Abstract
Background
Alcohol consumption during pregnancy can damage the developing fetus, illustrated by central nervous system dysfunction and deficits in motor and cognitive abilities. Binge drinking has been associated with an increased risk of fetal alcohol spectrum disorders, likely due to increased episodes of ethanol withdrawal. We hypothesized that overactivity of the N-methyl-D-aspartate (NMDA) receptor during ethanol withdrawal leads to excitotoxic cell death in the developing brain. Consistent with this, administration of NMDA receptor antagonists (e.g. MK-801) during withdrawal can attenuate ethanol's teratogenic effects. The aim of this study was to determine if administration of memantine, an NMDA receptor antagonist, during ethanol withdrawal could effectively attenuate ethanol-related deficits, without the adverse side effects associated with other NMDA receptor antagonists.
Methods
Sprague-Dawley pups were exposed to 6.0 g/kg ethanol or isocaloric maltose solution via intubation on postnatal day 6, a period of brain development equivalent to a portion of the 3rd trimester. Twenty-four and 36 hours after ethanol, subjects were injected with 0, 10 or 15 mg/kg memantine, totaling doses of 0, 20, or 30 mg/kg. Motor coordination was tested on a parallel bar task and the total number of cerebellar Purkinje cells was estimated using unbiased stereology.
Results
Alcohol exposure induced significant parallel bar motor incoordination and reduced Purkinje cell number. Memantine administration significantly attenuated both ethanol-associated motor deficits and cerebellar cell loss in a dose-dependent manner.
Conclusions
Memantine was neuroprotective when administered during ethanol withdrawal. These data provide further support that ethanol withdrawal contributes to fetal alcohol spectrum disorders.
Keywords: fetal alcohol, treatment, NMDA, excitotoxicity, cerebellum
Introduction
Consumption of alcohol during any stage of pregnancy can result in damage to the developing fetus. Although heavy prenatal alcohol exposure may manifest as a set of abnormalities defined as fetal alcohol syndrome (FAS), the severity and range of outcomes varies, producing what is now referred to as fetal alcohol spectrum disorders (FASD). Central nervous system (CNS) damage is the most devastating outcome in FASD, illustrated by a variety of structural and behavioral abnormalities (Abel and Sokol, 1987; Miller, 1993; Miller, 1996; Riley and McGee, 2005). Developmental ethanol exposure in animal models produces CNS dysfunction similar to the alcohol-related neurodevelopmental deficits observed in children born to drinking mothers (Clarren et al., 1978; Goodlett and Horn, 2001; Ponnappa and Rubin, 2000). Such ethanol-induced CNS damage in both humans and animals is expressed as long-lasting behavioral problems which include overactivity, motor dysfunction, social difficulties and learning deficits (Driscoll et al., 1990; Riley and McGee, 2005; Sokol et al., 2003).
Both animal and clinical studies report that binge drinking during pregnancy is associated with an increased risk of FASD (Bonthius and West, 1990; Streissguth et al., 1994), likely due to the high blood alcohol concentrations. However, high blood alcohol levels associated with binge drinking may also be linked with increased episodes of ethanol withdrawal (Goodlett et al., 1990; Trevisan et al., 1998; West et al., 1990). We have hypothesized that N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity occurs during these withdrawal episodes, contributing to the neuropathology and behavioral alterations associated with prenatal alcohol exposure (Thomas and Riley, 1998).
Acutely, alcohol inhibits the NMDA receptor, one of several receptor subtypes that are activated by the neurotransmitter, glutamate. Alcohol's inhibition of the NMDA receptor likely contributes to its sedative and intoxicating effects (Crews et al., 1996). However, this action, in turn, may produce an adaptive neurocompensatory response, either as an increase in the number of NMDA receptors or an increase in the amount of glutamate released, contributing to an acute tolerance to alcohol's intoxicating effects (Lovinger, 1993). Therefore, when alcohol is eliminated from the body, during periods of alcohol withdrawal, there is overactivity of the NMDA receptors or a rebound excitability (Grant and Lovinger, 1995). The resulting overstimulation of the NMDA receptors results in an excess of calcium entering the cell, causing excitotoxic cell death (Tsai and Coyle, 1998).
Consistent with this hypothesis, we have demonstrated that blocking NMDA receptors with noncompetitive antagonists such as MK-801 (Thomas et al., 2002; Thomas et al., 1997) or eliprodil (Thomas et al., 2004), an antagonist that acts at the polyamine modulatory site of the NMDA receptor, can attenuate some of ethanol's adverse effects on behavioral development in the rat. We have also found that the beneficial effects are time-dependent (Thomas et al., 2001), verifying that MK- 801, for example, is only effective when administered during the withdrawal phase when excitotoxic cell death is occurring.
MK-801 is a potent antagonist that acts at the phencyclidine site within the NMDA receptor-gated channel. This action can produce psychotomimetic and amnestic side effects (Sanger, 1992; Svensson, 2000; Verma and Moghaddam, 1996; Wedzony et al., 2000), as well as neurotoxicity (Ikonomidou et al., 1999). Memantine, an NMDA receptor antagonist currently being used clinically for Alzheimer's Disease, may be a drug that is more selective in its action (Rogawski et al., 2000). It is hypothesized to reduce excitotoxicity without blocking normal glutamate neurotransmission and in this way, may not produce the range of adverse side effects observed with other NMDA receptor antagonists like MK-801. Moreover, memantine has been shown to protect against ethanol withdrawal symptoms both in adult (Bienkowski et al., 2001; Lukoyanov and Paula-Barbosa, 2001) and developing rats (Stepanyan et al., 2008).
The present study examines the effects of administering multiple doses of memantine during the withdrawal phase in the neonatal rat pup, a period of rapid brain development that correlates with the human third trimester. Rat pups were exposed to a heavy binge of ethanol on postnatal day 6, a period marked by a high sensitivity to both ethanol-induced teratogenicity (Goodlett et al., 1990) and NMDA receptor-mediated excitotoxicity (Ikonomidou et al., 2000; Ikonomidou et al., 1999; Ikonomidou et al., 1989; Watanabe et al., 1994). Given that memantine peaks in concentration soon after the injection (20-30 mins; (Parsons et al., 2007)) and has a relatively short half-life (3-5 hours), memantine was given at both 24 and 36 hours following ethanol exposure. Administration of MK-801 (which has a much longer half life) at either of these timepoints has mitigating effects after an ethanol binge (Thomas et al., 2001). In addition, withdrawal symptoms and increases in glutamate release have been found up to 36 hours post-ethanol (Rossetti and Carboni, 1995), and increases in NMDA receptors can persevere for at least 24 hours after ethanol withdrawal (Sanna et al., 1993; Thomas and Morrisett, 2000).
One brain region that is particularly sensitive to ethanol during this time is the cerebellum (Bonthius and West, 1990; Goodlett et al., 1990), and it remains one of the more extensively studied brain regions affected by developmental ethanol exposure (Norman et al., 2009; O'Hare et al., 2005; Sowell et al., 1996). The cerebellum contributes to both motor and cognitive abilities (Smeyne et al., 1995) and ethanol-related damage to the cerebellum likely contributes to the behavioral pattern observed among individuals with FASD. For example, deficits in posture, balance and gait are common (Meyer et al., 1990; Riley and McGee, 2005; Roebuck et al., 1998), as are deficits in classical eyeblink conditioning (Jacobson et al., 2008). Animal models of FASD have demonstrated similar cerebellar-associated functional deficits with developmental ethanol exposure inducing shorter stride lengths and ataxia (Hannigan and Riley, 1988) deficits in motor coordination and balance (Goodlett and Lundahl, 1996; Thomas et al., 1998), and impairments in classical eyeblink conditioning (Brown et al., 2009).
In the present study, subjects were tested on a cerebellar-associated motor coordination task – the parallel bars. To determine if memantine was also neuroprotective, cerebellar Purkinje cell number was estimated using unbiased stereological procedures.
Materials and Methods
Subjects
Subjects were Sprague-Dawley rat offspring from the breeding colony at the Center for Behavioral Teratology, San Diego State University. Briefly, a Sprague-Dawley male and female were housed together overnight. The presence of a sperm plug on the following morning indicated mating and was designated as gestational day (GD) 0. Pregnant dams were then singly housed in a temperature- and humidity-controlled room with food and water ad libitum. On the morning following birth, litters were pseudorandomly culled to 8 pups, with 4 males and 4 females when possible.
Treatment
On PD 6, pups were randomly assigned to one of six treatment groups (ethanol (EtOH) vs. maltose control (Cont) × (0, 20 or 30 mg/kg memantine)). To control for potential litter effects, not more than one sex pair per litter was assigned to any treatment group. On PD 6, EtOH subjects received 6 g/kg (13.6% v/v) ethanol in a binge-like manner via intragastric intubation. The maltose control (Cont) subjects were intubated with a milk diet made equicaloric with a maltose solution. Specifically, subjects received two intubations of 3 g/kg ethanol in a milk formula, separated by two hours. To control for reductions in nursing that occurs in ethanol-treated subjects, EtOH subjects received two additional intubations of a nutritionally balanced milk formula every two hours following the ethanol intubations. The Cont subjects were sham intubated during these two additional feedings. All subjects were returned to the dam between treatment intubations.
Twenty-four hours after the initial EtOH intubation, all subjects received either 0, 10 or 15 mg/kg memantine (Sigma, St Louis, MO) via intraperitoneal injection. They received a second memantine injection of 0, 10 or 15 mg/kg memantine 12 hours later, or 36 hours after the initial EtOH intubation, creating a total memantine dose of 0, 20 or 30 mg/kg. On PD 7, subjects' paws were injected with India ink so that individual subjects could be identified, yet the investigator remained blind to treatment condition.
Blood Ethanol Concentration
Twenty μl of blood was collected from the tail of each subject, both ethanol-exposed and controls, 1.5 hrs after the second EtOH feeding, to determine the peak blood ethanol concentration. Blood samples were centrifuged and supernatant collected. Samples were analyzed by the Analox Ethanol Analyzer (Model AM1, Lunenberg, MA) for blood ethanol content.
Body Weights
Body weights were recorded daily from PD 6-21. Subjects remained with the dam in between treatments and were weaned on PD 21, remaining group housed. From PD 21, body weights were recorded every 5 days, beginning on PD 25. Subjects were also separated by sex on PD28, remaining group housed.
Parallel Bar
On PD 30-32, subjects were tested on a parallel bar motor coordination task (Thomas et al., 1998; Thomas et al., 1996), with testing conducted by an experimenter blind to treatment group. The parallel bars were two steel rods (0.5-cm diameter each, 91 cm long) held between two platforms (15.3 × 17.8 cm). The rods were fastened on each platform onto a rack of 28 grooved slots (0.5 cm apart), allowing the distance between the rods to be altered. The platforms stood 63 cm above a floor of wood chip bedding. The subject was initially placed on each platform for 30 sec. Then, the subject was carefully placed on the rods halfway between the platforms, with both left paws on one bar and both right paws on the other. Four successive alternating steps with the hind legs on the rods constituted a successful traversal. If the subject placed two hind paws on one rod, fell, or swung under the rods, the trial was deemed unsuccessful. The initial distance between the rods was set at 3.5 cm. Subjects were tested for up to five consecutive trials at a given width with an intertrial interval of 5-10 sec. Once successful at a given width, the distance between the rods was increased by 0.5 cm. If unsuccessful after five consecutive trials, testing for the day was terminated. Subjects were tested a maximum of 15 trials a day for 3 consecutive days. Each day, the distance between the rods was set at the last successful distance for each individual animal. The maximum distance successfully traveled each day, as well as the ratio of successful to total traversals, served as performance measures.
Cerebellar Purkinje Cell Counts
After parallel bar testing, subjects were tested on additional behavioral tasks, which will be discussed in a separate manuscript. On PD 60, subjects were deeply anesthetized with 100 mg/kg sodium pentobarbital (Vortech Pharmaceuticals, Dearborn, MI), and perfused intracardially with 0.9% saline, followed by a fixative solution containing 1.0% (w/v) paraformaldehyde (Sigma, St Louis, MO) and 1.25% (v/v) glutaraldehyde (Fisher Scientific, Pittsburgh, PA) in 0.1 M phosphate buffer (Sigma, St Louis, MO; pH 7.4). Each brain was removed and separated from the spinal cord at the pyramidal decussation of the medulla oblongata. Cuts between the superior and inferior colliculi and through the cerebellar peduncles isolated the cerebellum from the rest of the brain. Whole brain, forebrain and cerebellar weights were recorded. Brain tissues were then stored in the fixative solution at 4°C, before further analysis.
Since there were no sex differences in behavioral performance, only the cerebella from male rats were processed for Purkinje cell counts. Each cerebellum was cut sagittally down the midline and then embedded in Technovit 7100 (Kulzer Histo-technik 7100; Hereus Kulzer, Wehrheim, Germany) according to the manufacturer's recommendations. Tissue blocks were cut into 30 μm-thick parasagittal sections using a Rotary Microtome (Leica RM2165, Nussloch, Germany). Every 20th section was collected, floated on a water bath at 45°C, mounted on Superfrost Plus glass slides (Thermo Scientific, Portsmouth, NH) and dried at room temperature. After drying, sections were stained with cresyl violet (Sigma, St Louis, MO) for 20 mins.
The Purkinje cell number was estimated utilizing the combined optical disector/fractionator method, the experimenter being blind to treatment group. The optical disector/fractionator method is one of the most straightforward and powerful stereological cell counting techniques and, coupled with systematic random sampling of the entire volume of the target structure, produces both an unbiased and efficient method for counting cells (Gundersen, 1986; Sterio, 1984). Cell counting was accomplished with a computerized stereological imaging system, the Stereo Investigator (MicroBrightfield, Inc, Colchester, VT). This utilized an Olympus BX50 microscope (Olympus Optical, Tokyo, Japan) with an Optiscan automated stage (Prior Scientific Inc, Rockland, MA). A 60× oil immersion lens with a numerical aperture of 1.25 was used.
An appropriate random sampling strategy generated by the software fulfilled the guideline of counting 100-200 Purkinje cells within each cerebellum (West et al., 1991). At each stop, step size being 1000 μm × 1000 μm, Purkinje cells were counted within an optical disector. Briefly, the software superimposed a two-dimensional counting frame (x,y) onto the tissue image. The third dimension (z) was provided by moving the plane of focus throughout the depth of the section, the electronic microcator indicating depth (in μm) in the z-axis. The optical disector used in this study had the following measurements: x=146.4 μm, y=105.3 μm, z=15 μm. Purkinje cells in this three dimensional space were counted based on the presence of a discrete nucleus as the unique counting particle per cell (for further methodological details, see (Goodlett and Eilers, 1997; Goodlett and Johnson, 1997)).
Data Analyses
Data were analyzed using SPSS software (Version 15, SPSS Science, Chicago, IL). Dependent measures included body weights, blood ethanol concentration, parallel bar performance, and Purkinje cell number. All data were analyzed with ANOVAs with a 2 (ethanol, maltose) × 3 (0, 20, 30 mg/kg memantine) × 2 (Male, Female) between-subjects design, with the exception of blood ethanol concentration and Purkinje cell counts. The blood ethanol concentration was analyzed with a 3 (memantine) × 2 (sex) design; the Purkinje cell counts were analyzed with a 2 (ethanol, maltose) × 3 (0, 20, 30 mg/kg memantine) design. Day served as a repeated within-subject variable for body weight and parallel bar performance. Post hoc comparisons were conducted with Neuman-Keuls analyses (p's <0.05).
Results
Body Weights
Because variability increases with growing body weight, particularly after PD25 where sex differences are more likely to observed, data collected during PD 6-12 and PD25-55 were analyzed separately. Specifically, weight changes during PD 6-12 are shown in Table 1.
Table 1.
Effects of neonatal treatment on body weight (mean ± SEM) from postnatal day (PD) 6-12. Ethanol-exposed subjects lagged in growth compared to controls beginning on PD 7, but began to catch up by weaning. Importantly, there were no significant effects of memantine on body growth.
EtOH+0 = ethanol-exposed, 0 mg/kg memantine; EtOH+20 = ethanol-exposed, 20mg/kg memantine; EtOH+30 = ethanol-exposed, 30 mg/kg memantine; Cont+0 = maltose control, 0 mg/kg memantine; Cont+20 = maltose control, 20 mg/kg memantine; Cont+30 = maltose control, 30 mg/kg memantine
| Treatment | N | Postnatal Day | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Male | ||||||||
| EtOH + 0 | 10 | 17.1+0.6 | 17.3+0.6 | 19.1+0.6 | 20.9+0.6 | 23.5+0.8 | 26.0+0.8 | 28.7+0.9 |
| EtOH + 20 | 10 | 16.2+0.7 | 16.5+0.7 | 18.2+0.6 | 20.2+0.7 | 22.7+0.7 | 25.1+0.7 | 27.5+0.7 |
| EtOH + 30 | 10 | 16.8+0.6 | 17.0+0.7 | 18.5+0.7 | 20.3+0.7 | 23.0+0.9 | 25.4+0.8 | 28.0+1.0 |
| Cont + 0 | 9 | 16.6+0.7 | 19.9+0.8 | 22.1+0.6 | 24.3+0.7 | 26.5+0.8 | 28.4+0.8 | 30.8+0.9 |
| Cont + 20 | 10 | 16.3+0.8 | 19.7+0.9 | 21.6+1.0 | 23.6+1.0 | 25.8+1.1 | 28.5+1.0 | 31.2+1.2 |
| Cont + 30 | 10 | 15.7+0.7 | 19.0+0.7 | 20.4+0.7 | 22.5+0.8 | 24.9+0.9 | 27.3+0.9 | 30.1+1.0 |
| Female | ||||||||
| EtOH + 0 | 10 | 15.5+0.6 | 15.8+0.5 | 19.1+0.6 | 20.9+0.6 | 23.5+0.8 | 26.0+0.8 | 28.7+0.9 |
| EtOH + 20 | 10 | 16.4+0.4 | 16.3+0.5 | 18.2+0.6 | 20.0+0.7 | 22.6+0.9 | 25.0+0.9 | 27.6+1.0 |
| EtOH + 30 | 9 | 15.6+0.7 | 15.5+0.6 | 17.0+0.7 | 18.4+0.7 | 20.8+0.8 | 23.2+0.8 | 25.7+0.9 |
| Cont + 0 | 8 | 16.2+0.6 | 19.4+0.7 | 21.3+0.9 | 23.2+0.9 | 25.1+1.1 | 27.1+1.1 | 29.6+1.2 |
| Cont + 20 | 11 | 16.0+0.5 | 19.4+0.6 | 21.3+0.6 | 23.5+0.6 | 26.0+0.8 | 28.4+0.8 | 31.2+0.7 |
| Cont + 30 | 9 | 15.9+0.4 | 19.4+0.5 | 21.0+0.6 | 23.3+0.7 | 25.7+0.7 | 28.1+0.7 | 30.9+0.8 |
During PD 6-12, there was a significant effect of day, due to growth in all treatment groups [F(6,624)=3478.0, p<0.001], and a significant day by ethanol interaction [F(6,624)=68.9, p<0.001]. A main effect of ethanol was also observed [F(1,104)=44.5, p<0.001]. Although there were no significant differences in body weight among groups on PD 6, beginning on PD 7, the ethanol-treated subjects lagged in growth when compared to controls.
During PD 25-55, besides a significant effect of day [F(6,624)=10.0, p<0.001] and a main effect of ethanol [F(1,104)=16.1, p<0.001], a significant sex effect was observed [F(1,104)=509.5, p<0.001], due to the heavier body weights of the males. A significant day by sex by ethanol interaction was likewise observed [F(6,624)=3.1, p<0.01]. Subsequent follow-up tests for each sex revealed that during PD25-55, ethanol-exposed females, but not males, weighed significantly less than controls during the entire period, although there was some catch-up in weight. Importantly, memantine treatment had no significant effect on body growth at any time.
Blood Ethanol Concentration
The mean blood ethanol concentrations were 396.9 ± 6.5, 399.2 ± 8.1 and 412.0 ± 6.8 mg/dl for ethanol-exposed rats receiving 0, 20 and 30 mg/kg memantine injections, respectively. There were no significant differences among the ethanol groups.
Parallel Bar
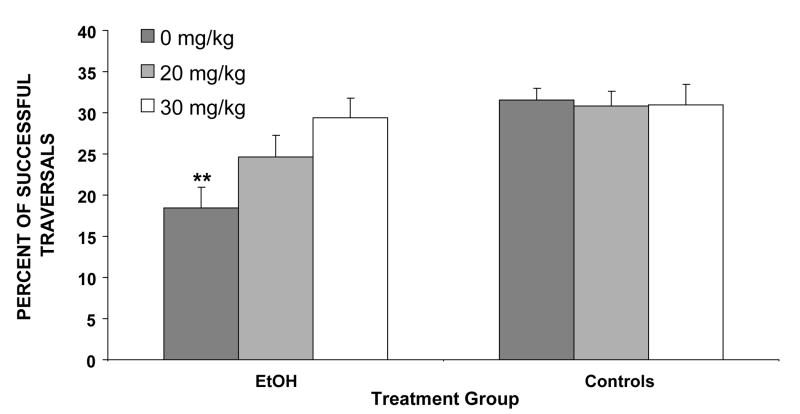
Exposure to ethanol on PD 6 produced significant deficits in motor performance. The severity of these ethanol-related motor coordination deficits was reduced by memantine treatment. The percentage of trials that were successfully traversed is shown in Figure 1. The EtOH + 0 rats were significantly less successful than the other treatment groups, requiring more trials to successfully traverse the parallel bars. This produced a main effect of ethanol [F(1,85)=14.4, p< 0.001] and a significant interaction of ethanol by memantine [F(2,85)=3.6, p< 0.05]. Follow-up comparisons demonstrated that the EtOH + 0 memantine subjects performed significantly worse than all other groups except the EtOH + 20 group. Most notably, performance of the EtOH + 30 subjects was significantly better than that of EtOH +0 subjects and did not differ significantly from that of the control subjects. Performance of the EtOH + 20 subjects was intermediate, not differing significantly from any other group. Finally, memantine had no significant effects on motor performance among controls.
Figure 1.
Subjects exposed to ethanol (EtOH) were less successful at traversing the parallel bars compared to the control groups, as shown by the percent of successful traversals (means + SEM). Performance of the EtOH + 30 memantine subjects was significantly better than that of the EtOH + 0 group and did not differ from that of controls.
** = significantly different from all other groups except EtOH + 20 memantine EtOH+0 = ethanol-exposed, 0 mg/kg memantine; EtOH+20 = ethanol-exposed, 20mg/kg memantine; EtOH+30 = ethanol-exposed, 30 mg/kg memantine; Cont+0 = maltose control, 0 mg/kg memantine; Cont+20 = maltose control, 20 mg/kg memantine; Cont+30 = maltose control, 30 mg/kg memantine
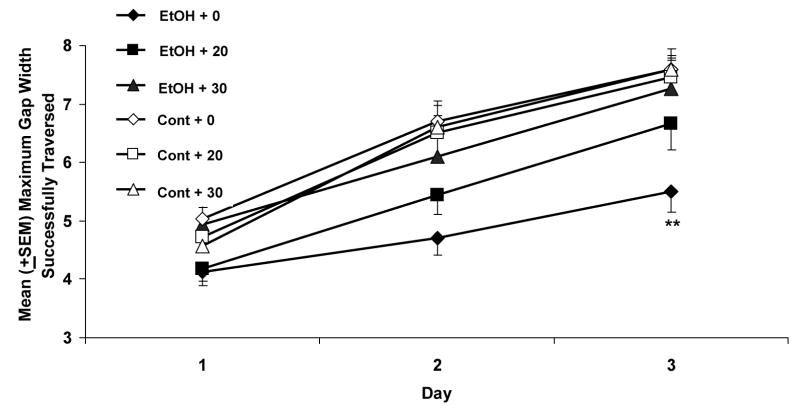
The maximum gap successfully traversed on the parallel bars is shown in Figure 2. The performance of all subjects showed gradual improvement over the three testing days [main effect of day [F(2,172)=244.7, p< 0.001]. Similar to the success percent, there was also a significant effect of ethanol [F(1,86)=12.9, p< 0.001], and an ethanol by memantine interaction [F(2,86)=3.5, p< 0.05]. Follow-up analyses indicated that the EtOH + 0 rats performed significantly worse than the EtOH + 30 memantine group, as well as control groups. In fact, the performance of the EtOH + 30 memantine group did not differ significantly from that of the control groups. Once again, the performance of the EtOH + 20 subjects was intermediate, not differing significantly from any other ethanol group. There were no significant effects of memantine on control subjects on any of the motor coordination measures.
Figure 2.
The maximum gap successfully traversed on the parallel bars. All groups showed improvements in motor performance over the three testing days; however the maximum gap attained by the subjects exposed to ethanol and treated with vehicle was significantly less wide when compared to the control groups. Similar to the percent of successful traversals, the EtOH + 30 memantine subjects performed significantly better than the EtOH+0 group, attaining a wider maximum gap, that did not differ significantly from that of controls
** = EtOH + 0 memantine significantly different from all other groups except EtOH + 20 memantine
Purkinje Cell Counts
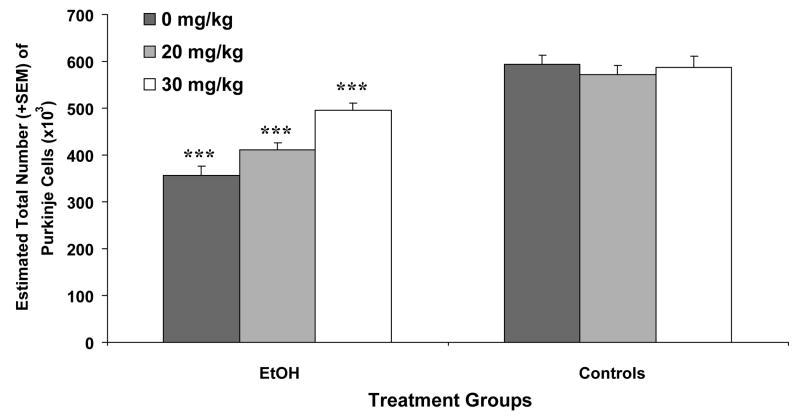
The estimated number of cerebellar Purkinje cells is shown in Figure 3. As seen, the ethanol-exposed subjects had fewer Purkinje cells compared to the control subjects, producing a significant effect of ethanol [F(1,54) = 98.9, p<0.001]. A significant interaction of ethanol by memantine [F(2,54) = 6.9, p< 0.01] was also observed, as well as a main effect of memantine [F(1,54) = 5.9, p< 0.01]. Follow-up comparisons demonstrated that the memantine treatment dose-dependently attenuated ethanol-related Purkinje cell loss. Firstly, ethanol-exposed subjects treated with 20 mg/kg memantine had significantly more Purkinje cells than ethanol-exposed subjects treated with vehicle, but significantly fewer than the ethanol-exposed subjects treated with 30 mg/kg memantine [main effect of memantine among EtOH Groups: F(2,31) = 14.3, p< 0.001]. Secondly, although the EtOH + 30 group had significantly more cerebellar Purkinje cells compared to the EtOH + 0 and EtOH + 20 groups, they still had significantly fewer Purkinje cells compared to the control groups.
Figure 3.
Mean (+ SEM) Purkinje cell number for each group. Ethanol exposure produced significant cell loss in all groups compared to controls. Memantine treatment significantly attenuated ethanol-induced Purkinje cell loss in a dose-dependent manner.
*** = significantly different from all other groups
Discussion
The present study demonstrates that administration of the NMDA receptor antagonist, memantine, can mitigate ethanol-induced motor deficits and associated cerebellar neuronal loss. An acute, high dose of ethanol during the brain growth spurt significantly altered behavioral development, leading to impaired motor coordination on the parallel bar task. Similarly, a single high dose exposure of ethanol during the neonatal period significantly reduced the total number of Purkinje cells within the cerebellum. Administration of memantine during the withdrawal period, 24 and 36 hours after a binge-like exposure to ethanol, ameliorated these ethanol-induced deficits in a dose-dependent manner. Importantly, memantine treatment by itself did not induce significant alterations on the measures observed in this study, illustrating that the low affinity and rapid off-rate kinetics of memantine make it a more clinically relevant treatment. (Rogawski et al., 2000).
One day of ethanol exposure, on PD 6, produced significant motor incoordination on the parallel bar task and a significantly reduced number of cerebellar Purkinje cells. First, ethanol-exposed subjects not treated with memantine had significantly fewer successful trials and were unable to traverse the wider gaps when compared with the maltose control groups. Certainly, performance of ethanol-exposed subjects did improve over the three days, but failed to reach the levels of the controls. This is consistent with earlier reports (Thomas et al., 1996), and further shows that a brief exposure to an ethanol binge, even during the mid-3rd trimester period, can induce long-term disruptions in development of behaviors that rely on the functional integrity of the cerebellum. Secondly, ethanol exposure on PD 6 significantly reduced Purkinje cell number estimates, thereby correlating well with the cerebellar-associated motor coordination task. This effect is consistent with numerous reports on ethanol-induced cerebellar cell loss from multiple days of exposure during the neonatal period (e.g. (Bonthius and West, 1990; West and Goodlett, 1990; West et al., 1990)). Purkinje cell loss is most severe following alcohol exposure during the early 3rd trimester equivalent (PD 4 and 5) (Maier et al., 1999; Thomas et al., 1998). Even a single-day binge exposure on PD 4 has been shown to significantly reduce Purkinje cell number (e.g. (Goodlett et al., 1990)). Similar long-term deficits in Purkinje cell number have also been observed following a single ethanol binge on PD 5 (Pauli et al., 1995). To date, this is the first report to show that a single-day ethanol binge on PD 6 itself results in a significant loss of Purkinje cells. Thus, these findings indicate that the Purkinje cells' temporal window of vulnerability includes PD 6 and further emphasizes that a single day binge exposure can produce permanent Purkinje cell loss.
Memantine treatment, when given during the withdrawal period, mitigated the ethanol-related motor deficits and Purkinje cell loss. On the motor task, although the 20 mg/kg memantine dose tended to improve performance, significant improvements were more readily observed with the 30 mg/kg dose. In fact, motor performance of the EtOH + 30 subjects was not significantly different from that of maltose control subjects. In comparison, a significant reduction in the amount of ethanol-induced cell loss was observed even with the lower dose of memantine (20 mg/kg) when compared to the EtOH + 0 subjects. It should be noted that there remained a significant reduction in the total number of Purkinje cells in the EtOH + 30 group, when compared to the maltose controls, indicating that complete neuroprotection was not afforded by memantine.
The above results are consistent with our hypothesis that NMDA receptor-mediated excitotoxic cell death occurs during the withdrawal phase that follows an ethanol binge. Acute cell death studies (e.g. (Light et al., 2002)) have reported a peak in ethanol-induced Purkinje cell apoptosis within 12 hrs of an ethanol binge on PD 4. This would presumably manifest as a long-term reduction in the total Purkinje cell number. Another wave of excitotoxic cell death, 24 - 36 hours after the ethanol binge, during the withdrawal period, would likewise result in further Purkinje cell loss. With administration of memantine during the withdrawal period to overcome this second wave of cell death, a further loss of Purkinje cells would no longer result. However, the initial acute cell death directly resulting from the ethanol binge would not be affected by the administration of memantine, thus accounting for the remaining significant deficit in Purkinje cell number in the EtOH + 30 group, when compared to the controls.
Most interestingly, the motor performance of ethanol-exposed subjects treated with 30 mg/kg memantine did not differ significantly from that of control subjects. This was despite a significant reduction in the number of Purkinje cells present in the EtOH + 30 subjects. From this, it would appear that complete amelioration of alcohol-induced Purkinje cell loss is not necessary for full ‘recovery’ of cerebellar-associated behavioral deficits. Instead, there may be a threshold in the number of Purkinje cells that are required for satisfactory parallel bar performance. It is also possible that the motor deficits are related to damage to other CNS motor areas or other components of cerebellar circuitry.
Though the cerebellar Purkinje cells are of particular interest given their position in cerebellar circuitry, ethanol can also lead to loss and dysfunction of other cerebellar neurons, as well as many glial populations including the Bergmann glia (Dikranian et al., 2005; Gonzalez-Burgos and Alejandre-Gomez, 2005). In fact, administration of ethanol during the early postnatal period can lead to reductions of cerebellar granule cells (Bonthius and West, 1990; Goodlett and Eilers, 1997; Hamre and West, 1993), deep cerebellar nuclei (Green et al., 2002) and the inferior olivary nucleus (Napper and West, 1995), as well as neurodegeneration of cerebellar-related neurons in the brainstem (Dikranian et al., 2005). Neurodegeneration can be observed in these cell populations even following single ethanol exposure periods (Dikranian et al., 2005;(Goodlett and Eilers, 1997; Marcussen et al., 1994), such as those used in the present study. Moreover, developmental alcohol exposure can influence the structure and function of surviving cells, reducing myelin (Zoeller et al., 1994), reducing synaptic connections (Klintsova et al., 2002), altering receptor and ion channel function (Gruol and Parsons, 1996; Incerti et al., 2010; Servais et al., 2007), and impairing plasticity (Servais et al., 2007). It is possible that any of these alcohol effects could contribute to motor deficits and/or be affected by memantine administration. Moreover, although we have found a strong correlation between parallel bar motor performance and cerebellar Purkinje cell number (Thomas et al., 1998), it is also possible that the parallel bar motor performance also relies on other motor systems, including the motor cortex and basal ganglia, and that neuroprotection occurs in these neuronal populations.
It is interesting that memantine induces such robust neuroprotection in the cerebellum and on motor performance. Based on the temporal vulnerability to developmental alcohol exposure and NMDA receptor-mediated excitotoxicity, previous studies investigating withdrawal-related excitotoxicity during development focused primarily on the hippocampus (e.g. Thomas et al., 2004; Stepanyan et al., 2008; Wilkins et al., 2006)). However, the developmental changes in the expression of the NMDA receptors in the cerebellum are well characterized. For example, from embryonic day 13, NMDA receptor subtypes begin to be primarily expressed in the Purkinje cell layer; after which they are expressed from PD 1 in the external granule cell layer, and from PD 7 in the internal granule cell layer (Akazawa et al., 1994; Sarna and Hawkes, 2003; Watanabe et al., 1994). The onset of NMDA receptor expression occurs just prior to the respective peaks in proliferation of each cell population (Altman, 1972; Altman and Bayer, 1985; Hatten and Heintz, 1995), with NMDA receptor expression levels increasing with age until PD 14. Expression levels then decrease to levels found in the adult (Naassila and Daoust, 2002; Watanabe et al., 1994). Specifically, NMDA receptor subtypes NR2B and NMDAR1 are consistently expressed at high levels within the Purkinje cell layer during embryonic and postnatal development. Their expression then decreases towards the end of the first postnatal week (an interesting finding since cerebellar Purkinje cells become less sensitive to alcohol-related cell death after the first postnatal week), but can still be seen in the Purkinje cell population in the adult cerebellum (Watanabe et al., 1992; Watanabe et al., 1994; Zhong et al., 1995). Importantly, NR2B subtypes are sensitive to ethanol in both developing and adult tissue (e.g. (Allgaier, 2002)) and are expressed in large numbers in the Purkinje cell population on PD 6 (Watanabe et al., 1992; Watanabe et al., 1994; Zhong et al., 1995). In the third postnatal week, there is a developmental change from expression of NR2B to NR2C subunits, NR2C being expressed predominately in the adult cerebellum (Zhong et al., 1995). However, mature Purkinje neurons were thought not to express functional NMDA receptors; in fact, they have been found at climbing fiber-Purkinje cell synapses from PD 21 (Llansola et al., 2005; Piochon et al., 2007). Incidentally, the NR2D subunit is also expressed in the Purkinje cells for the first 8 postnatal days (Akazawa et al., 1994; Haberny et al., 2002).
These data suggest that overactivation of NMDA receptors contribute to Purkinje cell loss; However, to our knowledge, the acute effects of alcohol on Purkinje cell NMDA receptors have not been examined in the neonate, although several studies have shown ethanol can inhibit NMDA receptors, thus leading to a subsequent upregulation of NMDA receptor subunits, within the cerebellar granule cells following ethanol exposure in adult or developing rats, typically using in vitro models (Bhave et al., 1999; Hoffman et al., 1995; Raeder et al., 2008; Snell et al., 1996). Even if there are no changes to NMDAR number, there may be increases in glutamate release during withdrawal, as demonstrated by developmental alcohol exposure in the hippocampus (Mameli et al., 2005).
In fact, similar to our findings, Lewis et al., (2007) (Lewis et al., 2007) showed that the administration of agmatine, an NMDA receptor antagonist, attenuated cerebellar-associated motor balance deficits (subjects were required to traverse a single dowel rod). While agmatine also displays several actions on the CNS, it preferentially targets the polyamine site of the NMDA receptor, thereby modulating the receptor's actions (Gibson et al., 2003); memantine blocks the NMDA receptor but leaves the channel relatively quickly in order to allow normal glutamate neurotransmission (Rogawski et al., 2000). Unlike our study, agmatine was administered on the last day of ethanol treatment, after several consecutive days of binge-like exposure to ethanol from PD 1 to 8, thereby assessing a clinically relevant paradigm for newborns undergoing withdrawal at birth. Nevertheless, collectively, these studies suggest that blockade of NMDA receptors during withdrawal can protect against alcohol-related motor deficits. The present study further shows that administration of an NMDA receptor antagonist protects against cerebellar cell death.
That being said, it is also possible that memantine is neuroprotective via alternative mechanisms. For example, memantine can reduce microglia-related inflammation and increase the release of neurotrophic factors from astroglia (Jantas and Lason, 2009; Wu et al., 2009). Memantine also acts on other neurotransmitter systems, including serotonergic systems, and administration of serotonergic agonists being found to be protective against alcohol-related teratogenicity (e.g. (Druse et al., 2004)). Other sites of action include blockade of the α7, α4/beta2, and α9/α10 nicotinic receptors, though at levels not considered to be therapeutically relevant (Parsons et al., 2007). Importantly, memantine's apparent predominant mechanism is blockade of NMDA receptors, and memantine can directly inhibit ethanol's upregulation of NMDA receptors in the hippocampus (Maler et al., 2005), protect against ethanol-withdrawal related cell death in the developing hippocampus, and reduce withdrawal-related seizures (Strepnayn, 2006).-
Certainly, it is not known whether MK-801 or eliprodil have similar mitigating effects on the developing cerebellum. Thus, it is not clear how memantine compares to these other NMDA receptor antagonists on motor areas of the brain. However, given the rapid off-rate kinetics and lower affinity properties of memantine (Rogawski et al., 2000), memantine should have a protective effect without any additional toxic effects, making it a more ideal clinical treatment for ethanol's neurotoxic effects.
In sum, the current study further illustrates that binge drinking is especially harmful to the developing fetus, as it is accompanied by periods of withdrawal. Together, these findings support our hypothesis that withdrawal-related NMDA receptor-mediated excitotoxicity contributes to some of the brain damage and behavioral alterations associated with FASD. Treatments such as memantine, which may be relatively safe, could serve as potential treatments for FASD, reducing the severity of fetal alcohol effects.
Acknowledgments
This research was supported by funds from the National Institute on Alcohol Abuse and Alcoholism (AA06902) to EPR and from the Kaplan Fellowship from the Department of Psychology, San Diego State University to NIM.
All procedures included in this study were approved by the SDSU IACUC and are in accordance with the NIH Guide for Care and Use of Laboratory Animals.
References
- Abel EL, Sokol RJ. Incidence of fetal alcohol syndrome and economic impact of FAS-related anomalies. Drug Alcohol Depend. 1987;19(1):51–70. doi: 10.1016/0376-8716(87)90087-1. [DOI] [PubMed] [Google Scholar]
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347(1):150–60. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41(6):377–82. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145(3):353–97. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985;231(1):42–65. doi: 10.1002/cne.902310105. [DOI] [PubMed] [Google Scholar]
- Bhave SV, Snell LD, Tabakoff B, Hoffman PL. Ethanol sensitivity of NMDA receptor function in developing cerebellar granule neurons. Eur J Pharmacol. 1999;369(2):247–59. doi: 10.1016/s0014-2999(99)00071-0. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Krzascik P, Koros E, Kostowski W, Scinska A, Danysz W. Effects of a novel uncompetitive NMDA receptor antagonist, MRZ 2/579 on ethanol self-administration and ethanol withdrawal seizures in the rat. Eur J Pharmacol. 2001;413(1):81–9. doi: 10.1016/s0014-2999(01)00743-9. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14(1):107–18. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Brown KL, Burman MA, Duong HB, Stanton ME. Neonatal binge alcohol exposure produces dose dependent deficits in interstimulus interval discrimination eyeblink conditioning in juvenile rats. Brain Res. 2009;1248:162–75. doi: 10.1016/j.brainres.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Jr, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92(1):64–7. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Brain Res Dev Brain Res. 2005;155(1):1–13. doi: 10.1016/j.devbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12(3):231–7. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin NF, Gillespie RA, Dickson E, Atieh M, Pietrzak CA, Le PT. The serotonin-1A agonist ipsapirone prevents ethanol-associated death of total rhombencephalic neurons and prevents the reduction of fetal serotonin neurons. Brain Res Dev Brain Res. 2004;150(2):79–88. doi: 10.1016/j.devbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Harris BR, Prendergast MA, Hart SR, Blanchard JA, 2nd, Holley RC, Pedigo NW, Littleton JM. Polyamines contribute to ethanol withdrawal-induced neurotoxicity in rat hippocampal slice cultures through interactions with the NMDA receptor. Alcohol Clin Exp Res. 2003;27(7):1099–106. doi: 10.1097/01.ALC.0000075824.10502.DD. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M. Cerebellar granule cell and Bergmann glial cell maturation in the rat is disrupted by pre- and post-natal exposure to moderate levels of ethanol. Int J Dev Neurosci. 2005;23(4):383–8. doi: 10.1016/j.ijdevneu.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21(4):738–44. [PubMed] [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25(3):175–84. [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19(6):435–46. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Lundahl KR. Temporal determinants of neonatal alcohol-induced cerebellar damage and motor performance deficits. Pharmacol Biochem Behav. 1996;55(4):531–40. doi: 10.1016/s0091-3057(96)00248-1. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7(2):107–14. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3(3):155–64. [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002;956(2):302–11. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Parsons KL. Chronic alcohol reduces calcium signaling elicited by glutamate receptor stimulation in developing cerebellar neurons. Brain Res. 1996;728(2):166–74. doi: 10.1016/0006-8993(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci. 2002;68(1):9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17(3):610–22. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Riley EP. Prenatal ethanol alters gait in rats. Alcohol. 1988;5(6):451–4. doi: 10.1016/0741-8329(88)90081-x. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Iorio KR, Snell LD, Tabakoff B. Attenuation of glutamate-induced neurotoxicity in chronically ethanol-exposed cerebellar granule cells by NMDA receptor antagonists and ganglioside GM1. Alcohol Clin Exp Res. 1995;19(3):721–6. doi: 10.1111/j.1530-0277.1995.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Mosinger JL, Salles KS, Labruyere J, Olney JW. Sensitivity of the developing rat brain to hypobaric/ischemic damage parallels sensitivity to N-methyl-aspartate neurotoxicity. J Neurosci. 1989;9(8):2809–18. doi: 10.1523/JNEUROSCI.09-08-02809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incerti M, Vink J, Roberson R, Wood L, Abebe D, Spong CY. Reversal of alcohol-induced learning deficits in the young adult in a model of fetal alcohol syndrome. Obstet Gynecol. 2010;115(2 Pt 1):350–6. doi: 10.1097/AOG.0b013e3181cb59da. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–72. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jantas D, Lason W. Anti-apoptotic effect of memantine against staurosporine- and low-potassium-induced cell death in cerebellar granule cells: a development-dependent effect. Pharmacol Rep. 2009;61(5):827–937. doi: 10.1016/s1734-1140(09)70138-0. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Scamra C, Hoffman M, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Res. 2002;937(1-2):83–93. doi: 10.1016/s0006-8993(02)02492-7. [DOI] [PubMed] [Google Scholar]
- Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav. 2007;88(1):114–21. doi: 10.1016/j.pbb.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KE, Brown DP, Newton BW, Belcher SM, Kane CJ. Ethanol-induced alterations of neurotrophin receptor expression on Purkinje cells in the neonatal rat cerebellum. Brain Res. 2002;924(1):71–81. doi: 10.1016/s0006-8993(01)03224-3. [DOI] [PubMed] [Google Scholar]
- Llansola M, Sanchez-Perez A, Cauli O, Felipo V. Modulation of NMDA receptors in the cerebellum. 1. Properties of the NMDA receptor that modulate its function. Cerebellum. 2005;4(3):154–61. doi: 10.1080/14734220510007996. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin Exp Res. 1993;17(1):19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Paula-Barbosa MM. Memantine, but not dizocilpine, ameliorates cognitive deficits in adult rats withdrawn from chronic ingestion of alcohol. Neurosci Lett. 2001;309(1):45–8. doi: 10.1016/s0304-3940(01)02037-7. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res. 1999;23(4):726–34. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Maler JM, Esselmann H, Wiltfang J, Kunz N, Lewczuk P, Reulbach U, Bleich S, Ruther E, Kornhuber J. Memantine inhibits ethanol-induced NMDA receptor up-regulation in rat hippocampal neurons. Brain Res. 2005;1052(2):156–62. doi: 10.1016/j.brainres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25(35):8027–36. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcussen BL, Goodlett CR, Mahoney JC, West JR. Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis. Alcohol. 1994;11(2):147–56. doi: 10.1016/0741-8329(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Meyer LS, Kotch LE, Riley EP. Neonatal ethanol exposure: functional alterations associated with cerebellar growth retardation. Neurotoxicol Teratol. 1990;12(1):15–22. doi: 10.1016/0892-0362(90)90107-n. [DOI] [PubMed] [Google Scholar]
- Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res. 1993;17(2):304–14. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effect of early exposure to ethanol on the protein and DNA contents of specific brain regions in the rat. Brain Res. 1996;734(1-2):286–94. [PubMed] [Google Scholar]
- Naassila M, Daoust M. Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J Neurochem. 2002;80(5):850–60. doi: 10.1046/j.0022-3042.2002.00755.x. [DOI] [PubMed] [Google Scholar]
- Napper RM, West JR. Permanent neuronal cell loss in the inferior olive of adult rats exposed to alcohol during the brain growth spurt: a stereological investigation. Alcohol Clin Exp Res. 1995;19(5):1321–6. doi: 10.1111/j.1530-0277.1995.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15(3):209–17. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16(12):1285–90. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Pauli J, Wilce P, Bedi KS. Acute exposure to alcohol during early postnatal life causes a deficit in the total number of cerebellar Purkinje cells in the rat. J Comp Neurol. 1995;360(3):506–12. doi: 10.1002/cne.903600311. [DOI] [PubMed] [Google Scholar]
- Piochon C, Irinopoulou T, Brusciano D, Bailly Y, Mariani J, Levenes C. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J Neurosci. 2007;27(40):10797–809. doi: 10.1523/JNEUROSCI.2422-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnappa BC, Rubin E. Modeling alcohol's effects on organs in animal models. Alcohol Res Health. 2000;24(2):93–104. [PMC free article] [PubMed] [Google Scholar]
- Raeder H, Holter SM, Hartmann AM, Spanagel R, Moller HJ, Rujescu D. Expression of N-methyl-d-aspartate (NMDA) receptor subunits and splice variants in an animal model of long-term voluntary alcohol self-administration. Drug Alcohol Depend. 2008;96(1-2):16–21. doi: 10.1016/j.drugalcdep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230(6):357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):339–44. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Wasterlain CG, Mazarati AM. Re: Mazarati et al. …clinically available [antiepileptic drug] with a moderate affinity for the glycine site of the N-methyl-D-aspartate (NMDA) receptor. Epilepsia. 2000;41(7):918–9. doi: 10.1111/j.1528-1157.2000.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283(1-3):177–83. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Sanger DJ. NMDA antagonists disrupt timing behaviour in rats. Behav Pharmacol. 1992;3(6):593–600. [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, Concas A, Biggio G. Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcohol Clin Exp Res. 1993;17(1):115–23. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Sarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Prog Neurobiol. 2003;70(6):473–507. doi: 10.1016/s0301-0082(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci U S A. 2007;104(23):9858–63. doi: 10.1073/pnas.0607037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Chu T, Lewin A, Bian F, Sanlioglu S, Kunsch C, Lira SA, Oberdick J. Local control of granule cell generation by cerebellar Purkinje cells. Mol Cell Neurosci. 1995;6(3):230–51. doi: 10.1006/mcne.1995.1019. [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40(1):71–8. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. Jama. 2003;290(22):2996–9. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcohol Clin Exp Res. 1996;20(1):31–4. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Stepanyan TD, Farook JM, Kowalski A, Kaplan E, Barron S, Littleton JM. Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcohol Clin Exp Res. 2008;32(12):2128–35. doi: 10.1111/j.1530-0277.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134(Pt 2):127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcohol Clin Exp Res. 1994;18(2):248–54. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Svensson TH. Dysfunctional brain dopamine systems induced by psychotomimetic NMDA-receptor antagonists and the effects of antipsychotic drugs. Brain Res Brain Res Rev. 2000;31(2-3):320–9. doi: 10.1016/s0165-0173(99)00048-x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Fleming Sl, Riley EP. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol Clin Exp Res. 2001;25(5):764–73. [PubMed] [Google Scholar]
- Thomas JD, Fleming SL, Riley EP. Administration of low doses of MK-801 during ethanol withdrawal in the developing rat pup attenuates alcohol's teratogenic effects. Alcohol Clin Exp Res. 2002;26(8):1307–13. doi: 10.1097/01.ALC.0000025888.60664.D9. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004;175(2):189–95. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998;105(2):159–66. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Riley EP. Fetal alcohol syndrome: does alcohol withdrawal play a role? Alcohol Health Res World. 1998;22(1):47–53. [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29(5):433–52. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Weinert SP, Sharif S, Riley EP. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcohol Clin Exp Res. 1997;21(7):1218–25. [PubMed] [Google Scholar]
- Thomas MP, Morrisett RA. Dynamics of NMDAR-mediated neurotoxicity during chronic ethanol exposure and withdrawal. Neuropharmacology. 2000;39(2):218–26. doi: 10.1016/s0028-3908(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61–6. [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–84. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16(1):373–9. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3(12):1138–40. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal expressions of five NMDA receptor channel subunit mRNAs in the cerebellum. J Comp Neurol. 1994;343(4):513–9. doi: 10.1002/cne.903430402. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Mackowiak M, Zajaczkowski W, Fijal K, Chocyk A, Czyrak A. WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology. 2000;23(5):547–59. doi: 10.1016/S0893-133X(00)00150-0. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR. Teratogenic effects of alcohol on brain development. Ann Med. 1990;22(5):319–25. doi: 10.3109/07853899009147914. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin Exp Res. 1990;14(6):813–8. doi: 10.1111/j.1530-0277.1990.tb01820.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Jr, Prendergast MA, Blanchard J, Holley RC, Chambers ER, Littleton JM. Potential value of changes in cell markers in organotypic hippocampal cultures associated with chronic EtOH exposure and withdrawal: comparison with NMDA-induced changes. Alcohol Clin Exp Res. 2006;30(10):1768–80. doi: 10.1111/j.1530-0277.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Wu HM, Tzeng NS, Qian L, Wei SJ, Hu X, Chen SH, Rawls SM, Flood P, Hong JS, Lu RB. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 2009;34(10):2344–57. doi: 10.1038/npp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J Neurochem. 1995;64(2):531–9. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Butnariu OV, Fletcher DL, Riley EP. Limited postnatal ethanol exposure permanently alters the expression of mRNAS encoding myelin basic protein and myelin-associated glycoprotein in cerebellum. Alcohol Clin Exp Res. 1994;18(4):909–16. doi: 10.1111/j.1530-0277.1994.tb00059.x. [DOI] [PubMed] [Google Scholar]