaks, five of which were related to non-colonic enteric repairs. Colonic anastomosis leak rates were 16% overall (six of the 38 patients), 14% in group 1 (four of the 28 patients), and 20% in group 2 (two of the 10 patients). Compared with patients who did not leak, patients who leaked had a higher median age (37 versus 25 y; P = 0.05), greater likelihood of not achieving facial closure before post-injury day 5 (18% versus 2%; P = 0.003), and a longer hospital length of stay (46 versus 25 d; P = 0.003).
Conclusions
Outcomes after colonic injury in the setting of DCL were similar regardless of the surgical management strategy. Based on these findings, a strategy of diversion over anastomosis cannot be strongly recommended.
Keywords: Colon, Anastomosis, Damage control, Open abdomen
1. Introduction
After >2 decades of widespread use, the concept of damage control has fundamentally altered the management of severely injured patients [1–3]. The damage control process is characterized by a staged approach in which an abbreviated surgery is used to control the immediate threats of coagulopathy, hypothermia, and metabolic acidosis, followed byphysiological restoration in the intensive care unit (ICU) and eventual return to the operating room (OR) for definitive repair [4]. In patients with destructive abdominal injuries, the use of damage control laparotomy (DCL) is now widely accepted as the standard of care in critically injured patients [1,4–12]. However, DCL is not without significant short- and long-term complications, including intra-abdominal infections, enterocutaneous fistulae, and ventral hernias requiring complicated repair [12].
During the damage control process, an injured bowel is often left in discontinuity. On returning to the OR for definitive repair, the surgeon is left with an important decision: restore bowel continuity with a colonic anastomosis or create an ostomy for fecal diversion. Although there is evidence supporting a colonic anastomosis in the non-damage control setting [13–16], there remains limited data regarding the optimal approach to restoring bowel continuity in the patient undergoing DCL. Few studies have specifically evaluated colon wound management after DCL and those that have offer conflicting results [17–23]. In these series, leak rates were variable, ranging from 0% to 27%, and a myriad of risk factors were associated with the development of anastomotic leak, including higher 12-h heart rate, elevated base deficit, left-sided injury, greater transfusion requirements, and abdominal closure after post-injury day 5.
With significant morbidity associated with failed repair, the decision to construct an anastomosis versus ostomy has major implications. We hypothesized that the colonic anastomosis would result in a greater number of complications and worse clinical outcomes than fecal diversion in DCL patients. The objectives of this study were to assess the safety of colonic resection and anastomosis versus fecal diversion in trauma patients requiring DCL and identify the potential risk factors for anastomotic leakage.
2. Methods
This study was approved by the Institutional Review Board of University of Pennsylvania. Patients were initially identified by query of our institutional Pennsylvania Trauma Outcome Study trauma registry over the period of 2000–2010. The study inclusion criteria included age >18 y, traumatic colon injury, and an initial operation consisting of DCL. DCL was defined as an emergent laparotomy in which temporary wound closure methods are used with the intention of returning to the OR for definitive repair after correction of physiological abnormalities. Patients were excluded if they died within 48 h of admission, if they did not undergo colonic anastomosis or fecal diversion to repair their colonic injuries, or if there were insufficient data available (Figure). Additional clinical data not available from the trauma registry were obtained via a comprehensive chart review, which included the review of all operative and daily progress notes, radiology reports, and discharge documentation. There was no specific hospital-wide protocol for the management of traumatic colon injuries in place at the time of this study. As such, all treatment decisions were made on a case-by-case basis by the operating surgeon.
Fig.
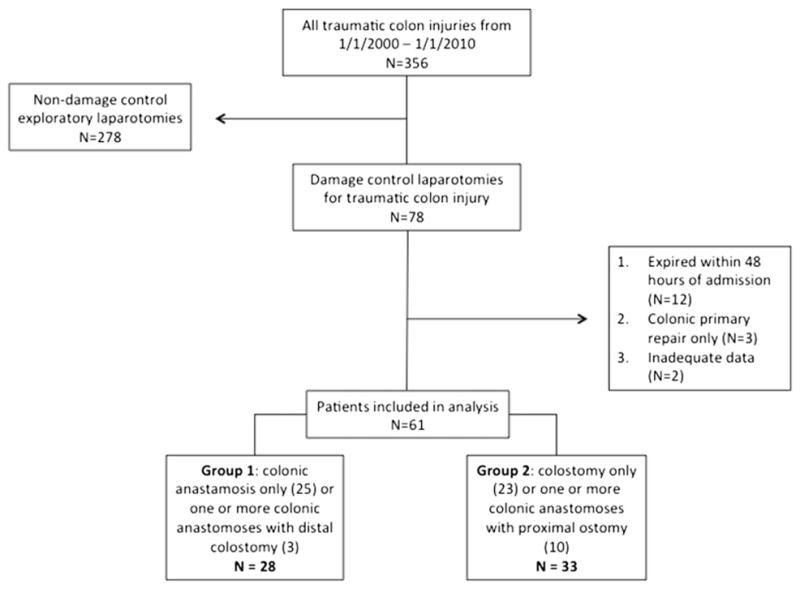
Study population.
Demographic data included age, race, sex, and mechanism of injury. The injury severity was classified via Injury Severity Score (ISS) [24] and abdominal Abbreviated Injury Scale (AIS) score [25]. Physiological derangement was assessed by vital signs on admission (heart rate and systolic blood pressure), laboratory values during the first 24 h of admission (lactate, hemoglobin, and international normalized ratio), lowest body temperature during the first operative procedure, and transfusion and resuscitation requirements during the first operative procedure. The surgical approach was characterized by the total number and timing of abdominal surgeries, including repairs of both the large bowel and small bowel (SB). The total number and location of four types of surgical repair were included: (1) primary repairs (defined as bowel injury managed by suture repair), (2) resection and anastomosis constructed solely with suture, (3) resection and anastomosis constructed primarily with mechanical stapling devices, and (4) ostomies.
Primary outcomes of interest included anastomotic leak, intra-abdominal abscess, and the development of enter-ocutaneous fistulae. If a patient had an anastomotic leak and an adjacent abscess, only the leak was counted as a complication. Secondary outcomes of interest included hospital length of stay, ICU length of stay (ICU LOS), in-hospital mortality, and the status and timing of abdominal closure. When evaluating the relationship between anastomotic leak and the duration of fascial non-closure, a cutoff of 5 d was chosen based on the previous literature [23].
Two primary analyses were conducted. First, patients were divided into two groups for comparison based on the primary surgical management strategy (i.e., anastomosis versus diversion, respectively): those undergoing one or more colonic anastomoses with or without distal colostomy (group 1) and those undergoing colostomy only or one or more colonic anastomoses with a protecting proximal ostomy (group 2). Three patients underwent primary repair as their only colon intervention (primary repair group) and were excluded from comparative analysis. Second, patients were divided into two groups for comparison based on the development of anastomotic leak (leak versus no leak group).
For comparison between groups, the Wilcoxon rank sum test was used for continuous variables, whereas χ2 or Fisher exact test was used for categorical variables as appropriate. Only 12 patients met the primary endpoint of the study (anastomotic leak), limiting the utility of multivariable logistic regression. Statistical significance was set at P < 0.05 (two sided). Analysis was performed using SPSS software (v19; IBM SPSS Statistics, Chicago, IL).
3. Results
During the 10-y study period, 78 patients with traumatic colon injury met the inclusion criteria. Seventeen patients were excluded; 12 patients died within 48 h of admission, three underwent a colonic primary repair as their sole means of injury management, and two had insufficient data. Sixty-one patients were included in the final analysis. Group 1 (those undergoing one or more colonic anastomoses with or without distal colostomy) included 28 patients and group 2 (those undergoing colostomy only or one or more colonic anastomoses with a protecting proximal ostomy) 33 patients. Of the 28 patients in group 1, three had their colonic anastomosis proximal to their colonic anastomosis. Of the 33 patients in group 2, 10 underwent concurrent colonic anastomosis distal to the ostomy (referred to hereafter as defunctionalized anastomoses) (Figure). Fifty-six patients (92%) suffered from penetrating trauma and five from blunt injury.
No differences were observed in age, ISS, maximum abdominal AIS, vital signs at admission, transfusion and resuscitation requirements, or OR temperature between groups 1 and 2 (Table 1). Only the peak lactate during the first 24 h of admission reached statistical significance, with group 2 patients being more acidotic than group 1 (3.8 versus 5.4; P = 0.01). Similarly, no differences were observed in the total number of abdominal surgeries, rates of fascial closure at discharge, ICU LOS, and in-patient mortality between the two groups (Table 2). The timing of definitive colonic repairs varied, with anastomosis or diversion occurring during the first abdominal surgery in 16% of patients, at the second in 56% of patients (i.e., first return to the OR with an open abdomen), and at the third in 27% of patients. Overall, 34 (53%) patients underwent definitive fascial closure before discharge and seven (11%) died during hospitalization.
Table 1.
Comparison of baseline demographics, injury severity, and acuity between group 1 and group 2 patients.
| Group 1 (n = 28) | Group 2 (n = 33) | P | |
|---|---|---|---|
| Demographics | |||
| Age, (y) | 25 (21–39) | 26 (21–43) | 0.48 |
| Race, n (%) | |||
| Caucasian | 1 (4) | 2 (6) | 0.78 |
| African American | 26 (93) | 29 (88) | |
| Asian | 1 (4) | 1 (3) | |
| Other or unknown | 0 (0) | 1 (3) | |
| Male, n (%) | 27 (97) | 30 (91) | 0.62 |
| Injury | |||
| Mechanism of injury, n (%) | |||
| Blunt | 0 (0) | 5 (15) | 0.06 |
| Penetrating | 28 (100) | 28 (85) | 0.87 |
| ISS | 25 (17–33) | 26 (19–38) | 0.18 |
| Abdominal AIS | 4 (3–4) | 4 (3–4) | 0.84 |
| Admission GCS | 15 (14–15) | 15 (11–15) | 0.75 |
| Pancreatic injury, n (%) | 3 (11) | 1 (3) | 0.33 |
| Duodenal injury, n (%) | 2 (7) | 5 (15) | 0.44 |
| SB anastomosis, n (%) | 15 (54) | 20 (61) | 0.61 |
| Physiology | |||
| Admission heart rate | 104 (81–128) | 96 (80–115) | 0.53 |
| Admission systolic blood pressure | 120 (95–143) | 113 (90–136) | 0.53 |
| Lab values | |||
| Peak lactate in the first 24 h | 3.8 (3.4–6.3) | 5.4 (4.3–7.8) | 0.01 |
| Lowest hemoglobin in the first 24 h | 8.2 (7–8.8) | 8.7 (6.8–10.3) | 0.69 |
| Peak INR in the first 24 h | 1.4 (1.3–1.7) | 1.5 (1.2–1.7) | 0.93 |
| Transfusion and resuscitation | |||
| PRBC (U)* | 6 (3–9) | 4 (2–6) | 0.18 |
| Platelets (U)* | 0 (0–4) | 0 (0–1) | 0.28 |
| FFP (U)* | 4 (0–6) | 3 (0–4) | 0.65 |
| Crystalloid (L)* | 6 (4–8) | 6 (5–9) | 0.85 |
| Lowest OR temperature (°C)* | 35.5 (34.6–36.2) | 35.8 (35.2–36.2) | 0.46 |
GCS = glasgow coma scale; INR = international normalized ratio; PRBC = packed red blood cells; FFP = fresh frozen plasma; OR = operating room.
Median and interquartile range reported for continuous variables.
P value is based on the Wilcoxon rank sum test for continuous variables and χ2 or Fisher exact test for categorical variables.
During the first abdominal surgery.
Table 2.
Surgical complications and outcomes between group 1 and group 2 patients.
| Group 1 (n = 28) | Group 2 (n = 33) | P | |
|---|---|---|---|
| Complications | |||
| Intra-abdominal abscess, n (%) | 11 (39) | 11 (33) | 0.79 |
| Fistula, n (%) | 5 (18) | 5 (15) | 1.0 |
| Anastomotic leaks, n (%) | 6 (21) | 5 (15) | 0.74 |
| Colonic | 4 | 2 | — |
| SB | 0 | 3 | — |
| Non-colon primary repair | 2 | 0 | — |
| Outcome | |||
| Total abdominal surgeries | 3 (2–6) | 3 (3–7) | 0.21 |
| Fascia closed at discharge, n (%) | 14 (50) | 20 (61) | 0.45 |
| Fascia closure day* | 2 (1–3) | 2 (2–9) | 0.13 |
| HLOS | 29 (20–46) | 23 (15–43) | 0.89 |
| ICU | 12 (7–27) | 11 (6–25) | 0.84 |
| In-patient mortality, n (%) | 3 (11) | 4 (12) | 1.0 |
HLOS = hospital length of stay.
Median and interquartile range reported for continuous variables.
P value is based on χ2 or Fisher exact test.
In those whose fascia was closed before discharge.
Rates of complications related to surgical management were similar between groups (Table 2). Overall, 22 (36%) patients developed an intra-abdominal abscess, 10 (16%) developed an enterocutaneous fistula, and 11 (18%) suffered an anastomotic leak. Of the 11 leaks, six occurred at colonic anastomoses, three at SB anastomoses, and two at non-colon primary repairs (stomach and duodenum).
Overall, there were a total of 38 patients who underwent one or more colonic anastomoses. Of these, six (16%) developed a leak. The anastomosis leak rate per repair was 14% (six of the 44 total anastomoses), with six patients undergoing two anastomoses each. In group 1, the colonic anastomosis leak rate was 14% per patient (four of the 28 patients) and 13% (four of the 31 patients) per suture line. In group 2, the defunctionalized colonic anastomosis leak rate was 20% per patient (two of the 10 patients) and 20% per suture line (two of the 10 patients). Of the three SB anastomosis leaks that occurred, all were in group 2 (three of the 35 anastomoses, 9%). The median day to diagnosis of a leak was 9 d (range, 1–15 d). All leaks occurred in the setting of an open abdomen, before any attempts at abdominal closure.
Table 3 details the types and locations of repairs and the leak characteristics of the 11 patients whose anastomotic repairs failed. Of the six colonic anastomosis leaks, three occurred at anastomoses between transverse colon (three of the 17 anastomoses, 18% leak rate), two at anastomoses between ileum and ascending colon (two of the 13 anastomoses, 15%), and one at an anastomosis between ileum and descending colon (one of the 14 anastomoses, 7%). Two of the six colonic leaks developed in patients with defunctionalized anastomoses. Four leaks were associated with staples and two with suture repairs.
Table 3.
Description of patients with anastomotic leak by primary colon management strategy.
| No. | Primary repair | Anastomosis | Ostomy | Location of leak | Day of leak | Colon anastomosis leak | Defunctionalized colon anastomosis leak |
|---|---|---|---|---|---|---|---|
| GROUP 1
| |||||||
| 1 | — |
|
Transverse colon | Transverse colon → colon anastomosis (suture) | 9 | Yes | No |
| 2 |
|
SB → ascending colon (staple) | — | 1. SB → ascending colon anastomosis (staple) | 8 | Yes | No |
| 3 | — |
|
— | 2. SB primary repair Transverse colon → colon anastomosis (staple) | 8 | Yes | No |
| 4 | SB | Transverse colon → colon (staple) | — | Transverse colon → colon anastomosis (staple) | 10 | Yes | No |
| 5 | Duodenum |
|
— | Duodenum primary repair | 11 | No | No |
| 6 | Stomach | Descending colon → colon (staple) | — | Stomach primary repair | 15 | No | No |
|
| |||||||
| GROUP 2
| |||||||
| 1 | Duodenum |
|
Ascending colon | Stomach → SB anastomosis (staple) | 1 | No | No |
| 2 | — | SB → SB (suture) | Transverse colon | SB → SB anastomosis (suture) | 4 | No | No |
| 3 | — | SB/ascending colon (suture) | SB | SB → ascending colon anastomosis (suture) | 6 | Yes | Yes |
| 4 | — |
|
SB |
|
2 | Yes | Yes |
| 5 |
|
SB → SB (suture) | Ascending colon | SB → SB anastomosis (suture) | 11 | No | No |
In the univariate analysis, patients who leaked were significantly older than those who did not (37 versus 25 y; P = 0.05). ISS, AIS, laboratory values, and transfusion requirements did not differ significantly between those who leaked and those who did not (Table 4). Compared with patients who did not leak, patients who leaked required more abdominal surgeries (6 versus 3; P = 0.01), were less likely to achieve fascial closure (36% versus 66%; P = 0.05), had longer ICU LOS (26 versus 11 d; P = 0.004), and had longer hospital length of stay (46 versus 25 d; P = 0.003). A greater likelihood of not achieving facial closure before post-injury day 5 was associated with a 16.80 times higher likelihood of developing a leak (18% versus 2%; P = 0.03; 95% confidence interval, 2–196). The in-patient mortality was not significantly different between the two groups (18% in the leak group versus 12% in the no leak group; P = 0.64).
Table 4.
Comparison of patients with anastomotic leak with those with no leak.
| Leak (n = 11) | No leak (n = 50) | P | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, (y) | 37 (25–52) | 25 (20–39) | 0.05 |
| Penetrating injury, n (%) | 11 (100) | 45 (90) | 0.33 |
| ISS | 24 (17–26) | 26 (18–34) | 0.08 |
| Maximum abdominal AIS | 4 (3–4) | 4 (3–4) | 0.84 |
| Peak lactate in the first 24 h | 4.5 (3–5.6) | 4.5 (3.5–7.8) | 0.32 |
| Lowest hemoglobin in the first 24 h | 8.9 (7.7–10.2) | 8.3 (7–8.9) | 0.21 |
| Peak INR in the first 24 h | 1.3 (1.2–1.6) | 1.5 (1.4–1.7) | 0.07 |
| PRBC (U)* | 5 (3–11) | 4 (2–8) | 0.28 |
| Platelets (U)* | 2 (0–6) | 0 (0–1) | 0.06 |
| FFP (U)* | 4 (2–6) | 3 (0–5) | 0.16 |
| Crystalloid (L)* | 7 (4–10) | 6 (5–8) | 0.59 |
| Lowest OR temperature (°C)* | 35.5 (35.5–36.3) | 35.6 (34.6–36.2) | 0.49 |
| Outcomes | |||
| Total abdominal surgeries | 6 (5–7) | 3 (2–6) | 0.01 |
| Fascia closed at discharge, n (%) | 4 (36) | 33 (66) | 0.05 |
| Fascia closure day† | 26 (7–35) | 2 (1–5) | 0.03 |
| HLOS | 46 (38–62) | 25 (14–40) | 0.003 |
| ICU LOS | 26 (19–34) | 11 (6–24) | 0.004 |
| In-patient mortality, n (%) | 2 (18) | 6 (12) | 0.64 |
INR = international normalized ratio; PRBC = packed red blood cells; FFP = fresh frozen plasma; OR = operating room; HLOS = hospital length of stay.
Median and interquartile range reported for continuous variables.
P value is based on the Wilcoxon rank sum test for continuous variables and χ2 or Fisher exact test for categorical variables.
During the first abdominal surgery.
In those whose fascia was closed before discharge.
4. Discussion
Although there is evidence supporting a colonic anastomosis in the non-damage control setting, the optimal approach to restoring bowel continuity in the patient undergoing DCL remains controversial. In this study, the overall colonic anastomotic leak rate was 16%. Leaks were associated with significantly greater morbidity, including increased ICU LOS, total number of abdominal surgeries, and a decreased likelihood of fascial closure. Risk factors associated with the development of anastomotic leak included older age and failure to achieve fascial closure before post-injury day 5. Importantly, half of all leaks in this study occurred at SB anastomosis with a smaller proportion occurring distal to a diverting ostomy. Although the results of anastomotic leak were significant, the risk of anastomotic failure was similar in both primary colon management strategies.
In a non-damage control setting, colonic anastomosis leak rates have been shown to be as low as 1%–3% [13] and as high as 42% [26]. Patients at the greatest risk of anastomotic failure include those with preexisting medical comorbidities, large transfusion and/or resuscitation requirements, and a high degree of fecal contamination [16,26,27]. To this end, guidelines from the Eastern Association for the Surgery of Trauma recommend colostomy for destructive colon injuries in the setting of other significant injuries, shock, or peritonitis [28]. However, these guidelines were created before the widespread popularization of the damage control surgery. With its staged approach and focus on physiological restoration, the damage control offers surgeons the opportunity for delayed anastomotic repair, even in the setting of severe abdominal injuries. Despite a better understanding of the damage control process, the risk factors associated with failure of colonic anastomoses in the damage control setting remain unclear.
Since 2007, seven retrospective studies have examined the surgical approach to traumatic colon injury in damage control patients (Table 5). In these series, leak rates are variable, ranging from 0% to 27% (weighted average, 16%). The largest study by Burlew et al. found an 18% leak rate among 65 patients undergoing colonic resection and anastomosis in the damage control setting. Anastomotic failure was associated with higher 12-h heart rate and base deficit, left-sided injuries, and an abdominal closure after post-injury day 5 [23]. Ott et al. [21] reported a 27% leak rate among 44 patients, with greater risk associated with increased transfusion requirements and the presence of left colonic injury. In contrast, Weinberg et al. [18] found no predictors significantly associated with the 12% leak rate among their 33 patients. In our study, we report an anastomotic leak rate of 16% with older age and fascial closure after 5 d being significant risk factors.
Table 5.
Studies to date evaluating colon wound management in the damage control setting.
Central to the question of whether anastomosis is safe in the damage control setting is the potential effect of an open abdomen on normal physiology and wound healing. In our study, a greater likelihood of not achieving facial closure before post-injury day 5 was associated with a 16.80 times higher likelihood of developing a leak (P = 0.03; 95% confidence interval, 2–196). Although this may suggest that an open abdomen has direct deleterious effects on anastomotic healing, it may also reflect the overall physiological status of the injured patient. A critically ill patient may be less likely to have their abdomen closed and more prone to develop an anastomotic leak. As such, the two processes may not be directly related but instead the result of a common underlying cause such as septicemia, poor nutritional status, or widespread edema. When comparing those who leaked with those who did not, the only significant difference in baseline characteristics was age. This suggests that at initial presentation, patients who leaked were as ill as those who did not, at least by available quantitative measures such as ISS, packed red blood cells and crystalloid requirements, and lowest OR temperature. Furthermore, in this study, all leaks were diagnosed in an open abdomen with a median day to diagnosis of 9 d. Although a vigorous systemic stress and inflammatory response after severe injury are well recognized, it is unclear whether the open abdomen exacerbates the host defense response and how this might impact anastomotic healing [29,30]. More research is needed to elucidate this potential relationship.
Unlike previous studies, our cohort had a significant number of colonic anastomoses with proximal diverting ostomies. These defunctionalized colonic anastomoses had a leak rate of 20% (two of the 10 patients), suggesting that proximal diversion may not protect against clinically significant distal anastomotic failure. These findings contrast with the recent literature in elective colorectal surgery, suggesting that proximal colonic diversion reduces the rate of clinically relevant anastomotic leakage and reoperation [31–33]. Although defunctionalized anastomoses are spared the passage of intestinal contents, the risk of anastomotic breakdown in DCL patients may be more closely related to the physiologic derangements associated with severe injury. As such, creating an unnecessary anastomosis in the damage control abdomen with the intent of proximal diversion must be considered cautiously.
The location of a repair may also be an important risk factor for the development of anastomotic failure. Historically, left-sided colon injuries have been linked to greater leak rates than other sites of colonic anastomosis [34–36], although this is not always born out in the literature [37]. There are a number of factors that may explain a greater risk of breakdown in left-sided colon repairs. First, there is a greater concentration of mucosal bacteria and therefore a potentially greater risk of infectious complications in the descending colon. Second, when left-sided colon injuries are at or near the peritoneal reflection, the absence of peritoneum on the distal anastomotic target may contribute to poor anastomotic healing. Finally, the proximal descending colon (i.e., the splenic flexure) is a watershed area with a relatively poor blood supply. The relationship between left-sided repair and anastomotic failure has also been described in the damage control setting [21,23]. In contradistinction to these findings, we did not observe a relationship between location of colonic anastomosis and leak rate, although our ability to do so was limited by the small overall number of colonic anastomotic leaks.
When comparing diversion with anastomotic repair, it is important to note the challenges present when constructing an ostomy in the damage control abdomen. At the time of repair, the presence of bowel and abdominal wall edema in addition to poorly mobilizable mesentery may compromise stoma integrity. If a patient’s ostomy is eventually reversed, the necessity for additional abdominal procedures in a hostile scarred abdomen introduces a host of potential complications. Furthermore, the approach to ventral hernia repair—a common complication in the damage control abdomen—is compromised by the avoidance of permanent mesh because of the risk of infection.
As a retrospective study, this work has inherent limitations. At the time of the study, there was no specific hospital-wide protocol for the management of traumatic colon injuries. As such, all treatment decisions were made on a case-by-case basis by the operating surgeon, leaving open the possibility of provider bias in the management of the colonic injury. In addition, the complexity of the patients studied makes retrospective data collection and interpretation challenging. Although a Penetrating Abdominal Trauma Index score would have allowed for more accurate quantification of injury severity, we were unable to obtain sufficiently detailed information from operative notes for calculation. Moreover, although a multitude of factors could contribute to the risk of anastomotic failure, the small number of the primary endpoints of anastomotic leak precluded the use of multivariable logistic regression to control for all factors potentially associated with anastomotic leaks. Finally, all but five patients suffered from penetrating trauma, decreasing the generaliz-ability to traumatic colon injuries caused by blunt force. The decision was made not to exclude or differentiate the patients who suffered from blunt trauma because of the small sample size of the study.
In conclusion, we found that the anastomotic leak rate was 16% in patients undergoing primary resection and anastomosis for colonic injury in the setting of damage control surgery. In our small study, outcomes, including anastomotic failure, were similar in both primary colon management strategies, suggesting clinical equipoise between study groups. Based on these findings, a primary management strategy of diversion over anastomosis cannot be strongly recommended in patients suffering from penetrating colon trauma. However, colonic anastomotic leaks carried significant morbidity and occurred at both SB and large bowel anastomoses, including those distal to a diverting ostomy. In patients with a persistently open abdomen, the risk of anastomotic leak was increased. Additional research is warranted to understand this important association. Given the relative rarity of this constellation of injury, it is unlikely that a prospective randomized trial will be successfully conducted. However, further multicenter prospective trials could lead to improved management paradigms for patients undergoing DCL and a better understanding of how the open abdomen impacts anastomotic repair.
References
- 1.Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375. [PubMed] [Google Scholar]
- 2.Shapiro MB, Jenkins DH, Schwab CW, Rotondo MF. Damage control: collective review. J Trauma. 2000;49:969. doi: 10.1097/00005373-200011000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Peitzman AB. Damage-control laparotomy. Curr Opin Crit Care. 2006;12:346. doi: 10.1097/01.ccx.0000235213.63988.9a. [DOI] [PubMed] [Google Scholar]
- 4.Rotundo MR, Zonies DH. The damage control sequence and underlying logic. Surg Clin North Am. 1997;77:761. doi: 10.1016/s0039-6109(05)70582-x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JW, Gracias VH, Schwab CW, et al. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51:261. doi: 10.1097/00005373-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp KW, Locicero RJ. Abdominal packing for surgically uncontrollable hemorrhage. Ann Surg. 1992;215:467. doi: 10.1097/00000658-199205000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burch JM, Ortiz VB, Richardson RJ, Martin RR, Mattox KL, Jordan GL. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg. 1992;215:476. doi: 10.1097/00000658-199205000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saifi J, Fortune JB, Graca L, Shah DM. Benefits of intra-abdominal pack placement for the management of nonmechanical hemorrhage. Arch Surg. 1990;125:119. doi: 10.1001/archsurg.1990.01410130125019. [DOI] [PubMed] [Google Scholar]
- 10.Hirshberg A, Wall MJ, Mattox KL. Planned reoperation for trauma: a two-year experience with 124 consecutive patients. J Trauma. 1994;37:365. [PubMed] [Google Scholar]
- 11.Cirocchi R, Abraha I, Montedori A, et al. Damage control surgery for abdominal trauma. Cochrane Database Syst Rev. 2010;20:CD007438. doi: 10.1002/14651858.CD007438.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Brenner M, Bochicchio G, Bochicchio K, et al. Long-term impact of damage control laparotomy: a prospective study. Arch Surg. 2011;146:395. doi: 10.1001/archsurg.2010.284. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RL, Singer M. Primary repair for penetrating colon injuries. Cochrane Database Syst Rev. 2003:Art. No.: CD002247. doi: 10.1002/14651858.CD002247. http://dx.doi.org/10.1002/14651858.CD002247. [DOI] [PubMed]
- 14.Sasaki LS, Allaben RD, Gobwala R, Mittal VK. Primary repair of colon injuries: a prospective randomized study. J Trauma. 1995;39:895. doi: 10.1097/00005373-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez RP, Merlotti GJ, Holevar MR. Colostomy in penetrating colon injury: is it necessary? J Trauma. 1996;41:271. doi: 10.1097/00005373-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Demetriades D, Murray JA, Chan L, et al. Penetrating colon injuries requiring resection: diversion or primary anastomosis? An AAST prospective multicenter study. J Trauma. 2001;50:765. doi: 10.1097/00005373-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Miller PR, Chang MC, Hoth JJ, Holmes JH, 4th, Meredith JW. Colonic resection in the setting of damage control laparotomy: is delayed anastomosis safe? Am Surg. 2007;73:606. doi: 10.1177/000313480707300613. discussion 609–610. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg JA, Griffin RL, Vandromme MJ, et al. Management of colon wounds in the setting of damage control laparotomy: a cautionary tale. J Trauma. 2009;67:929. doi: 10.1097/TA.0b013e3181991ab0. [DOI] [PubMed] [Google Scholar]
- 19.Kashuk JL, Cothren CC, Moore EE, Johnson JL, Biffl WL, Barnett CC. Primary repair of civilian colon injuries is safe in the damage control scenario. Surgery. 2009;146:663. doi: 10.1016/j.surg.2009.06.042. discussion 668–670. [DOI] [PubMed] [Google Scholar]
- 20.Vertrees A, Wakefield M, Pickett C, et al. Outcomes of primary repair and primary anastamosis in war-related colon injuries. J Trauma. 2009;66:1286. doi: 10.1097/TA.0b013e31819ea3fc. [DOI] [PubMed] [Google Scholar]
- 21.Ott MM, Norris PR, Diaz JJ, et al. Colon anastamosis after damage control laparotomy: recommendations from 174 trauma colectomies. J Trauma. 2011;70:595. doi: 10.1097/TA.0b013e31820b5dbf. [DOI] [PubMed] [Google Scholar]
- 22.Ordonez C, Pino L, Badiel M, et al. Safety of performing a delayed anastomosis during damage control laparotomy in patients with destructive colon injuries. J Trauma. 2011;71:1512. doi: 10.1097/TA.0b013e31823d0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burlew CC, Moore EE, Cuschieri J, et al. WTA Study Group. Sew it up! A Western Trauma Association multi-institutional study of enteric injury management in the postinjury open abdomen. J Trauma. 2011;70:273. doi: 10.1097/TA.0b013e3182050eb7. [DOI] [PubMed] [Google Scholar]
- 24.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187. [PubMed] [Google Scholar]
- 25.Copes WS, Sacco WJ, Champion HR, Bain LW. Progress in characterising anatomic injury. Proceedings of the 33rd Annual Meeting of the Association for the Advancement of Automotive Medicine; Baltimore, MD. [Google Scholar]
- 26.Stewart RM, Fabian TC, Croce MA, Pritchard FE, Minard G, Kudsk KA. Is resection with primary anastomosis following destructive colon wounds always safe? Am Surg. 1994;168:316. doi: 10.1016/s0002-9610(05)80156-4. [DOI] [PubMed] [Google Scholar]
- 27.Schnuriger B, Inaba K, Wu T, Eberle B, Belzberg H, Demetriades D. Crystalloids after primary colon resection and anastomosis at initial trauma laparotomy: excessive volumes are associated with anastomotic leakage. J Trauma. 2011;70:603. doi: 10.1097/TA.0b013e3182092abb. [DOI] [PubMed] [Google Scholar]
- 28.Pasquale M, Fabian TC. Practice management guidelines for trauma from the Eastern Association for the Surgery of Trauma. J Trauma. 1998;44:941. doi: 10.1097/00005373-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Lenz A, Franklin G, Cheadle W. Systemic inflammation after trauma. Injury. 2007;38:1336. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Hüser N, Michalski CW, Erkan M, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. 2008;248:52. doi: 10.1097/SLA.0b013e318176bf65. [DOI] [PubMed] [Google Scholar]
- 32.Chude GG, Rayate NV, Patris V, et al. Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology. 2008;55:1562. [PubMed] [Google Scholar]
- 33.Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009;96:462. doi: 10.1002/bjs.6594. [DOI] [PubMed] [Google Scholar]
- 34.Veyrie N, Ata T, Muscari F, et al. Anastomotic leakage after elective right versus left colectomy for cancer: prevalence and independent risk factors. J Am Coll Surg. 2007;205:785. doi: 10.1016/j.jamcollsurg.2007.06.284. [DOI] [PubMed] [Google Scholar]
- 35.Golub R, Golub RW, Cantu R, Jr, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997;184:364. [PubMed] [Google Scholar]
- 36.Bokey EL, Chapuis PH, Fung C, et al. Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum. 1995;38:480. doi: 10.1007/BF02148847. [DOI] [PubMed] [Google Scholar]
- 37.Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, Valleur P. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg. 2002;26:499. doi: 10.1007/s00268-001-0256-4. [DOI] [PubMed] [Google Scholar]


