Abstract
Background: Previous studies have reported beneficial effects of a Mediterranean diet rich in monounsaturated fatty acids (MUFAs) on coronary artery disease (CAD) risk. However, these findings remain inconsistent because some experimental studies have suggested atherogenic and lipotoxicity effects of long-chain and very-long-chain MUFAs on cardiomyocytes.
Objective: We examined whether red blood cell (RBC) long-chain and very-long-chain MUFAs are associated with risk of CAD in the Physicians’ Health Study.
Design: The ancillary study used a prospective nested case-control design to select 1000 cases of incident CAD and 1000 control subjects matched for age, year of birth, and time of blood collection. RBC MUFAs were measured by using gas chromatography, and CAD was validated by an endpoint committee. Conditional logistic regression was used to estimate RRs.
Results: The mean (±SD) age was 68.7 ± 8.7 y. In a multivariable model that was controlled for matching factors and established CAD risk factors and RBC saturated and omega-3 (n−3) fatty acids, ORs for CAD associated with each SD increase of 20:1n−9 and log 22:1n−9 were 0.89 (95% CI: 0.80, 1.00; P = 0.0441) and 0.83 (95% CI: 0.72, 0.95; P = 0.0086). However, only the 22:1n−9–CAD relation remained statistically significant after Bonferroni correction (P < 0.0125). RBC cis 18:1n−9 and 24:1n−9 were not associated with CAD risk.
Conclusion: Our data suggest an inverse association of RBC 22:1n−9 but not 20:1n−9, 18:1n−9, or 24:1n−9 with CAD risk after Bonferroni correction in the Physicians’ Health Study.
INTRODUCTION
MUFAs may have cardioprotective effects because several epidemiologic studies reported that olive oil enriched with MUFAs lowered LDL cholesterol concentrations and coronary artery disease (CAD)5 risk (1, 2). However, reports from epidemiologic studies and meta-analyses have not consistently supported the hypothesis that MUFA consumption lowers risk of CAD (3–5). Although previous randomized clinical trials evaluated the effect of dietary MUFAs on CAD risk factors such as LDL cholesterol, little is known about the effects of dietary MUFAs on CAD risk in a community setting. Furthermore, some previous studies reported that long-chain MUFAs and very-long-chain MUFAs may lead to cardiac lipotoxicity and coronary atherosclerosis (6, 7). These results suggest that long-chain MUFAs could have unfavorable influence on CAD via inflammation, apoptosis of myocardium, and dyslipidemia (ie, LDL particle enrichment with cholesteryl oleate) (7–9). Our group has previously reported a positive relation of red blood cell (RBC) cis palmitoleic (16:1n–7) acid with CAD and an inverse relation of cis vaccenic acid (18:1n–7) with CAD, which are 2 MUFAs from de novo lipogenesis (10). However, limited data are available on the association between other individual long-chain MUFAs and CAD risk. Therefore, we examined whether individual RBC long-chain and very-long-chain MUFAs are each associated with risk of CAD in participants in the Physicians’ Health Study (PHS).
SUBJECTS AND METHODS
Study population
The PHS I was a randomized, double-blind, placebo-controlled trial designed to test the effects of low-dose aspirin and β carotene on cardiovascular disease (CVD) and cancer in 22,071 US male physicians. The PHS II was a randomized trial designed to test benefits and risks of vitamins E and C, β carotene, and multivitamins in the prevention of cancer, CVD, age-related eye diseases, and cognitive function in 14,642 US male physicians aged ≥50 y at baseline. A detailed description of both studies has been published previously (11, 12). Both PHS I and II trials were registered at clinicaltrials.gov as NCT00000500 and NCT00270647, respectively.
With the use of a prospective nested case-control design, we randomly selected 1000 incident CAD cases who provided blood samples between 1995 and 2001 in the PHS I and PHS II for this ancillary study. For each case, we used a density sampling technique to randomly select one control subject who was alive and free of confirmed CAD at the time of the index case diagnosis and matched for age at blood collection (≤1 y), year of birth (≤2 y), and time of blood collection (≤3 mo).
Each case was eligible to serve as a control before CAD diagnosis. Similarly, each control was eligible to later become a CAD case to ensure that controls were representative of a total population that gave rise to the CAD cases (13). This study was conducted according to the guidelines in the Declaration of Helsinki. Each participant gave written informed consent, and the Brigham and Women's Hospital Institutional Review Board approved the study protocol (LD has full access to the data sets used for the current analyses; with a proper institutional review board approval and data-distribution agreement, these data sets can be obtained by external investigators).
Blood collection and storage
For the current project, blood was collected between 1995 and 2001. A detailed description of methods of blood collection and storage has been published previously (14).
Measurement of red blood fatty acid profiles
Baseline RBC samples from all cases and controls were handled identically throughout the sample collection, long-term storage, sample retrieval, and assays. All laboratory personnel were blinded to the case-control status of participants to minimize the ascertainment bias. Deidentified samples were mixed before shipment to the laboratory, and each test tube only contained a study identification number. The fatty acid content of RBC membranes was determined as follows: after osmotic hemolysis, RBC membranes were washed thrice with sodium chloride, an internal standard (heptadecanoate) was added to the cell pellet, and total lipids were extracted according to Folch's method (15) and followed by saponification and methylation (16). The resultant fatty acid methyl esters were dried down under nitrogen, resuspended in 100 μL hexane, transferred into amber gas chromatography vials, and stored at −20°C until the time of analysis (17, 18). RBC fatty acid methyl esters were analyzed by using an Autosystem XL gas chromatograph (Perkin Elmer) equipped with a 100-m × 0.2-mm inside diameter (film thickness: 0.25 μm) capillary column (SP-2560, Supelco). Injector and flame ionization detector temperatures were 250°C and 260°C, respectively. Helium was used as the carrier gas (2.5 mL/min), and the split ratio was 2:1. The oven temperature was programmed at 80°C, held for 16 min, and increased to 190°C at a rate of 5°C/min. After 10 min, the temperature was increased to 210°C at a rate of 0.5°C/min and held for 4 min. The final temperature was 250°C and held for 2 min. Peaks of interest were identified by comparison with authentic fatty acid standards (Nu-Chek Prep Inc) and expressed as molar percentage proportions of fatty acids relative to the internal standard. We focused on fatty acid measurements, and a total 33 fatty acids were ascertained by gas chromatography. See Supplemental Figures 1 and 2 under “Supplemental data” in the online for a depiction of chromatograms of the fatty acid standard mixture and RBC fatty acid profile of a de-identified PHS subject, respectively. Interassay CVs were 3.46% for cis 18:1n–9, 3.84% for 20:1n–9, 9.05% for 22:1n–9, and 5.29% for 24:1n–9.
Ascertainment of incidents of CAD
We obtained information on the occurrence of major diseases including CAD through annual follow-up questionnaires. CAD was defined as a nonfatal myocardial infarction, fatal myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, coronary death, and sudden death. All cardiovascular events in the PHS have been adjudicated by an endpoint committee (12). The diagnosis of myocardial infarction was confirmed by using WHO criteria (19). Revascularization procedures were confirmed by hospital records.
Other variables
Information on age, height, body weight, BMI, cigarette smoking, exercise, parental history of myocardial infarction, history of hypertension, diabetes, and hypercholesterolemia was collected at baseline through annual questionnaires. A validated food-frequency questionnaire that consisted of 127 items was used to collect data on total energy intake and nutrients between 1997 and 2001 (20).
Statistics
The distributions of each RBC long-chain and very-long-chain MUFA, except 20:1n−9, was skewed to the right. Thus, for non-Gausian distributed biomarkers, we used the ln to normalize their distributions. The correlation in RBC MUFAs was evaluated by using Spearman's correlations. Baseline CAD risk factors were compared between cases or controls by using the t test for means and chi-square tests for proportions (the significance level was set at 0.05). We performed conditional logistic regression to examine the association between each RBC MUFA and CAD. ORs associated with each SD higher RBC MUFA were assessed. The initial model only controlled for matching factors (the year of birth, date of blood collection, and age at blood test; model 1), and the second model controlled for matching factors, BMI, smoking status (never, current, and past smokers), exercise (rarely or never, 1–3 times/mo, 1–4 times/wk, and 5–7 times/wk), and alcohol consumption (<1, 1–4, 5–7, and >7 times/wk), parental history of myocardial infarction (before age 50 y), treatment of hypertension, diabetes, and hypercholesterolemia (model 2). The final multivariate model was adjusted for all covariates in the second model and for RBC omega-3 fatty acids and SFAs (model 3). In addition, we performed conditional logistic regression adjusted for covariates in model 3 plus 16:1n–7 and 18:1n–7 to evaluate whether the association between long-chain MUFAs and risk of CAD was independent of MUFAs in de novo lipogenesis (16:1n–7 and 18:1n–7). We used Bonferroni correction for a final multivariate regression model to correct for inflation and used a P value of 0.0125 (0.05 ÷ 4) to indicate statistical significance. Similarly, to achieve simultaneous 95% CIs, we constructed 98.75% CIs for the point estimate of each individual long-chain MUFA. All analyses were completed with SAS software (version 9.2; SAS Institute). All P values were 2 sided.
To explore the shape of the association, we fitted restricted cubic splines with 4 knots at the 10th, 36.7th, 63.4th, and 90th percentiles by using each MUFA's 1st percentile value (0.10 for 1st percentile of 20:1n–9 and –3.91 for 1th percentile of log 22:1n–9) as the reference. The restricted cubic splines were completed with the use of STATA/MP version 12.
RESULTS
The mean (±SD) age of participants was 68.7 ± 8.7 y. Baseline characteristics of participants by case and control of each RBC long-chain MUFA are summarized in Table 1. RBC cis 18:1n−9, BMI, current smoking, history of hypercholesterolemia, diabetes, and parental history of myocardial infarction were higher in cases than controls. Spearman's correlation showed a weak to modest correlation (−0.05 to 0.36) across individual MUFAs (all P < 0.05; Figure 1).
TABLE 1.
Characteristics of 2000 participants according to cases and controls1
| Baseline characteristics | Controls (n = 1000) | Cases (n = 1000) | P |
| Age (y) | 68.7 ± 8.7 | 68.7 ± 8.7 | 0.999 |
| BMI (kg/m2) | 25.6 ± 3.3 | 26.3 ± 3.6 | <0.001 |
| RBC cis 18:1–9 (%) | 14.22 ± 1.63 | 14.39 ± 1.73 | 0.030 |
| RBC 20:1–9 (%) | 0.23 ± 0.06 | 0.22 ± 0.05 | 0.206 |
| RBC 22:1–9 (%) | 0.07 ± 0.03 | 0.06 ± 0.03 | 0.074 |
| RBC 24:1–9 (%) | 1.02 ± 0.30 | 1.01 ± 0.30 | 0.526 |
| RBC omega-3 fatty acids (%) | 6.10 ± 1.95 | 5.94 ± 1.95 | 0.064 |
| RBC SFAs (%) | 42.99 ± 3.69 | 43.00 ± 3.56 | 0.965 |
| Smoking status (%) | 0.004 | ||
| Current | 2.2 | 4.6 | |
| Past | 46 | 48.2 | |
| Exercise (%) | 0.127 | ||
| Rarely/never | 34.9 | 39.7 | |
| 1–3/mo | 2.5 | 2.9 | |
| 1–4/wk | 46.1 | 41.8 | |
| Alcohol intake (%) | 0.030 | ||
| <1 time/wk | 24.0 | 28.0 | |
| 1–4 times/wk | 20.3 | 22.9 | |
| 5–7 times/wk | 21.2 | 19.3 | |
| Total energy intake (kcal) | 1713 ± 518 | 1659 ± 514 | 0.033 |
| Energy from carbohydrate (%) | 50.2 ± 9.9 | 50.3 ± 9.8 | 0.791 |
| Energy from protein (%) | 18.2 ± 3.3 | 18.6 ± 3.4 | 0.012 |
| Energy from SFA (%) | 10.0 ± 3.0 | 10.2 ± 2.9 | 0.103 |
| Energy from MUFA (%) | 10.4 ± 3.1 | 10.6 ± 3.0 | 0.316 |
| Energy from PUFA (%) | 4.5 ± 1.3 | 4.5 ± 1.2 | 0.687 |
| Parental history of MI (%) | 7.2 | 13.2 | <0.001 |
| Hypertension (%) | 33.4 | 33.6 | 0.925 |
| Hypercholesterolemia (%) | 27.7 | 33.8 | 0.003 |
| Diabetes (%) | 6.0 | 11.6 | <0.001 |
All values are means ± SDs for continuous variables and percentages for categorical variables. Cases were men with coronary artery disease defined as nonfatal MI, fatal MI, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, coronary death, and sudden death. There were 22 subjects with missing data for exercise level and 325 subjects with missing data for dietary variables. P values were determined across cases and controls. The t test was used for continuous variables, and the χ 2 test was used for categorical variables. MI, myocardial infarction; RBC, red blood cell.
FIGURE 1.
Association of each MUFA shown by scatter plots with a table of Spearman's correlation. *P < 0.05 for Spearman correlation.
The RBC log 22:1n−9 showed significant inverse associations with CAD. The OR (95% CI) for CAD per 1-SD higher log 22:1n−9 was 0.84 (0.71, 1.00; P = 0.0125) in the multivariate model adjusted for matching factors and lifestyle risk factors (Table 2, model 2).
TABLE 2.
ORs (98.75% CIs) for coronary artery disease according to RBC long-chain MUFAs in the PHS1
| Per SD higher | Model 1 | P | Model 2 | P | Model 3 | P |
| Log cis 18:1n−9 | 1.11 (0.99, 1.25) | 0.0219 | 1.08 (0.95, 1.22) | 0.1399 | 1.08 (0.94, 1.24) | 0.1887 |
| 20:1n−9 | 0.93 (0.81, 1.06) | 0.1444 | 0.90 (0.78, 1.03) | 0.0539 | 0.89 (0.77, 1.03) | 0.0441 |
| Log 22:1n−9 | 0.83 (0.71, 0.98) | 0.0043 | 0.84 (0.71, 1.00) | 0.0125 | 0.83 (0.70, 0.99) | 0.0086 |
| Log 24:1n−9 | 0.96 (0.83, 1.12) | 0.4906 | 0.97 (0.83, 1.13) | 0.5968 | 0.96 (0.81, 1.14) | 0.5856 |
Model 1 was a conditional logistic regression matched for matching factors of year of birth, date of blood collection, and age at blood test. Coronary artery disease was defined as nonfatal myocardial infarction, fatal myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, coronary death, and sudden death. Model 2 was adjusted as for model 1 and for BMI, smoking status, exercise level, alcohol consumption, parental history of myocardial infarction, treatment of diabetes, treatment of hypertension, and treatment of hypercholesterolemia. Model 3 was adjusted as for model 2 and for RBC omega-3 fatty acids and RBC SFAs. PHS, Physicians’ Health Study; RBC, red blood cell.
The inverse association of log 22:1n−9 with CAD did not change after being additionally adjusted for RBC omega-3 fatty acids and SFA (Table 2, model 3). In this multivariate model, 20:1n−9 also showed significant association with CAD at the α = 0.05 level. The OR (95% CI) for CAD per 1-SD higher 20:1n−9 was 0.89 (0.80, 1.00; P = 0.0441). Furthermore, additional adjustment for 16:1n–7 and 18:1n–7 did not alter the results (data not shown).
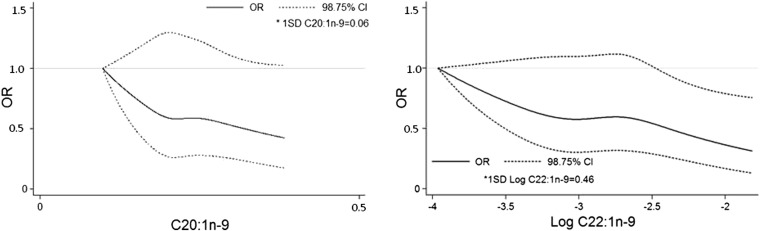
Cubic splines also revealed inverse associations of 20:1n−9 and log 22:1n−9 with CAD (Figure 2). However, after Bonferroni correction (P < 0.0125), only log 22:1n−9 showed a significant association with CAD [per 1-SD higher log 22:1n−9: OR (98.75% CI) of 0.83 (0.70, 0.99); P = 0.0086] (Table 2, model 3). In contrast, we did not observe significant associations between other RBC MUFAs (cis 18:1n−9 and 24:1n−9) and CAD risk after adjustment for confounding factors (all P > 0.05; Table 2). cis 18:1n−9 showed a significant positive association with CAD in the minimally adjusted model at the α = 0.05 level [OR (95% CI): 1.11 (1.02, 1.22); P = 0.0219; Table 2, model 1], but this result became nonsignificant after Bonferroni correction.
FIGURE 2.
Restricted cubic spline for coronary artery disease by 20:1n−9 and log 22:1n−9. Solid dark lines represents ORs, and dotted lines represent 98.75% CIs. Models were adjusted for matching factors and BMI, smoking status (never, current, and past smokers), exercise (rarely or never, 1–3 times/mo, 1–4 times/wk, and 5–7 times/wk), alcohol consumption (<1, 1–4, 5–7, and >7 times/wk), parental history of myocardial infarction (before age 50 y), treatment of hypertension, diabetes, hypercholesterolemia, and RBC omega-3 fatty acids and SFAs. Spline knots were placed at 10th, 36.7th, 63.4th, and 90th percentiles of each of the long-chain MUFAs, and the lowest 1st percentile (0.10 for 1st percentile of 20:1n–9 and –3.91 for 1st percentile of log 22:1n–9) served as the reference. The lowest 1% and the highest 99% of each long-chain MUFA were cut from the graphs. Coronary artery disease was defined as nonfatal myocardial infarction, fatal myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, coronary death, and sudden death.
DISCUSSION
In this prospective, nested case-control study, we showed inverse associations of RBC 22:1n−9 but not other MUFAs with CAD risk in US male physicians after Bonferroni correction for multiple testing.
RBC 20:1n−9 (gondoic acid), 22:1n−9 (erucic acid), and CAD risk
Our study showed 17% lower risk of CAD per 1-SD higher RBC 22:1n−9 (erucic acid) after adjustment for multiple covariates and Bonferroni correction (Table 2, model 3). To the best of our knowledge, this is the first study to evaluate the association of RBC 22:1n−9 with CAD risk. One epidemiologic study from India reported inverse association between CAD risk and the use of mustard oil (oil that is rich in MUFAs, especially in erucic acid) (21). However, mustard oil is also enriched with PUFAs. Therefore, it is not clear whether erucic acid consumption has protective effects on CAD. Although higher concentrations of erucic acid might lead to cardiolipotoxity, current data available in the literature are inadequate to confirm the role of erucid acid on CAD and elucidate underlying biologic pathways. Additional investigations are needed to evaluate cardioprotective effects of RBC erucic acid.
RBC 20:1n−9 (gondoic acid) showed significant inverse association with CAD before, but not after, Bonferroni correction. One study reported that the consumption of macadamia nuts (which are rich in oleic and palmitoleic acids) raised plasma concentrations of palmitoleic, oleic, and gondoic acids and favorably influenced oxidative stress, thrombosis, and inflammation (22). Hence, it is possible that a high concentration of RBC gondoic acid could confer cardioprotection. However, the study did not perform correction for multiple comparisons. The metabolism of gondoic acid has not been completely elucidated, which underscores the need for additional investigations of gondoic acid.
Although the clinical significance of the observed inverse relation of erucic acid with CAD was not obvious at this time, if replicated, our findings could provide novel pharmaceutical targets for the use of erucic acid in CAD prevention.
RBC cis 18:1n−9 (oleic acid), 24:1n−9 (nervonic acid), and CAD
Several studies have reported inconsistent associations between plasma cis 18:1n−9 ( oleic acid) concentrations and CAD (4, 23). Our findings of a nonsignificant association between RBC oleic acid and CAD risk are consistent with a lack of oleic-CHD relation in the Atherosclerosis Risk in Communities study (23). In contrast, a Swedish cohort reported a positive association between serum cholesteryl ester oleic acid and CVD mortality, with 18% higher risk of CVD death per 1-SD higher serum oleic acid [HR (95% CI): 1.18 (1.07, 1.30)], after adjustment for total cholesterol, BMI, smoking, physical activity, and hypertension (4). However, correction for multiple comparisons was not performed in the study. Also, the primary outcome of the study was not CAD but CVD mortality, and differences in methodology could partially explain these inconsistencies.
Our results also did not suggest an association between RBC 24:1n−9 (nervonic acid) and CAD. Nervonic acid is a component of membrane sphingolipids and phosphatidylethanolamines. One small cross-sectional study from Japan reported an inverse association between plasma nervonic acid and leptin (24). Additional data are needed to elucidate the role of nervonic acid on CAD risk.
Several studies suggested that the dietary replacement of SFA with MUFA (ie, oleic acid) reduces plasma LDL cholesterol without a reduction of HDL cholesterol (2), and this effect is regarded as one of the major reasons for the cardioprotective effect of MUFAs. However, a meta-analysis by Mensink et al (25) showed that the replacement of SFA with MUFA led to lower HDL cholesterol. Moreover, several epidemiologic studies reported no significant association between MUFAs and risk of CAD (5, 24). In the absence of large randomized clinical trials that evaluated effects of MUFA consumption on CAD prevention, the interpretation of observational data remains difficult.
Of note is that plasma or tissue MUFAs may not be the best estimate of dietary MUFA intake in general. Long-chain MUFAs such as erucic acid and gondoic acid are minor fatty acids mostly synthesized in vivo. The RBC content of MUFAs can be influenced by dietary and lifestyle factors (26). For instance, a low-fat diet might increase oleic acid in the blood lipid proportion (27), and exercise might be associated with higher concentrations of oleic acid of tissues (28).
Strengths and limitations
The current study had some limitations. First, we had only one measurement of RBC long-chain and very-long-chain MUFAs. Thus, we were not able to account for changes in RBC MUFAs over time during the follow-up period. Second, we could not exclude residual and unmeasured confounding as a partial explanation of our findings. Third, our sample consisted of only highly educated male physicians, which limited the generalizability of our findings to other socioeconomic or ethnic groups and women. Nevertheless, our study had several strengths, including a large sample size, matching on key confounders to minimize confounding, prospective study design, validation of incident CAD, and the use of reproducible biomarkers to quantify MUFA.
In conclusion, our data suggest an inverse association of RBC 22:1n−9 with CAD after adjustment for multiple comparisons. However, RBC cis 18:1n−9, 20:1n−9, and 24:1n−9 were not associated with CAD risk in this cohort of US male physicians. If our findings are replicated in other populations, erucic acid might provide novel drug targets for CAD prevention.
Supplementary Material
Acknowledgments
We are indebted to participants in the PHS for their outstanding commitments and cooperation, and we thank the entire PHS staff.
The authors’ responsibilities were as follows—LD: study design and conception, obtainment of funding, and supervision; NRM and AHL: measurement of MUFA; CM and LD: statistical analyses and drafting of the manuscript; and all authors: manuscript review for scientific content. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CAD, coronary artery disease; CVD, cardiovascular disease; PHS, Physicians’ Health Study; RBC, red blood cell.
REFERENCES
- 1.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9. [DOI] [PubMed] [Google Scholar]
- 2.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9. [DOI] [PubMed] [Google Scholar]
- 3.Degirolamo C, Rudel LL. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr Atheroscler Rep 2010;12:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warensjö E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 2008;88:203–9. [DOI] [PubMed] [Google Scholar]
- 5.Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremer J, Norum KR. Metabolism of very long-chain monounsaturated fatty acids (22:1) and the adaptation to their presence in the diet. J Lipid Res 1982;23:243–56. [PubMed] [Google Scholar]
- 7.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab 2012;15:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–58. [DOI] [PubMed] [Google Scholar]
- 9.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol 2009;53:317–33. [DOI] [PubMed] [Google Scholar]
- 10.Djoussé L, Matthan NR, Lichtenstein AH, Gaziano JM. Red blood cell membrane concentration of cis-palmitoleic and cis-vaccenic acids and risk of coronary heart disease. Am J Cardiol 2012;110:539–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II–a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol 2000;10:125–34. [DOI] [PubMed] [Google Scholar]
- 12.Steering Committee of the Physicians’ Health Study Research Group Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989;321:129–35. [DOI] [PubMed] [Google Scholar]
- 13.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 14.Djoussé L, Kurth T, Gaziano JM. Cystatin C and risk of heart failure in the Physicians’ Health Study (PHS). Am Heart J 2008;155:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 1964;5:600–8. [PubMed] [Google Scholar]
- 17.Matthan NR, Dillard A, Lecker JL, Ip B, Lichtenstein AH. Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B golden Syrian hamster. J Nutr 2009;139:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay DL, Chen CY, Yeum KJ, Matthan NR, Lichtenstein AH, Blumberg JB. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J 2010;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Report of the Fifth Working Group. Including a Second Revision of the Operating Protocol: Copenhagen, 26 –29 April 1971. Copenhagen, Denmark: Regional Office for Europe, World Health Organization, 1971. [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26; discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi T, Reddy KS, Vaz M, Spiegelman D, Prabhakaran D, Willett WC, Stampfer MJ, Ascherio A. Diet and risk of ischemic heart disease in India. Am J Clin Nutr 2004;79:582–92. [DOI] [PubMed] [Google Scholar]
- 22.Garg ML, Blake RJ, Wills RB, Clayton EH. Macadamia nut consumption modulates favourably risk factors for coronary artery disease in hypercholesterolemic subjects. Lipids 2007;42:583–7.. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Folsom AR, Eckfeldt JH. Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr Metab Cardiovasc Dis 2003;13:256–66. [DOI] [PubMed] [Google Scholar]
- 24.Oda E, Hatada K, Kimura J, Aizawa Y, Thanikachalam PV, Watanabe K. Relationships between serum unsaturated fatty acids and coronary risk factors: negative relations between nervonic acid and obesity-related risk factors. Int Heart J 2005;46:975–85. [DOI] [PubMed] [Google Scholar]
- 25.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr 1995;62:564–71. [DOI] [PubMed] [Google Scholar]
- 27.King IB, Lemaitre RN, Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr 2006;83:227–36. [DOI] [PubMed] [Google Scholar]
- 28.Helge JW, Wu BJ, Willer M, Daugaard JR, Storlien LH, Kiens B. Training affects muscle phospholipid fatty acid composition in humans. J Appl Physiol 2001;90:670–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.