Abstract
Background
C-reactive protein (CRP), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin are systemic inflammatory markers (IM) that positively correlate with cardiovascular (CV) risk. Despite the known CV effects of atypical antipsychotics, there is limited prospective data on IM changes during treatment.
Methods
IM outcomes were compared between antipsychotic treatment groups in the CATIE Schizophrenia Trial phase 1 using subjects with laboratory assessments at baseline and 3 months (n=789).
Results
There were significant treatment differences in CRP, E-selectin and ICAM-1 at 3 months, with a differential impact of baseline values on the CRP and ICAM-1 results. In overall comparisons, quetiapine and olanzapine had the highest median levels for CRP, and olanzapine for E-selectin and ICAM-1. Olanzapine was significantly different after baseline adjustment than perphenazine (p=0.001) for E-selectin, and, in those with low baseline CRP (< 1 mg/L), olanzapine was significantly different than perphenazine (p<0.001), risperidone (p<0.001) and ziprasidone (p=0.002) for CRP. Perphenazine had the lowest 3-month ICAM-1 levels in subjects with baseline ICAM-1 above the median, but the differences were not statistically significant vs. olanzapine (p=0.010), quetiapine (p=0.010) and risperidone (p=0.006) after controlling for multiple comparisons. The 18-month repeated measures CRP analysis confirmed the significantly higher values for olanzapine in those with low baseline CRP.
Conclusions
This analysis provides further evidence for differential antipsychotic metabolic liabilities as measured by changes in systemic inflammation. CRP may emerge as a useful target for CV risk outcomes in schizophrenia patients.
Keywords: antipsychotic, schizophrenia, cardiovascular risk, inflammation, C-reactive protein, ICAM-1, VCAM-1, E-selectin
Introduction
Schizophrenia is associated with twofold greater cardiovascular (CV) mortality (1), with life expectancies 25–30 years less than expected (2). Modifiable risk factors and undertreatment of CV risks (e.g. dyslipidemia) contribute to this public health problem (3). Increasingly, consensus panels insist that treatment of schizophrenia patients addresses medical illness to minimize common causes of excess mortality, especially CV disease (4).
Cardiovascular risk prediction has been greatly enhanced by the development of algorithms derived from prospective trials, such as the Framingham Heart Study (FHS) (5). Therapy for modifiable risk factors is crucial to risk reduction and focuses on decreasing low-density lipoprotein (LDL) cholesterol; however, there are aspects of CV risk not captured by cholesterol markers. Particularly problematic is the fact that 20% of FHS subjects experienced major CV events without evident major risk factors (6), that 46% of CV events in the Women’s Health Study occurred in those with serum LDL <130 mg/dL (7), and the lack of correlation between baseline LDL and myocardial infarction (MI) risk during statin treatment (8).
The search for means to refine CV risk assessment has centered on inflammatory markers (IM) such as intracellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), E-selectin, and C-reactive protein (CRP). Underlying this focus is evidence that atherosclerotic disease is a chronic inflammatory state involving components of vascular tissue (endothelial cells, smooth muscle cells) (9). Physiological stresses and inflammatory cell recruitment are the primary causes of vascular injury, and precede changes in most markers of vascular inflammation. The first step in monocyte recruitment is the presentation of E-selectin on endothelial cell surfaces in response to cytokines, oxidative stress from mechanical injury (e.g. turbulence, shear), or from phospholipids such as LDL and its oxidative byproducts (10). E-selectin binds to selective receptors on lymphocyte surfaces, slowing their movement along the endothelial surface (11). Subsequent adhesion of immune cells to vascular endothelium is mediated by increased expression of cellular adhesion molecules such as ICAM-1 and VCAM-1, the upregulation of which also occurs as a response to mechanical or oxidative stress (9,12). The expression of ICAM-1 appears more sensitive to LDL and oxidative LDL byproducts than mechanical injury, while VCAM-1 expression is increased by disturbed flow, other cytokines, and cholesterol (13,14). Since these three markers have limited expression in normal endothelium, increases in soluble forms measured in plasma serve as atherosclerosis markers (15).
Elevated IM levels are seen in various states associated with increased CV risk, including metabolic disturbances in glucose-insulin homeostasis (15–17). Serum ICAM-1 and VCAM-1 levels are elevated in patients with coronary heart disease (18), with higher ICAM-1 levels predictive of greater CV risk (19), and increased levels of both ICAM-1 and VCAM-1 associated with greater carotid atherosclerotic disease burden (20). Importantly, serum levels of soluble E-selectin and ICAM-1 positively correlate with central adiposity, and successful lifestyle modification that decreases body mass also lowers levels of both markers (21), and of C-reactive protein (CRP) (22).
CRP is a circulating acute phase reactant produced in hepatic cells in response to interleukin-6 and leptin, and also in arterial smooth muscle cells, with the highest expression in areas of atherosclerotic plaque (23). While CRP is an inflammatory marker, it accentuates atherosclerotic injury through multiple mechanisms (24) including binding to, and subsequent interference with leptin’s activity at target receptors (25). The CV risk associated with CRP is continuous, but risk stratification typically divides patients into tertiles: <1 mg/L (lowest risk), 1–3 mg/L, and >3 mg/L. The highest tertile is associated with twofold greater risk (RR 1.9; 95% CI 1.5–2.3) for nonfatal MI and CV death compared to the lowest tertile after controlling for traditional factors. Markedly elevated values (>>10 mg/L) are typically ignored and repeated later, as they may reflect acute conditions (e.g. infection) and not baseline systemic inflammation levels (26). Persistent values >15 mg/L are rarely encountered.
CRP quantification of using high sensitivity assays (hS-CRP) has shown superior predictive power by itself than other IM (27), and the use of CRP provides added CV risk information after controlling for traditional CV risk factors (7). In global risk models, hS-CRP is second to systolic blood pressure and ahead of smoking as a CV risk predictor (28,29). The hS-CRP results from large statin trials also question whether the CV benefits from lipid reduction are driven by LDL changes or changes in CRP. Patients in a large randomized pravastatin vs. atorvastatin trial with lower post-treatment CRP experienced fewer CV events than those with higher CRP, regardless of LDL levels (30); moreover, agents which reduce LDL but increase CRP (e.g. hormone replacement therapy) are associated with increased CV risk. Convincing evidence for CRP as a mediator of CV risk has also been recently demonstrated by results of the JUPITER trial. This randomized, placebo-controlled study found that statin-induced CRP reduction was associated with significantly reduced CV event risk among individuals with low baseline LDL (<130 mg/dL) but elevated CRP (≥2 mg/L) (31).
Despite the cardiometabolic risk associated with atypical antipsychotics, there is limited published information on IM changes in response to antipsychotics with varying metabolic liabilities. Preliminary data from a 5-month randomized risperidone vs. olanzapine trial (n=42) found few patients with CRP >1 mg/L at any time point, and no between-drug differences in CRP at any time point by independent t-test, or repeated measures analysis of drug by time effects (32). However, a cross-sectional analysis of IM in 88 chronic schizophrenia patients found higher mean CRP levels: clozapine 5.2±5.0 mg/L (n=29), olanzapine 4.9±2.9 mg/L (n=29), typical antipsychotics 6.9±4.2 mg/L (n=30) (33). While varying assay methods and subject demographics create difficulties in comparing CRP levels between the two trials, CRP levels in the latter study correlated positively with central adiposity and negatively with serum high-density lipoprotein (HDL) cholesterol levels, thus demonstrating a consistent association between dysmetabolic states and increased systemic inflammation.
The large sample and randomized nature of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial phase 1 offers an opportunity to examine antipsychotic effects on IM. The a priori hypothesis for this analysis is that there will be significant between-drug differences in 3-month IM changes. Secondary analyses will examine the association between IM changes and changes in individual metabolic parameters.
Methods and Materials
Recruitment and enrollment criteria for the CATIE Schizophrenia Trial have been previously described (34). Blood for IM was collected at screening and months 3, 6, 12, 18 and end of phase 1 (up to 18 months). Plasma was analyzed for soluble levels of CRP, ICAM-1, VCAM-1 and E-selectin on the Bioplex Xmap system (Bio Rad) using Luminex bead-based assays. CRP levels were determined with an Invitrogen/Biosource (Carlsbad, CA) kit as a single-plex assay with initial plasma dilution of 1:500 per the manufacturer's instructions. Subject samples were compared to a serial 1:3 dilution standard curve of CRP (10.97–8,000 pg/mL). ICAM-1, VCAM-1 and E-selectin levels were determined with a Linco/Millipore (Chicago, IL) kit as a three-plex assay with initial plasma dilution of 1:100 per the manufacturer’s instructions. Subject samples were compared to a serial 1:5 dilution standard curve of ICAM-1, VCAM-1 and E-selectin (80–250,000 pg/mL). For both assays, four-point logarithmic curve fits were performed on all standard curves of expected concentration versus median fluorescence intensity per bead set. The minimum detectable level of CRP was 2 pg/mL, of ICAM-1 9 pg/mL, of VCAM-1 16 pg/mL, and of E-selectin 79 pg/mL. Seventy-four distinct assays were performed over a 4-month period with the following median interassay coefficients of variation (6 test measurements per run): CRP-4.1%, ICAM-3.8%, VCAM-5.5% and E-selectin-3.7%. Reported final results were corrected for the initial sample dilution in either assay. Samples with results outside this range were assayed after further dilution, and results were extrapolated.
The primary analysis was conducted in subjects with non-extrapolated IM values at baseline and 3 months. The 3-month value was chosen as the time point for the analyses to maximize subject retention, while providing a physiologically meaningful period to assess antipsychotic impact. E-selectin, ICAM-1, VCAM-1 are sensitive to prandial effects, so only subjects with fasting laboratory measures (last meal >8 hours) at baseline and 3 months could be used (n=268). CRP has a half-life of 19 hours, with limited diurnal and prandial effects (36), so all non-extrapolated specimens were used in the primary analyses, regardless of fasting status (n=789) except for 2 samples with CRP ≥15 mg/L, due to their probable association with acute infectious or inflammatory processes. Only CRP and E-selectin had extrapolated data. A supportive analysis included the extrapolated data, but with values truncated to the lowest or highest nonextrapolated value. The exclusion of extrapolated values from the primary analysis removed 28 subjects (3.4% of 817) for CRP and 72 subjects (27% of 268) for E-selectin. As a result of the skewed distribution for CRP, E-selectin, and ICAM-1, the data for these IM underwent log transformation prior to analysis.
Analysis of variance (ANOVA) and Spearman correlations were used to examine the relationship between IM levels and metabolic syndrome (MS) criteria. Initial treatment group comparisons of IM at baseline and 3 months were made using (ANOVA) (4 df). Adjusted comparisons of IM at 3 months were performed using a 4 df Analysis of Covariance (ANCOVA), which adjusted for baseline IM values and allowed for consideration of age, gender, other baseline antipsychotic medications, and two CATIE design measures (study entrance after the ziprasidone treatment option was added, and having tardive dyskinesia at baseline) (35). For all treatment group comparisons, if the analysis yielded an overall treatment group difference (p<0.05), the 10 possible pairwise comparisons between treatments were performed with a Bonferroni correction, yielding an α of .05/10 = 0.005 as the threshold for significance. Due to the conservative nature of the Bonferroni correction, p-values between 0.005 and 0.01 are noted for the reader’s discretion.
Potential interactions between baseline covariates and treatment group were explored and, if identified, treatment groups were compared within levels of the covariate using additional ANCOVA models. If an interaction was identified between baseline level of CRP and treatment group, treatment comparisons were made with additional models for those with low (<1 mg/L) and moderate/high (≥1 mg/L) baseline CRP values. This cutoff point was selected a priori because of its clinical significance to CV risk. Unlike CRP, for which well-established risk cutpoints exist based on population norms, these do not exist for the other markers. Therefore, when necessary, other cutpoints were based on the sample median.
Because IM data at 3 months was not available for all randomized patients, a sensitivity analysis of the primary treatment group comparison was performed, and ANCOVA models further adjusted for baseline covariates that were found to be predictive of patient dropout. These significant covariates (p<0.10) were identified with logistic regression models that predicted which patients had baseline and 3-month laboratory data, both overall and limited to fasting samples.
The ability to use all gathered data for CRP permitted a secondary mixed model analysis to be performed using non-extrapolated values collected at any post-baseline visit, combined into time intervals corresponding to 3, 6, 12 and 18 months as in prior CATIE metabolic analyses (37). Due to the paucity of fasting samples, mixed model analyses could not be performed for the other markers. CRP change from baseline was compared across treatment groups with a mixed model including terms for baseline CRP, time (treated as a classification variable), and terms representing baseline-by-time, treatment-by-time, and baseline-bytreatment interactions. A random subject effect and a spatial power covariance structure were used to adjust standard errors for correlation of observations within individual. No treatment-bytime interaction was found, and therefore treatment effects were averaged over visits for comparisons.
Results
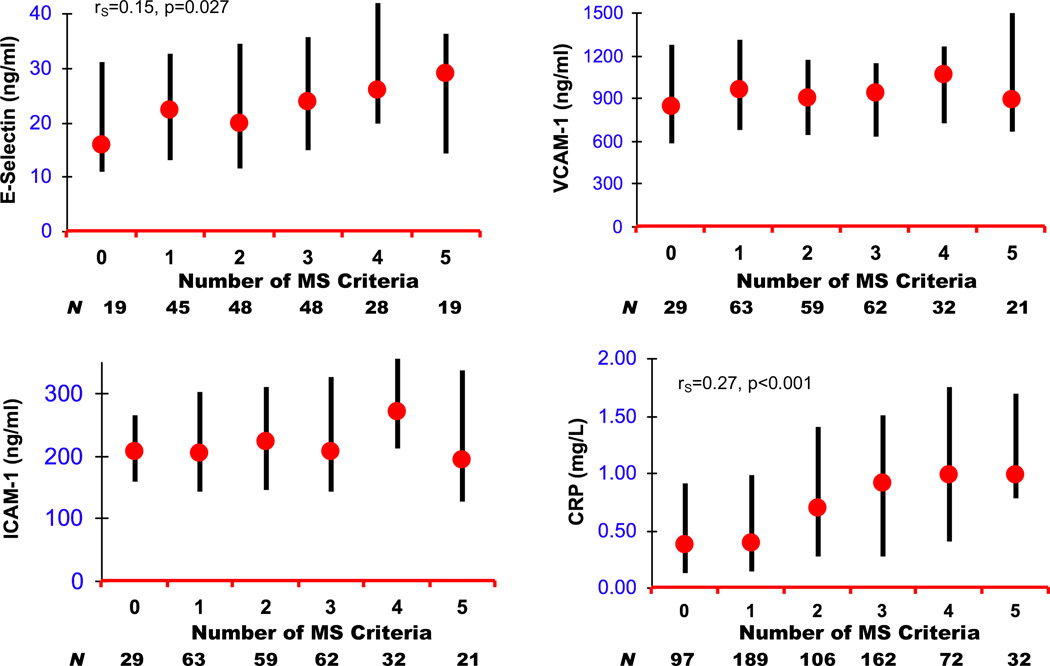
Subjects with fasting values at baseline and 3-month assessments (fasting cohort) were similar on all demographic variables with subjects who had nonfasting laboratory values at one or both time points (Table 1). Those with baseline and 3-month CRP values were also similar to other CATIE phase 1 subjects, but were older (+1.4 years) than those without follow-up CRP (T1,347=2.25, p=0.025). At 3 months, median CRP levels were significantly higher in patients who met MS criteria (Table 2), and higher CRP values positively correlated with greater number of MS criteria (rS=0.27, p<0.001) (Figure 1). Among the other IM, only fasting E-selectin levels were correlated with greater number of MS criteria (rS=0.15, p=0.027).
Table 1.
Demographics of CATIE Subjects with Baseline and 3-month Fasting ICAM-1, VCAM-1 and E-selectin Values (Fasting Cohort), and Baseline and 3-Month C-Reactive Protein Values (CRP) *
| Parameter | Fasting Cohort |
CRP |
|---|---|---|
| Age | 40.6 ± 11.1 (N=268) |
41.2 ± 10.8 (N=789) |
| Gender (% Male) | 78.4% (N=268) |
74.5% (N=789) |
| Race (% White) | 64.6% (N=268) |
62.8% (N=788) |
| Ethnicity (% Hispanic) | 11.6% (N=268) |
11.3% (N=789) |
| Years of Education | 12.3 ± 2.0 (N=266) |
12.1 ± 2.3 (N=785) |
| Years Since First Antipsychotic Treatment | 14.2 ± 10.7 (N=259) |
14.5 ± 10.9 (N=759) |
| Smoking | 58.9% (N=260) |
59.2% (N=770) |
| Hypertension | 34.7% (N=268) |
33.2% (N=789) |
| Diabetes | 11.6% (N=268) |
13.2% (N=789) |
| Body Mass Index (kg/m2) | 29.5 ± 6.9 (N=265) |
30.0 ± 6.9 (N=780) |
Note: Table entries are Mean ± SD or %.
Excluding extrapolated data
Table 2.
Median Marker Levels (Interquartile Range) By Presence or Absence of Metabolic Syndrome Criteria† and Smoking Status at 3 Months1
| CRP (mg/L) | E-Selectin (ng/mL) | ICAM-1 (ng/mL) | VCAM-1 (ng/mL) | |
|---|---|---|---|---|
| Central Obesity | ||||
| Present |
0.95 (0.40–1.66) n=382 |
23.7 (15.6–36.5) n=99 |
246.5 (144.7–337.7) N=118 |
925.0 (668.6–1216.8) N=118 |
| Absent |
0.42 (0.16–1.03) n=390 |
21.9 (12.6–33.0) n=105 |
209.9 (152.2–294.2) N=145 |
933.0 (676.1–1289.9) N=145 |
| Test Statistic; p-value†† | F1,770 =56.44; p<0.001 | F1,202 =2.90; p=0.090 | F1,261 =2.01; p=0.158 | F1,261 =0.062; p=0.803 |
| Elevated Triglycerides | ||||
| Present |
0.79 (0.33–1.47) N=188 |
24.1 (14.9–35.5) N=111 |
237.4 (155.4–335.6) N=128 |
971.9 (678.2–1275.5) N=128 |
| Absent |
0.46 (0.18–1.23) n=360 |
21.3 (12.5–34.7) N=98 |
207.9 (134.2–305.2) N=140 |
904.2 (639.1–1268.7) N=140 |
| Test Statistic; p-value†† | F1,546 =8.78; p=0.003 | F1,207 =1.01; p=0.316 | F1,266 =4.23; p=0.041 | F1,266 =0.77; p=0.382 |
| Low HDL | ||||
| Present |
0.74 (0.28–1.53) n=450 |
21.8 (14.0–35.9) N=116 |
235.5 (147.2–331.0) N=147 |
914.5 (639.7–1199.9) N=147 |
| Absent |
0.48 (0.17–1.20) n=330 |
23.7 (13.0–32.9) N=93 |
212.1 (148.2–311.7) N=121 |
960.4 (693.4–1302.4) N=121 |
| Test Statistic; p-value†† | F1,778 =13.89; p<0.001 | F1,207 =0.00; p=0.972 | F1,266 =6.50; p=0.421 | F1,266 =0.57; p=0.450 |
| Elevated Glucose | ||||
| Present |
0.79 (0.29–1.47) n=164 |
26.8 (14.3–41.1) N=67 |
208.3 (148.2–332.8) N=83 |
965.2 (705.1–1341.4) N=83 |
| Absent |
0.59 (0.20–1.31) n=503 |
21.6 (13.0–32.6) N=141 |
224.7 (145.7–316.9) N=184 |
909.3 (636.3–1227.3) N=184 |
| Test Statistic; p-value†† | F1,665 =4.48; p=0.035 | F1,206 =3.95; p=0.048 | F1,265 =0.61; p=0.435 | F1,265 =1.70; p=0.193 |
| Elevated BP | ||||
| Present |
0.86 (0.32–1.55) n=382 |
25.2 (14.3–36.5) N=103 |
231.7 (148.0–327.2) N=128 |
958.7 (690.0–1275.5) N=128 |
| Absent |
0.50 (0.19–1.18) n=400 |
21.0 (12.1–33.8) N=103 |
211.3 (145.6–302.6) N=137 |
899.9 (658.1–1275.5) N=137 |
| Test Statistic; p-value†† | F1,780 =17.98; p<0.001 | F1,204 =2.58; p=0.110 | F1,263 =0.54; p=0.463 | F1,263 =1.36; p=0.244 |
| Metabolic Syndrome * | ||||
| Present |
0.93 (0.40–1.64) N=266 |
25.0 (15.6–37.9) N=95 |
237.3 (147.9–337.7) N=115 |
969.0 (672.6–1216.8) N=115 |
| Absent |
0.42 (0.16–1.14) n=392 |
20.6 (12.2–32.9) N=112 |
211.3 (144.4–298.7) N=151 |
905.9 (664.4–1293.4) N=151 |
| Test Statistic; p-value†† | F1,656 =40.13; p<0.001 | F1,205 =5.09; p=0.025 | F1,264 =1.76; p=0.186 | F1,264 =0.28; p=0.595 |
| Current Smoker ** | ||||
| Yes |
0.69 (0.25–1.41) n=470 |
21.9 (13.1–32.9) N=119 |
246.4 (161.2–327.8) N=15 |
928.3 (668.6V1220.3) N=157 |
| No |
0.61 (0.21–1.32) n=309 |
24.1 (13.7–36.5) N=89 |
199.5 (138.4–311.7) N=109 |
937.5 (693.4–1299.1) N=109 |
| Test Statistic; p-value†† | F1,777 =1.24; p=0.266 | F1,206 =0.86; p=0.355 | F1,264 =2.50; p=0.115 | F1,264 =2.53; p=0.113 |
Excluding extrapolated data
Metabolic Syndrome Criteria
Central Obesity: Male and waist circumference > 40 inches, or female and waist circumference > 35 inches
Elevated Triglycerides: Fasting triglycerides ≥ 150 mg/dL
Low HDL: Male and HDL <40 mg/dL, or female and HDL <50 mg/dL
Elevated Glucose: Fasting glucose ≥ 100 mg/dL or random glucose value ≥ 200 mg/dl, or on insulin or hypoglycemic medication
Elevated BP: ≥ 130/85 mm Hg or on antihypertensive medication
P-values based on ANOVA on the log transformation, except for VCAM-1, which was normally distributed and not transformed.
Metabolic syndrome diagnosis based on all subjects with sufficient data to be classified (i.e. met ≥ 3 criteria).
Patients were considered current smokers if they smoked five or more cigarettes daily over the previous week.
Figure 1.
Distribution of Median and Interquartile Range for Inflammatory Marker Values According to Number of Metabolic Syndrome (MS) Criteria at 3 months
The olanzapine and quetiapine groups had the highest median CRP at the 3-month assessment, and the greatest numerical increase from baseline (Table 3). In the primary analysis, there was a significant overall treatment difference in 3-month CRP values after adjustment for the significant effect of baseline CRP and gender (p=0.013). Pairwise comparison revealed a statistically significant difference between olanzapine and risperidone (p=0.004). Given the differential impact of baseline CRP on the relationship between treatment and 3-month outcome (F4,779=2.56, p=0.037), the same ANCOVA model was repeated separately for those subjects with low baseline CV risk (CRP <1 mg/L, n=500) and higher risk (CRP ≥1 mg/L, n=289). For subjects with lower baseline CRP, pairwise treatment comparison found significant differences for olanzapine vs. perphenazine (p<0.001), vs. risperidone (p<0.001), and vs. ziprasidone (p=0.002) (see Table 4). The overall test of treatment difference was not significant for patients with higher CRP values at baseline (p=0.511). The supportive analysis, including extrapolated values truncated at the lower or higher nonextrapolated value, did not find a significant overall treatment difference for CRP (F4,810=1.98, p=0.095). However, for those subjects with low baseline risk, significant differences (with Bonferroni correction) for olanzapine vs. perphenazine (T231=3.37, p=0.001) and vs. risperidone (T245=3.39, p=0.001), but not ziprasidone (T195=2.46, p=0.014), were confirmed.
Table 3.
Median Baseline and 3-Month Marker Levels (Interquartile Range) By Treatment1
| CRP (mg/L) | E-Selectin (ng/mL) | ICAM-1 (ng/mL) | VCAM-1 (ng/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | |
| Olanzapine | (n=202) | (n=202) | (n=54) | (n=54) | (n=70) | (n=70) | (n=70) | (n=70) |
| Level | 0.63 (0.17–1.36) | 0.77 (0.26–1.57) | 29.2 (18.5–46.7) | 28.4 (19.3–40.5) | 360 (231–479) | 270 (173 – 347) | 1330 (915–1668) | 928 (713–1308) |
| Change | 0.09 (−0.44–0.50) | −1.4 (−23.8 – 10.8) | −78 (−201 – 49) | −240 (−598 – 51) | ||||
| Perphenazine | (n=143) | (n=143) | (n=36) | (n=36) | (n=51) | (n=51) | (n=51) | (n=51) |
| Level | 0.54 (0.19–1.34) | 0.51 (0.22–1.30) | 36.3 (21.7–60.3) | 15.9 (11.7–34.0) | 317 (218–424) | 211 (134 – 262) | 1336 (850–1866) | 964 (624–1278) |
| Change | −0.06 (−0.31–0.30) | −11.7 (−28.3 – 1.2) | −119 (−211 – −23) | −359 (−904 – 90) | ||||
| Quetiapine | (n=180) | (n=180) | (n=40) | (n=40) | (n=65) | (n=65) | (n=65) | (n=65) |
| Level | 0.71 (0.30–1.41) | 0.77 (0.37–1.38) | 25.7 (16.4–36.2) | 26.4 (15.8–41.3) | 245 (176–357) | 215 (161–328) | 1125 (842–1553) | 1089 (755–1418) |
| Change | 0.04 (−0.39–0.48) | +0.9 (−9.2 – 11.1) | −32 (−112 – 70) | −55 (−414 – 204) | ||||
| Risperidone | (n=178) | (n=178) | (n=32) | (n=32) | (n=51) | (n=51) | (n=51) | (n=51) |
| Level | 0.53 (0.17–1.51) | 0.53 (0.15–1.40) | 29.8 (23.2–42.8) | 19.9 (13.1–31.5) | 223 (152–307) | 201 (150–326) | 685 (453–921) | 878 (604–1074) |
| Change | −0.03 (−0.46–0.30) | −9.7 (−19.4 – 1.7) | +1 (−70 – 102) | +133 (−35 – 480) | ||||
| Ziprasidone | (n=86) | (n=86) | (n=17) | (n=17) | (n=31) | (n=31) | (n=31) | (n=31) |
| Level | 0.64 (0.23–1.23) | 0.60 (0.22–1.04) | 25.7 (15.7–40.1) | 21.0 (13.3–25.5) | 188 (113–280) | 194 (118–263) | 533 (370–750) | 705 (576–1008) |
| Change | −0.06 (−0.36–0.26) | −4.6 (−20.9 – 2.6) | −2 (−85 – 82) | +214 (−132 – 470) | ||||
| Overall Difference: | ||||||||
| Unadjusted † | ||||||||
| Test Statistic; p-value | F4,784=0.54; p=0.706 | F4,784=2.83; p=0.024 | F4,174=1.42; p=0.231 | F4,174=2.45; p=0.048 | F4,263=7.28; p<0.001 | F4,263=2.30; p=0.059 | F4,263=25.85; p<0.001 | F4,263=3.82; p=0.005 |
| Adjusted † | ||||||||
| Test Statistic; p-value | F4,782=3.20; p=0.013* | F4,173=3.96; p=0.004** | F3,231=4.65; p=0.004*** | F4,262=1.77; p=0.136 | ||||
Descriptive statistics excluding extrapolated CRP and E-selectin data
An ANCOVA on the log transformation was used to compare treatments, except for VCAM-1, which was normally distributed and not transformed. The adjusted models included the log transformation of the baseline IM values (except for VCAM-1, which was not transformed), as well as gender in the CRP model, and a ziprasidone cohort effect in the ICAM-1 model. These were the only significant covariates of those considered for inclusion.
Between group comparison at 3 months was statistically significant for olanzapine vs. risperidone (T379=2.89, p=0.004).
Between group comparison at 3 months was statistically significant for olanzapine vs. perphenazine (T89=3.28, p=0.001) and numerically different (but not statistically significant with Bonferroni correction) for olanzapine vs. risperidone (T85=2.59, p=0.010).
Between group comparison at 3 months statistically significant for perphenazine vs. quetiapine (T115=3.22, p=0.002), risperidone (T101=3.26, p=0.001), and numerically different (but not statistically significant with Bonferroni correction) for perphenazine vs. olanzapine (T120=2.76, p=0.006). A significant ziprasidone cohort effect was found for this model (F1,231=7.98, p=0.005). No significant differences were found between ziprasidone and the other treatments in the ziprasidone cohort (N=143).
Table 4.
Median 3-Month Marker Levels and Change from Baseline (Interquartile Range) By Treatment Stratified on Baseline Marker Level
| CRP (mg/L) | ICAM-1 (ng/mL) | |||
|---|---|---|---|---|
| Baseline <1 mg/L | Baseline ≥1 mg/L | Baseline < 273 ng/ml |
Baseline ≥ 273 ng/ml |
|
| Olanzapine | (n=127) | (n=75) | (n=24) | (n=46) |
| Level | 0.61 (0.19–1.23) | 1.27 (0.50–1.89) | 221 (120–298) | 305 (205–440) |
| Change | 0.27 (−0.01–0.85) | −0.72 (−1.29–0.13) | 44 (−40–102) | −174 (−260- −52) |
| Perphenazine | (n=95) | (n=48) | (n=18) | (n=33) |
| Level | 0.31 (0.15–0.77) | 1.31 (0.78–2.10) | 194 (132–250) | 241 (148–297) |
| Change | 0.04 (−0.15–0.32) | −0.39 (−1.08–0.18) | 6 (−48–41) | −190 (−339- −118) |
| Quetiapine | (n=111) | (n=69) | (n=34) | (n=31) |
| Level | 0.51 (0.23–0.99) | 1.23 (0.78–1.88) | 197 (152–249) | 299 (188–374) |
| Change | 0.13 (−0.07–0.53) | −0.44 (−1.27–0.29) | 35 (−41–85) | −118 (−188- −32) |
| Risperidone | (n=109) | (n=69) | (n=36) | (n=15) |
| Level | 0.26 (0.12–0.78) | 1.14 (0.54–1.94) | 189 (146–279) | 358 (199–550) |
| Change | 0.04 (−0.09–0.46) | −0.73 (−1.60–0.04) | 7 (−36–100) | −12 (−187–179) |
| Ziprasidone | (n=58) | (n=28) | (n=23) | (n=8) |
| Level | 0.43 (0.14–0.87) | 0.91 (0.41–2.19) | 146 (91–237) | 273 (179–448) |
| Change | 0.11 (−0.16–0.38) | −0.56 (−1.39- −0.09) | 15 (−69–82) | −113 (−195–40) |
|
Overall Treatment Difference† |
||||
| Test Statistic; p-value | F4,493=5.57; p<0.001* | F4,282=0.82; p=0.511 | F4,129=1.26; p=0.287 | F3,119=3.82; p=0.012** |
An ANCOVA on the log transformation was used to compare treatments. The adjusted models included the log transformation of the baseline IM values, as well as gender in the CRP model and a ziprasidone cohort effect in the ICAM-1 model where baseline ≥ 273 ng/ml. These were the only significant covariates of those considered for inclusion. E-Selectin and VCAM-1 are not included in table due to absence of significant treatment by baseline interaction.
Between group comparisons at 3 months were statistically significant for olanzapine vs. perphenazine (T221=4.02, p<0.001), risperidone (T235=3.57, p<0.001), and ziprasidone (T184=3.17, p=0.002).
Between group comparison at 3 months were numerically different (but not statistically significant with Bonferroni correction) for perphenazine vs. olanzapine (T78=2.61, p=0.010), quetiapine (T63=2.60, p=0.010), and risperidone (T47=2.80, p=0.006). A significant ziprasidone cohort effect was found for this model (F1,119=6.04, p=0.015). No significant differences were found between ziprasidone and the other treatments in the ziprasidone cohort (N=63).
There were also significant treatment differences for fasting E-selectin in the 3-month analysis. Olanzapine and quetiapine patients had the highest median E-selectin value at 3 months, and treatment pairwise comparison was statistically significant for olanzapine vs. perphenazine (p=0.001) and noteworthy for olanzapine vs. risperidone (p=0.010), although not significant after Bonferroni correction (Table 3). The supportive analysis of E-selectin outcomes including truncated extrapolated values confirmed the significant between-group differences for olanzapine vs. perphenazine (T97=2.91, p=0.004), and found noteworthy differences for olanzapine vs. risperidone (T116=2.65, p=0.009), for quetiapine vs. perphenazine (T91=2.80, p=0.006) and for quetiapine vs. risperidone (T109=2.72, p=0.007). Due to a significant effect for TD status in this model (F1,201=4.67, p=0.032), all comparisons involving perphenazine excluded patients with TD.
For ICAM-1, there were significant differences for perphenazine vs. quetiapine (p=0.002), and vs. risperidone (p=0.001) and a noteworthy difference, but not significant after Bonferroni correction, vs. olanzapine (p=0.006). As with CRP, there was a differential effect of baseline ICAM-1 value on treatment effects (F4,258=2.87, p=0.024), with trends for treatment differences occurring only in those patients with baseline ICAM-1 above the median (≥273 ng/ml); however, these differences fell short of statistical significance due to Bonferroni correction: perphenazine vs. quetiapine (p=0.010), vs. olanzapine (p=0.010), and vs. risperidone (p=0.006) (Table 4). There were no significant between group differences for VCAM-1 in the 3-month analysis after adjusting for baseline levels (Table 3).
In a supportive logistic regression analysis, age, white race, and baseline total Positive and Negative Syndrome Scale (PANSS) score were found to be significant predictors of having baseline and 3-month laboratory values, while gender and baseline PANSS score were associated with having fasting laboratory values at baseline and 3 months. Inclusion of these covariates in the ANCOVA models revealed that none of the predictors were significantly associated with change in IM at 3 months (p>0.05 for all), yielding results consistent with the original findings.
Given the association between metabolic dysfunction and IM, we examined the Spearman correlation between 3-month changes in each of the four IM and changes from baseline in metabolic parameters: adiposity measures (waist circumference, body mass index {BMI}), systolic and diastolic BP, fasting lipids (total cholesterol, triglycerides and HDL) and fasting glucose. Only data from the fasting cohort was used for the 3 IM that are sensitive to prandial effects (E-selectin, ICAM-1, VCAM-1) and for correlations between CRP and metabolic parameters that require fasting samples (glucose, lipids). Among these correlations, there were small associations between 3-month change in E-selectin with waist circumference (rS=0.20, p=0.007) and BMI (rS=0.26, p<0.001). CRP itself does not require fasting samples, so all available data (fasting and random samples) were used in the correlation analysis for change in CRP and change in adiposity and blood pressure measures. The 3-month change in CRP was weakly correlated with both change in BMI (rS=0.073, p=0.044) and change in HDL (rS=−0.11, p=0.004). After adjusting for baseline IM value, the correlations with CRP and E-selectin were nonsignificant.
To account for prior antipsychotic medications, especially the large number taking olanzapine (24%) or risperidone (21%) at study baseline who were randomized to the same medication in phase 1 (8% and 5%, respectively), prior antipsychotic was considered as a potential covariate in the rank ANCOVA model for each IM at 3-months. However, in all cases, it was neither statistically significant nor did it alter the treatment effects.
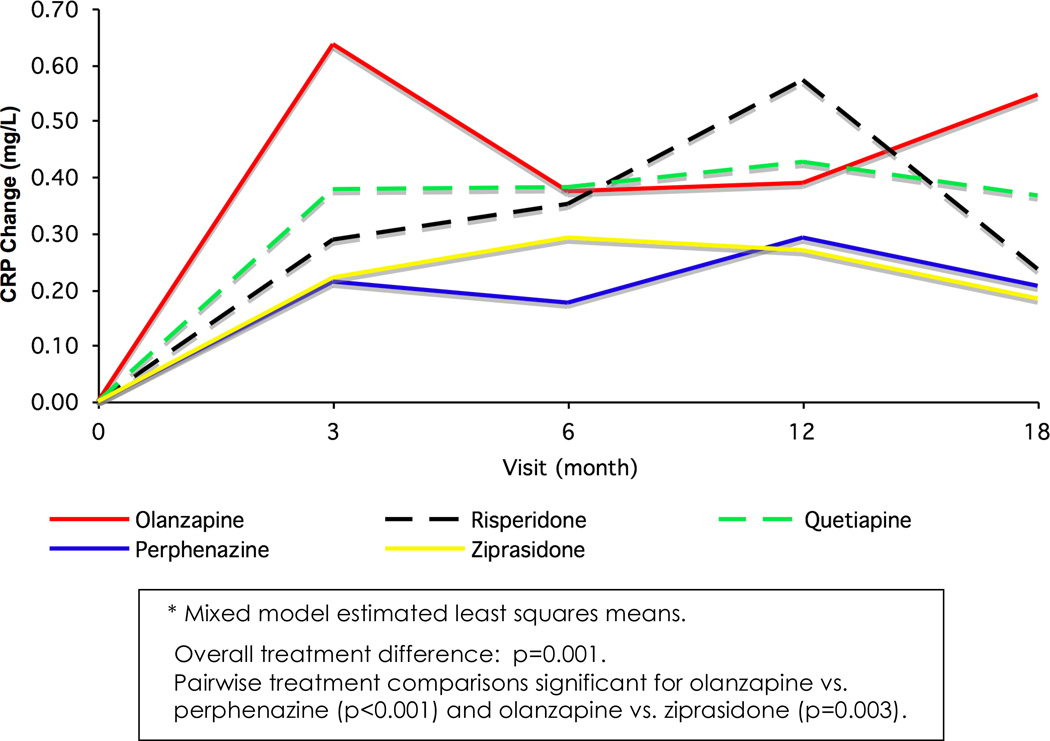
The repeated measures analysis of change in CRP up to 18 months found an overall significant treatment effect over time (F4,1086=2.86; p=0.023), with pairwise comparisons revealing a trend for higher values in olanzapine versus perphenazine (T1,058=2.57; p=0.010). As with the 3-month analysis, an interaction between treatment and baseline CRP was identified (F4,986=4.68; p=0.001), and treatment comparisons were made stratifying on baseline CRP risk level. There were significant between group differences among 694 subjects with CRP values <1mg/L at baseline (F4,671=4.51, p=0.001) (Figure 2, Table 5). Pairwise treatment comparisons found significant differences for olanzapine vs. perphenazine (T657=3.83, p<0.001) and vs. ziprasidone (T681=3.03, p=0.003). There was no treatment difference in the cohort with baseline CRP ≥1 mg/L (F4,387=0.25; p=0.910, n=394).
Figure 2.
Repeated Measures Model of C-Reactive Protein (CRP) Changes After Adjustment for Baseline CRP (Subjects With Baseline CRP < 1 mg/L)*
Table 5.
Sample Sizes by Visit and Treatment for Subjects with Baseline CRP < 1 mg/L
| Visit | 3 | 6 | 12 | 18 |
|---|---|---|---|---|
| Olanzapine | 154 | 114 | 83 | 71 |
| Perphenazine | 124 | 78 | 46 | 34 |
| Quetiapine | 143 | 89 | 60 | 40 |
| Risperidone | 144 | 97 | 71 | 53 |
| Ziprasidone | 81 | 46 | 35 | 20 |
| TOTAL | 646 | 424 | 295 | 218 |
Discussion
Presented here is the first analysis of data from a randomized study to examine the comparative impact of multiple antipsychotic therapies on changes in systemic inflammation. While certain metabolic effects from antipsychotic exposure are known, this is the first attempt to examine other pathophysiological elements that may underlie excess CV morbidity and mortality in chronic schizophrenia patients, and may be associated with antipsychotic treatment, particularly second-generation agents. The importance of CRP for CV risk prediction has been firmly established (31), but this is the first data set to confirm that atypical antipsychotic therapy may have differential effects on other IM, with olanzapine consistently emerging as a treatment with higher risk across several IM. Over the 3-month time frame, only the VCAM-1 results did not reveal any differences between antipsychotics.
As seen in larger samples (38), there is a correlation among CATIE subjects between increasing numbers of MS components and measures of systemic inflammation, specifically levels of E-selectin and CRP, so the expectation is that antipsychotics which adversely affect MS risk will result in concomitant changes in these IM. Olanzapine is associated with deleterious metabolic effects (39), while earlier CATIE analyses indicated that perphenazine, risperidone and ziprasidone are relatively more benign (37), so the findings seen here are consistent with the metabolic properties of these medications. Little historical metabolic outcomes data was available for perphenazine, but the findings from this and other CATIE studies confirm that perphenazine treatment is associated with reduction in markers of CV risk, including CRP and E-selectin (34,37). There were also noteworthy numerical differences when 3-month ICAM-1 levels for perphenazine were compared to those of other treatments among subjects with high baseline ICAM-1 levels, although these fell short of significance due to Bonferroni correction. It is also worth pointing out that the quetiapine cohort the numerically highest 3-month VCAM-1 level and the numerically highest 3-month CRP level (which it shared with olanzapine), and was the only treatment in which the median E-selectin level at 3 months was not lower than baseline.
Aside from the link between psychotropic-induced weight gain and central histamine H1 antagonism (40), with additive effects of 5HT2C antagonism (or 5HT2C receptor polymorphisms) for medications with high H1 affinity (41,42), the mechanisms underlying antipsychotic-induced dyslipidemia and hyperglycemia are unclear; nonetheless, these data indicate that alterations in IM are likely to accompany antipsychotic changes. The absence of strong correlations between change in any IM and change in metabolic variables may be due to a number of factors. Small sample sizes for the fasting analyses are one contributor. As shown in prior 3-month CATIE metabolic analyses (37), the mean changes in metabolic syndrome parameters across all drug arms was modest (waist circumference −0.4 – +0.7 inches; systolic BP −2.9 – −0.5 mmHg; diastolic BP −1.4 – +0.1 mmHg; HDL −2.3 – −0.1 mg/dL; fasting glucose −1.1 – +4.7 mg/dL; fasting triglycerides −32.1 – +21.5 mg/dL), so these small changes individually may not correlate with IM alterations; however, the fact that significant associations were found between certain IM and medication suggests that the combined effects of antipsychotic-induced changes on multiple metabolic parameters, or possibly direct drug effects on cytokine regulation, can accrue significant physiological impact. Given the impact of CRP on metabolic functioning, the findings of these analyses also open the door to the possibility that certain antipsychotic metabolic effects are mediated through inflammatory cytokines such as CRP (24). Further research might clarify why significant antipsychotic effects on CRP were only seen in those with low baseline CRP, while greater effects on ICAM-1 were only found for those with high baseline ICAM-1.
These data clearly indicate that, among subjects with lower CRP levels, changes in antipsychotic therapy can improve or worsen CV risk as early as 3 months after treatment initiation through changes in CRP; moreover, there may be concomitant changes in other IM, the clinical significance of which is still being elucidated. Clinicians are thus provided with another reason to be judicious in the use of antipsychotics, and to be diligent in metabolic monitoring. For patients with metabolic abnormalities who cannot switch medications, the limited studies on statin treatment in dyslipidemic schizophrenia patients show expected improvements in LDL cholesterol (43,44). In general population studies for patients with type 2 DM or coronary heart disease, statin therapy has been proven to reduce LDL cholesterol and IM (15,30). Unfortunately, the published literature is silent on whether statin treatment achieves comparable IM reduction in antipsychotic treated patients. Given the association between insulin resistance, central adiposity and systemic inflammation (45–47), medications that improve insulin sensitivity have been proposed as one way to address elevated IM in the context of antipsychotic mediated metabolic dysfunction; however, two placebo-controlled studies of adjunctive metformin during olanzapine treatment failed to establish any significant impact on CRP (48,49), although the metabolic benefits noted in some trials (50) might certainly influence other IM.
One limitation of this analysis derives from the fact that, at study baseline, nearly half of CATIE subjects were taking either olanzapine or risperidone, and 13% were randomized to the same medication in phase 1, diminishing the extent of any change from baseline noted in those medication arms. In addition, analyses of fasting IM, and in particular E-selectin, were hindered by small sample sizes, as were stratified analyses used to explore treatment effects in the presence of interaction with baseline IM values. In general, there was also reduced power to find differences between ziprasidone and the other treatments due to its addition to CATIE after 40% of subjects had been enrolled. While certain baseline variables were predictive of drop-out, these did not have a significant influence on IM outcomes; however, unequal subject attrition for assigned treatments may have injected some bias, as more data was available for olanzapine-treated subjects than for other medication assignments. The assay method employed for these analyses is used for research purposes only, so the magnitude of any treatment effect found here may differ from that using other forms of laboratory analysis.
Nonetheless, differences exist between various antipsychotics on measures of systemic inflammation, and emerge after relatively brief exposure. When antipsychotic switching is not an option, the available evidence points towards lifestyle modification and standard pharmacotherapy as the best means to address elevated IM in the context of antipsychotic therapy. For many patients with schizophrenia, the greatest impediments to effective medical management of antipsychotic treatment are the failure to perform routine laboratory monitoring (51) and undertreatment of common risk conditions (3). The present data not only reinforce the need for aggressive metabolic monitoring during antipsychotic treatment, but also foreshadow the importance of inflammation in our understanding of CV risk changes during antipsychotic therapy (52).
Acknowledgement
The CATIE Trials were supported by National Institute of Mental health (NIMH) grant #N01MH90001. The NIMH and study principal investigators are responsible for the design and conduct of the trial, and the primary analyses. There is no industry involvement in these activities.
We wish to acknowledge the contributions of all investigators, study personnel and subjects from all of the CATIE Schizophrenia Trial sites. We would also like to acknowledge Dr. Gregory D. Sempowski and Mr. Jeffery Hale of the Duke University Human Vaccine Institute for performing the multiplex biomarker assays. Dr. Sempowski's laboratory is partially supported by the Duke Center for Translational Research (AI51445) and the Duke Translational Medicine Institute (CTSA-UL1-RR-24128) to facilitate translational research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Jonathan M. Meyer, M.D.: Dr. Meyer reports having received research support from Bristol-Myers Squibb and Pfizer, Inc., and has received speaking or advising fees from AstraZeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, Pfizer, Inc., Vanda, and Wyeth.
Vicki G. Davis, DrPH: Dr. Vicki Davis reports that she is an employee of the Collaborative Studies Coordinating Center in the Department of Biostatistics, University of North Carolina.
Donald C. Goff, M.D.: Dr. Goff has received compensation within the past three years from: AstraZenca, Cephalon, Bristol-Myers-Squibb, Eli Lilly, Glaxo Smith Kline, Janssen Pharmaceuticals, Merck, Organon, Pfizer, Inc., Solvay, Wyeth, Dainippon Sumitomo Pharma, XenoPort, Vox, DiMedix, SG Cowen, Advanced Health Media, American Psychiatric Association, Primedia, Behavioral Options, Axio, Verusmed, the Nelson Group, Letters and Science, Centron, Imedex, Oakstone Publishing, Synapse, NARSAD, NIMH, Xytis, Organon, the Sidney Baer Foundation, and MedReviews, LLC.
Joseph P. McEvoy, M.D.: Dr. McEvoy reports having received research funding from AstraZeneca Pharmaceuticals LP, Forest Research Institute, Eli Lilly and Co., Janssen Pharmaceutica, and Pfizer, Inc.; consulting or advisory board fees from Eli Lilly and Co., Organon, Pfizer, Inc. and Bristol-Myers Squibb; and lecture fees from Eli Lilly and Co., Janssen Pharmaceutica, and Bristol-Myers Squibb.
Henry A. Nasrallah, M.D.: Dr. Nasrallah reports receiving fees for consulting, advising or speaking for Abbott Labs, AstraZeneca Pharmaceuticals LP, Janssen Pharmaceutica, Pfizer, Inc. and Solvay, research grant support from AstraZeneca Pharmaceuticals LP, GlaxoSmithKline, Janssen Pharmaceutica, Eli Lilly and Co., Pfizer, Inc., Sanofi-Avenits, and NIMH.
Sonia M. Davis, DrPH: Dr. Sonia Davis reports that she is an employee of Quintiles, Inc.
John Hsiao, M.D.: reported no biomedical financial interests or potential conflicts of interest.
Marvin S. Swartz, M.D.: Dr. Swartz reports having received research support from Eli Lilly and Co., and speaking or advising fees from Eli Lilly and Co., Pfizer, Inc., and AstraZeneca Pharmaceuticals LP.
T. Scott Stroup, M.D., M.P.H.: Dr. Stroup reports that he has received compensation within the past 2 years from: AstraZeneca, Janssen, Eli Lilly, Lundbeck, and Solvay.
Jeffrey A. Lieberman, M.D.: Dr. Lieberman serves as a consultant and/or advisor for Astra Zeneca, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Pfizer and Wyeth; and as a member of the Data Safety Management Board (DSMB) for Solvay and Wyeth. He does not receive financial compensation or salary support for his participation as a consultant or as a member of a board. He receives grant support from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck, Pfizer and Wyeth; and he holds a patent from Repligen.
Contributor Information
Jonathan M. Meyer, Assistant Professor – Department of Psychiatry, University of California, San Diego, Staff Psychiatrist – VA San Diego Healthcare System, jmmeyer@ucsd.edu
Joseph P. McEvoy, Associate Professor – Department of Psychiatry and Behavioral Sciences, Duke University, Clinical Research, John Umstead Hospital, 1003 12th Street, Butner, NC 27509, jpmcevoy@duke.edu
Vicki G. Davis, Research Investigator – Department of Biostatistics, Collaborative Studies Coordinating Center, University of North Carolina at Chapel Hill, Bank of America Center, 137 E. Franklin Street, Suite 400, Chapel Hill, NC 27514-4145, Vicki.Davis@mail.cscc.unc.edu.
Donald C. Goff, Associate Professor - Department of Psychiatry, Harvard University, Director, Schizophrenia Program, Massachusetts General Hospital, Freedom Trail Clinic - Lindemann Mental Health Center, 25 Staniford St., Boston, MA 02114, goff@psych.mgh.harvard.edu
Henry A. Nasrallah, Professor of Psychiatry and Neuroscience, University of Cincinnati, 231 Albert Sabin Way, PO Box 670559, Cincinnati, OH 45267-0559, nasralha@ucmail.uc.edu
Sonia M. Davis, Director of Biostatistics, Quintiles Inc., 5927 South Miami Blvd, Morrisville, NC 27560, sonia.davis@quintiles.com.
John K. Hsiao, Chief - Adult Psychopharmacology Program, Division of Services and Interventions Research, National Institute of Mental Health, Bethesda, MD 20892-9635, jh23f@nih.gov
Marvin S. Swartz, Professor – Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3173, Duke University Medical Center, Durham, NC 27710, swart001@mc.duke.edu
T. Scott Stroup, Associate Professor – Department of Psychiatry, University of North Carolina at Chapel Hill, Campus Box 7160, Chapel Hill, NC 27599-7160, scott_stroup@med.unc.edu
Jeffrey A. Lieberman, Professor and Chairman, Department of Psychiatry, Columbia University, Psychiatric Institute, 1051 Riverside Drive, New York, NY 10032, JL2616@columbia.edu
References
- 1.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- 2.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing Chronic Diseases. 2006;3:1–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, Lieberman JA. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophrenia Research. 2006;86:15–22. doi: 10.1016/j.schres.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, et al. Physical health monitoring of patients with schizophrenia. American Journal of Psychiatry. 2004;161:1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. New England Journal of Medicine. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 8.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 9.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arteriosclerosis, Thrombosis & Vascular Biology. 2007;27:2292–2302. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 10.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Current Opinion in Cell Biology. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 11.Stocker CJ, Sugars KL, Harari OA, Landis RC, Morley BJ, Haskard DO. TNF-alpha, IL-4, and IFN-gamma regulate differential expression of P- and E-selectin expression by porcine aortic endothelial cells. Journal of Immunology. 2000;164:3309–3315. doi: 10.4049/jimmunol.164.6.3309. [DOI] [PubMed] [Google Scholar]
- 12.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. Journal of Pathology. 1993;171:223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 13.Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Current Opinion in Lipidology. 2003;14:421–430. doi: 10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Matsuura E, Kobayashi K, Tabuchi M, Lopez LR. Oxidative modification of low-density lipoprotein and immune regulation of atherosclerosis. Progress in Lipid Research. 2006;45:466–486. doi: 10.1016/j.plipres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, et al. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53:701–710. doi: 10.2337/diabetes.53.3.701. [DOI] [PubMed] [Google Scholar]
- 16.Aronson D, Bartha P, Zinder O, Kerner A, Shitman E, Markiewicz W, et al. Association between fasting glucose and C-reactive protein in middle-aged subjects. Diabetic Medicine. 2004;21:39–44. doi: 10.1046/j.1464-5491.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- 17.Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 18.Haught WH, Mansour M, Rothlein R, Kishimoto TK, Mainolfi EA, Hendricks JB, et al. Alterations in circulating intercellular adhesion molecule-1 and L-selectin: further evidence for chronic inflammation in ischemic heart disease. American Heart Journal. 1996;132:1–8. doi: 10.1016/s0002-8703(96)90383-x. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM. Intercellular adhesion molecule (ICAM-1) and the risks of developing atherosclerotic disease. European Heart Journal. 1998;19:1119–1121. doi: 10.1053/euhj.1998.1101. [DOI] [PubMed] [Google Scholar]
- 20.Rohde LE, Lee RT, Rivero J, Jamacochian M, Arroyo LH, Briggs W, et al. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arteriosclerosis, Thrombosis & Vascular Biology. 1998;18:1765–1770. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 21.Ito H, Ohshima A, Inoue M, Ohto N, Nakasuga K, Kaji Y, et al. Weight reduction decreases soluble cellular adhesion molecules in obese women. Clinical & Experimental Pharmacology & Physiology. 2002;29:399–404. doi: 10.1046/j.1440-1681.2002.03672.x. [DOI] [PubMed] [Google Scholar]
- 22.Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism: Clinical & Experimental. 2004;53:377–381. doi: 10.1016/j.metabol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. American Journal of Pathology. 2001;158:1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabata A, Kuroki M, Ueba H, Hashimoto S, Umemoto T, Wada H, et al. C-reactive protein induces endothelial cell apoptosis and matrix metalloproteinase-9 production in human mononuclear cells: Implications for the destabilization of atherosclerotic plaque. Atherosclerosis. 2008;196:129–135. doi: 10.1016/j.atherosclerosis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nature Medicine. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 26.Bassuck SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Current Problems in Cardiology. 2004;29:439–493. [PubMed] [Google Scholar]
- 27.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 28.Boekholdt SM, Hack CE, Sandhu MS, Luben R, Bingham SA, Wareham NJ, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006;187:415–422. doi: 10.1016/j.atherosclerosis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Annals of Internal Medicine. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. New England Journal of Medicine. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 32.Smith RC, Viviano TF, Zorbas P, Mattute N, Kelly E. Effects of olanzapine and risperidone on C-reactive protein and interleukin-6 in patients with schizophrenia. Schizophrenia Research. 2007;33:461. [Google Scholar]
- 33.Carrizo E, Fernandez V, Quintero J, Connell L, Rodriguez Z, Mosquera M, et al. Coagulation and inflammation markers during atypical or typical antipsychotic treatment in schizophrenia patients and drug-free first-degree relatives. Schizophrenia Research. 2008;103:83–93. doi: 10.1016/j.schres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 35.Koch GG, Amara IA, Davis GW, Gillings DB. A review of some statistical methods for covariance analysis of categorical data. Biometrics. 1982;38:563–595. [PubMed] [Google Scholar]
- 36.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clinical Chemistry. 2001;47:426–430. [PubMed] [Google Scholar]
- 37.Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, Davis SM, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophrenia Research. 2008;101:273–286. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 39.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19:1–95. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 40.Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Luca V, Muller DJ, Hwang R, Lieberman JA, Volavka J, Meltzer HY, Kennedy JL. HTR2C haplotypes and antipsychotics-induced weight gain: X-linked multimarker analysis. Human Psychopharmacology. 2007;22:463–467. doi: 10.1002/hup.868. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds GP, Hill MJ, Kirk SL. The 5-HT2C receptor and antipsychotic induced weight gain - mechanisms and genetics. Journal of Psychopharmacology. 2006;20:15–18. doi: 10.1177/1359786806066040. [DOI] [PubMed] [Google Scholar]
- 43.De Hert M, Kalnicka D, van Winkel R, Wampers M, Hanssens L, Van Eyck D, et al. Treatment with rosuvastatin for severe dyslipidemia in patients with schizophrenia and schizoaffective disorder. Journal of Clinical Psychiatry. 2006;67:1889–1896. doi: 10.4088/jcp.v67n1208. [DOI] [PubMed] [Google Scholar]
- 44.Hanssens L, De Hert M, Kalnicka D, van Winkel R, Wampers M, Van Eyck D, et al. Pharmacological treatment of severe dyslipidaemia in patients with schizophrenia. International Clinical Psychopharmacology. 2007;22:43–49. doi: 10.1097/YIC.0b013e3280113d3b. [DOI] [PubMed] [Google Scholar]
- 45.Troseid M, Lappegard KT, Mollnes TE, Arnesen H, Seljeflot I. Changes in serum levels of E-selectin correlate to improved glycaemic control and reduced obesity in subjects with the metabolic syndrome. Scandinavian Journal of Clinical & Laboratory Investigation. 2005;65:283–290. doi: 10.1080/00365510510013811. [DOI] [PubMed] [Google Scholar]
- 46.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome. Journal of the American College of Cardiology. 2005;46:1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 47.Song Y, Manson JE, Tinker L, Rifai N, Cook NR, Hu FB, et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007;56:1898–1904. doi: 10.2337/db07-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baptista T, Rangel N, Fernandez V, Carrizo E, El Fakih Y, Uzcategui E, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophrenia Research. 2007;93:99–108. doi: 10.1016/j.schres.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Baptista T, Sandia I, Lacruz A, Rangel N, de Mendoza S, Beaulieu S, et al. Insulin counter-regulatory factors, fibrinogen and C-reactive protein during olanzapine administration: effects of the antidiabetic metformin. International Clinical Psychopharmacology. 2007;22:69–76. doi: 10.1097/YIC.0b013e32801182e6. [DOI] [PubMed] [Google Scholar]
- 50.Wu R-R, Zhao J-P, Jin H, Shao P, Fang M-S, Guo X-F, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299:185–193. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 51.Buckley PF, Miller DD, Singer BE, Arena J, Stirewalt EM. Clinicians’ recognition of the metabolic adverse effects of antipsychotic medications. Schizophrenia Research. 2005;79:281–288. doi: 10.1016/j.schres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Muller N, Schwarz MJ. COX-2 Inhibition in schizophrenia and major depression. Current Pharmaceutical Design. 2008;14:1452–1465. doi: 10.2174/138161208784480243. [DOI] [PubMed] [Google Scholar]