Abstract
Background: Calcium supplementation of pregnant Gambian women with a low calcium intake results in lower maternal bone mineral content in the subsequent lactation.
Objective: The objective was to investigate whether the lower bone mineral content persists long term.
Design: All women in the calcium supplementation trial (International Trial Registry ISRCTN96502494) who had been scanned with dual-energy X-ray absorptiometry at 52 wk of lactation (L52; n = 79) were invited for follow-up when neither pregnant nor lactating for ≥3 mo (NPNL) or at 52 wk postpartum in a future lactation (F52). Bone scans and anthropometric and dietary assessments were conducted.
Results: Sixty-eight women participated (35 at both NPNL and F52 and 33 at only one time point): n = 59 NPNL (n = 31 calcium, n = 28 placebo) and n = 44 F52 (n = 24 calcium, n = 20 placebo). The mean (±SD) time from L52 was 4.9 ± 1.9 y for NPNL and 5.0 ± 1.3 y for F52. Size-adjusted bone mineral content (SA-BMC) was greater at NPNL than at L52 in the placebo group (P ≤ 0.001) but not in the calcium group (P for time-by-group interaction: lumbar spine, 0.002; total hip, 0.03; whole body, 0.03). No significant changes in SA-BMC from L52 to F52 were observed in either group. Consequently, the lower SA-BMC in the calcium group at L52 persisted at NPNL and F52 (P ≤ 0.001): NPNL (lumbar spine, −7.5 ± 0.7%; total hip, −10.5 ± 1.0%; whole body, −3.6 ± 0.5%) and F52 (lumbar spine, −6.2 ± 0.9%; total hip, −10.3 ± 1.4%; whole body, −3.2 ± 0.6%).
Conclusion: In rural Gambian women with a low-calcium diet, a calcium supplement of 1500 mg/d during pregnancy resulted in lower maternal bone mineral content in the subsequent lactation that persisted long term. This trial was registered at www/controlled-trials.com/mrct/ as ISRCTN96502494.
INTRODUCTION
In a double-blind, randomized, placebo-controlled trial of calcium carbonate supplementation (1500 mg Ca/d) in pregnant Gambian women accustomed to a low calcium intake (∼300–400 mg/d), we reported that the women who had been in the calcium group had a lower bone mineral content (BMC)4 at the hip and greater bone mobilization from the spine in the succeeding lactation (1). This finding was contrary to expectations and may have indicated that the calcium supplement had altered the mother's ability to adapt to a low calcium intake. Alternatively, because the women were still demand breastfeeding at 52 wk of lactation (L52) when the last bone measurement was made, it is possible that the differences between the groups were temporary and would disappear after the women had completed that period of lactation.
During human pregnancy and lactation, the calcium requirement for fetal bone growth and mineralization in pregnancy is met mainly by increases in maternal calcium absorption and metabolic changes that mobilize skeletal mineral (2–4). In lactation, the calcium requirement for breast milk production is largely met by mobilization of maternal bone mineral and renal calcium conservation (2, 4, 5). Maternal BMC and bone mineral density (BMD) are known to decrease during pregnancy and the first months of lactation, particularly at the lumbar spine and hip (2). Studies of reproductive women with dietary calcium close to recommended intakes have shown that the changes in BMC and BMD during pregnancy and lactation are independent of current calcium intake and that maternal bone mineral is replenished in late lactation or after breastfeeding stops (3, 4, 6). Such changes can, therefore, be regarded as physiologic (2, 4, 6, 7). However, for women such as those in rural areas of The Gambia, a low calcium intake may result in greater maternal bone mobilization during pregnancy and lactation and may be insufficient to fully replenish the maternal skeleton after breastfeeding is stopped.
We therefore anticipated that the calcium pregnancy supplement in the Gambian trial would have either reduced the amount of maternal bone mineral mobilized during the subsequent lactation or would have had no effect, but mobilization actually increased (1). The aim of this study was to conduct follow-up measurements on those women who had been scanned by using dual-energy X-ray absorptiometry (DXA) during the trial to determine whether the effects of the pregnancy supplement on maternal bone outcomes persisted after the index lactation. It is common for women in The Gambia, who breastfeed each child for ∼2 y, to become pregnant again while still breastfeeding or shortly afterward. The follow-up measurements, therefore, were scheduled for a time when either each woman was neither pregnant nor lactating for ≥3 mo (NPNL) or when she was 52 wk postpartum in a future lactation (F52).
SUBJECTS AND METHODS
Participants and study design
The 79 women from the villages of Keneba and Manduar, West Kiang, The Gambia, who had taken part in a trial of pregnancy calcium supplementation and blood pressure (International Trial Registry: ISRCTN96502494) and who had DXA scans at L52 as part of a substudy (1) were eligible for the study. They were invited for follow-up when they were NPNL or at F52. All women who agreed to participate when they met one of these criteria were invited to return for a second set of measurements if subsequently they met the other criterion within the study period. The joint Medical Research Council (MRC) Gambia and the Gambian Government Ethics Committee approved the follow-up study. All participants gave written informed consent after the study was explained to them in their local language.
Detailed descriptions of the original substudy of the calcium trial, the flowchart of recruitment, the inclusion and exclusion criteria, the supplementation protocol, the losses of participants and the results of the trial on maternal bone outcomes and breast milk calcium concentrations, and on infant outcomes were published previously (1, 8). In brief, women were randomly assigned, double-blind and in a permuted block of 4, to receive a supplement that contained 1500 mg Ca/d (3 calcium carbonate tablets; Calcichew; Nycomed Pharma AS; distributed by Shire Pharmaceuticals) or matched placebo (microcrystalline cellulose and lactose; Nycomed Pharma AS) from 20 wk of pregnancy to delivery. The placebo and calcium tablets were well accepted, and 97% of the participants consumed ≥95% of the tablets. There were no reports of adverse effects. The mean (±SD) duration of the supplement in pregnancy was 136 ± 15 d. Supplementation was stopped at delivery, and no supplements were consumed during lactation. The women and the staff involved in data collection remained blinded to the group designations throughout the trial, in the intervening years, and during the follow-up study.
Data collections and procedures
BMC (in g) and bone area (BA; in cm2) were measured at the whole body, lumbar spine (lumbar vertebrae 1–4), and hip (total, shaft, trochanter, and neck). The 3 hip regions (trochanter, femoral neck, and femoral shaft) were also considered separately. When necessary, the femoral neck box size was decreased from the standard width of 15 mm for women with short femoral necks; in such instances, the same setting was used for all subsequent hip scans in the longitudinal series. The measurements were made by using the same DXA that had been used during the trial (Lunar DPX+, software version 4.7b; Lunar Corporation). The bone outcome variables were BMC, BA, areal BMD (in g/cm2), and size-adjusted BMC [SA-BMC = BMC adjusted for BA, weight, and height (9); see Statistical analysis]. All scans were scrutinized for quality by one experienced member of the study team (MAL). The following scans were excluded on this basis: 1 whole body, 3 lumbar spine, and 6 hip scans. The calibration and performance of the DXA were regularly monitored and showed stability and precision. The CV of spine phantom measurements over the period encompassing the trial and follow-up study was 0.6%, and there was no evidence of significant drift. A preliminary account of the within-individual changes in bone measures over time in the placebo group was published elsewhere (10).
The women were weighed to the nearest 0.1 kg, while they were wearing light clothes and no shoes, by using scales that were checked regularly (Seca). Height was measured to the nearest 0.1 cm on a stadiometer (supplied by Chasmors Ltd) by trained female members of staff who followed standardized protocols. The accuracy of the stadiometer equipment was checked at the beginning of each session by using a calibrated pole. The measurements were made after the removal of any head dress and while the subjects were standing with flat feet and positioned to ensure a horizontal Frankfort plane.
Accurate information on age and parity of the women at each time point was obtained from clinic antenatal records and the register of all births in the local community. These records have been kept for many years at MRC Keneba (11).
A 2-d weighed dietary record was obtained at each time point by using a method similar to that described in previous Gambian studies (8). The coded records were analyzed by using an in-house electronic nutrient database for Gambian foods (10), which contains food-composition data obtained by direct measurement combined with information of recipes and ingredients (12). Calcium from drinking water was not quantified because the calcium concentration of water in this region is low (<10 mg/L) (13).
Statistical analysis
Data were analyzed by using Data Desk 6.3 software (Data Description Inc). Descriptive characteristics for continuous variables are presented as means ± SDs and as percentage changes between time points ± SEs within each group. For ordered categorical variables, the data are summarized as medians and IQRs. Size-adjustment of BMC was achieved by using multivariate regression with the variables transformed to natural logarithms (9). To calculate individual values for BMC at each skeletal site after adjustment for bone and body size (SA-BMC), the residuals from loge-loge regression models of BMC with BA, body weight, and height were obtained and added to mean loge(BMC), and the antilogarithm was obtained.
Long-term effects of the calcium pregnancy supplement were investigated by using repeated-measures ANOVA or ANCOVA with Scheffe post hoc tests to compare the change in BMC, BA, BMD, and SA-BMC since L52. This was achieved through the use of hierarchical linear models that included supplement trial group (calcium and placebo), subject (nested by supplement group), and time point (L52, NPNL, and F52). Data were transformed into natural logarithms to investigate proportional effects (9). With the dependent variable in natural logarithms, the coefficient for a discrete variable, once multiplied by 100, approximates closely to a percentage difference as defined by (difference/mean) × 100 (14). Differences between the groups in change over time were tested for by including a time point–by-group interaction term. For SA-BMC hierarchical models, unadjusted loge(BMC) was used as the dependent variable, loge(BA) and loge(weight) were included as independent variables, and parsimonious models were produced by backward elimination of nonsignificant variables. The significance level was set at P = 0.05. Adjustment for the time interval between the scans, the number of pregnancies since L52, and, for those subjects measured at both F52 and NPNL, the chronologic order of these measurements, did not materially alter the results. For simplicity, these variables were omitted from the models presented in the article.
RESULTS
Of the 79 eligible women, 68 agreed to participate, of whom 59 (calcium: n = 31; placebo: n = 28) were measured at NPNL and 44 (calcium: n = 24; placebo: n = 20) at F52. Thirty-five women were measured at both time points; F52 preceded NPNL for 22 women, and NPNL preceded F52 for 13 women. At L52, the participants had a mean (±SD) age of 29 ± 8.0 y, height of 1.61 ± 0.6 m, and median (IQR) parity of 4 (2–6.5). The median (IQR) increase in parity between L52 and NPNL was 1 (0–1): a change of 0 in 33.9%, 1 in 45.8%, and 2 in 20.3%. At F52 the median (IQR) increase in parity was 1 (1–2): a change of 1 in 54.5%, 2 in 41.0%, and 3 in 4.5%. The means (±SDs) and ranges for the time interval from L52 to NPNL and F52 were 4.9 ± 1.4 (2.7–8.2) y and 5.0 ± 1.3 (2.4–7.5) y, respectively. Calcium intakes at NPNL and F52 were 329 ± 159 and 330 ± 149 mg/d, respectively. No significant differences in any of these variables were observed between the calcium and placebo groups at either time point.
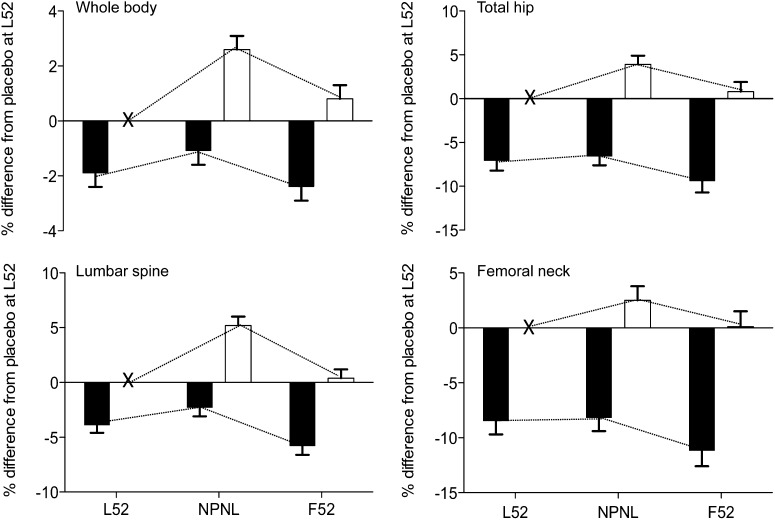
The weight and bone measures at the whole body, lumbar spine, total hip, and hip regions of women in the calcium and placebo groups at the 3 time points are shown in Tables 1 and 2. The changes in weight and bone mineral measures from L52 are presented, by group, in Tables 3 and 4. The magnitude of the differences between the calcium and placebo groups at each time point are detailed in Tables 5 and 6. The differences between the groups in change from L52 in SA-BMC at NPNL and F52 at the lumbar spine, whole body, total hip, and femoral neck are provided in Figure 1.
TABLE 1.
Weight and bone measures at the whole body and lumbar spine at L52, NPNL, and F521
| L52 |
NPNL |
F52 |
||||
| Calcium group(n = 35) | Placebo group(n = 33) | Calcium group(n = 31) | Placebo group(n = 28) | Calcium group(n = 24) | Placebo group(n = 20) | |
| Weight (kg) | 52.9 ± 5.9 | 54.5 ± 8.9 | 55.3 ± 6.1 | 56.1 ± 9.1 | 54.3 ± 5.7 | 54.2 ± 7.3 |
| Whole body2 | ||||||
| BMC (g) | 2120 ± 254 | 2212 ± 3123 | 2167 ± 229 | 2319 ± 4033 | 2072 ± 249 | 2211 ± 2963 |
| BA (cm2) | 2000 ± 181 | 2036 ± 2084 | 2017 ± 149 | 2074 ± 2563 | 1986 ± 174 | 2034 ± 2245 |
| BMD (g/cm2) | 1.058 ± 0.058 | 1.083 ± 0.0673 | 1.073 ± 0.065 | 1.112 ± 0.0783 | 1.042 ± 0.061 | 1.086 ± 0.0673 |
| SA-BMC (g) | 2131 ± 98 | 2167 ± 1183 | 2191 ± 126 | 2250 ± 1183 | 2087 ± 115 | 2165 ± 1313 |
| Lumbar spine6 | ||||||
| BMC (g) | 45.7 ± 6.6 | 47.7 ± 9.65 | 48.4 ± 6.3 | 52.6 ± 11.73 | 45.8 ± 6.8 | 49.5 ± 9.93 |
| BA (cm2) | 47.9 ± 4.6 | 47.8 ± 5.6 | 49.0 ± 4.3 | 49.5 ± 5.8 | 48.8 ± 4.5 | 48.3 ± 5.6 |
| BMD (g/cm2) | 0.954 ± 0.101 | 0.991 ± 0.1113 | 0.987 ± 0.097 | 1.054 ± 0.1353 | 0.936 ± 0.096 | 1.019 ± 0.1133 |
| SA-BMC (g) | 45.4 ± 4.9 | 47.1 ± 4.63 | 48.5 ± 4.5 | 51.4 ± 5.53 | 45.1 ± 4.5 | 49.3 ± 4.93 |
All values are means ± SDs. Differences between groups were tested with Scheffe post hoc tests from hierarchical repeated-measures ANOVA or ANCOVA models with continuous variables in natural logarithms that involved subjects nested by group, time, and group × time interaction terms. BA, bone area; BMC, bone mineral content; BMD, bone mineral density; F52, 52 wk postpartum in a later lactation; L52, 52 wk postpartum of the index lactation; NPNL, neither pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted BMC [derived by including BA, body weight, and height in the loge-loge model; evaluating the residual for each subject; adding the residual to mean loge(BMC) value; and calculating the antilogarithm].
The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 34 and 33 at L52, 31 and 28 at NPNL, and 24 and 20 at F52.
Significant differences between the calcium and placebo groups: 3P ≤ 0.001, 4P ≤ 0.05, 5P ≤ 0.01.
The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 35 and 33 at L52, 30 and 27 at NPNL, and 23 and 20 at F52.
TABLE 2.
Bone measures at the hip at L52, NPNL, and F521
| L52 |
NPNL |
F52 |
||||
| Calcium group(n = 34) | Placebo group(n = 31) | Calcium group(n = 30) | Placebo group(n = 28) | Calcium group(n = 22) | Placebo group(n = 20) | |
| Total hip | ||||||
| BMC (g) | 25.5 ± 3.8 | 29.1 ± 3.92 | 26.1 ± 3.8 | 29.7 ± 5.02 | 25.4 ± 3.7 | 28.3 ± 5.12 |
| BA (cm2) | 27.3 ± 2.2 | 28.6 ± 2.22 | 27.7 ± 2.1 | 28.2 ± 1.93 | 27.5 ± 2.1 | 28.5 ± 2.62 |
| BMD (g/cm2) | 0.930 ± 0.100 | 1.015 ± 0.0962 | 0.941 ± 0.108 | 1.051 ± 0.1332 | 0.919 ± 0.089 | 0.990 ± 0.1222 |
| SA-BMC (g) | 26.2 ± 2.4 | 27.9 ± 2.62 | 26.3 ± 2.8 | 29.1 ± 3.22 | 25.8 ± 2.3 | 27.4 ± 3.32 |
| Femoral neck | ||||||
| BMC (g) | 3.96 ± 0.68 | 4.36 ± 0.722 | 3.98 ± 0.76 | 4.58 ± 0.682 | 3.96 ± 0.66 | 4.22 ± 0.772 |
| BA (cm2) | 4.35 ± 0.55 | 4.38 ± 0.49 | 4.36 ± 0.58 | 4.54 ± 0.433 | 4.47 ± 0.57 | 4.38 ± 0.55 |
| BMD (g/cm2) | 0.909 ± 0.095 | 0.993 ± 0.0962 | 0.912 ± 0.121 | 1.009 ± 0.1162 | 0.886 ± 0.095 | 0.964 ± 0.1102 |
| SA-BMC (g) | 3.95 ± 0.37 | 4.28 ± 0.412 | 4.03 ± 0.49 | 4.44 ± 0.492 | 3.89 ± 0.42 | 4.22 ± 0.482 |
| Trochanter | ||||||
| BMC (g) | 6.86 ± 1.77 | 8.30 ± 1.862 | 7.25 ± 1.67 | 8.16 ± 2.182 | 6.99 ± 1.70 | 8.07 ± 2.572 |
| BA (cm2) | 9.47 ± 1.74 | 10.51 ± 1.562 | 9.71 ± 1.41 | 9.84 ± 1.54 | 9.63 ± 1.55 | 10.38 ± 2.084 |
| BMD (g/cm2) | 0.717 ± 0.094 | 0.784 ± 0.0882 | 0.740 ± 0.093 | 0.819 ± 0.1182 | 0.719 ± 0.080 | 0.774 ± 0.1092 |
| SA-BMC (g) | 7.19 ± 0.75 | 7.46 ± 0.682 | 7.13 ± 0.70 | 7.88 ± 0.882 | 7.11 ± 0.65 | 7.42 ± 0.692 |
| Shaft | ||||||
| BMC (g) | 14.7 ± 1.8 | 16.4 ± 1.82 | 14.9 ± 1.8 | 17.0 ± 2.42 | 14.4 ± 1.7 | 16.1 ± 2.22 |
| BA (cm2) | 13.5 ± 0.8 | 13.7 ± 0.92 | 13.6 ± 0.9 | 13.8 ± 0.92 | 13.4 ± 0.8 | 13.9 ± 1.04 |
| BMD (g/cm2) | 1.086 ± 0.130 | 1.198 ± 0.1282 | 1.094 ± 0.139 | 1.233 ± 0.1782 | 1.075 ± 0.124 | 1.158 ± 0.1602 |
| SA-BMC (g) | 14.8 ± 1.5 | 16.2 ± 1.62 | 14.9 ± 1.7 | 16.8 ± 1.92 | 14.6 ± 1.6 | 15.9 ± 2.12 |
All values are means ± SDs. Differences between groups were tested with Scheffe post hoc tests from hierarchical repeated-measures ANOVA or ANCOVA models with continuous variables in natural logarithms that involved subjects nested by group, time, and group × time interaction terms. BA, bone area; BMC, bone mineral content; BMD, bone mineral density; F52, 52 wk postpartum in a later lactation; L52, 52 wk postpartum of the index lactation; NPNL, neither pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted BMC [derived by including BA, body weight, and height in the loge-loge model; evaluating the residual for each subject; adding the residual to mean loge(BMC) value; and calculating the antilogarithm].
Significant differences between the calcium and placebo groups: 2P ≤ 0.001, 3P ≤ 0.05, 4P ≤ 0.01.
TABLE 3.
Changes from L52 in maternal body weight and bone measures at the whole body and lumbar spine at NPNL and F521
| Percentage change from L52 |
|||||||
| NPNL |
F52v |
p2 |
|||||
| Calcium group | Placebo group | Calcium group | Placebo group | Time | Group | Time × group interaction | |
| Weight (kg)3 | 4.0 ± 1.54 | 3.4 ± 1.6 | 3.4 ± 1.7 | 1.0 ± 1.8 | 0.004 | ≤0.0001 | 0.6 |
| Whole body5,6 | |||||||
| BMC (g) | 1.8 ± 0.9 | 5.0 ± 0.97 | −0.2 ± 1.0 | 2.1 ± 1.1 | ≤0.0001 | ≤0.0001 | 0.04 |
| BA (cm2) | 0.8 ± 0.7 | 2.2 ± 0.74 | 0.2 ± 0.8 | 1.1 ± 0.9 | 0.02 | ≤0.0001 | 0.4 |
| BMD (g/cm2) | 1.0 ± 0.5 | 2.9 ± 0.57 | −0.4 ± 0.5 | 0.9 ± 0.5 | ≤0.0001 | ≤0.0001 | 0.02 |
| SA-BMC (g) | 1.0 ± 0.5 | 2.7 ± 0.57 | −0.4 ± 0.5 | 0.8 ± 0.5 | ≤0.0001 | ≤0.0001 | 0.03 |
| Lumbar spine5,8 | |||||||
| BMC (g) | 4.8 ± 1.07 | 8.8 ± 1.07 | −0.1 ± 1.1 | 2.4 ± 1.2 | ≤0.0001 | ≤0.0001 | 0.02 |
| BA (cm2) | 2.0 ± 0.47 | 2.3 ± 0.57 | 1.1 ± 0.5 | 1.2 ± 0.5 | ≤0.0001 | ≤0.0001 | 0.9 |
| BMD (g/cm2) | 2.8 ± 0.77 | 6.6 ± 0.87 | −1.2 ± 0.8 | 1.2 ± 0.9 | ≤0.0001 | ≤0.0001 | 0.002 |
| SA-BMC (g) | 1.6 ± 0.8 | 5.2 ± 0.87 | −1.8 ± 0.8 | 0.4 ± 0.8 | ≤0.0001 | ≤0.0001 | 0.002 |
All values are means ± SEs. The percentages were derived from hierarchical repeated-measures ANOVA and ANCOVA models with continuous variables in natural logarithms that involved subjects nested by group, time, and time × group interaction terms. Body weight was not a significantly independent variable of bone mineral status at the whole body and lumbar spine and was removed from the models presented. BA, bone area; BMC, bone mineral content; BMD, bone mineral density; F52, 52 wk postpartum in a later lactation; L52, 52 wk postpartum of the index lactation; NPNL, neither pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted BMC derived by including bone area and body weight in the hierarchical ANCOVA model.
P values are for each component of the interaction model.
The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 35 and 33 at L52, 31 and 28 at NPNL, and 24 and 20 at F52.
Significant changes from L52 to NPNL and F52 within each group: 4P ≤ 0.05, 7P ≤ 0.001.
Measures were obtained by using dual-energy X-ray absorptiometry.
The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 34 and 33 at L52, 31 and 28 at NPNL, and 24 and 20 at F52.
The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 35 and 33 at L52, 30 and 27 at NPNL, and 23 and 20 at F52.
TABLE 4.
Changes in maternal bone measures at the hip at NPNL and F521
| Percentage change from L52 |
|||||||
| NPNL |
F52 |
P2 |
|||||
| Calcium group | Placebo group | Calcium group | Placebo group | Time | Group | Time × group interaction | |
| Total hip3,4 | |||||||
| BMC (g) | 2.9 ± 1.2 | 3.7 ± 1.35 | −2.1 ± 1.4 | 0.6 ± 1.5 | ≤0.0001 | ≤0.0001 | 0.4 |
| BA (cm2) | 1.8 ± 0.8 | −0.9 ± 0.8 | −0.4 ± 0.9 | −0.5 ± 0.9 | 0.4 | ≤0.0001 | 0.05 |
| BMD (g/cm2) | 1.2 ± 1.0 | 4.7 ± 1.06 | −1.6 ± 1.1 | 1.1 ± 1.2 | ≤0.0001 | ≤0.0001 | 0.04 |
| SA-BMC (g) | 0.5 ± 1.0 | 3.9 ± 1.06 | −2.4 ± 1.1 | 0.8 ± 1.1 | 0.001 | ≤0.0001 | 0.03 |
| Femoral neck | |||||||
| BMC (g) | 2.2 ± 1.3 | 4.8 ± 1.47 | −1.2 ± 1.5 | 0.9 ± 1.6 | 0.0007 | ≤0.0001 | 0.4 |
| BA (cm2) | 2.0 ± 0.9 | 2.5 ± 0.95 | 1.4 ± 1.0 | 1.1 ± 1.1 | 0.003 | ≤0.0001 | 0.9 |
| BMD (g/cm2) | 0.2 ± 1.2 | 2.4 ± 1.3 | −2.6 ± 1.4 | −0.2 ± 1.5 | 0.04 | ≤0.0001 | 0.4 |
| SA-BMC (g) | 0.3 ± 1.2 | 2.5 ± 1.3 | −2.7 ± 1.3 | −0.1 ± 1.4 | 0.03 | ≤0.0001 | 0.3 |
| Trochanter | |||||||
| BMC (g) | 6.6 ± 2.65 | 1.1 ± 2.7 | −1.3 ± 2.9 | 0.4 ± 3.1 | 0.07 | ≤0.0001 | 0.2 |
| BA (cm2) | 3.8 ± 2.3 | −4.8 ± 2.4 | −0.9 ± 2.6 | −2.2 ± 2.8 | 0.7 | ≤0.0001 | 0.03 |
| BMD (g/cm2) | 2.7 ± 1.2 | 5.9 ± 1.36 | −0.4 ± 1.4 | 2.6 ± 1.5 | ≤0.0001 | ≤0.0001 | 0.1 |
| SA-BMC (g) | 1.9 ± 1.3 | 5.1 ± 1.36 | −1.2 ± 1.4 | 2.3 ± 1.4 | 0.0006 | ≤0.0001 | 0.1 |
| Shaft | |||||||
| BMC (g) | 1.6 ± 1.1 | 4.6 ± 1.26 | −2.3 ± 1.3 | 0.5 ± 1.4 | ≤0.0001 | ≤0.0001 | 0.1 |
| BA (cm2) | 0.5 ± 0.6 | 0.6 ± 0.6 | −0.5 ± 0.6 | −0.1 ± 0.7 | 0.2 | ≤0.0001 | 0.9 |
| BMD (g/cm2) | 1.1 ± 1.0 | 4.0 ± 1.16 | −1.7 ± 1.1 | 0.5 ± 1.2 | 0.0004 | ≤0.0001 | 0.1 |
| SA-BMC (g) | 0.3 ± 1.0 | 3.2 ± 1.07 | −2.5 ± 1.1 | 0.2 ± 1.2 | 0.004 | ≤0.0001 | 0.07 |
All values are means ± SEs. The percentages were derived from hierarchical repeated-measures ANOVA and ANCOVA models with continuous variables in natural logarithms that involved subjects nested by group, time, and time × group interaction terms. Body weight was a significantly independent variable of bone mineral status at hip from the models presented. BA, bone area; BMC, bone mineral content; BMD, bone mineral density; F52, 52 wk postpartum in a later lactation; L52, 52 wk postpartum of the index lactation; NPNL, neither pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted BMC derived by including bone area and body weight in the hierarchical ANCOVA model.
P values are for each component of the interaction model.
The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 34 and 31 at L52, 30 and 28 at NPNL, and 22 and 20 at F52.
Measures were obtained by using dual-energy X-ray absorptiometry.
Significant changes from L52 to NPNL and F52 within each group: 5P ≤ 0.05, 6P ≤ 0.001, 7P ≤ 0.01.
TABLE 5.
Differences in maternal body weight and bone measures at the whole body and lumbar spine between the calcium and placebo groups at each of the 3 time points1
| Percentage difference between calcium and placebo groups |
||||||
| L52 |
NPNL |
F52 |
||||
| Calcium vs placebo group | P2 | Calcium vs placebo group | P2 | Calcium vs placebo group | P2 | |
| Weight (kg)3 | −2.4 ± 1.5 | 0.3 | −1.9 ± 1.6 | 0.5 | −0.1 ± 2.0 | 1.0 |
| Whole body4,5 | ||||||
| BMC (g) | −4.0 ± 0.9 | ≤0.0001 | −7.2 ± 1.0 | ≤0.0001 | −6.2 ± 1.2 | ≤0.0001 |
| BA (cm2) | −1.9 ± 0.7 | 0.03 | −3.3 ± 0.8 | 0.0002 | −2.8 ± 0.9 | 0.01 |
| BMD (g/cm2) | −2.1 ± 0.4 | ≤0.0001 | −3.9 ± 0.5 | ≤0.0001 | −3.4 ± 0.6 | ≤0.0001 |
| SA-BMC (g) | −1.9 ± 0.5 | ≤0.0001 | −3.6 ± 0.5 | ≤0.0001 | −3.2 ± 0.6 | ≤0.0001 |
| Lumbar spine4,6 | ||||||
| BMC (g) | −3.4 ± 0.9 | 0.002 | −7.5 ± 1.1 | ≤0.0001 | −5.8 ± 1.3 | ≤0.0001 |
| BA (cm2) | 0.3 ± 0.4 | 0.7 | 0.1 ± 0.5 | 1.0 | 0.2 ± 0.6 | 0.9 |
| BMD (g/cm2) | −3.7 ± 0.7 | ≤0.0001 | −7.5 ± 0.8 | ≤0.0001 | −6.1 ± 1.0 | ≤0.0001 |
| SA-BMC (g) | −3.9 ± 0.7 | ≤0.0001 | −7.5 ± 0.7 | ≤0.0001 | −6.2 ± 0.9 | ≤0.0001 |
1All values are means ± SEs. The differences between the calcium and placebo groups (ie, calcium group minus placebo group) at each time point were derived from hierarchical repeated-measures ANOVA and ANCOVA models with continuous variables in natural logarithms that involved subjects nested by group, time, and time × group interaction terms. Body weight was not a significantly independent variable of bone mineral status at the whole body and lumbar spine from the models presented and was therefore removed. BA, bone area; BMC, bone mineral content; BMD, bone mineral density; F52, 52 wk postpartum in a later lactation; L52, 52 wk postpartum of the index lactation; NPNL, neither pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted BMC derived by including bone area and body weight in the hierarchical ANCOVA model.
2P values are for the differences between calcium and placebo at each time point.
3The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 35 and 33 at L52, 31 and 28 at NPNL, and 24 and 20 at F52.
4Measures were obtained by dual-energy X-ray absorptiometry.
5The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 34 and 33 at L52, 31 and 28 at NPNL, and 24 and 20 at F52.
6The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 35 and 33 at L52, 30 and 27 at NPNL, and 23 and 20 at F52.
TABLE 6.
Differences in maternal bone measures at the hip between the calcium and placebo groups at 3 time points1
| Percentage difference between calcium and placebo groups |
||||||
| L52 |
NPNL |
F52 |
||||
| Calcium vs placebo group | P2 | Calcium vs placebo group | P2 | Calcium vs placebo group | P2 | |
| Total hip3,3,4 | ||||||
| BMC (g) | −12.4 ± 1.2 | ≤0.0001 | −13.2 ± 1.3 | ≤0.0001 | −15.0 ± 1.6 | ≤0.0001 |
| BA (cm2) | −5.1 ± 0.8 | ≤0.0001 | −2.4 ± 0.8 | 0.02 | −5.0 ± 1.0 | ≤0.0001 |
| BMD (g/cm2) | −7.3 ± 1.0 | ≤0.0001 | −10.8 ± 1.0 | ≤0.0001 | −10.0 ± 1.3 | ≤0.0001 |
| SA-BMC (g) | −7.1 ± 1.1 | ≤0.0001 | −10.5 ± 1.0 | ≤0.0001 | −10.3 ± 1.4 | ≤0.0001 |
| Femoral neck | ||||||
| BMC (g) | −10.3 ± 1.3 | ≤0.0001 | −12.9 ± 1.4 | ≤0.0001 | −12.4 ± 1.8 | ≤0.0001 |
| BA (cm2) | −2.1 ± 0.9 | 0.06 | −2.7 ± 0.9 | 0.02 | −1.8 ± 1.2 | 0.3 |
| BMD (g/cm2) | −8.1 ± 1.2 | ≤0.0001 | −10.3 ± 1.3 | ≤0.0001 | −10.5 ± 1.6 | ≤0.0001 |
| SA-BMC (g) | −8.5 ± 1.2 | ≤0.0001 | −10.7 ± 1.3 | ≤0.0001 | −11.1 ± 1.6 | ≤0.0001 |
| Trochanter | ||||||
| BMC (g) | −18.4 ± 2.5 | ≤0.0001 | −12.9 ± 2.7 | ≤0.0001 | −20.1 ± 3.4 | ≤0.0001 |
| BA (cm2) | −10.9 ± 2.2 | ≤0.0001 | −2.3 ± 2.4 | 0.6 | −9.6 ± 3.0 | 0.009 |
| BMD (g/cm2) | −7.4 ± 1.2 | ≤0.0001 | −10.6 ± 1.3 | ≤0.0001 | −10.4 ± 1.6 | ≤0.0001 |
| SA-BMC (g) | −7.0 ± 1.3 | ≤0.0001 | −10.2 ± 1.3 | ≤0.0001 | −10.5 ± 1.7 | ≤0.0001 |
| Shaft | ||||||
| BMC (g) | −10.5 ± 1.1 | ≤0.0001 | −13.4 ± 1.2 | ≤0.0001 | −13.2 ± 1.5 | ≤0.0001 |
| BA (cm2) | −2.2 ± 0.6 | 0.0007 | −2.2 ± 0.6 | 0.001 | −2.7 ± 0.7 | 0.002 |
| BMD (g/cm2) | −8.3 ± 1.0 | ≤0.0001 | −11.2 ± 1.1 | ≤0.0001 | −10.5 ± 1.3 | ≤0.0001 |
| SA-BMC (g) | −7.8 ± 1.0 | ≤0.0001 | −10.7 ± 1.1 | ≤0.0001 | −10.5 ± 1.4 | ≤0.0001 |
1All values are means ± SEs. The differences between the calcium and placebo groups (ie, calcium group minus placebo group) at each time point were derived from hierarchical repeated-measures ANOVA and ANCOVA models with continuous variables in natural logarithms that involved subjects nested by group, time, and time × group interaction terms. Body weight was a significantly independent variable of bone mineral status at hip from the models presented. BA, bone area; BMC, bone mineral content; BMD, bone mineral density; F52, 52 wk postpartum in a later lactation; L52, 52 wk postpartum of the index lactation; NPNL, not pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted BMC derived by including bone area and body weight in the hierarchical ANCOVA model.
2P values are the differences between calcium and placebo at each of the 3 time points.
3Measures were obtained by dual-energy X-ray absorptiometry.
4The numbers of subjects in the calcium and placebo groups, respectively, were as follows: 34 and 31 at L52, 30 and 28 at NPNL, and 22 and 20 at F52.
FIGURE 1.
Effect of calcium supplementation during pregnancy on SA-BMC of the whole body, lumbar spine, total hip, and femoral neck at L52, NPNL, and F52. Values are the mean (±SE) percentage differences in SA-BMC relative to the placebo group at L52 in the calcium group (black bars) and placebo group (white bars). Dotted lines represent the time trend observed within each group. An “X” on the x axes denotes the value in the placebo group at L52, which was used as the reference and set to zero. Results were obtained from Scheffe post hoc tests for time × group interaction terms in hierarchical repeated-measures ANCOVA models that included subject (nested by group), time, group, and time × group interaction. The difference between the calcium and placebo groups was significant at every time point in each set of scans, P ≤ 0.0001. The numbers of subjects at L52, NPNL, and F52, respectively, were as follows: lumbar spine, 35, 30, and 23 in the calcium group and 33, 27, and 20 in the placebo group; whole body, 34, 31, and 24 in the calcium group and 33, 28, and 20 in the placebo group; total hip and femoral neck, 34, 30, and 22 in the calcium group and 31, 28, and 20 in the placebo group. F52, 52 wk postpartum in a later lactation; L52, 52 wk postpartum of the index lactation; NPNL, neither pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted (for bone area and weight) bone mineral content.
No significant differences in maternal weight were found between the calcium and placebo groups at any time point (Table 1). There was a tendency for weight to be higher at NPNL and F52 than at L52 in both groups, significantly so at NPNL in the calcium group, although the interaction was not significant (Table 3). In the placebo group, increases in BMC, BMD, and SA-BMC at NPNL ranged from 1% to 6%. These increases were significant, except for BMD and SA-BMC at the femoral neck and unadjusted BMC at the trochanter (Tables 3 and 4). BA also increased in the placebo group at the whole body, lumbar spine, and femoral neck (Tables 3 and 4). In contrast, the increases in BMC, BMD, and SA-BMC in the calcium group at NPNL were either small and not significant, or, at the lumbar spine, were approximately half those of the placebo group. BA in the calcium group increased significantly only at the lumbar spine. No significant changes between L52 and F52 were found in any bone variable in either group, although there was a tendency for lower SA-BMC values at the lumbar spine and total hip in the calcium group (P = 0.07 and P = 0.09, respectively).
The differences in the changes from L52 to NPNL between the calcium and placebo groups in BMC, BMD, and SA-BMC at the whole body and lumbar spine and in BMD and SA-BMC at the total hip were confirmed by significant time × group interaction terms (Tables 3 and 4). No significant time × group interactions were observed for weight or BA, except at the total hip and trochanter, which reflected apparent increases in the calcium group and decreases in the placebo group at NPNL—none of which were statistically significant.
The lower BMC, BMD, and SA-BMC values in the calcium group at L52, and the group differences in changes within individuals over time resulted in significantly lower values in the calcium group at NPNL and F52, of a magnitude similar to or greater than at L52 (Tables 5 and 6). Significant group differences were also found in BA at L52, at all sites except the lumbar spine, which were mostly still evident at NPNL and F52.
Weight was a significant predictor of BMC only at the total hip and, therefore, contributed to the longitudinal SA-BMC models only at this site. However, the removal of weight from the total hip model had only a small effect on the magnitude and significance of the time × group interaction and marginally increased the difference between the groups (data not presented).
DISCUSSION
This was a follow-up study designed to test whether the effects of a calcium supplement consumed in pregnancy on maternal bone measures during the following lactation (1) disappeared in subsequent years. The participants had taken part in a trial of the potential benefits of calcium supplementation in pregnancy on maternal blood pressure and preeclampsia risk reduction and had participated in a detailed substudy of the effects on maternal bone health and on infant growth and bone mineral accretion (8). We showed that supplementing pregnant Gambian women with calcium had no significant effect on maternal blood pressure (15) or breast milk calcium concentrations in the subsequent lactation period (8). In addition, no evidence of benefit to infant weight, length, or bone mineral accretion in the first year of life was observed (8, 15). The expectation was that the calcium supplement would increase maternal SA-BMC and/or reduce lactational bone mobilization or would have no effect. However, the calcium-supplemented women were found to have lower hip bone mineral postpartum and to have greater decreases in bone mineral of the lumbar spine and distal radius from 2 to 52 wk of lactation (1). In addition, the calcium-supplemented women were shown to have a smaller hip BA postpartum, which suggests diminished expansion of the hip during pregnancy (1).
In the follow-up study, 68 of the 79 women with a DXA scan at 52 wk postpartum had 1 or 2 sets of repeat scans between 2 and 8 y later, when they were NPNL and/or at 52 wk postpartum in another lactation. This study showed that BMC at NPNL in the placebo group was significantly and substantially greater than at L52 and remained so after adjustment for changes in BA and body weight at all sites measured. This indicates, as we reported earlier (10), that skeletal mineral is accreted in Gambian women after a period of breastfeeding in a manner similar to that in Western women, despite their much lower calcium intakes (2, 6, 16–18). In addition, no significant differences in the bone measures obtained at 52 wk were found in the 2 lactation periods. This adds to the evidence that multiple pregnancies and long lactation periods in African women with low calcium intakes are not associated with skeletal mineral depletion (10, 19).
In the calcium group there were also no significant within-mother differences in bone measures between 52 wk in the 2 lactation periods. In contrast, the increases in bone mineral from L52 to NPNL in the calcium group were less than those in the placebo group and often small and not significant. In consequence, the lower bone mineral values at the lumbar spine, hip, and whole body in the calcium group observed in the index lactation were sustained for 2–8 y. These differences were amplified when the women were NPNL, which showed that the effects of the supplement on the maternal skeleton, which were in a direction opposite from that anticipated, persisted beyond the index lactation.
The mechanisms responsible for these unexpected findings are unknown. We speculated in our report, describing the effects on maternal bone measures in the index lactation (1), that the calcium supplement had diminished periosteal apposition at the hip during pregnancy and had altered the mother's ability to adapt to a low calcium intake. Recent animal and cell studies have strengthened earlier evidence of a central role for osteocytes in the regulation of calcium homeostasis (20) and in the release and replacement by osteolytic perilacunar/canalicular remodeling of bone mineral mobilized during lactation (20–22). It is therefore plausible that a subpopulation of osteocytes, newly embedded in bone during the period of high bone turnover in the second half of pregnancy, develop a “memory” of the prevailing calcium environment that they retain throughout their life span [estimated as up to 25 y (20)], as they do for the local strain environment (23). This would provide a mechanism for the effects on bone mobilization observed after supplementation during and after the index lactation in the Gambian study—effects that persisted for several years. If this were the case, then it would be anticipated that the effects of the calcium supplement will be diluted by subsequent pregnancies because newly embedded osteocytes would retain a memory of the low calcium environment prevailing at the time. Continued longitudinal follow-up of these women will be needed to investigate this hypothesis further.
In conclusion, the results from this study suggest that rural Gambian women who are accustomed to a low calcium intake have physiologic mechanisms that enable them to replenish the bone mineral that is mobilized during pregnancy and lactation, but that a period of calcium supplementation during pregnancy disrupts this process, which results long term in lower skeletal mineral content. Whether this poses an increased risk to bone health in later life will require further follow-up studies. These findings indicate that breastfeeding by Gambian mothers with a low calcium intake does not compromise maternal bone health. However, unexpectedly, calcium supplementation in pregnancy may have had unintended consequences by disrupting the mother's ability to conserve calcium. Many differences in genetics, diet, sunshine exposure, physical activity, and other aspects of lifestyle exist between rural Gambian and Western women, and further research will be needed to determine the applicability of these results to women in other populations. However, this study, combined with our findings of an earlier adolescent growth spurt and shorter adult stature in Gambian boys who had been supplemented with calcium for 12 mo when they were 8–12 y of age as part of a supplementation trial of prepubertal children (24), cautions against applying dietary recommendations based on Western populations to countries such as The Gambia without supporting evidence.
Acknowledgments
We thank the women who participated in this study and the staff of MRC Keneba, The Gambia, particularly Michael Mendy, Mustapha Ceesay, Mariama Jammeh, Fatou Manneh, Buba Ceesay, Morikebba Sanyang, and Lamin Jammeh. We also are grateful for the contributions of staff at MRC Human Nutrition Research, Cambridge, especially Sheila Levitt, Celia Prynne, and Jenny Thompson. We also thank Professor Nigel Loveridge for expert scientific discussions on osteocyte function.
The authors’ responsibilities were as follows—LMAJ and AP (Principal Investigators): designed the study, analyzed the data, interpreted the results, and drafted the manuscript; YS: conducted the fieldwork and DXA scans and contributed to the data analysis; GRG and MAL: provided senior scientific oversight of the data collection and bone densitometry and critically reviewed the manuscript; TJC: provided expert statistical input and critically reviewed the manuscript; and AP: had overall responsibility for the decision to publish. All authors had full access to the data and approved the final manuscript. The sources of funding had no role in the study design, collection, analysis, or interpretation of the data or decision to publish. None of the authors reported a financial or personal conflict of interest. This work was supported by the UK Medical Research Council under program numbers U105960371, U123261351, and MR/J004839/1.
Footnotes
Abbreviations used: BA, bone area; BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; F52, 52 wk postpartum in a future lactation; L52, 52 wk of lactation; MRC, Medical Research Council; NPNL, neither pregnant nor lactating for ≥3 mo; SA-BMC, size-adjusted bone mineral content.
REFERENCES
- 1.Jarjou LMA, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. Am J Clin Nutr 2010;92:450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olausson H, Goldberg GR, Laskey MA, Schoenmakers I, Jarjou LMA, Prentice A. Calcium economy in human pregnancy and lactation. Nutr Res Rev 2012;25:40–67. [DOI] [PubMed] [Google Scholar]
- 3.Olausson H, Laskey MA, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. Am J Clin Nutr 2008;88:1032–9. [DOI] [PubMed] [Google Scholar]
- 4.Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. J Nutr 2003;133:1693S–9S. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am 2011;40:795–826. [DOI] [PubMed] [Google Scholar]
- 6.Laskey MA, Prentice A. Bone mineral changes during and after lactation. Obstet Gynecol 1999;94:608–15. [DOI] [PubMed] [Google Scholar]
- 7.Prentice A. Calcium supplementation during breast-feeding. N Engl J Med 1997;337:558–9. [DOI] [PubMed] [Google Scholar]
- 8.Jarjou LMA, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, Cole TJ. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr 2006;83:657–66. [DOI] [PubMed] [Google Scholar]
- 9.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr 1994;60:837–42. [DOI] [PubMed] [Google Scholar]
- 10.Sawo Y, Jarjou LM, Goldberg GR, Laskey MA, Prentice A. Bone mineral changes after lactation in Gambian women accustomed to a low calcium intake. Eur J Clin Nutr (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayco-Solon P, Moore SE, Fulford AJ, Prentice AM. Fifty-year mortality trends in three rural African villages. Trop Med Int Health 2004;9:1151–60. [DOI] [PubMed] [Google Scholar]
- 12.Prynne CJ, Paul AA. Food composition table for use in The Gambia. Cambridge, UK: Medical Research Council, Human Nutrition Research, 2011. [Google Scholar]
- 13.Prentice A, Laskey MA, Shaw J, Hudson GJ, Day KC, Jarjou LMA, Dibba B, Paul AA. The calcium and phosphorus intakes of rural Gambian women during pregnancy and lactation. Br J Nutr 1993;69:885–96. [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med 2000;19:3109–25. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg GR, Jarjou LMA, Cole TJ, Prentice A. Randomized, placebo-controlled, calcium supplementation trial in pregnant Gambian women accustomed to a low calcium intake: effects on maternal blood pressure and infant growth. Am J Clin Nutr (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akesson A, Vahter M, Berglund M, Eklof T, Bremme K, Bjellerup P. Bone turnover from early pregnancy to postweaning. Acta Obstet Gynecol Scand 2004;83:1049–55. [DOI] [PubMed] [Google Scholar]
- 17.Holmberg-Marttila D, Sievanen H, Laippala P, Tuimala R. Factors underlying changes in bone mineral during postpartum amenorrhea and lactation. Osteoporos Int 2000;11:570–6. [DOI] [PubMed] [Google Scholar]
- 18.Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci 1998;94:405–12. [DOI] [PubMed] [Google Scholar]
- 19.Walker ARP. The human requirement of calcium: should low intakes be supplemented? Am J Clin Nutr 1972;25:518–30. [DOI] [PubMed] [Google Scholar]
- 20.Atkins GJ, Findlay DM. Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int 2012;23:2067–79. [DOI] [PubMed] [Google Scholar]
- 21.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jähn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res 2012;27:1018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone 2009;44:11–6. [DOI] [PubMed] [Google Scholar]
- 23.Skerry TM, Bitensky L, Chayen J, Lanyon LE. Loading-related reorientation of bone proteoglycan in vivo. Strain memory in bone tissue? J Orthop Res 1988;6:547–51. [DOI] [PubMed] [Google Scholar]
- 24.Prentice A, Dibba B, Sawo Y, Cole TJ. The effect of prepubertal calcium carbonate supplementation on the age of peak height velocity in Gambian adolescents. Am J Clin Nutr 2012;96:1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]