Abstract
Background
Malaria immunity is commonly believed to wane in the absence of Plasmodium falciparum exposure, based on limited epidemiological data and short-lived antibody responses in some longitudinal studies in endemic areas.
Methods
A cross-sectional study was conducted among sub-Saharan African adults residing in Spain for 1 up to 38 years (immigrants) with clinical malaria (n=55) or without malaria (n=37), naïve adults (travelers) with a first clinical malaria episode (n=20) and life-long malaria exposed adults from Mozambique (semi-immune adults) without malaria (n=27) or with clinical malaria (n=50). Blood samples were collected and IgG levels against the erythrocytic antigens AMA-1 and MSP-142 (3D7 and FVO strains), EBA-175 and DBL-α were determined by Luminex. IgG levels against antigens on the surface of infected erythrocytes (IEs) were measured by flow cytometry.
Results
Immigrants without malaria had lower IgG levels than healthy semi-immune adults regardless of the antigen tested (P≤0.026), but no correlation was found between IgG levels and time since migration. Upon reinfection, immigrants with malaria had higher levels of IgG against all antigens than immigrants without malaria. However, the magnitude of the response compared to semi-immune adults with malaria depended on the antigen tested. Thus, immigrants had higher IgG levels against AMA-1 and MSP-142 (P≤0.015), similar levels against EBA-175 and DBL-α, and lower levels against IEs (P≤0.016). Immigrants had higher IgG levels against all antigens tested compared to travelers (P≤0.001), both with malaria.
Conclusions
Upon cessation of malaria exposure, IgG responses to malaria-specific antigens were maintained to a large extent, although the conservation and the magnitude of the recall response depended on the nature of the antigen. Studies on immigrant populations can shed light on the factors that determine the duration of malaria specific antibody responses and its effect on protection, with important implications for future vaccine design and public health control measures.
Introduction
Maintenance of long-term memory responses is critical for achieving protective immunity against many pathogens. The understanding of differential immuno-reactivity to malaria and maintenance of these immune responses is fundamental for the development and design of immunogenic strategies for disease control and eradication. In malaria endemic areas, immunity is acquired gradually with age and continuous exposure, first to severe disease and ultimately to clinical malaria and high parasitemia [1]. Nevertheless, it is thought that upon cessation of exposure to Plasmodium falciparum infection immunity wanes rapidly, and this is in contrast with the long-term antibody-mediated immunity that follows one or few exposures to antigens from other infectious microbes [2].
The control of P. falciparum infections is complex, and requires the combined action of antibodies (Ab) and cell-mediated immune responses against both pre-erythrocytic and blood stages; and these two effector mechanisms are required for both anti-parasitic as well as clinical immunity [3,4]. The relevance of Ab responses in malaria protection was established several decades ago by immunoglobulin G (IgG) passive transfer experiments [5,6], and different mechanisms of immunity have been proposed. Potential Ab effector actions include: blockade of hepatocyte invasion by sporozoites and red blood cell invasion by merozoites; Ab-dependent cellular killing through interaction of target-bound Ab with certain Fc receptors from cell surfaces; opsonization of infected erythrocytes (IE) inducing phagocytic clearance; and neutralization of the parasite glycosylphosphatidylinositol, inhibiting the induction of the inflammatory cytokine cascade [3]. P. falciparum antigens targeted by naturally acquired IgG associated with immunity include the merozoite proteins: apical membrane antigen 1 (AMA-1), the 42-kDa fragment from the C terminus of merozoite surface protein 1 (MSP-142), and the 175 kDa erythrocyte binding antigen (EBA-175), all three involved in erythrocyte invasion [7–11]. In addition, variant surface antigens (VSA) expressed on IE membranes are also targets of naturally acquired Ab responses associated with immunity [12]. The P. falciparum erythrocyte membrane protein 1 (PfEMP-1) is the major antigen of this VSA family, containing several domains that mediate cytoadherence to host cells, like the Duffy binding-like alpha (DBL-α) domain that is involved in rosetting [13].
Despite the common perception that immune memory to malaria is short-lived in the absence of exposure, most clinical evidence suggests that immunity may last for long periods of time. Immigrants maintain some immunity to clinical malaria, and have milder episodes than naïve adults with a malaria primary infection [14–21]. Also in areas of low or unstable malaria transmission such as Madagascar, adults previously exposed to malaria, even several decades before, were protected against clinical disease during malaria epidemics [22–24].
However, data on the longevity of protective immune responses are scarce. Ab responses to malarial antigens are often thought to be short-lived [25–30], although this has mostly been reported in children in areas where malaria is endemic [27–31]. On the contrary, in adults, long-lived IgG responses have been detected [32–35]. It appears that frequent reinfection is required to maintain high levels of circulating Ab, thus in highly endemic areas Ab levels are stable [36,37], but in low or unstable transmission areas Ab levels diminish quickly after an infection [28,38], showing seasonal variation [26,27,39–41]. Conversely, memory B cells (MBC) can persist with reduced transmission [42–45]. However, a study reported the presence of Ab, but only very low frequencies of malaria-specific MBC in children, suggesting a low induction of malaria-specific circulating MBC as a reason for short-lived anti-malarial Ab responses [46].
The aim of this study was to measure P. falciparum-specific Ab responses as indicative of loss or maintenance of immunity in immigrants who have not been exposed to malaria for long periods of time. We compared IgG levels against a panel of blood stage antigens thought to be involved in malaria immunity between previously exposed immigrants, continuously exposed adults and naïve travelers, with or without a clinical malaria episode, using Luminex and flow cytometry.
Materials and Methods
Ethics Statement
Written informed consent was obtained from participants before sample collection. Approval for the protocols was obtained from the Hospital Clínic of Barcelona Ethics Review Committee and the National Mozambican Ethics Review Committee. Parasitemic individuals were treated according to standard national guidelines at the time of the studies.
Study design, subjects and sample collection
Three groups of participants were recruited for this study: (i) sub-Saharan African adults originally from malaria endemic areas residing in Spain (immigrants) without clinical malaria (n=37) or with clinical malaria (n=55) upon return from travel; (ii) adults from a sub-Saharan Africa endemic area with life-long exposure to P. falciparum infection (semi-immune adults), with (n=50) or without clinical malaria (n=27); and (iii) naïve adults from a non-endemic area returning from a sub-Saharan Africa malaria endemic region with a first malaria episode (travelers, n=20).
Immigrants were recruited at the Tropical Medicine Units of Hospital Clínic de Barcelona (Barcelona, Spain), Hospital Arnau de Vilanova (Lleida, Spain) and Hospital Santa Caterina de Salt (Girona, Spain) between 2005 and 2009. Travelers were recruited at the Tropical Medicine Unit of the Hospital Clínic de Barcelona (Barcelona, Spain) [47]. Fifty-five immigrants and 20 travelers were diagnosed with P. falciparum clinical malaria after traveling to an African country. Clinical malaria was defined as the presence of asexual P. falciparum parasites on Giemsa-stained blood smears detected by light microscopy, together with fever. P. falciparum parasitemia in blood was measured as the percentage of parasitized red blood cells. Blood samples from acute malaria episodes (day 0) and at convalescence after malaria treatment (day 7) were collected by venipuncture into vacutainers without anticoagulant for serum cryopreservation at -80° C. In addition, blood samples from 37 immigrants visiting the Tropical Medicine Units without malaria were also collected. These immigrants were healthy companions or those presenting with non-malaria diseases. Most of them had a febrile syndrome or traveler diarrhea but the following conditions were also diagnosed: giardiasis, katayama syndrome, mononucleosis syndrome EBV, pneumonia, pruritus eczema, anxiety disorder, appendicitis, dermatitis, toxic syndrome, viral infection, ketoacidosis, diabetes, headache, spontaneous abortion, bacterial lung abscess and HIV infection. Clinical and demographical data were recorded in standardized questionnaires. Data on Ab levels from travelers have been previously published [47], but are re-analysed here for comparison to the immigrant group.
Semi-immune adults were from Manhiça District in Mozambique, where malaria transmission is perennial, with some seasonality and moderate intensity. Fifty semi-immune adults with P. falciparum clinical malaria were recruited at the Manhiça District Hospital in the context of a hospital-based study conducted at the Centro de Investigação em Saúde de Manhiça in 2006 [13]. Clinical malaria was defined as the presence of asexual P. falciparum parasites on blood smears, together with fever. Blood films were Giemsa-stained, and examined using a light microscope and parasite density in blood was measured as parasites/µL. Additionally, 27 healthy semi-immune adults were recruited in a cross-sectional study in the Manhiça District between 2005 and 2006. They were adults who had not lived in a city and were not parasitemic by microscopy at the moment of collecting the blood samples. Blood samples were collected by venipuncture into heparinized tubes, and plasma samples were cryopreserved at -80° C.
Recombinant proteins
AMA-1 from the 3D7 strain [48], the receptor-binding region F2 of EBA-175 from the CAMP strain [49] and the DBL-α domain of a PfEMP-1 involved in rosetting [13] were produced as recombinant proteins at ICGEB. AMA-1 from FVO strain, and MSP-142 from 3D7 and FVO strains [50,51] were produced as recombinant proteins at WRAIR.
Antibody levels to recombinant proteins
IgG responses to P. falciparum antigens were determined using Luminex xMAPTM (Luminex Corp., Austin, Texas, USA) and the Bio-Plex 100 platform (Bio-Rad, Hercules, California, USA) as previously described [47,52]. A pool of plasma samples from hyper-immune Mozambican adult volunteers (n=33), and 4 plasma samples from non-exposed European adults (n=42) were added in duplicates to each plate as positive and negative controls, respectively. In addition, curves of a pool of plasma samples from hyper-immune adults were added in each experiment (Figure S1). Study samples were tested in duplicates at the dilutions 1/1,000 and 1/30,000, but only the dilution 1/1,000 was chosen for the statistical analyses because of a wider quantitative dynamic range. In some wells no serum/plasma was added as a control of background. Plates were read using Bio-Plex Manager version 4.0, and at least 100 microspheres per analyte were acquired per sample. Median fluorescent intensity with background fluorescence subtracted was exported, and arbitrary units (AU) concentration for each Ab was calculated by dividing the median fluorescent intensity of each sample by the median fluorescent intensity of the positive control run in each plate.
Antibodies to IEs surface antigens
Two laboratory clones (CS2, R29), three isolates collected from travelers to endemic regions (IETrav1, IETrav2, IETrav3), two pediatric isolates from Mozambican children, one with uncomplicated malaria (IECh1) and another with severe malaria (IECh2), and one isolate from a pregnant Mozambican woman (IEWoman), each of whom had the O blood group, were tested for recognition by IgG using flow cytometry as previously described [47]. Cryopreserved ring-stage parasites were thawed in a sorbitol gradient and grown to late trophozoites. Study samples were tested blindly in a single assay against each parasite. A pool of plasma samples from hyper-immune Mozambican adults (n=11) and 10 samples from non-exposed European adults were included as positive and negative controls, respectively. Data from 1,000 positive events was acquired with a Becton-Dickinson (BD) FACSCalibur flow cytometer. Reactivity against IE surface antigens was expressed as the difference between the geometric mean fluorescent intensity (MFI) of IEs and the MFI of uninfected erythrocytes. Representative flow cytometry data are showed in Figure S2.
Statistical methods
Recognition of recombinant proteins by Ab was considered positive if AU values were above the mean of the negative controls plus 2 standard deviations. Threshold values for seroprevalence were: 238.02 AU for AMA-1 3D7; 1134.73 AU for AMA-1 FVO; 921.18 AU for MSP-142 3D7; 638.33 AU for MSP-142 FVO; 3110.36 AU for EBA-175; 1572.55 AU for DBL-α. Recognition of parasites by Ab was considered positive if MFI values were above the mean of the negative controls plus 3 standard deviations. Threshold values for seroprevalence were: 36.88 MFI for IETrav1, 38.55 MFI for IETrav2, 24.75 MFI IETrav3, 14.23 MFI for CS2, 19.92 MFI for R29, 8.69 MFI for ECh1, 1.98 MFI for IECh2, and 5.54 MFI for IEWoman. Comparisons between groups for categorical variables were done using χ2 test or Fisher’s exact test. Continuous variables were analyzed using the non-parametric Kruskal Wallis test or the Wilcoxon Rank Sum test. Correlations between IgG levels and years since migration were assessed by Spearman’s rank coefficient. All p-values were two-sided and considered statistically significant when < 0.05. All data collected were analyzed using Stata version 11.0 (Stata Corporation, College Station, Texas, USA).
Results
Characteristics of the study participants
The characteristics of study participants are summarized in Table 1. Immigrants with malaria were originally from Cameroon (n=3, 5.5%), Ghana (n=8, 14.6%), Guinea-Conakry (n=4, 7.3%), Equatorial Guinea (n=12, 21.8%), Gambia (n=8, 14.6%), Mali (n= 4, 7.3%), Mauritania (n=1, 1.8%), Mozambique (n=1, 1.8%), Nigeria (n=6, 10.9%) and Senegal (n=7, 12.7%). Immigrants without malaria were from Benin (n=1, 2.7%), Burkina Faso (n=2, 5.41%), Cameroon (n=3, 8.11%), Guinea-Conakry (n=3, 8.11%), Equatorial Guinea (n=3, 8.11%), Gambia (n=3, 8.11%), Kenya (n=1, 2.7%), Mali (n=9, 24.32%), Mauritania (n=1, 2.7%), Mozambique (n=1, 2.7%), Nigeria (n=3, 8.11%), Senegal (n=5, 13.61%) and Sudan (n=1, 2.7%). Most of the semi-immune adults without malaria were males, whereas there were more females among the semi-immune adults with malaria than in the other groups (P=0.001). Visiting countries were very heterogeneous among immigrants and travelers. Immigrants were those returning from visiting their countries of origin. Travelers came from Burkina Faso & Mali & Senegal (n=1, 5.0%), Burkina Faso (n=3, 15.0%), Burkina Faso & Mali & Ghana & Togo (n=1, 5.0%), Ivory Coast (n=1, 5.0%), Guinea-Conakry (n=1, 5.0%), Equatorial Guinea (n=3, 15.0%), Gambia & Senegal (n=1, 5.0%), Madagascar (n=1, 5.0%), Mali (n=1, 5.0%), Mozambique (n=2, 10.0%), Mozambique & South Africa (n=1, 5.0%), Senegal (n=3, 15.0%) and Sierra Leone & Senegal (n=1, 5.0%). Immigrants with malaria had lived a median of 7 years in Spain, whereas immigrants without malaria had lived a median of 5 years (P= 0.0362, Table 1). However, 10% of immigrants with malaria were returning from their first trip to their original country, 21% had previously returned 1 to 2 times, 50% had returned 3 to 4 times and 19% had returned more than 5 times. Forty-seven percent of immigrants without malaria had never returned to their original country, 15% had returned 1 to 2 times, 12% had returned 3 to 4 times and 27% had returned more than 5 times. No significant differences were detected in parasitemia between immigrants and travelers (P=0.0890, Table 1).
Table 1. Characteristics of the study participants.
|
Immigrants
|
Travelers |
Semi-Immune
|
|||
|---|---|---|---|---|---|
| Characteristics | Malaria | No Malaria | Malaria | Malaria | No Malaria |
| N | |||||
| Day 0 | 55 | 37 | 20 | 50 | 27 |
| Day 7 | 29 | 0 | 12 | 0 | 0 |
| Age, median IQR (years)a | 34 (29-; 43) | 36 (30; 44) | 32 (28.5; 38.5) | 33 (27; 42) | 41 (39, 43) |
| Sexb, n (%) | |||||
| Males | 40 (73) | 23 (62) | 15 (75) | 28 (56) | 26 (96) |
| Origin area, n (%) | |||||
| Europe | 0 (0) | 0 (0) | 17 (85) | 0 (0) | 0 (0) |
| Africa | 54 (100) | 37 (100) | 0 (0) | 50 (100) | 27 (100) |
| Others | 0 (0) | 0 (0) | 3 (15) | 0 (0) | 0 (0) |
| Time since immigration, median IQR (years)c | 7 (5; 14) | 5 (2; 9) | na | na | na |
| Number of previous returns, n (%) | |||||
| 0 | 5 (10) | 16 (47) | na | na | na |
| 1-2 | 11 (21) | 5 (15) | na | na | na |
| 3-4 | 26 (50) | 4 (12) | na | na | na |
| >5 | 10 (19) | 9 (27) | na | na | na |
| Parasitemia by microscopy | |||||
| median IQR (%)d | 0.4 (0.02; 1.5) | na | 0.075 (0.01;0.8) | nd | na |
| median IQR (parasites/μl) | nd | na | nd | 34783.5 (7721, 55122) | na |
Abbreviations: IQR, interquartile range; nd, not determined; na, not applicable
P=0.0515 Kruskal Wallis test. Data missing from 2 semi-immune adults without malaria
P=0.001 χ2
P= 0.0362 Wilcoxon Rank Sum test. Data available from 52 immigrants with malaria and 34 without malaria.
P=0.0890 Wilcoxon Rank Sum test.
Antibodies in immigrants without malaria
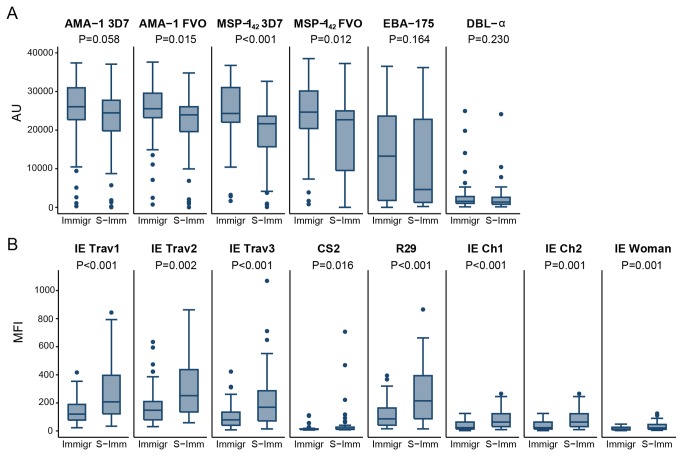
IgG levels and seroprevalence against erythrocytic antigens associated with immunity, and against a set of IE of different origins, were determined in immigrants and compared to semi-immune adults from Mozambique to assess if the cessation of exposure had an effect on basal Ab levels. IgG levels (Figure 1) and seroprevalences (Table 2) against all recombinant proteins tested were lower in non-infected immigrants compared to healthy semi-immune adults, with the exception of AMA-1 3D7 that the difference between groups was not statistically significant, and the seroprevalence of DBL-α that was similar in both groups.
Figure 1. IgG antibody responses to merozoite antigens in immigrants (Immigr) and semi-immune adults (S-Immune) without malaria.

Data are presented as boxplots that illustrate the medians and the 25th and 75th quartiles, and the whiskers represent the 10% and 90% percentiles. Outliers are marked with circles. P-values were calculated using the Wilcoxon Rank Sum test. Cutoff values for seroprevalences were 238.02 AU for AMA-1 3D7; 1134.73 AU for AMA-1 FVO; 921.18 AU for MSP-142 3D7; 638.33 AU for MSP-142 FVO; 3110.36 AU for EBA-175; 1572.55 AU for DBL-α.
Table 2. IgG seroprevalence in immigrants and semi-immune adults without malaria.
|
Immigrants (n=37)
|
Semi-Immune (n=27)
|
||||
|---|---|---|---|---|---|
| n | % | n | % | P-value* | |
| AMA-1 3D7 | 35 | 95 | 27 | 100 | 0.504 |
| AMA-1 FVO | 30 | 81 | 27 | 100 | 0.018 |
| MSP-142 3D7 | 31 | 84 | 27 | 100 | 0.035 |
| MSP-142 FVO | 30 | 81 | 27 | 100 | 0.018 |
| EBA-175 | 12 | 32 | 17 | 63 | 0.022 |
| DBL-α | 7 | 19 | 12 | 44 | 0.051 |
Fisher’s exact test
Antibodies in immigrants upon malaria reinfection
Ab levels and prevalence were determined in immigrants and semi-immune adults, both with an acute episode of clinical malaria (Figure 2, Table 3). Immigrants showed higher Ab levels against the merozoite antigens AMA-1 and MSP-142 (Figure 2A). There were no differences in Ab responses to EBA-175 and DBL-α. However, immigrants had significantly lower levels of Ab against all IEs compared to semi-immune adults (Figure 2B). No differences were detected in seroprevalence between immigrants and semi-immune adults with the exception of IECh1, which was higher in the later (Table 3).
Figure 2. IgG antibody responses to merozoite antigens (A) and P. falciparum Infected Erythrocytes (B) in immigrants (Immigr) and Semi-Immune adults (S-Imm) with clinical malaria.
Data are presented as boxplots that illustrate the medians and the 25th and 75th quartiles, and the whiskers represent the 10% and 90% percentiles. Outliers are marked with circles. P-values were calculated using the Wilcoxon Rank Sum test. Cutoff values for seroprevalences were 238.02 AU for AMA-1 3D7, 1134.73 AU for AMA-1 FVO, 921.18 AU for MSP-142 3D7, 638.33 AU for MSP-142 FVO,3110.36 AU for EBA-175, 1572.55 AU for DBL-α, 36.88 MFI for IETrav1, 38.55 MFI for IETrav2, 24.75 MFI IETrav3, 14.23 MFI for CS2, 19.92 MFI for R29, 8.69 MFI for ECh1, 1.98 MFI for IECh2, and 5.54 MFI for IEWoman.
Table 3. IgG seroprevalence in immigrants and semi-immune adults with a clinical malaria episode, and naïve adults with a primary infection.
|
Immigrants (n=55)
|
Semi-Immune (n=50)
|
Travelers (n=20)
|
P-value*
|
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Immigrants vs Semi-Immune | Immigrants vs Travelers | |
| AMA-1 3D7 | 54 | 98 | 48 | 96 | 9 | 45 | 0.604 | <0.001 |
| AMA-1 FVO | 54 | 98 | 47 | 94 | 6 | 30 | 0.345 | <0.001 |
| MSP-142 3D7 | 55 | 100 | 47 | 94 | 10 | 50 | 0.105 | <0.001 |
| MSP-142 FVO | 54 | 98 | 46 | 92 | 8 | 40 | 0.189 | <0.001 |
| EBA-175 | 34 | 62 | 25 | 50 | 0 | 0 | 0.243 | <0.001 |
| DBL-α | 22 | 40 | 14 | 28 | 3 | 15 | 0.222 | 0.054 |
| IETrav1 | 51 | 93 | 49 | 98 | 4 | 20 | 0.366 | <0.001 |
| IETrav2 | 52 | 95 | 50 | 100 | 7 | 35 | 0.245 | <0.001 |
| IETrav3 | 49 | 89 | 49 | 98 | 1 | 5 | 0.115 | <0.001 |
| CS2 | 15 | 27 | 22 | 44 | 0 | 0 | 0.102 | 0.008 |
| R29 | 50 | 91 | 48 | 96 | 7 | 35 | 0.441 | <0.001 |
| IECh1 | 49 | 89 | 50 | 100 | 1 | 5 | 0.028 | <0.001 |
| IEWoman | 47 | 85 | 47 | 94 | 1 | 5 | 0.207 | <0.001 |
| IECh2 | 50 | 91 | 50 | 100 | 2 | 10 | 0.058 | <0.001 |
Fisher’s exact test
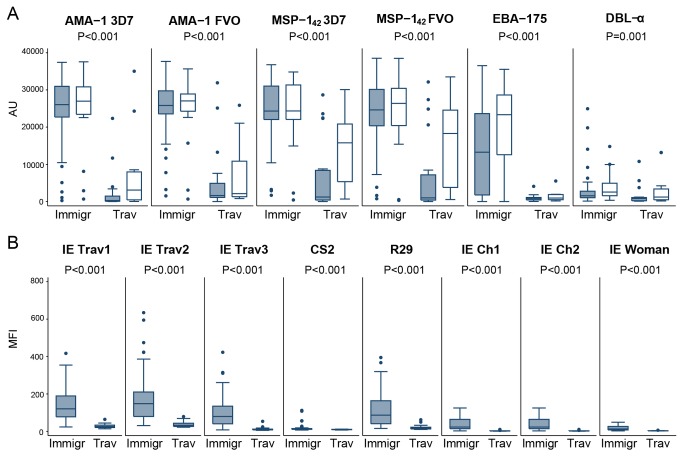
Next, Ab responses in immigrants were compared with Ab responses in travelers during an acute malaria episode and in convalescence (Figure 3, Table 3). Overall, infected immigrants had higher Ab levels and seroprevalences against erythrocytic antigens (Figure 3A, Table 3) and IEs (Figure 3B, Table 3) compared to travelers.
Figure 3. IgG antibody responses to merozoite antigens (A) and P. falciparum Infected Erythrocytes (B) in immigrants (Immigr) and travelers (Trav) with clinical malaria.
IgG levels were determined at the acute episode of malaria (day 0, black boxes) and at convalescence (day 7, white boxes). Data are presented as boxplots that illustrate the medians and the 25th and 75th quartiles, and the whiskers represent the 10% and 90% percentiles. Outliers are marked with circles. P-values were calculated using the Wilcoxon Rank Sum test. Cutoff values for seroprevalences were 238.02 AU for AMA-1 3D7, 1134.73 AU for AMA-1 FVO, 921.18 AU for MSP-142 3D7, 638.33 AU for MSP-142 FVO,3110.36 AU for EBA-175, 1572.55 AU for DBL-α, 36.88 MFI for IETrav1, 38.55 MFI for IETrav2, 24.75 MFI IETrav3, 14.23 MFI for CS2, 19.92 MFI for R29, 8.69 MFI for ECh1, 1.98 MFI for IECh2, and 5.54 MFI for IEWoman.
Effect of time since cessation of exposure on antibody levels
The effect of time since immigration on Ab levels in individuals without malaria or with a clinical malaria episode was assessed. None of the Ab against the antigens tested showed any correlation with the time since migration (data not shown) in immigrants with malaria or without malaria. Similarly, no significant differences were found in IgG levels or seroprevalences between individuals that had migrated from their malaria endemic country of origin 5 or fewer years ago, and those that had migrated more than 5 years ago, without malaria (Table S1) or with clinical malaria (Table S2).
Discussion
In this study we found that after long periods without continuous malaria exposure, immigrants from endemic areas without malaria still presented seropositivities of 32% to 98% for erythrocytic antigens considered as leading vaccine candidates, and immigrants with clinical malaria had similar seroprevalences than semi-immune adults also with a clinical episode However, immigrants without malaria had lower levels of IgGs to all antigens tested compared to healthy adults with continuous life-long exposure to malaria. Although this may suggest that there is some loss of immunity, in this cohort we did not observe an association between time since migration and Ab levels, which may indicate that IgG are longer-lived than usually perceived, at least in adults. In addition, we cannot discard that the healthy adults recruited in Mozambique may have been recently exposed.
Immigrants with clinical malaria had higher IgG levels compared to immigrants without malaria and compared to naïve adults with a primary infection. This may indicate that even at day of presentation to the clinics with malaria, there is boosting of Ab against a wide repertoire of antigens. Rapid boosting of Ab responses to various P. falciparum antigens has been reported after re-exposure to malaria following prolonged periods of either sustained control or low transmission, in both children and adults [35,40], suggesting that there is maintenance of immune memory and the capacity to respond quickly to reinfection. Nevertheless, previous exposure could account for part of this difference between the two groups of immigrants. Migrants who contracted malaria upon return to their countries of origin could have been historically more exposed than migrants without clinical malaria (i.e. because there is higher malaria transmission intensity in those areas). However, we think this is unlikely since the subjects were form highly diverse areas. In addition, differences with the group of travelers could be due to different times of presentation to clinical attention: partially immune immigrants could have a smoldering blood stage infection for weeks whereas travelers would require clinical attention within a few days of the start of the blood stage infection, before their antibody response peaked. This, however, argues in favor of our hypothesis that there is maintenance of immunity.
Interestingly, the magnitude of Ab responses in immigrants with clinical malaria compared to Mozambican semi-immune adults with clinical malaria varied according to the type of antigen. Three different response patterns could be identified. First, immigrants had higher levels of IgG against the merozoite antigens AMA-1 and MSP-142, which are relatively polymorphic/dimorphic [11,53] and highly immunogenic, although many studies have associated them with malaria exposure rather than protection [54–56]. In contrast, both study groups had similar levels of Ab against EBA-175 and DBL-α. EBA-175 is more conserved and less immunogenic than AMA-1 and MSP-142, but more consistently associated with protection from clinical malaria [8,54,55] in our previous studies. DBL-α is also less immunogenic and relatively polymorphic considering that it is a PfEMP-1 domain, which is a highly diverse protein [13]. In fact, it is the most conserved PfEMP-1 domain, probably due to its function in mediating rosetting, and Ab against it have been associated with protection against malaria severity [57]. Finally, immigrants had lower levels of IgG against VSA on IEs that are the most polymorphic and poorly immunogenic upon initial exposures to parasite infections [58]. Therefore, these results together with our previous data on Ab responses to these antigens in young Mozambican children [8,52,58] suggest that, after extended periods in the absence of P. falciparum exposure, the magnitude of Ab recall responses upon reinfection depend on the nature of the antigen, as does the pattern of acquisition of such responses upon initial parasite exposure. Nevertheless, genetic and/or environmental differences between migrants who were originally from very diverse countries, and semi-immune adults who were from a unique African endemic area, could also account for some of the differences observed between these groups.
The finding that clinical malaria in immigrants induced higher Ab levels for the more immunogenic antigens may be attributable to a B cell response inclined toward merozoite antigens instead of a broad B cell response against a wider repertoire of different antigens. In contrast, the semi-immune group had greater Ab reactivity against multiple variants of IE antigens, indicating that the response under continuous exposure may be directed toward control of parasite variants [12]. Immunity to IEs is slowly acquired in infancy due to the high polymorphism of parasite antigens expressed on the surface of the erythrocytes, resulting in the need of cumulative exposures to acquire an Ab repertoire able to recognize antigenically distinct PfEMP-1 molecules [59,60]. The interruption of malaria exposure in immigrants, and therefore the interruption of acquisition of immunity to VSA, may explain why they have lower Ab levels and why they do not respond to the same extent as continuously exposed semi-immune adults upon re-exposure against these antigens. However, since we do not have data on their antibody levels at the time of migration we cannot discard that the lower levels are due to a loss of immunity.
In contrast, the maintenance of Ab responses against DBL-α and EBA-175, which have been shown to be important in protection from clinical and severe malaria, could represent evidence that immunity to the most severe manifestations of clinical malaria is largely maintained, as clinical data appear to show [14–20]. Data comparing IgG levels in immigrants with malaria against IgG levels in naïve adults with a primary infection represent further evidence of maintenance of immunity, as immigrants had higher IgG levels against all antigens and IEs. This is consistent with clinical observations that migrants rarely get severe/deadly malaria compared to travelers [14–21].
Although our results show that there are P. falciparum-specific Ab memory responses after long periods without exposure to malaria, the effect of these responses on immunity is not clear. We do not know if these Ab responses would lead to protection from infection or clinical/severe malaria upon reinfection. However, immigrants returning with clinical malaria have not been protected from infection or clinical malaria in this occasion, but this does not imply per se that they have no immunity to malaria. It could be that the progression of the disease was different than the naïve or the semi-immune adults. Unfortunately, we do not have data on the progression of the disease. In addition, we cannot conclude that they have lost immunity since even continuously exposed adults may have clinical malaria, which is what we observed also with the group of semi-immune adults in Mozambique. We are probably facing two different layers of immunity, in which the maintenance of basal levels of Ab is key in the initial control of a reinfection, whereas the capacity to generate effector responses (new Ab and MBC) may lead to prevention of malaria pathogenicity and severity.
Previous findings on long-lived malaria-specific MBC support maintenance of memory responses. Malaria-specific MBC were detected in adults from a low endemicity setting in Thailand and found to persist for more than 7 years without ongoing exposure [44]. In The Gambia, the median number of malaria-specific MBC was similar to the median number of diphtheria-specific MBC, suggesting that the malaria-specific circulating MBC pool is of similar magnitude to that of other antigens [45]. However, it is not clear whether Ab levels correlate with MBC, and the maintenance of the plasma cell pool may be independent of MBC [61]. P. falciparum Ab and MBC have been shown to correlate when there is recent exposure, but correlation does not seem to persist after long periods of non-exposure [32,39,42,45]. The role and contribution of MBC in Ab levels of a recall response during a malaria infection needs to be addressed more thoroughly.
While Ab responses to erythrocytic antigens seem to be maintained to a large extent, cytokine profiling in these same individuals showed that, upon loss of exposure, control of pro-inflammatory response and tolerance to P. falciparum may be reduced [62]. No correlation was found between plasma cytokine or chemokine concentrations and Ab levels. However, other Ab responses involved in “anti-disease immunity” or tolerance [63] may be directly implicated in the cellular immune response, and may be more short-lived than the Ab responses measured in this study.
The results of this work highlight the usefulness of migrant populations’ studies to understand the maintenance of immunity to malaria and to disentangle determinants and mediators, allowing the study of memory immune responses without the interference of natural malaria exposure. Nevertheless, several limitations were faced. We could not control for the immune status of the migrants when arriving to the non-endemic area, or for the number of returns to their country of origin with possible malaria exposure and boost of the immune responses. However, none of them had a malaria episode after their migration and the exposure may have been minimal due to the few times they returned and the nature of these short trips. Longitudinal migrant cohort studies would resolve the limitations of the present study and would allow for more precise assessment of long-term immunological maintenance. Further research into the factors that determine the duration of specific Ab responses and the underlying immune mechanisms will be important for vaccine design to induce levels of immunity that are more protective and sustained than those attained by current vaccine candidates [64]. Public health will ultimately benefit from a better understanding of the mechanisms involved in the maintenance of immunity upon loss of exposure, since malaria control strategies leading to diminished malaria transmission may decrease the population’s immunity with detrimental consequences in the case of malaria resurgence.
Supporting Information
Luminex IgG curves for each tested antigen made with a pool of plasma samples from hyper-immune Mozambican adult volunteers. Data is representative from one experiment in duplicates.
(DOCX)
Representative flow cytometry data of antibodies to IEs surface antigens assay. Panels A, B, and C show the dot plots for the lab parasite R29 and panels D, F and G the dot plots for one field isolate (D, F, G). Samples tested were the pool of plasma samples from hyper-immune Mozambican adult volunteers (A, D), the pool from non-exposed European adults (B, E) and plasma from one migrant patient (C, F).
(DOCX)
Plasma IgG levels and seroprevalence in immigrants without malaria who have been ≤ 5 years or > 5 years in a non-endemic area.
(DOCX)
Plasma IgG levels and seroprevalence in immigrants with a clinical a malaria episode who have been ≤ 5 years or > 5 years in a non-endemic area.
(DOCX)
Acknowledgments
We wish to thank all the volunteers for participating in the study; the staff of the Manhiça District Hospital, the Hospital Arnau de Vilanova, the Hospital Santa Caterina de Salt and the Hospital Clínic de Barcelona; clinical officers, field supervisors and data entry clerks; Eduard Rovira-Vallbona, Mauricio H. Rodríguez, Lázaro Mussacate Quimice and Nelito Ernesto José for their contribution to sample processing.
Funding Statement
The study received financial support from the Ministerio de Ciencia e Innovación (SAF2008-00743, salary support RYC-2008-02631 to C.D.); the Instituto de Salud Carlos III (PI050275, PS0901113, salary support CD10/00156 to G.M. and CP-4/00220 to A.M.); the Agència de Gestió d’Ajuts Universitaris i de Recerca (2009SGR934, 2009SGR385, salary support 2010FI_B 00168 to J.C.); and the Fundación Ramón Areces. The Centro de Investigação em Saúde de Manhiça receives core support from the Spanish Agency for International Cooperation and Development (AECID). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Doolan DL, Dobaño C, Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22: 13–36. doi:10.1128/CMR.00025-08. PubMed: 19136431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amanna IJ, Carlson NE, Slifka MK (2007) Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357: 1903–1915. doi:10.1056/NEJMoa066092. PubMed: 17989383. [DOI] [PubMed] [Google Scholar]
- 3. Langhorne J, Ndungu FM, Sponaas A-M, Marsh K (2008) Immunity to malaria: more questions than answers. Nat Immunol 9: 725–732. doi:10.1038/ni.f.205. PubMed: 18563083. [DOI] [PubMed] [Google Scholar]
- 4. Spence PJ, Langhorne J (2012) T cell control of malaria pathogenesis. Curr Top Microbiol Immunol, 355: 3–7. PubMed: 21809194. [DOI] [PubMed] [Google Scholar]
- 5. Cohen S, McGregor IA, Carrington S (1961) Gamma-globulin and acquired immunity to human malaria. Nature 192: 733–737. doi:10.1038/192733a0. PubMed: 13880318. [DOI] [PubMed] [Google Scholar]
- 6. Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H et al. (1991) Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45: 297–308. PubMed: 1928564. [DOI] [PubMed] [Google Scholar]
- 7. Richards JS, Stanisic DI, Fowkes FJI, Tavul L, Dabod E et al. (2010) Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51: e50–e60. doi:10.1086/656413. PubMed: 20843207. [DOI] [PubMed] [Google Scholar]
- 8. Dobaño C, Quelhas D, Quintó L, Puyol L, Serra-Casas E et al. (2012) Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin Vaccine Immunol 19: 157–166. doi:10.1128/CVI.05523-11. PubMed: 22169088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodoo D, Aikins A, Kusi KA, Lamptey H, Remarque E et al. (2008) Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J 7: 142. doi:10.1186/1475-2875-7-142. PubMed: 18664257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conway DJ, Cavanagh DR, Tanabe K, Roper C, Mikes ZS et al. (2000) A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med 6: 689–692. doi:10.1038/76272. PubMed: 10835687. [DOI] [PubMed] [Google Scholar]
- 11. Polley SD, Mwangi T, Kocken CHM, Thomas AW, Dutta S et al. (2004) Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23: 718–728. doi:10.1016/j.vaccine.2004.05.031. PubMed: 15542195. [DOI] [PubMed] [Google Scholar]
- 12. Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI et al. (1998) Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med 4: 358–360. doi:10.1038/nm0398-358. PubMed: 9500614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayor A, Rovira-Vallbona E, Srivastava A, Sharma SK, Pati SS et al. (2009) Functional and immunological characterization of a Duffy binding-like alpha domain from Plasmodium falciparum erythrocyte membrane protein 1 that mediates rosetting. Infect Immun 77: 3857–3863. doi:10.1128/IAI.00049-09. PubMed: 19546191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matteelli a, Colombini P, Gulletta M, Castelli F, Carosi G (1999) Epidemiological features and case management practices of imported malaria in northern Italy 1991-1995. Trop Med Int Health 4: 653–657. doi:10.1046/j.1365-3156.1999.00468.x. PubMed: 10583898. [DOI] [PubMed] [Google Scholar]
- 15. Jelinek T, Schulte C, Behrens R, Grobusch MP, Coulaud JP et al. (2002) Imported Falciparum malaria in Europe: sentinel surveillance data from the European network on surveillance of imported infectious diseases. Clin Infect Dis 34: 572–576. doi:10.1086/338235. PubMed: 11803507. [DOI] [PubMed] [Google Scholar]
- 16. MacMullin G, Mackenzie R, Lau R, Khang J, Zhang H et al. (2012) Host immune response in returning travellers infected with malaria. Malar J 11: 148. doi:10.1186/1475-2875-11-148. PubMed: 22554058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mascarello M, Allegranzi B, Angheben A, Anselmi M, Concia E et al. (2008) Imported malaria in adults and children: epidemiological and clinical characteristics of 380 consecutive cases observed in Verona, Italy. J Travel Med 15: 229–236. doi:10.1111/j.1708-8305.2008.00204.x. PubMed: 18666922. [DOI] [PubMed] [Google Scholar]
- 18. Bouchaud O, Cot M, Kony S, Durand R, Schiemann R et al. (2005) Do African immigrants living in France have long-term malarial immunity? Am J Trop Med Hyg 72: 21–25. PubMed: 15728861. [PubMed] [Google Scholar]
- 19. Salvadó E, Pinazo MJ, Muñoz J, Alonso D, Naniche D et al. (2008) Clinical presentation and complications of Plasmodium falciparum malaria in two populations: travelers and immigrants. Enferm Infecc Microbiol Clin 26: 282–284. doi:10.1157/13120415. PubMed: 18479645. [DOI] [PubMed] [Google Scholar]
- 20. Monge-Maillo B, Norman F, Pérez-Molina JA, Díaz-Menéndez M, Rubio JM et al. (2012) Plasmodium falciparum in asymptomatic immigrants from sub-Saharan Africa, Spain. Parasite Immunol 18: 356–357. PubMed: 22305463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. González A, Nicolás JM, Muñoz J, Castro P, Mas J et al (2009) Severe imported malaria in adults: retrospective study of 20 cases. Am J Trop Med Hyg 81: 595–599 [DOI] [PubMed]
- 22. Kleinschmidt I, Sharp B (2001) Patterns in age-specific malaria incidence in a population exposed to low levels of malaria transmission intensity. Trop Med Int Health 6: 986–991. doi:10.1046/j.1365-3156.2001.00817.x. PubMed: 11737835. [DOI] [PubMed] [Google Scholar]
- 23. Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S et al. (1997) The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg 91: 256–262. doi:10.1016/S0035-9203(97)90066-3. PubMed: 9231189. [DOI] [PubMed] [Google Scholar]
- 24. Deloron P, Chougnet C (1992) Is immunity to malaria really short-lived? Parasitol Today 8: 375–378. doi:10.1016/0169-4758(92)90174-Z. PubMed: 15463545. [DOI] [PubMed] [Google Scholar]
- 25. Cavanagh DR, Elhassan IM, Roper C, Robinson VJ, Giha H et al. (1998) A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol 161: 347–359. PubMed: 9647243. [PubMed] [Google Scholar]
- 26. Giha HA, Staalsoe T, Dodoo D, Elhassan IM, Roper C et al. (1999) Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect Immun 67: 4092–4098. PubMed: 10417178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Früh K, Doumbo O, Müller HM, Koita O, McBride J et al. (1991) Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun 59: 1319–1324. PubMed: 2004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kinyanjui SM, Conway DJ, Lanar DE, Marsh K (2007) IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J 6: 82. doi:10.1186/1475-2875-6-82. PubMed: 17598897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramasamy R, Nagendran K, Ramasamy MS (1994) Antibodies to epitopes on merozoite and sporozoite surface antigens as serologic markers of malaria transmission: studies at a site in the dry zone of Sri Lanka. Am J Trop Med Hyg 50: 537–547. PubMed: 7515593. [DOI] [PubMed] [Google Scholar]
- 30. Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE et al. (2008) Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun 76: 1748–1755. doi:10.1128/IAI.01333-07. PubMed: 18212081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torres KJ, Clark EH, Hernandez JN, Soto-Cornejo KE, Gamboa D et al. (2008) Antibody response dynamics to the Plasmodium falciparum conserved vaccine candidate antigen, merozoite surface protein-1 C-terminal 19kD (MSP1-19kD), in Peruvians exposed to hypoendemic malaria transmission. Malar J 7: 173. doi:10.1186/1475-2875-7-173. PubMed: 18782451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH et al. (2010) Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLOS Pathog 6: e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SLR et al. (2005) Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 102: 5108–5113. doi:10.1073/pnas.0408725102. PubMed: 15792998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Udhayakumar V, Kariuki S, Kolczack M, Girma M, Roberts JM et al. (2001) Longitudinal study of natural immune responses to the Plasmodium falciparum apical membrane antigen (AMA-1) in a holoendemic region of malaria in western Kenya: Asembo Bay Cohort Project VIII. Am J Trop Med Hyg 65: 100–107. PubMed: 11508382. [DOI] [PubMed] [Google Scholar]
- 35. Migot F, Chougnet C, Raharimalala L, Astagneau P, Lepers JP et al. (1993) Human immune responses to the Plasmodium falciparum ring-infected erythrocyte surface antigen (Pf155/RESA) after a decrease in malaria transmission in Madagascar. Am J Trop Med Hyg 48: 432–439. PubMed: 8470778. [DOI] [PubMed] [Google Scholar]
- 36. Dodoo D, Theander TG, Kurtzhals JA, Koram K, Riley E et al. (1999) Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect Immun 67: 2131–2137. PubMed: 10225865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perraut R, Mercereau-Puijalon O, Diouf B, Tall A, Guillotte M et al. (2000) Seasonal fluctuation of antibody levels to Plasmodium falciparum parasitized red blood cell-associated antigens in two Senegalese villages with different transmission conditions. Am J Trop Med Hyg 62: 746–751. PubMed: 11304067. [DOI] [PubMed] [Google Scholar]
- 38. Jakobsen PH, Morris-Jones SD, Hviid L, Theander TG, Høier-Madsen M et al. (1993) Anti-phospholipid antibodies in patients with Plasmodium falciparum malaria. Immunology 79: 653–657. PubMed: 8406592. [PMC free article] [PubMed] [Google Scholar]
- 39. Weiss GE, Traore B, Kayentao K, Ongoiba A, Doumbo S et al. (2010) The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLOS Pathog 6: e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vande Waa JA, Jensen JB, Akood MA, Bayoumi R (1984) Longitudinal study on the in vitro immune response to Plasmodium falciparum in Sudan. Infect Immun 45: 505–510. PubMed: 6378799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chougnet C, Deloron P, Lepers JP, Tallet S, Rason MD et al. (1990) Humoral and cell-mediated immune responses to the Plasmodium falciparum antigens PF155/RESA and CS protein: seasonal variations in a population recently reexposed to endemic malaria. Am J Trop Med Hyg 43: 234–242. PubMed: 2221217. [DOI] [PubMed] [Google Scholar]
- 42. Ndungu FM, Olotu A, Mwacharo J, Nyonda M, Apfeld J et al. (2012) Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc Natl Acad Sci U S A 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Migot F, Chougnet C, Raharimalala L, Astagneau P, Lepers JP et al. (1993) Human immune responses to the Plasmodium falciparum ring-infected erythrocyte surface antigen (Pf155/RESA) after a decrease in malaria transmission in Madagascar. Am J Trop Med Hyg 48: 432–439. PubMed: 8470778. [DOI] [PubMed] [Google Scholar]
- 44. Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH et al. (2010) Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLOS Pathog 6: e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nogaro SI, Hafalla JC, Walther B, Remarque EJ, Tetteh KKa et al. (2011) The Breadth, but Not the Magnitude, of Circulating Memory B Cell Responses to P. falciparum Increases with Age/Exposure in an Area of Low Transmission. PLOS ONE 6: e25582. doi:10.1371/journal.pone.0025582. PubMed: 21991321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dorfman JR, Bejon P, Ndungu FM, Langhorne J, Kortok MM et al. (2005) B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis 191: 1623–1630. doi:10.1086/429671. PubMed: 15838788. [DOI] [PubMed] [Google Scholar]
- 47. Moncunill G, Mayor A, Jiménez A, Nhabomba A, Puyol L et al. (2013) Cytokine and Antibody Responses to Plasmodium falciparum in Naïve Individuals during a First Malaria Episode: Effect of Age and Malaria Exposure. PLOS ONE 8: e55756. doi:10.1371/journal.pone.0055756. PubMed: 23437061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kocken CHM, Withers-Martinez C, Dubbeld MA, van der Wel A, Hackett F et al. (2002) High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect Immun 70: 4471–4476. doi:10.1128/IAI.70.8.4471-4476.2002. PubMed: 12117958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pandey KC, Singh S, Pattnaik P, Pillai CR, Pillai U et al. (2002) Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol Biochem Parasitol 123: 23–33. doi:10.1016/S0166-6851(02)00122-6. PubMed: 12165386. [DOI] [PubMed] [Google Scholar]
- 50. Angov E (2003) Development and pre-clinical analysis of a Plasmodium falciparum Merozoite Surface Protein-142 malaria vaccine. Mol Biochem Parasitol 128: 195–204. doi:10.1016/S0166-6851(03)00077-X. PubMed: 12742586. [DOI] [PubMed] [Google Scholar]
- 51. Angov E, Hillier CJ, Kincaid RL, Lyon JA (2008) Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLOS ONE 3: e2189. doi:10.1371/journal.pone.0002189. PubMed: 18478103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campo JJ, Dobaño C, Sacarlal J, Guinovart C, Mayor A et al. (2011) Impact of the RTS,S Malaria Vaccine Candidate on Naturally Acquired Antibody Responses to Multiple Asexual Blood Stage Antigens. PLOS ONE 6: e25779. doi:10.1371/journal.pone.0025779. PubMed: 22022448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanabe K, Mackay M, Goman M, Scaife JG (1987) Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol 195: 273–287. doi:10.1016/0022-2836(87)90649-8. PubMed: 3079521. [DOI] [PubMed] [Google Scholar]
- 54. Campo JJ, Dobaño C, Sacarlal J, Guinovart C, Mayor A et al. (2011) Impact of the RTS,S Malaria Vaccine Candidate on Naturally Acquired Antibody Responses to Multiple Asexual Blood Stage Antigens. PLOS ONE 6: e25779. doi:10.1371/journal.pone.0025779. PubMed: 22022448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quelhas D, Puyol L, Quintó L, Serra-Casas E, Nhampossa T et al. (2008) Impact of intermittent preventive treatment with sulfadoxine-pyrimethamine on antibody responses to erythrocytic-stage Plasmodium falciparum antigens in infants in Mozambique. Clin Vaccine Immunol 15: 1282–1291. doi:10.1128/CVI.00044-08. PubMed: 18495848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Serra-Casas E, Menéndez C, Bardají A, Quintó L, Dobaño C et al. (2010) The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. J Infect Dis 201: 123–131. doi:10.1086/648595. PubMed: 19954383. [DOI] [PubMed] [Google Scholar]
- 57. Rovira-Vallbona E, Moncunill G, Bassat Q, Aguilar R, Machevo S et al. (2012) Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J 11: 181. doi:10.1186/1475-2875-11-181. PubMed: 22646809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Quelhas D, Jiménez A, Quintó L, Serra-Casas E, Mayor A et al. (2011) IgG against Plasmodium falciparum variant surface antigens and growth inhibitory antibodies in Mozambican children receiving intermittent preventive treatment with sulfadoxine-pyrimethamine. Immunobiology 216: 793–802. doi:10.1016/j.imbio.2010.12.010. PubMed: 21257227. [DOI] [PubMed] [Google Scholar]
- 59. Cham GKK, Turner L, Lusingu J, Vestergaard L, Mmbando BP et al. (2009) Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J Immunol 183: 3356–3363. doi:10.4049/jimmunol.0901331. PubMed: 19675168. [DOI] [PubMed] [Google Scholar]
- 60. Bull PC, Berriman M, Kyes S, Quail Ma, Hall N et al. (2005) Plasmodium falciparum variant surface antigen expression patterns during malaria. PLOS Pathog 1: e26. doi:10.1371/journal.ppat.0010026. PubMed: 16304608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ahuja A, Anderson SM, Khalil A, Shlomchik MJ (2008) Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A 105: 4802–4807. doi:10.1073/pnas.0800555105. PubMed: 18339801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moncunill G, Mayor A, Bardají A, Puyol L, Nhabomba A et al. (2013) Cytokine profiling in immigrants with clinical malaria after extended periods of interrupted exposure to Plasmodium falciparum. PLOS ONE 8: e73360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boutlis CS, Yeo TW, Anstey NM (2006) Malaria tolerance--for whom the cell tolls? Trends Parasitol 22: 371–377. doi:10.1016/j.pt.2006.06.002. PubMed: 16784889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Bgno Methogo et al. (2012) A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367: 2284–2295. doi:10.1056/NEJMoa1208394. PubMed: 23136909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Luminex IgG curves for each tested antigen made with a pool of plasma samples from hyper-immune Mozambican adult volunteers. Data is representative from one experiment in duplicates.
(DOCX)
Representative flow cytometry data of antibodies to IEs surface antigens assay. Panels A, B, and C show the dot plots for the lab parasite R29 and panels D, F and G the dot plots for one field isolate (D, F, G). Samples tested were the pool of plasma samples from hyper-immune Mozambican adult volunteers (A, D), the pool from non-exposed European adults (B, E) and plasma from one migrant patient (C, F).
(DOCX)
Plasma IgG levels and seroprevalence in immigrants without malaria who have been ≤ 5 years or > 5 years in a non-endemic area.
(DOCX)
Plasma IgG levels and seroprevalence in immigrants with a clinical a malaria episode who have been ≤ 5 years or > 5 years in a non-endemic area.
(DOCX)