Abstract
Insulin-like growth factor (IGF)-1 is a well characterized growth factor that plays a role in the regulation of myocardial structure and function. Using an ex vivo murine model, Davani and coworkers, in this issue of Critical Care, demonstrate that IGF-1 confers cardiac protection against ischemia via mitochondria-dependent mechanisms. Those investigators used the ratio of mitochondrial to nuclear DNA to demonstrate that IGF-1, which prevents reduction in this ratio during reperfusion, provides cytoprotection. This commentary also reviews mechanisms of IGF-1 function and provides a graphic representation of IGF-1 signaling mechanisms in potential crosstalk relations with mediators of inflammation in the heart (specifically tumor necrosis factor-α).
Keywords: heart, inflammation, injury, ischemia, signaling
Introduction
Insulin-like growth factor (IGF)-1 is a well characterized growth factor for a variety of cells and plays a role in the regulation of myocardial structure and function. There is evidence that IGF-1 improves cardiac performance and muscle survival in heart subjected to ischemia/reperfusion [1,2]. Therefore, elucidating the IGF-1 signaling pathways, especially in relation to cell survival, may help to promote the potential use of IGF-1 in the treatment of heart disease.
Using an ex vivo murine model, Davani and coworkers [1], in this issue of Critical Care, demonstrate that IGF-1 confers cardiac protection from reperfusion injury via mitochondria-dependent mechanisms. Because of its vital role in myocardial energy production, the quantity of functional mitochondria is essential to myocardial activity and health. Davani and coworkers propose that the ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA), which is increased during ischemia and reduced with reperfusion, is a very sensitive marker of cardiac injury. They then use the mtDNA : nDNA ratio to demonstrate that IGF-1, which prevents the reduction in mtDNA : nDNA ratio that occurs during reperfusion, confers myocardial cytoprotection. The mtDNA : nDNA ratio emphasizes the importance of mitochondria-related mechanisms in reperfusion injury, and it also provides a novel reference for evaluating cardiac injury.
Insulin-like growth factor-1 mediated cell survival in myocardial tissue
Insulin-like growth factor-1 signaling pathways in myocardial tissue
Two distinct forms of cell death, namely necrosis and apoptosis, are involved in the survival effect of IGF-1 in the cardiovascular system. IGF-1 not only inhibits necrosis via preservation of mitochondrial function, specifically by inhibiting membrane permeability and cytochrome C release in mitochondria, but also it reduces apoptosis through the inhibition of death signals generated by mitochondria [3].
IGF-1 survival signals are mediated by binding to its receptor, the type 1 IGF receptor. This receptor is a heterotetramer containing cytosolic substrates (insulin receptor substrate [IRS], Shc, and Gab-1), which serve as docking proteins for downstream events. It has been demonstrated that IGF-1 mediated survival correlates with the activation of the protein kinase Akt, and this pathway was disrupted by applying wortmannin, a specific inhibitor of phosphoinositol-3 (PI3)-kinase, in different models of cardiac ischemia [4,5]. Moreover, the antiapoptotic effects of IGF-1 are abolished if Akt activation is suppressed during ischemia/reperfusion injury in transgenic mouse hearts that overexpress IGF-1 [6]. Recent studies revealed that IGF-1 induced myocardial protection from ischemia/reperfusion injury is associated with attenuated Bax induction and caspase 3 activation [3,7]. There is also evidence that P70 S6 kinase is involved in the survival signal of IGF-1 in H9c2 cells, and a PI3-kinase → Akt → P70 S6 kinase pathway exists in cardiomyocytes [8].
However, IGF-1 induced myocardial protection cannot be explained by the PI3-kinase/Akt pathway alone. In addition to inhibition of Bax expression, the protective effect of IGF-1 is associated with the inhibition of C-jun N-terminal protein kinase activation [9]. Moreover, Yamashita and coworkers [6] showed that inhibition of p38 mitogen-activated protein kinase activation was accompanied by suppression of Akt activation during ischemia/reperfusion in the IGF-1 transgenic heart.
Therefore, IGF-1 receptor binding serves as an extracellular signal to stimulate its intracellular substrate IRS, which leads to activation of PI3-kinase. The products of the PI3-kinase reaction then activate Akt. Activated Akt kinase plays a crucial role in antiapoptosis by modulating different signal downstream events (Fig. 1).
Figure 1.
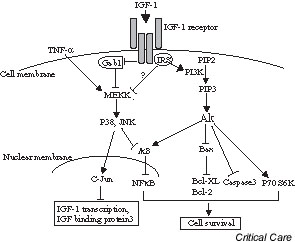
Model of insulin-like growth factor (IGF)-1 mediated signal transduction pathways to regulate cell survival in myocardial tissue. IRS, insulin receptor substrate; JNK, C-jun N-terminal protein kinase; MEKK, mitogen-activated protein kinase kinase kinase; NFκB, nuclear factor-κB; PI3, phosphoinositol-3; PIP, phosphoinositol-4-phosphate; TNF, tumor necrosis factor.
Insulin-like growth factor-1 and tumor necrosis factor
Tumor necrosis factor (TNF)-α is a proinflammatory cytokine that has been implicated in the pathogenesis of cardiovascular disease. Myocardial TNF-α is an autocrine contributor to myocardial contractile dysfunction and cardiomyocyte death in ischemia/reperfusion injury, sepsis, chronic heart failure, viral myocarditis, and cardiac allograft rejection [10].
Accumulating evidence shows that crosstalk of TNF-α and IGF-1 signaling pathways may modulate biologic functions in cardiovascular tissue. Patients with chronic heart failure exhibit an inverse relationship between IGF-1 and TNF levels [11]. Drugs that increase the concentration of TNF-α decrease IGF-1 concentration in the rat heart [12]. It has been demonstrated that TNF-α suppresses IGF-1 mRNA expression and upregulates IGF-binding protein 3 [13]. Moreover, TNF-α attenuation of IGF-1 may be involved in phosphorylation of the c-Jun pathway [14]. On the other hand, it has been suggested that the cytoprotection associated with IGF-1 is correlated with downregulation of nuclear factor-κB activation induced by TNF-α [15].
IGF-1 is important for the growth and survival of cardiovascular tissue. However, long-term IGF-1 treatment downregulates the expression of Gab1 associated with MEKK3, which enhances TNF-induced c-Jun and nuclear factor-κB activation, as well as adhesion molecule expression in endothelial cells [9]. Long-term IGF-1 treatment thus leads to hypersensitivity to TNF-mediated signaling events.
Effects of insulin-like growth factor-1 on myocardial function
IGF-1 is not only an important survival factor for the heart, but it also plays a role in improving cardiac function. IGF-1 has been shown to improve perfusion pressure and left ventricular compliance in ischemia/reperfusion by decreasing interstitial edema, preserving the myocardial tissue lattice, and protecting mitochondrial integrity [1]. IGF-1 mediated protection of cardiac contractility may be involved in the PI3 kinase pathway and in activation of protein kinase C [16]. IGF-1 induces increases in intracellular calcium [17] and myofilament calcium sensitivity, both of which improve cardiac contractility [16].
Conclusion
IGF-1 is an important growth factor for the survival and function of the heart. Accordingly, much focus has been given to IGF-1 as a potential therapeutic agent. Indeed, it has been shown that IGF-1 has a cytoprotective effect in different models of cardiac ischemic injury. However, the role of IGF-1 in cardiovascular disease is still not clear. Recent evidence suggests that long-term exposure to IGF-1 may actually be detrimental to the heart. It is clear that further investigation of the IGF-1 signaling network is needed before its clinical application can be declared.
Competing interests
None declared.
Abbreviations
IGF = insulin-like growth factor; IRS = insulin receptor substrate; mtDNA = mitochondrial DNA; nDNA = nuclear DNA; PI3 = phosphoinositol-3; TNF = tumor necrosis factor.
See related Research article: http://ccforum.com/content/7/6/R176
References
- Davani EY, Brumme Z, Singhera GK, Cote HCF, Harrigan PR, Dorscheid DR. Insulin-like growth factor-1 protects ischemic murine myocardium from ischemia/reperfusion associated injury. Crit Care. 2003;7:R176–R183. doi: 10.1186/cc2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci USA. 1995;92:8031–8035. doi: 10.1073/pnas.92.17.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T, Otani H, Nakao Y, Hattori R, Osako M, Imamura H. IGF-I differentially regulates Bcl-xL and Bax and confers myocardial protection in the rat heart. Am J Physiol Heart Circ Physiol. 2001;280:H1191–H1200. doi: 10.1152/ajpheart.2001.280.3.H1191. [DOI] [PubMed] [Google Scholar]
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H, Yamamura T, Nakao Y, Hattori R, Kawaguchi H, Osako M, Imamura H. Insulin-like growth factor-I improves recovery of cardiac performance during reperfusion in isolated rat heart by a wortmannin-sensitive mechanism. J Cardiovasc Pharmacol. 2000;35:275–281. doi: 10.1097/00005344-200002000-00015. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res. 2001;88:609–614. doi: 10.1161/01.res.88.6.609. [DOI] [PubMed] [Google Scholar]
- Wu W, Lee W, Wu Y, Chen D, Liu T, Sharma PM, Wang PH. Expression of constitutively active phosphatidylinositol 3 kinase inhibits activation of caspase 3 and apoptosis of cardiac muscle cells. J Biol Chem. 2000;275:40113–40119. doi: 10.1074/jbc.M004108200. [DOI] [PubMed] [Google Scholar]
- Hong F, Kwon SJ, Jhun BS, Kim SS, Ha J, Kim SJ, Sohn NW, Kang C, Kang I. Insulin-like growth factor-1 protects H9c2 cardiac myoblasts from oxidative stress-induced apoptosis via phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Life Sci. 2001;68:1095–1105. doi: 10.1016/S0024-3205(00)01012-2. [DOI] [PubMed] [Google Scholar]
- Che W, Lerner-Marmarosh N, Huang Q, Osawa M, Ohta S, Yoshizumi M, Glassman M, Lee JD, Yan C, Berk BC, Abe J. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circ Res. 2002;90:1222–1230. doi: 10.1161/01.RES.0000021127.83364.7D. [DOI] [PubMed] [Google Scholar]
- Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Pflaum CD, Clark AL, Strasburger CJ, Hooper J, Poole-Wilson PA, Coats AJ, Anker SD. Deficient insulin-like growth factor I in chronic heart failure predicts altered body composition, anabolic deficiency, cytokine and neurohormonal activation. J Am Coll Cardiol. 1998;32:393–397. doi: 10.1016/S0735-1097(98)00226-5. [DOI] [PubMed] [Google Scholar]
- Fan J, Li YH, Bagby GJ, Lang CH. Modulation of inflammation-induced changes in insulin-like growth factor (IGF)-I and IGF binding protein-1 by anti-TNF antibody. Shock. 1995;4:21–26. doi: 10.1097/00024382-199507000-00003. [DOI] [PubMed] [Google Scholar]
- Anwar A, Zahid AA, Scheidegger KJ, Brink M, Delafontaine P. Tumor necrosis factor-alpha regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vascular smooth muscle. Circulation. 2002;105:1220–1225. doi: 10.1161/hc1002.105187. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Tumor necrosis factor-alpha decreases insulin-like growth factor-I messenger ribonucleic acid expression in C2C12 myoblasts via a Jun N-terminal kinase pathway. Endocrinology. 2003;144:1770–1779. doi: 10.1210/en.2002-220808. [DOI] [PubMed] [Google Scholar]
- Vallee S, Fouchier F, Bremond P, Briand C, Marvaldi J, Champion S. Insulin-like growth factor-1 downregulates nuclear factor kappa B activation and upregulates interleukin-8 gene expression induced by tumor necrosis factor alpha. Biochem Biophys Res Commun. 2003;305:831–839. doi: 10.1016/S0006-291X(03)00866-0. [DOI] [PubMed] [Google Scholar]
- Cittadini A, Ishiguro Y, Stromer H, Spindler M, Moses AC, Clark R, Douglas PS, Ingwall JS, Morgan JP. Insulin-like growth factor-1 but not growth hormone augments mammalian myocardial contractility by sensitizing the myofilament to Ca2+ through a wortmannin-sensitive pathway: studies in rat and ferret isolated muscles. Circ Res. 1998;83:50–59. doi: 10.1161/01.res.83.1.50. [DOI] [PubMed] [Google Scholar]
- Ren J, Walsh MF, Hamaty M, Sowers JR, Brown RA. Altered inotropic response to IGF-I in diabetic rat heart: influence of intracellular Ca2+ and NO. Am J Physiol. 1998;275:H823–H830. doi: 10.1152/ajpheart.1998.275.3.H823. [DOI] [PubMed] [Google Scholar]


