Abstract
During growth on one-carbon (C1) compounds, the aerobic α-proteobacterium Methylobacterium extorquens AM1 synthesizes the tetrahydromethanopterin (H4MPT) derivative dephospho-H4MPT as a C1 carrier in addition to tetrahydrofolate. The enzymes involved in dephospho-H4MPT biosynthesis have not been identified in bacteria. In archaea, the final step in the proposed pathway of H4MPT biosynthesis is the reduction of dihydromethanopterin (H2MPT) to H4MPT, a reaction analogous to the reaction of the bacterial dihydrofolate reductase. A gene encoding a dihydrofolate reductase homolog has previously been reported for M. extorquens and assigned as the putative H2MPT reductase gene (dmrA). In the present work, we describe the biochemical characterization of H2MPT reductase (DmrA), which is encoded by dmrA. The gene was expressed with a six-histidine tag in Escherichia coli, and the recombinant protein was purified by nickel affinity chromatography and gel filtration. Purified DmrA catalyzed the NAD(P)H-dependent reduction of H2MPT with a specific activity of 2.8 μmol of NADPH oxidized per min per mg of protein at 30°C and pH 5.3. Dihydrofolate was not a substrate for DmrA at the physiological pH of 6.8. While the existence of an H2MPT reductase has been proposed previously, this is the first biochemical evidence for such an enzyme in any organism, including archaea. Curiously, no DmrA homologs have been identified in the genomes of known methanogenic archaea, suggesting that bacteria and archaea produce two evolutionarily distinct forms of dihydromethanopterin reductase. This may be a consequence of different electron donors, NAD(P)H versus reduced F420, used, respectively, in bacteria and methanogenic archaea.
The aerobic α-proteobacterium Methylobacterium extorquens AM1 is capable of growth on one-carbon (C1) and selected multicarbon compounds. During the catabolism of C1 compounds, M. extorquens synthesizes an analog of the coenzyme tetrahydromethanopterin (H4MPT) called dephospho-H4MPT (Fig. 1) in addition to tetrahydrofolate (H4F) (7). Although H4MPT was initially identified as a C1 carrier in methanogenic archaea (10, 19), analogs of H4MPT and H4MPT-dependent enzymes have been identified in sulfur-dependent hyperthermophilic archaea and methylotrophic proteobacteria (7, 13, 21, 24, 37, 39, 40). While H4F and H4MPT possess common structural features, they differ in other structural respects, and they play distinct functional roles (22). In particular, in M. extorquens, H4F appears to be primarily involved in carbon assimilation and anabolism, while dephospho-H4MPT is required for the catabolism of C1 growth substrates (6, 7). In M. extorquens, the genes encoding a number of dephospho-H4MPT-dependent enzymes are clustered together in a DNA region required for growth on C1 compounds (7). Several of these bacterial enzymes are homologous to archaeal enzymes involved in C1 metabolism during methanogenesis (7, 26, 27, 28).
FIG. 1.
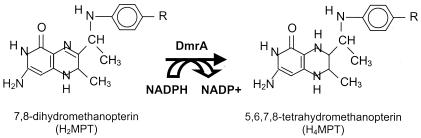
The reaction catalyzed by dihydromethanopterin reductase (DmrA). Analogs of H2MPT can be reduced by DmrA using NADPH as the electron donor. In H2MPT the side group (R) consists of ribitol, ribose, and α-hydroxyglutaryl phosphate. H2SPT from M. thermophila contains an additional terminal glutamate linked to H2MPT (22). In M. extorquens, the H2MPT derivative (dephospho-H2MPT) completely lacks the α-hydroxyglutaryl phosphate and terminal glutamyl residues (7).
The pathway for the biosynthesis of dephospho-H4MPT in M. extorquens and other bacteria is not known. However, an 18-step pathway for H4MPT biosynthesis in methanogenic archaea has been proposed (41). Thus far, 4 of the 18 putative H4MPT biosynthetic enzymes and their corresponding genes have been identified (15, 16, 31, 44). Interestingly, in M. extorquens a gene encoding a homolog of a key H4MPT biosynthetic enzyme, ribofuranosylaminobenzene 5′-phosphate synthase, has been found within the cluster of genes coding for H4MPT-dependent enzymes (7, 31). Therefore, it is likely that the biosynthesis of H4MPT analogs in archaea and bacteria share some common enzymatic steps.
The last step in the proposed pathway of H4MPT biosynthesis in archaea is the reduction of dihydromethanopterin (H2MPT) to H4MPT, catalyzed by a putative H2MPT reductase (Fig. 1) (41). This step is analogous to the well-characterized reaction of bacterial dihydrofolate (H2F) reductases (DHFRs) in H4F biosynthesis (4). Marx et al. (23) have recently discovered a gene in M. extorquens (the dmrA gene) that codes for a DHFR homolog. Deletion of this gene produces a mutant that is highly sensitive to methanol and formaldehyde toxicity and is deficient in the ability to grow on C1 compounds. These properties are consistent with the phenotype of a mutant deficient in dephospho-H4MPT-dependent metabolism (17, 38). Using an enzymatic assay that detects dephospho-H4MPT as well as H4MPT in cell extracts, our laboratory has determined that the dmrA mutant is incapable of producing dephospho-H4MPT, suggesting a defect in the pathway of dephospho-H4MPT biosynthesis (S. A. Wyles and M. E. Rasche, unpublished results). Based on the sequence similarity of the DmrA protein to DHFR and the inability of dmrA mutants to produce dephospho-H4MPT, it has been proposed that the dmrA gene codes for the putative H2MPT reductase (DmrA) (23). In the present work, we have tested this hypothesis directly by expressing dmrA with a six-histidine tag in Escherichia coli and developing an assay to measure H2MPT reductase activity in vitro. This is the first biochemical characterization of a H2MPT reductase from any microorganism.
MATERIALS AND METHODS
Heterologous expression of dmrA in E. coli.
The 405-bp dmrA gene from M. extorquens encodes a protein with a predicted molecular mass of 17 kDa (23). A plasmid containing the dmrA gene in the pCR 2.1 vector (Invitrogen, Carlsbad, Calif.) was kindly provided by Christopher Marx and Mary Lidstrom. The dmrA gene was subcloned into the NdeI and BamHI sites of the pET15b(+) expression vector (Novagen, Inc., Madison Wis.), which incorporates an N-terminal six-histidine (His6) tag during production of the recombinant protein. Since the dmrA gene contains an internal BamHI site, the pCR2.1 plasmid containing dmrA was cut with NdeI and BglII, producing a cohesive end that was compatible with BamHI. The vector pET15b(+) was digested with NdeI and BamHI, and the DNA fragments were separated on a 0.8% agarose gel. The appropriate pET15b and dmrA fragments were excised from the gel, purified using a gel extraction kit (Qiagen, Valencia, Calif.), and ligated with T4 DNA ligase (New England Biolabs, Beverly, Mass.). The new plasmid (pMC26) was transformed into electrocompetent E. coli DH5α cells (30).
For production of the His6-DmrA protein, pMC26 was transformed into chemically competent BL21(DE3) cells (Stratagene, La Jolla, Calif.). The cells were grown at 37°C with shaking (200 rpm) in 1 liter of M9 minimal medium (30) supplemented with glucose (final concentration, 0.4%) and ampicillin (125 μg/ml). When the optical density at 600 nm reached 0.4 to 0.5, the temperature was lowered to 15°C for 45 min. Subsequently, gene expression was induced with the addition of isopropyl thio-β-d-galactoside (Inalco Pharmaceuticals, San Luis Obispo, Calif.) to a concentration of 1 mM. The cells were grown at 15°C for 16 h, harvested by centrifugation (5,000 × g, 15 min, 4°C), and frozen at −20°C.
Purification of His6-DmrA.
The His6-DmrA protein was purified aerobically using nickel affinity chromatography and gel filtration. Cells (4 g) were suspended in 8 ml of lysis buffer (50 mM sodium phosphate, 300 mM sodium chloride, 15 mM 2-mercaptoethanol, 20 mM imidazole [pH 8.0]) and disrupted using a French press at 20,000 lb/in2. The mixture was centrifuged at 27,000 × g for 30 min at 4°C, and the supernatant was filtered through a 0.45-μm-pore-size filter (Millipore, Bedford, Mass.). To the resulting filtrate (8 ml), 2 ml of 50% nickel-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen, Valencia, Calif.) was added. The mixture was placed on a rocking shaker for 2 h at 4°C and poured into an empty 10-ml column (Bio-Rad, Hercules, Calif.). The Ni-NTA agarose was then washed three times with 5 ml of lysis buffer containing 30 mM imidazole, pH 8.0. The desired protein was eluted with 4 ml of elution buffer (50 mM sodium phosphate, 300 mM sodium chloride, 15 mM 2-mercaptoethanol, 100 mM imidazole [pH 8.0]). DmrA partially purified by Ni-NTA agarose was stable for 3 to 4 days at 4°C and for at least 3 months at −20°C when stored in the presence of 25% (vol/vol) glycerol.
For gel filtration analysis, the 4-ml fraction containing His6-DmrA was concentrated to 100 μl with a Centricon microconcentrator (3,000-molecular-weight cutoff; Millipore), and the retentate was diluted to 325 μl with 50 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (pH 7.0), 10 mM MgCl2, 150 mM KCl, and 2 mM dithiothreitol. A portion of the sample (250 μl) was applied to a Superdex 75 gel filtration column (Amersham-Pharmacia Biotech, Piscataway, N.J.) equilibrated with the same buffer. The His6-DmrA protein was eluted between 11 and 12 ml, corresponding to an apparent molecular mass of 38.5 kDa. The molecular mass markers were bovine serum albumin (67 kDa), ovalbumin (43 kDa), chymotrypsin (25 kDa), and RNase A (13.7 kDa) (Amersham-Pharmacia Biotech). His6-DmrA that was purified to homogeneity by Ni-NTA chromatography and gel filtration was stable at 4°C under anaerobic conditions for 24 h; however, the highly purified enzyme was not stable to freezing at −20°C under the conditions tested.
Protein purity was determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12) with Coomassie brilliant blue R-250 as the stain (Bio-Rad, Hercules, Calif.). Protein concentrations were determined using the protein dye binding assay (5) (Bio-Rad) with bovine serum albumin as the standard.
Preparation of H2MPT and dihydrosarcinapterin (H2SPT) from methanogen cell extracts.
Cells of the methanogens Methanothermobacter thermautotrophicus ΔH (formerly Methanobacterium thermoautotrophicum ΔH) (provided by Ralph Wolfe) and Methanosarcina thermophila TM-1 (31) were used as sources of H2MPT and H2SPT, respectively. Purification steps were performed in an anaerobic chamber (Coy Products, Inc., Grass Lake, Mich.) containing 2% hydrogen and 98% nitrogen, and samples were protected from light to avoid inactivation of the light-sensitive coenzyme. M. thermophila cells were grown as described previously (31) and frozen in liquid nitrogen. Frozen cells (5 g) were purged with H2 gas for 5 min in a sealed 37-ml glass vial and thawed in the presence of hydrogen to enzymatically reduce oxidized pterins to the fully reduced forms (H4MPT and tetrahydrosarcinapterin [H4SPT]). Anoxic buffer (10 ml of 30 mM sodium acetate [pH 4.0], 200 mM 2-mercaptoethanol) was added, and the vial was wrapped in foil and autoclaved for 15 min. After cooling, the mixture was centrifuged at 13,000 × g for 20 min at 25°C in the anaerobic chamber to remove precipitated proteins. The supernatant (8 ml) containing H4MPT or H4SPT was mixed with 8 ml of buffer A (50 mM morpholinepropanesulfonic acid [pH 6.8], 150 mM 2-mercaptoethanol) and applied to a 2-ml DEAE Sephadex A-25 column (Amersham-Pharmacia Biotech) wrapped in foil. The column was washed with buffer A, and coenzymes were eluted with a 0 to 1.5 M NaCl gradient in buffer A. H4MPT from M. thermautotrophicus ΔH was eluted at 200 mM NaCl, and H4SPT from M. thermophila was eluted at 500 mM NaCl. H4MPT and H4SPT were partially oxidized by incubating the fractions aerobically at 4°C in the dark for 14 h. Air oxidation of H4MPT or H4SPT produced a mixture containing the dihydro and tetrahydro forms of the coenzyme, as indicated by changes in the absorbance spectrum between 200 and 400 nm (10). The characteristic 302-nm absorbance of the tetrahydropterins decreased gradually upon exposure to air, and a 280-nm peak increased, indicative of the more oxidized form.
DmrA assay.
An enzymatic assay for DmrA (Fig. 1) was developed, based on modifications of the DHFR assay (1, 18, 32). All procedures were conducted in an anaerobic chamber or in sealed 3-ml glass cuvettes. The standard reaction mixture (1.8 ml) consisted of 36 μg of His6-DmrA and an anoxic solution containing 500 mM sodium acetate (pH 5.3), 20 mM sodium ascorbate, and 1 mM EDTA. To this solution, 200 μl of the DEAE-Sephadex fractions containing either H2MPT or H2SPT was added, and the cuvette was incubated for 20 min at 30°C in the absence of light. The reaction was initiated with 20 μl of 10 mM NADPH, which produced an initial absorbance at 340 nm (A340) of approximately 0.6. The decrease in A340 due to the oxidation of NADPH (ɛ340 = 6.22 · mM−1 · cm−1) (9) in the presence of H2MPT or H2SPT was measured. One unit of activity is defined as 1 μmol of NADPH oxidized per min.
We also made attempts to measure the product H4SPT by using reverse-phase high-pressure liquid chromatography (HPLC); however, we were unable to resolve H2SPT and H4SPT by HPLC using a variety of different conditions. In addition, H2SPT and H4SPT could not be quantified spectrally at 280 and 302 nm because of interference from the absorbance due to ascorbate, NADPH, and NADP+ in the assay. Thus, for this initial characterization of DmrA, NADPH oxidation in the presence of H2SPT or H2MPT was monitored using a procedure based on the DHFR assay (18).
The effect of temperature on enzyme activity was measured between 20 and 50°C. The pH optimum was determined using an anoxic mixed buffer containing 100 mM sodium acetate, 100 mM morpholineethanesulfonic acid, and 100 mM bistrispropane, over a pH range from 4.3 to 7.8. To test whether reduced methyl viologen serves as an electron donor for the DmrA reaction, NADPH was replaced with 5 mM reduced methyl viologen. The methyl viologen solution was initially reduced with dithionite to produce an absorbance at 578 nm of 0.6.
Chemicals and enzymes.
Restriction enzymes and ligase were purchased from New England Biolabs (Beverly, Mass.). NADPH, NADH, methyl viologen, bistrispropane, and H2F were from Sigma Chemical Corp. (St. Louis, Mo.). Gases were obtained from Strate Welding (Gainesville, Fla.). All other chemicals were of reagent grade and were purchased from Fisher Scientific (Suwanee, Ga.).
RESULTS
Amino acid sequence comparisons.
Amino acid sequence alignments showed that the proteins with the highest level of similarity to the putative H2MPT reductase (DmrA) from M. extorquens AM1 were DHFRs from M. extorquens (34% identity, 53% similarity), Vibrio cholerae (31% identity, 44% similarity), and Lactobacillus casei (26% identity, 44% similarity) (23, 36). While the overall identity among the proteins was relatively low, a careful comparison of the DmrA sequence to the L. casei DHFR sequence showed that 14 of the 18 amino acids important for NADPH binding in DHFR from L. casei (3, 11, 23) were identical or highly similar to the corresponding amino acids in DmrA. This suggested that NADPH could be the hydride donor for the DmrA reaction. A critical acidic amino acid that is conserved in DHFRs from many organisms was also found in DmrA at position 35. In DHFR, this aspartic acid plays an important role in binding the pterin ring of folate and structurally related inhibitors (4). Interestingly, although H4MPT was first characterized in methanogenic archaea, no archaeal homologs of DmrA could be identified using sequence alignments (2).
Overproduction and purification of the DmrA protein.
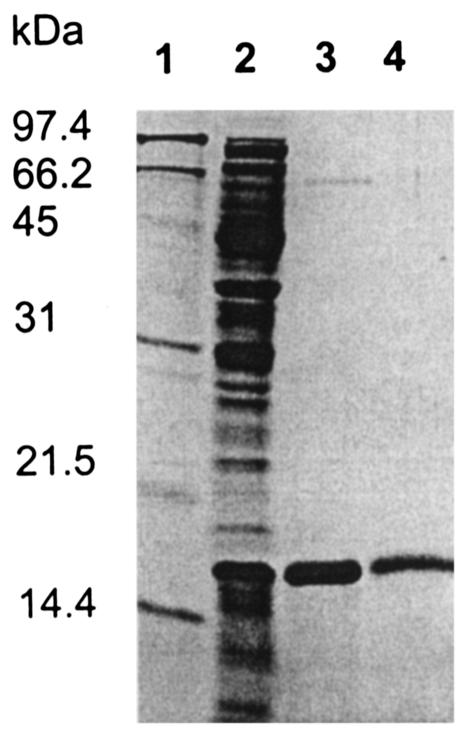
The sequence similarity of DmrA to DHFRs and the inability of dmrA mutants to synthesize dephospho-H4MPT led to the proposal that DmrA functions as a putative H2MPT reductase (23). To test this hypothesis biochemically, we cloned the dmrA gene into an overexpression vector that adds an amino-terminal His6 tag to DmrA (His6-DmrA) during protein synthesis in E. coli. When E. coli cells containing pMC26 were grown at 37°C in Luria-Bertani medium and induced with isopropyl thio-β-d-galactoside, His6-DmrA was produced in an insoluble form as indicated by SDS-PAGE analysis (data not shown). However, when cells were grown in M9 minimal medium with glucose and induced at 15°C, a soluble 17-kDa protein was produced in relatively high amounts (Fig. 2, lane 2). The 17-kDa His6-DmrA protein was purified to homogeneity using nickel affinity chromatography and gel filtration (Fig. 2, lanes 3 and 4; Table 1). The purified enzyme was eluted from the Superdex 75 column with an apparent molecular mass of 38.5 kDa, indicating that the protein is a homodimer.
FIG. 2.
SDS-PAGE of His6-DmrA overproduced in E. coli. Protein samples were boiled in the presence of 7.5% 2-mercaptoethanol in SDS-PAGE sample buffer and loaded onto a 15% polyacrylamide gel. The gel was stained using Coomassie brilliant blue R-250 (Bio-Rad). Lane 1, molecular mass marker; lane 2, cell extract; lane 3, His6-DmrA after Ni-NTA agarose purification; lane 4, His6-DmrA after Superdex 75 purification. The molecular mass markers are 97.4, 66.2, 45, 31, 21.5, and 14.4 kDa.
TABLE 1.
Purification of His6-DmrA overproduced in E. coli
| Purification step | Total activitya (μmol/min) | Sp acta (μmol/ min/mg of protein) | Yield (%) | Fold purification |
|---|---|---|---|---|
| Cell extract | 17 | 0.098 | 100 | 1 |
| Ni-NTA column | 6.2 | 1.9 | 36 | 19 |
| Superdex 75 | 0.20 | 2.8 | 1 | 28 |
DmrA assays were performed at pH 5.3 for 36 s at 30°C as described in Materials and Methods. Activity is expressed as micromoles of NADPH oxidized per minute in the presence of H2MPT from M. thermautotrophicus ΔH.
Enzymatic activity of His6-DmrA.
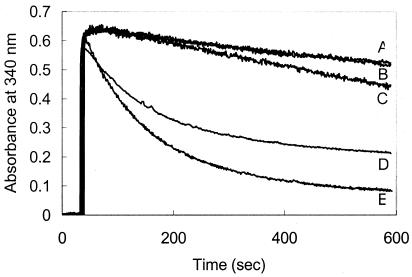
To determine whether His6-DmrA catalyzes the proposed H2MPT reductase reaction, His6-DmrA was purified by nickel column chromatography and combined with NADPH plus H2MPT from H2-treated M. thermautotrophicus ΔH in an acetate buffer at pH 5.3. Methanogenic archaea were used as a source of dihydropterins because they contain up to 20-fold-higher concentrations of methanopterin than M. extorquens cells (7). In the absence of His6-DmrA, the A340 decreased gradually (Fig. 3, trace A) due to the nonenzymatic decomposition of NADPH, which is somewhat unstable in acidic solutions (9). A similar nonenzymatic decrease in the A340 was reported previously for assays measuring the activity of DHFRs in acidic solutions (18). In contrast, when His6-DmrA was added to the reaction mixture, the A340 decreased rapidly (Fig. 3, trace E), providing evidence that His6-DmrA catalyzes the NADPH-dependent reduction of H2MPT. As a control, heat-inactivated protein was tested for activity in the presence of H2MPT; under these conditions, the rate of NADPH oxidation was the same as the background rate (Fig. 3, trace B). H2SPT, the H2MPT analog found in methanogens of the genus Methanosarcina, was also a substrate for His6-DmrA (Fig. 3, trace D). In contrast, when preparations of H2MPT and H2SPT were exposed to air for longer than 24 h at 4°C, activity in the DmrA assay was not detectable (data not shown), suggesting that under the conditions tested, the fully oxidized forms of methanopterin and sarcinapterin were not the principal substrates for DmrA.
FIG. 3.
DmrA activity in the presence of samples containing dihydropterins. The assays contained standard assay buffer (1.8 ml of 100 mM acetate [pH 5.3], 20 mM ascorbate, 1 mM EDTA), 200 μl of the indicated dihydropterin, and 36 μg of Ni-NTA-purified DmrA. Assays were initiated with a final concentration of 100 μM NADPH at 30°C as described in Materials and Methods. Trace A, 200 μl of H2MPT from M. thermautotrophicus ΔH without protein added; trace B, 200 μl of H2MPT with boiled protein; trace C, 200 μl of 1 mM H2F (final concentration, 100 μM) with active DmrA added; trace D, 200 μl of H2SPT from M. thermophila TM-1 with active DmrA; trace E, 200 μl of H2MPT with active DmrA.
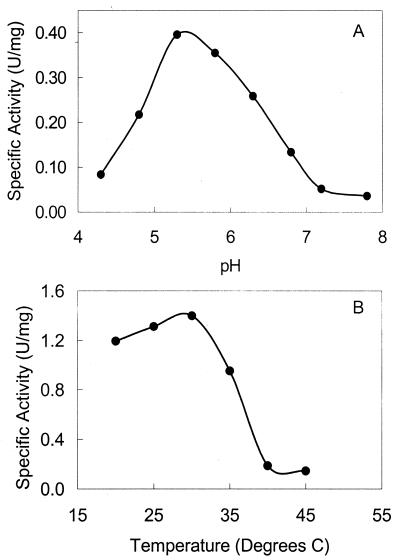
The pH optimum for DmrA was determined to be 5.3 (Fig. 4A), similar to the pH optima for many DHFRs from vertebrate sources (4). At pH 5.3, the temperature optimum for DmrA was 30°C (Fig. 4B), which corresponds to the optimal growth temperature for M. extorquens. The specific activity of the E. coli extract containing His6-DmrA was 0.098 U/mg of protein under these optimal enzymatic conditions (Table 1). After purification of His6-DmrA to homogeneity (Fig. 2, lane 4), the final specific activity was 2.8 U/mg of protein (Table 1). This is similar to the rates reported for H2F reduction by both bacterial and eukaryal DHFRs (4, 32, 43).
FIG. 4.
pH and temperature optima for DmrA. (A) The optimum pH for purified DmrA (36 μg of protein) was determined by monitoring the decrease in A340 at 30°C in a mixed buffer, as described in Materials and Methods. The velocity was determined during the initial part of the assay over the time period that the rate was constant. (B) The temperature optimum for DmrA was determined at the optimal pH in buffer containing 100 mM acetate, pH 5.3, 20 mM ascorbate, and 1 mM EDTA, as described in Materials and Methods.
To test whether H2F could be reduced by DmrA, H2MPT was replaced with H2F at concentrations up to 100 μM, which is 10 to 100 times higher than the reported Km values of H2F for DHFRs (4, 43). Under the physiological conditions of growth for M. extorquens (pH 6.8, 30°C) (25), no H2F-dependent NADPH oxidation activity was observed (data not shown). When H2F was tested under the conditions optimal for the enzymatic reaction of His6-DmrA (pH 5.3, 30°C), NADPH was oxidized at a low rate (0.119 U/mg of protein; Fig. 3, trace C). This activity was approximately 7% of the specific activity observed in the presence of H2MPT.
When NADH was used as the electron donor, the specific activity of DmrA was only 15% of the rate observed with NADPH. A similar preference for NADPH rather than NADH has been observed for DHFRs from both eukaryotes and bacteria (4, 8, 42). Reduced methyl viologen was examined as a possible electron donor, but it did not serve as a reductant for DmrA under the conditions tested (data not shown).
DISCUSSION
The present work provides biochemical evidence that the enzyme encoded by dmrA from M. extorquens catalyzes the NADPH-dependent reduction of H2MPT to H4MPT, as postulated by Marx et al. (23). This is the first H2MPT reductase to be characterized from any organism and the first biochemical demonstration of a H4MPT biosynthesis enzyme in bacteria. The conservation between the NADPH-binding residues of L. casei DHFR and DmrA (23) provided a clue that the hydride donor for DmrA was NADPH rather than F420H2, a reductant found in methanogenic archaea (10, 14). The histidine tag enabled rapid purification of the enzyme to homogeneity (Fig. 2 and Table 1). Purified His6-DmrA was a homodimer, which contrasts with the monomeric structure of DHFRs isolated from vertebrates and most bacteria (4). An exception is the thermostable DHFR from Thermotoga maritima, which is also a dimeric enzyme (43). It remains to be determined if the dimeric nature of His6-DmrA is an intrinsic property of the enzyme or a consequence of heterologous expression in E. coli.
The physiological substrate for DmrA is predicted to be the dephospho analog of H2MPT. This molecule lacks the hydroxyglutaryl phosphate residue of H2MPT as well as the terminal glutamate found in H2SPT (7). Since both H2MPT and H2SPT were substrates for DmrA, it is evident that the enzyme can accommodate substantial variations in the side chain of H2MPT. The ability to use different methanopterin derivatives is a common feature among H4MPT-dependent enzymes, including the NADP-dependent methylene-H4MPT dehydrogenase from M. extorquens (17) and several enzymes from methanogenic archaea (20, 29, 33, 34). However, modifications of the pterin ring and attached aminobenzene moiety (Fig. 1), which distinguish methanopterin derivatives from folates, substantially influence DmrA activity. Notably, H2F was not a substrate for DmrA at the physiological pH of 6.8 and exhibited only a low level of activity under the optimal in vitro conditions for the enzyme (pH 5.3). In DmrA, the presence of a conserved acidic amino acid (aspartate 35) that is important for pterin binding in DHFRs may contribute to the ability of DmrA to bind and reduce H2F at a low rate.
Previous genetic evidence supports a role for DmrA in the H4MPT-dependent catabolism of C1 compounds (23). dmrA mutants grow on succinate but not on C1 compounds, and the mutants are highly sensitive to methanol toxicity. This phenotype has been observed previously for M. extorquens mutants deficient in two enzymes of the H4MPT-dependent pathway, namely, formaldehyde-activating enzyme (38) and methylene-H4MPT dehydrogenase B (17). In addition, the dmrA mutant is incapable of producing dephospho-H4MPT, consistent with a deficiency in an enzyme required for dephospho-H4MPT biosynthesis (S. A. Wyles and M. E. Rasche, unpublished data). Finally, the genome of M. extorquens contains two additional DHFR analogs which appear to be involved in the reduction of H2F to H4F (6, 23). These results, combined with the biochemical data presented here, support the proposal that DmrA functions as H2MPT reductase, the last proposed enzyme of the H4MPT biosynthesis pathway.
Although many bacterial enzymes involved in H4MPT-dependent C1 metabolism are homologous to archaeal enzymes (7), no archaeal homologs of DmrA have been identified. This is perhaps not surprising if one considers that H2MPT reductase from methanogenic archaea is likely to use the methanogen cofactor F420H2 as an electron donor instead of NADPH. A similar phenomenon has been observed previously for the NAD-dependent methylene-H4MPT dehydrogenase B (MtdB) of M. extorquens, which also lacks an archaeal homolog but is closely related to an NADP-dependent methylene-tetrahydrofolate dehydrogenase from the same organism (7, 17). The archaeal version of methylene-H4MPT dehydrogenase uses F420H2 as an electron donor (35) and is not closely related in sequence to the bacterial counterpart. In addition, Pomper et al. (26) have reported a third bacterial H4MPT-dependent dehydrogenase that deviates from its archaeal counterpart. Transfer of a formyl group from dephospho-H4MPT to the coenzyme methanofuran in M. extorquens is catalyzed by a formyltransferase related to an archaeal homolog (26). However, instead of directly oxidizing the formyl group to CO2 as predicted by analogy to the archaeal formyltransferase, the bacterial enzyme releases formate without oxidation. Formate is then oxidized by a bacterial NAD-dependent formate dehydrogenase. Based on these three examples, it is anticipated that if additional dehydrogenases are required for H4MPT biosynthesis in M. extorquens, the enzymes will be NADPH dependent and homologous to bacterial genes rather than archaeal genes. This may be a general feature of bacterial dehydrogenases evolved for use in metabolic pathways derived from methanogenic archaea.
Characterization of H4MPT-dependent enzymes has led to speculation about the evolutionary events resulting in the existence of both H4MPT and H4F in methylotrophic bacteria. If clusters of archaeal-gene-like C1 metabolism genes were acquired by horizontal gene transfer, the three dehydrogenases described above may illustrate how host genes can be rapidly adapted to function in new metabolic pathways following interdomain horizontal gene transfer. Archaeal genes encoding proteins dependent on F420 instead of NAD(P) would have been nonfunctional in M. extorquens, which is not known to synthesize F420. To take advantage of the newly acquired genes for H4MPT-dependent enzymes, cells would need to develop strategies to replace the F420-dependent enzymes. In the case of DmrA and MtdB, M. extorquens appears to have adapted its own folate-dependent enzymes to function in H4MPT-dependent metabolism. One possible evolutionary scenario is that the DNA sequence encoding the F420 binding site of DmrA may have been lost and the remaining portion of the gene spliced with an NADPH-binding sequence through gene duplication and homologous recombination with a bacterial DHFR gene. Alternatively, accumulated mutations or rearrangements involving solely bacterial genes, such as a duplicated DHFR gene, might be sufficient to alter the substrate specificity from H2F to H2MPT. Identifying specific amino acids that distinguish between the preference of DmrA for H2MPT rather than H2F may provide insight into the evolutionary processes by which host genes are adapted to accommodate new metabolic pathways following horizontal gene transfer. Characterization of H2MPT reductases from archaea and bacteria will provide further insight into the evolutionary relationships among organisms that utilize methanopterin.
Acknowledgments
We are grateful to Mary Lidstrom and Christopher Marx for sharing their plasmids and M. extorquens mutants and for helpful suggestions. We also gratefully acknowledge Thomas Bobik for his valuable discussions and his contributions to the HPLC studies.
This work was supported by National Science Foundation grant number MCB-9876212 and the Florida Agricultural Experiment Station.
Footnotes
Florida Agricultural Experiment Station Journal Series number R-09922.
REFERENCES
- 1.Aiso, K., T. Nozaki, M. Shimoda, and E. Kokue. 1999. Assay of dihydrofolate reductase activity by monitoring tetrahydrofolate using high-performance liquid chromatography with electrochemical detection. Anal. Biochem. 272:143-148. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, D. J., C. R. Beddell, J. N. Champness, P. J. Goodford, F. E. Norrington, D. R. Smith, and D. K. Stammers. 1981. The binding of trimethoprim to bacterial dihydrofolate reductase. FEBS Lett. 126:49-52. [DOI] [PubMed] [Google Scholar]
- 4.Blakley, R. L. 1984. Dihydrofolate reductase, p. 191-253. In R. L. Blakley and S. J. Benkovic (ed.) Folates and pterins, vol. 1. John Wiley & Sons, New York, N.Y.
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chistoserdova, L., S.-W. Chen, A. Lapidus, and M. E. Lidstrom. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J. Bacteriol. 185:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chistoserdova, L., J. A. Vorholt, R. K. Thauer, and M. E. Lidstrom. 1998. C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic archaea. Science 281:99-102. [DOI] [PubMed] [Google Scholar]
- 8.Dann, J. G., G. Ostler, R. A. Bjur, R. W. King, P. Scudder, P. C. Turner, G. C. Roberts, and A. S. Burgen. 1976. Large-scale purification and characterization of dihydrofolate reductase from a methotrexate-resistant strain of Lactobacillus casei. Biochem. J. 157:559-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson, R. M. C., D. C. Elliot, W. H. Elliot, and K. M. Jones. 1986. Data for Biochemical Research, 3rd ed. Clarendon Press, Oxford, England.
- 10.DiMarco, A. A., T. A. Bobik, and R. S. Wolfe. 1990. Unusual coenzymes of methanogenesis. Annu. Rev. Biochem. 59:355-394. [DOI] [PubMed] [Google Scholar]
- 11.Filman, D. J., J. T. Bolin, D. A. Matthews, and J. Kraut. 1982. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. II. Environment of bound NADPH and implications for catalysis. J. Biol. Chem. 257:13663-13672. [PubMed] [Google Scholar]
- 12.Garfin, D. E. 1990. One-dimensional gel electrophoresis. Methods Enzymol. 182:425-441. [DOI] [PubMed] [Google Scholar]
- 13.Goenrich, M., J. Bursy, E. Hubner, D. Linder, A. C. Schwartz, and J. A. Vorholt. 2002. Purification and characterization of the methylene tetrahydromethanopterin dehydrogenase MtdB and the methylene tetrahydrofolate dehydrogenase FolD from Hyphomicrobium zavarzinii ZV580. Arch. Microbiol. 177:299-303. [DOI] [PubMed] [Google Scholar]
- 14.Gorris, L. G., and C. van der Drift. 1994. Cofactor contents of methanogenic bacteria reviewed. Biofactors 4:139-145. [PubMed] [Google Scholar]
- 15.Graham, D. E., H. Xu, and R. H. White. 2002. A member of a new class of GTP cyclo-hydrolases produces formylaminopyrimidine nucleotide monophosphates. Biochemistry 41:15074-15084. [DOI] [PubMed] [Google Scholar]
- 16.Graupner, M., H. Xu, and R. H. White. 2000. Identification of an archaeal 2-hydroxy acid dehydrogenase catalyzing reactions involved in coenzyme biosynthesis in methanoarchaea. J. Bacteriol. 182:3688-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagemeier, C. H., L. Chistoserdova, M. E. Lidstrom, R. K. Thauer, and J. A. Vorholt. 2000. Characterization of a second methylene tetrahydromethanopterin dehydrogenase from Methylobacterium extorquens AM1. Eur. J. Biochem. 267:3762-3769. [DOI] [PubMed] [Google Scholar]
- 18.Hillcoat, B. L., P. F. Nixon, and R. L. Blakley. 1967. Effect of substrate decomposition on the spectrophotometric assay of dihydrofolate reductase. Anal. Biochem. 21:178-189. [DOI] [PubMed] [Google Scholar]
- 19.Keltjens, J. T., M. J. Huberts, W. H. Laarhoven, and G. D. Vogels. 1983. Structural elements of methanopterin, a novel pterin present in Methanobacterium thermoautotrophicum. Eur. J. Biochem. 130:537-544. [DOI] [PubMed] [Google Scholar]
- 20.Keltjens, J. T., A. J. Brugman, J. M. Kesseleer, B. W. te Brommelstroet, C. van der Drift, and G. D. Vogels. 1992. 5-Formyl-5,6,7,8-tetrahydromethanopterin is the intermediate in the process of methanogenesis in Methanosarcina barkeri. Biofactors 3:249-255. [PubMed] [Google Scholar]
- 21.Lin, X., and R. H. White. 1988. Structure of solfapterin (erythro-neopterin-3′-d-2-deoxy-2-aminoglucopyranoside) isolated from the thermophilic archaebacterium Sulfolobus solfataricus. J. Bacteriol. 170:1396-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maden, B. E. H. 2000. Tetrahydrofolate and tetrahydromethanopterin compared: functionally distinct carriers in C1 metabolism. Biochem. J. 350:609-629. [PMC free article] [PubMed] [Google Scholar]
- 23.Marx, C. J., B. N. O'Brien, J. Breezee, and M. E. Lidstrom. 2003. Novel methylotrophy genes of Methylobacterium extorquens AM1 identified by using transposon mutagenesis including a putative dihydromethanopterin reductase. J. Bacteriol. 185:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Möller-Zinkhan, D., G. Börner, and R. K. Thauer. 1989. Function of methanofuran, tetrahydromethanopterin, and coenzyme F420 in Archaeoglobus fulgidus. Arch. Microbiol. 152:362-368. [Google Scholar]
- 25.Peel, D., and J. R. Quayle. 1961. Microbial growth on C1 compounds. Isolation and characterization of Pseudomonas AM1. Biochem. J. 81:465-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomper, B. K., O. Saurel, A. Milon, and J. A. Vorholt. 2002. Generation of formate by the formyltransferase/hydrolase complex (Fhc) from Methylobacterium extorquens AM1. FEBS Lett. 523:133-137. [DOI] [PubMed] [Google Scholar]
- 27.Pomper, B. K., and J. A. Vorholt. 2001. Characterization of formyltransferase from Methylobacterium extorquens AM1. Eur. J. Biochem. 269:4769-4775. [DOI] [PubMed] [Google Scholar]
- 28.Pomper, B. K., J. A. Vorholt, L. Chistoserdova, M. E. Lidstrom, and R. K. Thauer. 1999. A methenyl tetrahydromethanopterin cyclo-hydrolase and a methenyl tetrahydrofolate cyclo-hydrolase in Methylobacterium extorquens AM1. Eur. J. Biochem. 261:475-480. [DOI] [PubMed] [Google Scholar]
- 29.Raemakers-Franken, P. C., A. J. Kortstee, C. van der Drift, and G. D. Vogels. 1990. Methanogenesis involving a novel carrier of C1 compounds in Methanogenium tationis. J. Bacteriol. 172:1157-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Scott, J. W., and M. E. Rasche. 2002. Purification, overproduction, and partial characterization of beta-RFAP synthase, a key enzyme in the pathway of methanopterin biosynthesis. J. Bacteriol. 184:4442-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tahar, R., P. Eldin de Pécoulas, L. K. Bascoa, M. Chiadmic, and A. Mazabraud. 2001. Kinetic properties of dihydrofolate reductase from wild-type and mutant Plasmodium vivax expressed in Escherichia coli. Mol. Biochem. Parasitol. 113:241-249. [DOI] [PubMed] [Google Scholar]
- 33.te Brommelstroet, B. W., C. M. Hensgens, W. J. Geerts, J. T. Keltjens, C. van der Drift, and G. D. Vogels. 1990. Purification and properties of 5,10-methenyltetrahydromethanopterin cyclo-hydrolase from Methanosarcina barkeri. J. Bacteriol. 172:564-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.te Brommelstroet, B. W., W. J. Geerts, J. T. Keltjens, C. van der Drift, and G. D. Vogels. 1991. Purification and properties of 5,10-methylenetetrahydromethanopterin dehydrogenase and 5,10-methylenetetrahydromethanopterin reductase, two coenzyme F420-dependent enzymes, from Methanosarcina barkeri. Biochim. Biophys. Acta 1079:293-302. [DOI] [PubMed] [Google Scholar]
- 35.Thauer, R. K., R. Hedderich, and R. Fischer. 1993. Reactions and enzymes involved in methanogenesis from CO2 and H2, p. 209-252. In J. G. Ferry (ed.), Methanogenesis: ecology, physiology, biochemistry, & genetics. Chapman & Hall, New York, N.Y.
- 36.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorholt, J. A., L. Chistoserdova, S. M. Stolyar, R. K. Thauer, and M. E. Lidstrom. 1999. Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclo-hydrolases. J. Bacteriol. 181:5750-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vorholt, J. A., C. J. Marx, M. E. Lidstrom, and R. K. Thauer. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J. Bacteriol. 182:6645-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, R. H. 1991. Distribution of folates and modified folates in extremely thermophilic bacteria. J. Bacteriol. 173:1987-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White, R. H. 1993. Structures of the modified folates in the thermophilic archaebacteria Pyrococcus furiosus. Biochemistry 32:745-753. [DOI] [PubMed] [Google Scholar]
- 41.White, R. H. 2001. Biosynthesis of the methanogenic cofactors. Vitam. Horm. 61:299-337. [DOI] [PubMed] [Google Scholar]
- 42.Williams, T. J., T. K. Lee, and R. B. Dunlap. 1977. Dihydrofolate reductase from amethopterin-resistant Lactobacillus casei. Effects of pH, salts, temperature, and source of NADPH on enzyme activity and substrate specificity studies. Arch. Biochem. Biophys. 181:569-579. [DOI] [PubMed] [Google Scholar]
- 43.Wilquet, V., J. A. Gaspar, M. van de Lande, M. Van de Casteele, C. Legrain, E. M. Meiering, and N. Glansdorff. 1998. Purification and characterization of recombinant Thermotoga maritima dihydrofolate reductase. Eur. J. Biochem. 255:628-637. [DOI] [PubMed] [Google Scholar]
- 44.Xu, H., R. Aurora, G. D. Rose, and R. H. White. 1999. Identifying two ancient enzymes in Archaea using predicted secondary structure alignment. Nat. Struct. Biol. 6:750-754. [DOI] [PubMed] [Google Scholar]