Abstract
With a few exceptions, vaccines for viruses that cause hemorrhagic fever remain unavailable or lack well-documented efficacy. In the past decade this has not been due to a lack of the ability to develop vaccine platforms against highly pathogenic viruses, but rather the lack of will/interest to invest in platforms that have the potential to become successful vaccines. The two exceptions to this are vaccines against Dengue virus and Rift Valley Fever virus, which recently have seen significant progress in putting forward new and improved vaccines, respectively. Experimental vaccines for filoviruses and Lassa virus do exist but are hindered by a lack of financial interest and only partially or ill-defined correlates/mechanisms of protection that could be assessed in clinical trials.
Introduction
Several families of RNA viruses have members that can cause viral hemorrhagic fever (VHF) in humans: Arenaviruses, Bunyaviruses, Filoviruses, Flaviviruses and possibly a newly discovered not yet isolated Rhabdovirus [1]. Live-attenuated and inactivated whole virus vaccines are available for some VHFs and in some cases these vaccines are highly effective and in widespread use within specific countries. However, regulatory procedures usually mean they are unavailable outside of the source country as they often cannot meet the requirements to proceed to either clinical trials or licensing in the majority of western countries. Globalization, international travel and climate change are increasing the number of individuals at risk for VHFs, suggesting that at some point the mechanisms for moving vaccines against VHFs to clinical use are going to have to change.
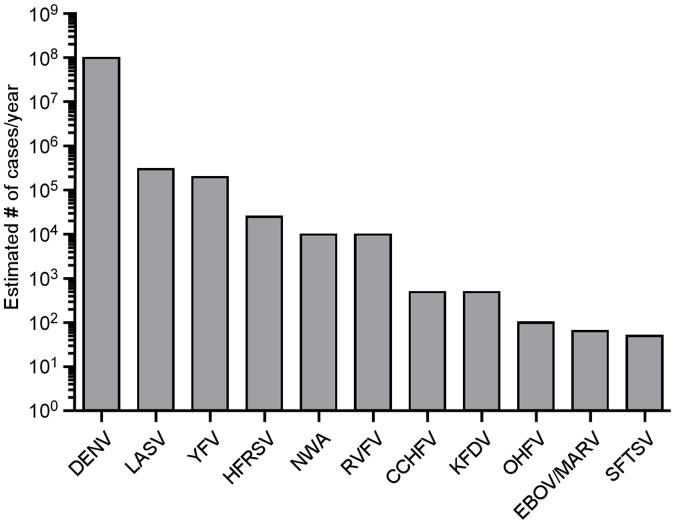
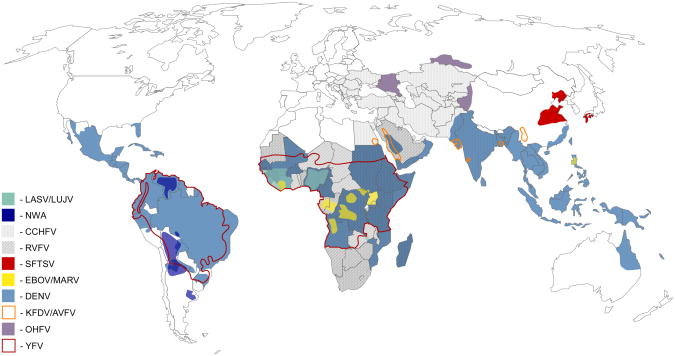
While most VHFs can be considered neglected tropical diseases, the combined public health impact of all VHFs combined is substantial. While the total number of lab confirmed VHF cases is relatively small, there are an estimated 100 million cases of Dengue virus (DENV) infection per year, with approximately 500,000 infections from all other VHFs combined (Figure 1). Moreover, more than a third of the world's population lives in areas that are at risk for VHFs (Figure 2). In the case of tick-transmitted viruses, incidence levels are less likely to increase quickly; however, the identification of severe fever with thrombocytopenia syndrome virus (SFTSV) in China in 2009 and its subsequent identification in Japan in 2012 serves as a reminder that novel viruses continue to emerge [2]. With the expansion of tick ranges due to climate change further spread of viruses and the emergence of novel viruses is possible. More concerning is the spread of mosquitos that are capable of transmitting DENV, yellow fever virus (YFV) and rift valley fever virus (RVFV). Given their already significant public and animal health impact and their potential for spread, more resources should be devoted to pushing proven experimental vaccines into clinical trials.
Figure 1. Estimated global burden of viral hemorrhagic fevers.
The number of estimated cases per year of Dengue virus (DENV), Lassa virus (LASV), Yellow fever virus (YFV), hemorrhagic fever with renal syndrome viruses (HFRSV), New-world arenaviruses (NWA), Rift Valley fever virus (RVFV), Crimean-Congo hemorrhagic fever virus (CCHFV), Kyasanur Forest disease virus (KFDV), Omsk hemorrhagic fever virus (OHFV), Ebola (EBOV) and Marburg virus (MARV), and Severe fever with thrombocytopenia syndrome virus (SFTSV).
Figure 2. Risk zones for hemorrhagic fever viruses.
Regions with current risk or past occurrence of the following viral hemorrhagic fevers: Dengue hemorrhagic fever (DHF), Crimean-Congo hemorrhagic fever (CCHF), Omsk hemorrhagic fever (OHF), Rift Valley hemorrhagic fever (RVF), Yellow fever (YF), Severe fever with thrombocytopenia syndrome (SFTS), Kyasanur Forest disease (KFD).
Arenaviruses
Old world arenaviruses (OWA) that result in VHF include Lassa virus (LASV) and Lujo virus. Lujo virus has recently been identified as a new genetically distinct OWA; however, no vaccines have been developed to date [3]. LASV remains one of the most neglected of the tropical viral diseases and next to DENV and YFV has the most significant impact on human health. LASV is endemic to West Africa with an estimated 300,000 infections per year and fatality rate of approximately 2% [4]. It is transmitted to humans via its rodent reservoir Mastomys natalensis through inhalation of contaminated droplets/dust or ingestion of contaminated food (Table 1) [4]. Currently there are no licensed vaccines for the prevention of LASV. A single-dose vaccine would be ideal for use in endemic areas as the infrastructure in these regions is limited [5]. For LASV it is thought that cell-mediated immunity plays a major role in recovery and protection, thus favoring the development of live-attenuated vaccines [5].
Table 1.
Summary of hemorrhagic fever viruses and the status of available vaccines (in use, clinical trial or experimental).
| Hemorrhagic Fever Viruses | Vector/Host Species | Vaccine in Use or [Clinical trial] | Experimental Vaccine | |
|---|---|---|---|---|
| Arenavirus | Chapare virus | Unknown | No | No |
| Guanarito virus | Sigmodon alstoni, Zygodontomys brevicauda | No | No | |
| Junín virus | Calomys musculinus | Candid#1 | Yes | |
| Lassa virus | Mastomys sp. | No | Yes | |
| Lujo virus | Unknown | No | No | |
| Machupo virus | C. callosus | No | No | |
| Sabia virus | Huesped desconocido | No | No | |
|
| ||||
| Bunyavirus | Crimean-Congo Hemorrhagic Fever virus | Hyalomma sp. | Inactivated | Yes |
| HFRS viruses | Apodemus sp., Rattus norvegicus, Clethrionomys glareolus | Hantavax | Yes | |
| Rift Valley virus | Aedes sp., Culex sp., Anopheles sp. | Inactivated, MP-12, Smithburn, Clone13 | Yes | |
| Severe Fever with Thrombocytopenia Syndrome virus | Haemaphysalis longicornis | No | No | |
|
| ||||
| Filovirus | Ebola virus | *Epomops franqueti, Hypsignathus monstrosus, Myonycteris torquata, Micropteropus pusillus, Mops condylurus, Rousettus aegyptiacus | [DNA, Adenovirus 5] | Yes |
| Marburg virus | R.aegyptiacus, H. monstrosus | No | Yes | |
|
| ||||
| Flavivirus | Dengue virus | Aedes sp. | [tetravalent YFV17D-based] | Yes |
| Kyasanur Forest | Hemaphysalis sp. | Inactivated | No | |
| Disease virus Omsk Hemorrhagic fever virus | Dermacentor reticulatus | Inactivated (discont.), [FSME-IMMUN (TBEV vaccine)] | No | |
| Yellow fever virus | Aedes sp. | 17D, 17DD | Yes | |
Vector species have only been identified for the Zaire species of Ebola virus.
While inactivated, peptide epitope and alphavirus replicon-based vaccines have been generated for LASV; the utility of these in nonhuman primate model is lacking [5]. A live-attenuated vaccine based on recombinant vesicular stomatitis virus (rVSV) expressing the glycoprotein was protective in cynomolgus macaques; however, the correlates of protection have not been established [6]. Virus-like particles containing the glycoprotein, nucleoprotein and Z matrix protein were immunogenic in mice but efficacy data are not available [7].
A LASV/Mopeia virus reassortant (ML29) containing the glycoprotein and nucleoprotein of LASV and the RNA polymerase and zinc-binding protein of Mopeia virus, a related but apathogenic arenavirus, was protective in marmosets [8]. In guinea pigs, ML29 provided protection against challenge from genetically diverse LASV isolates and also provided 80% protection when administered 48h post-infection [5]. Furthermore, ML29 has recently been shown not to cause disease in Simian immunodeficiency virus-infected rhesus macaques, supporting its safety [9]. The YFV vaccine strain 17D has also been genetically manipulated to express the LASV glycoprotein as a soluble product; and while it protected 80% of guinea pigs [10] it completely failed to protect marmosets and is genetically unstable [5].
New world arenaviruses (NWA) that result in VHF include the following viruses and their respective disease: Junín virus (Argentine HF), Machupo virus (Bolivian HF), Guanarito virus (Venezuelan HF), Sabia virus (Brazilian HF) and Chapare virus (not defined). Each virus has its own unique rodent reservoir (Table 1). Together they cause thousands of cases per year with up to a 20% case-fatality rate (Figure 1) [11]. A live-attenuated version of Junín virus, called Candid#1, is used in Argentina but is not recommended for use in pregnant women and children [12]. This vaccine represents a good example of a successful national/local vaccine that has not been approved for use in other countries. Molecular characterization into the attenuation of Candid#1 has indicated that mutations in glycoprotein G2 are responsible for its attenuation in mice [13]. An alphavirus replicon expressing the Junín glycoprotein provided protection following two immunizations in guinea pigs [14]. Junín virus-like particles (VLPs) containing Z protein were immunogenic in mice, but have been used to demonstrate efficacy from challenge [15]. Experimental vaccines for the other NWA are not available and partial cross-protection with Candid#1 has only been reported for Machupo virus but need further evaluation [12].
Bunyaviruses
Crimean-Congo Hemorrhagic Fever Virus (CCHFV) is a tick-borne Nairovirus that is distributed throughout Asia, the Middle East, south-eastern Europe, the Balkans and Africa (Figure 2). It can be transmitted directly through tick bites (main vector Hyalomma sp.) or through contact with tissues or blood from infected animal and patients (Table 1). Cattle, sheep, horses, goats and swine are susceptible to CCHFV as are small wild-life species such as hedgehogs and hares. Despite its wide distribution, historically CCHFV has caused only small outbreaks [16]; however, outbreaks have been occurring with increasing frequency and size in the past decade especially in Turkey (>5000 confirmed cases since 2002), Iran and the Balkans (Figures 1 & 2) [17].
Currently there is a chloroform/heat inactivated suckling mouse brain derived vaccine. It is used exclusively in Bulgaria in higher risk individuals where it has resulted in a four-fold reduction in the number of reported CCHFV cases [18]. This vaccine is not approved in other countries. Recently, it has been shown that vaccination with the CCHFV glycoproteins Gn and Gc in either a DNA vaccine [19] or purified from transgenic plants induced antibody responses in mice [20]; however, due to a lack of an animal model protection could not be determined. This limitation has recently been overcome with the development of two immunocompromised adult mouse models (STAT-1−/− and IFNAR−/−).
Old world hantavirus (HFRS/NE)
The old world hantaviruses (Hantaan, Seoul, Puumala and Dobrava viruses) are the causative agent of hemorrhagic fever with renal syndrome (HFRS). While hantavirus distribution is considered to be worldwide, HFRS-causing hantaviruses appear to be restricted to Asia and Eastern Russia, although less severe forms termed nephropathia epidemica (NE) are found in Europe. China alone has recorded over 1.5 million cases of HFRS with 46,000 deaths in the last 60 years (Figure 1 & 2) [21]. In contrast to the other bunyaviruses that cause hemorrhagic fever, these viruses are rodent-borne and transmission is through aerosol exposure to urine, feces or saliva (Table 1) [22].
There are multiple different inactivated vaccines that are currently in use. A formalin-inactivated Korean Hantaan virus derived from suckling mouse brain (Hantavax) elicits a good humoral immune response, but the protective efficacy has not been established despite wide-spread use [23]. Cell culture derived inactivated Hantaan or Seoul virus vaccines have been used in Korea, North Korea and China [24]. A formalin-inactivated bivalent vaccine containing both Hantaan and Seoul viruses derived from Syrian Golden hamster kidney cells has also been produced and used in China [25]. Hantavax and the bivalent vaccine elicited positive antibody response in 97% of individuals one month after booster (75% of individuals have neutralizing response). This response waned over one year to approximately 40% with a positive antibody response which was returned to near 100% following a booster [26]. The vaccines used in Korea and China appear to have reduced the number of HFRS cases since implementation [21,26,27]. DNA vaccines for Hantaan and Puumala have also been tested in human trials and elicited good antibody responses in approximately 50% of recipients [28].
Rift Valley Fever virus (RVFV) is a mosquito-borne Phlebovirus that has spread across most of Africa and into the Arabian Peninsula (Figure 2) [29]. Given the ability of RVFV to use multiple mosquito vectors (Aedes, Culex, Anopheles sp. and other species), some of which are present in Europe and North America, and its wide host range (sheep, cattle, goats, water buffalo and humans) further spread out of Africa/Arabia is certainly possible (Table 1) [30]. Outbreaks have resulted in tens to hundreds of thousands of human cases (Figure 1) and affected millions of livestock. Ruminant livestock, especially sheep and cattle, can have up to 70% neonatal mortality and 20-30% adult mortality [29]. Human cases are typically self-limiting, but 1-2% of cases involve more serious syndromes with a case-fatality rate of 10-20% in these individuals [29]. Contact with infected animal tissue and fluids, is thought to be a significant risk factor for severe and fatal human infections. Therefore, vaccination of livestock appears to be an ideal intervention point for preventing human disease and reducing the economic impact of RVFV.
Currently, there are no approved vaccines against RVFV for general use in humans, although the live-attenuated MP-12 vaccine has been used [31]; however, there are multiple livestock vaccines that are used in endemic regions and during outbreaks [29]. In order to be able to export animals it is essential to be able to serologically differentiate between infected and vaccinated animals (DIVA). This has further complicated vaccine development, in addition to the limitations of currently used vaccines including requirement for multiple doses, difficulties in manufacturing or post-vaccination abortion and teratogenicity [31]. Multiple live-attenuated vaccines are currently under development for use in livestock. A derivative of Clone 13 named R566, which contains the S segment of Clone 13 and the M and L segments of MP-12, has been developed; however, it may have reduced efficacy compared to Clone 13 [32]. Recombinant strain ZH501, lacking both NSs and NSm, was also protective, did not appear to be teratogenic and fulfills DIVA [33].
Alphavirus-based vaccines have also been developed using Sindbis and Venezuelan equine encephalitis virus replicons expressing Gn or Gn/Gc [34-36]. Other attenuated virus vectors including vaccinia [37] and Newcastle Disease virus [38] expressing Gn/Gc provided protection in mice and sheep, respectively. Sheep were protected against both Lumpy skin disease virus and RVFV challenge when vaccinated with an attenuated Lumpy skin disease virus strain of Capripoxvirus expressing Gn and Gc or RVFV [39,40]. VLPs with [41,42] or without the nucleocapsid [43] protect mice but require multiple doses. A subunit vaccine containing purified Gn ectodomain was produced that has been tested in mice and lambs with success [38]. Vaccine induced neutralizing antibody responses (against Gn/Gc) appear to be of primary importance for protection against subsequent RVFV challenge [38].
Filoviruses
Ebola virus (EBOV) and Marburg virus (MARV) cause unpredictable outbreaks of severe VHF in humans and non-human primates in equatorial Africa (Figures 1 & 2) [44,45]. Transmission is typically due to direct contact with blood, secretions or tissues from infected patients or animals; although fruit bats are suspected to be the reservoir [44,45]. Case-fatality rates vary by virus species and strain but can be as high as 90% (Table 1) [44,45]. Multiple experimental vaccine platforms have demonstrated efficacy in the gold-standard macaque models following homologous challenge. This has included DNA/recombinant Adenovirus-5 (rAd5) [46], rVSV [47] and recombinant human parainfluenza virus-3 (rHPIV3) [48] all expressing the glycoprotein. rVSV has also demonstrated efficacy when used up to 48 hours post-infection [49]. With this success, the focus has shifted towards delineating the correlates/mechanisms of protection and generating multivalent vaccines against the most relevant species.
Currently, there are four human pathogenic EBOV and one MARV species. Generally, there is no cross-protection between species following vaccination; however, there is cross-protection within a species. As the individual filovirus species are not geographically contained, attempts to generate a single vaccine that would be cross-protective against multiple species are being developed [50]. A single-injection blended vaccine composed of multiple rVSV vaccines expressing different filovirus glycoproteins independently, protected macaques from challenge against any of the species included in the vaccine [49]. A two injection pan-filovirus blended complex adenovirus (CAdVax) expressing multiple glycoproteins and nucleoproteins was also protective against challenge from the included viruses [51]. Similarly, a multivalent vaccine candidate (EBO7) expressing the glycoproteins of two EBOV species in CAdVax provided protection against challenge with either species [52].
Both a blended DNA vaccine containing glycoproteins from two EBOV species and the glycoprotein and nucleoprotein form a single species and the rAd5-ZEBOV GP vaccine were shown to be safe in clinical trials [53]. For the rAd5 vaccine, where T-cell responses have been reported as the mechanism of protection [54], less than half of individuals elicited a desirable immune response [55]. Pre-existing immunity has been a concern for the rAd5 and rHPIV3 platforms; however, airway delivery of rAd5 ZEBOV GP can circumvent pre-existing immunity and confer complete protection in macaques [56]. Despite presumed safety concerns, the rVSV-based vaccine has been shown to cause not be neurovirulent [57], nor side effects in immunocompromised simian-human immunodeficiency virus (SHIV)-infected macaques [49]. Moreover, 67% of SHIV-infected macaques were protected from subsequent challenge, indicating rVSV should be safe and could even provide protection in immunocompromised individuals. For the rAd5, rVSV and rHPIV3 platforms, virus glycoprotein-specific total IgG response appears to correlate with protection in survivors [46-49]. IgG titers are predictive for survival in the rAd5 platform, despite data showing that T cell subsets (CD8+) were required for the mechanism of immunity [54,58]. Furthermore, antibodies were shown to play a critical role in protection for the rVSV vaccine [59].
Flaviviruses
Among the VHFs, Dengue Virus (DENV) has the single largest impact on public health, causing an estimated 50-100 million infections per year in over 100 countries with 500,000 people requiring hospitalization resulting in 12,500 deaths for severe dengue (Table 1; Figures 1 & 2). It is currently estimated that 50% of the world's population is at risk for infection with DENV. Vaccines for DENV have seen a recent surge in potential intervention strategies. Multiple vaccines for DENV are currently under investigation but due to the extensiveness of this effort these are reviewed elsewhere (cross-reference to DENV review). Live-attenuated DENV has been found to be genetically unstable and frequently causes dengue-like syndromes, recombinant virus vectors expressing dengue envelope proteins, purified inactivated viruses, recombinant subunit vaccines, VLPs and DNA vaccines have all been attempted [60,61].
A live-attenuated tetravalent vaccine that expresses the pre-membrane and envelope genes of each of the four DENV serotypes [62] within the 17D YFV vaccine has recently been tested in a clinical trial in healthy Thai children with an overall efficacy of ∼30% [63]. This poor finding was a result of very low efficacy against DENV 2, which was also the prevalent serotype during the study. Encouragingly, despite the concern over incomplete immune response against all four serotypes leading to disease enhancement; this was not observed, even with the incomplete protection against DENV 2. The lack of efficacy against DENV 2 has yet to be explained given that there was a satisfactory immune response to the serotype. The brings in to question whether a balanced immune response to all four DENV serotypes as assessed by neutralizing antibodies is a correct assumption for protection.
Kyasanur forest disease virus (KFDV) and Omsk hemorrhagic fever virus (OHFV) are related to the tick-borne encephalitis (TBE) serocomplex of viruses; however, infection in humans tends to be characterized by hemorrhagic syndrome whereas other members of the TBE complex result in primarily neurological manifestations. There are an estimated 400-500 cases per year of KFDV in India with a case-fatality rate of 1-3% (Figures 1 & 2) [64]. OHFV occurs in some regions of western Siberia in Russia with case-fatality rates between 0.4 and 2.5% (Figures 1 & 2) [65]. The probable tick vector for KFDV has been identified as Hemaphysalis sp.; however, other species have been demonstrated to be capable of KFDV transmission (Table 1). KFDV was thought to be localized to Karnataka State, but serosurveys have suggested cases also occur in other areas of India and the Andaman Islands. Moreover, variants of KFDV have been reported to cause disease in China, while the closely related Alkhurma hemorrhagic fever virus (AHFV) has also been reported in Saudi Arabia, Egypt and Sudan. Transmission of OHFV is mainly via Dermacentor reticulatus; however, humans are mainly infected following contact with infected muskrats (Ondatra zibethicus) (Table 1). Person-to-person transmission has not been noted [64,65].
Currently a formalin-inactivated chick embryo fibroblast-derived vaccine against KFDV is used on a two-dose schedule with boosts at 6-9 months and then every 5 years. The vaccine appears to be well-tolerated and provides a good level of protection (0.027% vs. 0.86% incidence) [64]. The TBEV vaccines used in Europe and Russia may provide protection against KFDV; however, they have not been tested in KFDV regions. Recent data supports that humans vaccinated with the TBEV vaccine FSME-IMMUN have cross-reactive neutralizing antibody responses against OHFV, albeit at a lower titer than against related TBEV viruses [66]. Data from mouse and African green monkeys found that, while the TBEV vaccine did not prevent OHFV infection, it reduced the viral spread and alterations in the blood [67], suggesting that this widely used vaccine may be useful against OHFV. Further studies would be necessary to demonstrate this, but given the safety profile of the TBEV vaccine there does not appear to any reason why this vaccine could not be used in the event of an OHFV outbreak [68].
Yellow fever virus (YFV) is a mosquito-borne flavivirus that is endemic and epidemic in South America and Sub-Saharan Africa (Table 1; Figure 2). It is a bi-phasic disease that initially causes flu-like symptoms which can progress to a toxic phase with increased bleeding tendency, liver damage and jaundice. There are approximately 1500 reported cases annually with a 20-50% case-fatality rate, but it is estimated that up to 200,000 cases may occur annually with a 15% case-fatality rate (Figure 1) [69,70]. Highly efficacious, live-attenuated strains of YFV, known as 17D-204 or 17DD, both of which are produced in eggs, are currently used worldwide for vaccination. Its use however, is contraindicated in persons who are immunocompromised, infants under 6 months and persons with allergies to eggs. The vaccine can cause both neurotropic and viscerotropic disease, which generally occurs after the first dose. The viscerotropic form of disease has a case-fatality rate of 65% with the incidence rising with increasing age of the vaccinee [71]. The reported rate of adverse effect of the 17D vaccine is higher (0.4−0.8/100,000) than for both the smallpox (0.29/100,000) and the oral polio (0.11/100,000), which are no longer widely used due to safety concerns [71].
For YFV, the effectiveness of the current vaccine is not in question but the development of vaccine with a better safety profile is currently the goal. The prME gene which encodes the membrane and envelope proteins from 17D was inserted into non-replicating modified vaccinia virus Ankara and the D4R-defective vaccinia virus and subsequently shown to provide protection in a mouse model of YFV [72]. A β-propiolactone-inactivated whole YFV vaccine (XRX-001) produced in Vero cells has shown to be efficacious in animal models and humans [74,75]. Given the large population that requires vaccination against YFV, transitioning to a safer vaccine, especially in groups that are at higher risk for complications should be considered.
Summary
Given the combined global impact of all VHFs, the lack of urgency to develop vaccines to these viruses is somewhat surprising. Global travel and expanding vector ranges due to climate change are driving expansion of the range of some of these viruses. Experimental vaccine platforms that have extensive evaluation in animal models certainly exist for several VHFs, but are not close to being used in clinical trials (i.e. LASV, EBOV and MARV). Current clinical and field trials of DENV and RVFV vaccines are exciting and demonstrate that when a large economic need is present vaccine trials are possible. A mechanism to address vaccines that have less of an immediate economic impact needs to be developed. The success of the Candid#1 as a vaccine for Argentine HF is the example of how a vaccine of limited utility can be generated with governmental support. As the correlate/mechanisms of protection become better defined for the different VHF vaccine approaches establishing clinical trials (especially phase II and III) that meet current standards should be easier and less controversial.
Highlights.
- Dengue vaccine trial shows limited protection against Dengue 2 but no safety concerns
- Development of Rift Valley Fever vaccines that are safe in pregnant livestock
- Defined correlates of protection for Ebola vaccine platforms could allow clinical trials
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Footnotes
Financial and Competing Interest Disclosure: DF claims no financial or competing interest. HF claims intellectual property regarding VSV-based filovirus vaccines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Grard G, Fair JN, Lee D, Slikas E, Steffen I, Muyembe JJ, Sittler T, Veeraraghavan N, Ruby JG, Wang C, et al. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa. PLoS Pathog. 2012;8:e1002924. doi: 10.1371/journal.ppat.1002924. The first description of Bas-Congo virus, proposed to be the first rhabdovirus that causes hemmorhagic fever in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. The first description and partial characterization of severe fever with thrombocytopenia syndrome virus (SFTSV) and the disease that it causes in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briese T, Paweska JT, McMullan LK, Hutchison SK, Street C, Palacios G, Khristova ML, Weyer J, Swanepoel R, Egholm M, et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009;5:e1000455. doi: 10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fichet-Calvet E, Rogers DJ. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3:e388. doi: 10.1371/journal.pntd.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukashevich IS. Advanced vaccine candidates for lassa Fever. Viruses. 2012;4:2514–2557. doi: 10.3390/v4112514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisbert TW, Jones S, Fritz EA, Shurtleff AC, Geisbert JB, Liebscher R, Grolla A, Stroher U, Fernando L, Daddario KM, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005;2:e183. doi: 10.1371/journal.pmed.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branco LM, Grove JN, Geske FJ, Boisen ML, Muncy IJ, Magliato SA, Henderson LA, Schoepp RJ, Cashman KA, Hensley LE, et al. Lassa virus-like particles displaying all major immunological determinants as a vaccine candidate for Lassa hemorrhagic fever. Virol J. 2010;7:279. doi: 10.1186/1743-422X-7-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukashevich IS, Carrion R, Jr, Salvato MS, Mansfield K, Brasky K, Zapata J, Cairo C, Goicochea M, Hoosien GE, Ticer A, et al. Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for Lassa fever in small non-human primates. Vaccine. 2008;26:5246–5254. doi: 10.1016/j.vaccine.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Zapata JC, Poonia B, Bryant J, Davis H, Ateh E, George L, Crasta O, Zhang Y, Slezak T, Jaing C, et al. An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit Lassa virus-specific immunity. Running title: Lassa vaccine in SIV-positive macaques. Virol J. 2013;10:52. doi: 10.1186/1743-422X-10-52. Demonstration of the safety of the ML29 vaccine plaform against Lassa virus infection in immunocompromised macaques. This addresses some of the theoretical concerns of using a replication competent vacccine in potentially immunocompromised people and further demonstrates the safety of this platform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Jiang X, Dalebout TJ, Bredenbeek PJ, Carrion R, Jr, Brasky K, Patterson J, Goicochea M, Bryant J, Salvato MS, Lukashevich IS. Yellow fever 17D-vectored vaccines expressing Lassa virus GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine. 2011;29:1248–1257. doi: 10.1016/j.vaccine.2010.11.079. Demonstrates that protection against Lassa virus challenge in mice and guina pigs is possible using a modified yellow fever vaccine platform that expresses portions of GP1 and GP2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrion R, Jr, Bredenbeek P, Jiang X, Tretyakova I, Pushko P, Lukashevich IS. Vaccine Platforms to Control Arenaviral Hemorrhagic Fevers. J Vaccines Vaccin. 2012;3 doi: 10.4172/2157-7560.1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosio A, Saavedra M, Mariani M, Gamboa G, Maiza A. Argentine hemorrhagic fever vaccines. Hum Vaccin. 2011;7:694–700. doi: 10.4161/hv.7.6.15198. [DOI] [PubMed] [Google Scholar]
- 13*.Albarino CG, Bird BH, Chakrabarti AK, Dodd KA, Flint M, Bergeron E, White DM, Nichol ST. The major determinant of attenuation in mice of the Candid1 vaccine for Argentine hemorrhagic fever is located in the G2 glycoprotein transmembrane domain. J Virol. 2011;85:10404–10408. doi: 10.1128/JVI.00856-11. Defines the genetic requirements for the attenuation of the Candid#1 vaccine, a necessary step to allow wider acceptance of this vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seregin AV, Yun NE, Poussard AL, Peng BH, Smith JK, Smith JN, Salazar M, Paessler S. TC83 replicon vectored vaccine provides protection against Junin virus in guinea pigs. Vaccine. 2010;28:4713–4718. doi: 10.1016/j.vaccine.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 15.Borio CS, Bilen MF, Arguelles MH, Goni SE, Iserte JA, Glikmann G, Lozano ME. Antigen vehiculization particles based on the Z protein of Junin virus. BMC Biotechnol. 2012;12:80. doi: 10.1186/1472-6750-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortekaas J, Ergonul O, Moormann RJ. Interventions against West Nile virus, Rift Valley fever virus, and Crimean-Congo hemorrhagic fever virus: where are we? Vector Borne Zoonotic Dis. 2010;10:709–718. doi: 10.1089/vbz.2010.0040. [DOI] [PubMed] [Google Scholar]
- 17.Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Mousavi-Jazi M, Karlberg H, Papa A, Christova I, Mirazimi A. Healthy individuals' immune response to the Bulgarian Crimean-Congo hemorrhagic fever virus vaccine. Vaccine. 2012;30:6225–6229. doi: 10.1016/j.vaccine.2012.08.003. Describes the first analysis of the immune response in humans to the inactivated Bulgarian Crimean- Congo hemorrhagic fever virus vaccine. Despite being in use for decades this is the first in depth analysis of the immune response to this vaccine. [DOI] [PubMed] [Google Scholar]
- 19.Spik K, Shurtleff A, McElroy AK, Guttieri MC, Hooper JW, SchmalJohn C. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine. 2006;24:4657–4666. doi: 10.1016/j.vaccine.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Ghiasi SM, Salmanian AH, Chinikar S, Zakeri S. Mice orally immunized with a transgenic plant expressing the glycoprotein of Crimean-Congo hemorrhagic fever virus. Clin Vaccine Immunol. 2011;18:2031–2037. doi: 10.1128/CVI.05352-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YZ, Zou Y, Fu ZF, Plyusnin A. Hantavirus infections in humans and animals, China. Emerg Infect Dis. 2010;16:1195–1203. doi: 10.3201/eid1608.090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardestam J, Karlsson M, Falk KI, Olsson G, Klingstrom J, Lundkvist A. Puumala hantavirus excretion kinetics in bank voles (Myodes glareolus) Emerg Infect Dis. 2008;14:1209–1215. doi: 10.3201/eid1408.080221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho HW, Howard CR. Antibody responses in humans to an inactivated hantavirus vaccine (Hantavax) Vaccine. 1999;17:2569–2575. doi: 10.1016/s0264-410x(99)00057-2. [DOI] [PubMed] [Google Scholar]
- 24.Maes P, Clement J, Van Ranst M. Recent approaches in hantavirus vaccine development. Expert Rev Vaccines. 2009;8:67–76. doi: 10.1586/14760584.8.1.67. [DOI] [PubMed] [Google Scholar]
- 25.Dong GM, Han L, An Q, Liu WX, Kong Y, Yang LH. Immunization effect of purified bivalent vaccine to haemorrhagic fever with renal syndrome manufactured from primary cultured hamster kidney cells. Chin Med J (Engl) 2005;118:766–768. [PubMed] [Google Scholar]
- 26.Cho HW, Howard CR, Lee HW. Review of an inactivated vaccine against hantaviruses. Intervirology. 2002;45:328–333. doi: 10.1159/000067925. [DOI] [PubMed] [Google Scholar]
- 27.Schmaljohn CS. Vaccines for hantaviruses: progress and issues. Expert Rev Vaccines. 2012;11:511–513. doi: 10.1586/erv.12.15. [DOI] [PubMed] [Google Scholar]
- 28.Boudreau EF, Josleyn M, Ullman D, Fisher D, Dalrymple L, Sellers-Myers K, Loudon P, Rusnak J, Rivard R, Schmaljohn C, et al. A Phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for hemorrhagic fever with renal syndrome. Vaccine. 2012;30:1951–1958. doi: 10.1016/j.vaccine.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Bird BH, Nichol ST. Breaking the chain: Rift Valley fever virus control via livestock vaccination. Curr Opin Virol. 2012;2:315–323. doi: 10.1016/j.coviro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Kortekaas J, de Boer SM, Kant J, Vloet RP, Antonis AF, Moormann RJ. Rift Valley fever virus immunity provided by a paramyxovirus vaccine vector. Vaccine. 2010;28:4394–4401. doi: 10.1016/j.vaccine.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 31.Indran SV, Ikegami T. Novel approaches to develop Rift Valley fever vaccines. Front Cell Infect Microbiol. 2012;2:131. doi: 10.3389/fcimb.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouloy M, Flick R. Reverse genetics technology for Rift Valley fever virus: current and future applications for the development of therapeutics and vaccines. Antiviral Res. 2009;84:101–118. doi: 10.1016/j.antiviral.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Bird BH, Maartens LH, Campbell S, Erasmus BJ, Erickson BR, Dodd KA, Spiropoulou CF, Cannon D, Drew CP, Knust B, et al. Rift Valley fever virus vaccine lacking the NSs and NSm genes is safe, nonteratogenic, and confers protection from viremia, pyrexia, and abortion following challenge in adult and pregnant sheep. J Virol. 2011;85:12901–12909. doi: 10.1128/JVI.06046-11. Development of a Rift Valley fever virus vaccine that prevents disease in sheep, and does not cause birth defects or abortions when given to pregnant sheep. This suggests that this platform can fulfill all of the desired requirements for a Rift Valley fever virus vaccine in livestock. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorchakov R, Volkova E, Yun N, Petrakova O, Linde NS, Paessler S, Frolova E, Frolov I. Comparative analysis of the alphavirus-based vectors expressing Rift Valley fever virus glycoproteins. Virology. 2007;366:212–225. doi: 10.1016/j.virol.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heise MT, Whitmore A, Thompson J, Parsons M, Grobbelaar AA, Kemp A, Paweska JT, Madric K, White LJ, Swanepoel R, et al. An alphavirus replicon-derived candidate vaccine against Rift Valley fever virus. Epidemiol Infect. 2009;137:1309–1318. doi: 10.1017/S0950268808001696. [DOI] [PubMed] [Google Scholar]
- 36.Bhardwaj N, Heise MT, Ross TM. Vaccination with DNA plasmids expressing Gn coupled to C3d or alphavirus replicons expressing gn protects mice against Rift Valley fever virus. PLoS Negl Trop Dis. 2010;4:e725. doi: 10.1371/journal.pntd.0000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papin JF, Verardi PH, Jones LA, Monge-Navarro F, Brault AC, Holbrook MR, Worthy MN, Freiberg AN, Yilma TD. Recombinant Rift Valley fever vaccines induce protective levels of antibody in baboons and resistance to lethal challenge in mice. Proc Natl Acad Sci U S A. 2011;108:14926–14931. doi: 10.1073/pnas.1112149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kortekaas J, Antonis AF, Kant J, Vloet RP, Vogel A, Oreshkova N, de Boer SM, Bosch BJ, Moormann RJ. Efficacy of three candidate Rift Valley fever vaccines in sheep. Vaccine. 2012;30:3423–3429. doi: 10.1016/j.vaccine.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Wallace DB, Ellis CE, Espach A, Smith SJ, Greyling RR, Viljoen GJ. Protective immune responses induced by different recombinant vaccine regimes to Rift Valley fever. Vaccine. 2006;24:7181–7189. doi: 10.1016/j.vaccine.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 40.Soi RK, Rurangirwa FR, McGuire TC, Rwambo PM, DeMartini JC, Crawford TB. Protection of sheep against Rift Valley fever virus and sheep poxvirus with a recombinant capripoxvirus vaccine. Clin Vaccine Immunol. 2010;17:1842–1849. doi: 10.1128/CVI.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandell RB, Koukuntla R, Mogler LJ, Carzoli AK, Freiberg AN, Holbrook MR, Martin BK, Staplin WR, Vahanian NN, Link CJ, et al. A replication-incompetent Rift Valley fever vaccine: chimeric virus-like particles protect mice and rats against lethal challenge. Virology. 2010;397:187–198. doi: 10.1016/j.virol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pichlmair A, Habjan M, Unger H, Weber F. Virus-like particles expressing the nucleocapsid gene as an efficient vaccine against Rift Valley fever virus. Vector Borne Zoonotic Dis. 2010;10:701–703. doi: 10.1089/vbz.2009.0248. [DOI] [PubMed] [Google Scholar]
- 43.de Boer SM, Kortekaas J, Antonis AF, Kant J, van Oploo JL, Rottier PJ, Moormann RJ, Bosch BJ. Rift Valley fever virus subunit vaccines confer complete protection against a lethal virus challenge. Vaccine. 2010;28:2330–2339. doi: 10.1016/j.vaccine.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 44.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehedi M, Groseth A, Feldmann H, Ebihara H. Clinical aspects of Marburg hemorrhagic fever. Future Virol. 2011;6:1091–1106. doi: 10.2217/fvl.11.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 47.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 48.Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, Murphy BR, Collins PL, Sanchez A. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol. 2007;81:6379–6388. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011;204(3):S1075–1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falzarano D, Geisbert TW, Feldmann H. Progress in filovirus vaccine development: evaluating the potential for clinical use. Expert Rev Vaccines. 2011;10:63–77. doi: 10.1586/erv.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swenson DL, Warfield KL, Larsen T, Alves DA, Coberley SS, Bavari S. Monovalent virus-like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev Vaccines. 2008;7:417–429. doi: 10.1586/14760584.7.4.417. [DOI] [PubMed] [Google Scholar]
- 52.Pratt WD, Wang D, Nichols DK, Luo M, Woraratanadharm J, Dye JM, Holman DH, Dong JY. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol. 2010;17:572–581. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, Bailer RT, Chakrabarti BK, Bailey MA, Gomez PL, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13:1267–1277. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, Honko A, Olinger G, Bailey M, Geisbert JB, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17:1128–1131. doi: 10.1038/nm.2447. Determined that the CD8+ cell response is required for protection against Ebola virus in the adenovirus 5 vaccine platform. This would allow correlates of protection to be monitored in a clinical trial. [DOI] [PubMed] [Google Scholar]
- 55.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 56.Richardson JS, Pillet S, Bello AJ, Kobinger GP. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J Virol. 2013 doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Mire CE, Miller AD, Carville A, Westmoreland SV, Geisbert JB, Mansfield KG, Feldmann H, Hensley LE, Geisbert TW. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl Trop Dis. 2012;6:e1567. doi: 10.1371/journal.pntd.0001567. Demonstrates the safety of the VSV vaccine platform in light of one of the primary safety concerns: neurovirulence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59**.Marzi A, Engelmann F, Feldamnn F, Haberthur K, Shupert WL, Brining D, Scott DP, Geisbert TW, Kawaoka Y, Katze MG, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 110:1893. doi: 10.1073/pnas.1209591110. Demonstrates that antibodies are required for protection from Ebola virus challenge with the vesicular stomatitis virus vaccine platform. Establishing the correlates of protection is essential for any future clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 61.Schmitz J, Roehrig J, Barrett A, Hombach J. Next generation dengue vaccines: a review of candidates in preclinical development. Vaccine. 2011;29:7276–7284. doi: 10.1016/j.vaccine.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Lang J. Development of Sanofi Pasteur tetravalent dengue vaccine. Rev Inst Med Trop Sao Paulo. 2012;54(18):S15–17. doi: 10.1590/s0036-46652012000700007. [DOI] [PubMed] [Google Scholar]
- 63**.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. Demonstrated that a tetravalent vaccine against all dengue virus serotypes appears to be safe but has low overall efficacy and demonstrated almost no protection against dengue 2. Importantly, no evidence of antibody-dependent enhancement was observed. [DOI] [PubMed] [Google Scholar]
- 64.Holbrook MR. Kyasanur forest disease. Antiviral Res. 2012;96:353–362. doi: 10.1016/j.antiviral.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruzek D, Yakimenko VV, Karan LS, Tkachev SE. Omsk haemorrhagic fever. Lancet. 2010;376:2104–2113. doi: 10.1016/S0140-6736(10)61120-8. [DOI] [PubMed] [Google Scholar]
- 66*.Orlinger KK, Hofmeister Y, Fritz R, Holzer GW, Falkner FG, Unger B, Loew-Baselli A, Poellabauer EM, Ehrlich HJ, Barrett PN, et al. A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J Infect Dis. 2011;203:1556–1564. doi: 10.1093/infdis/jir122. Demonstrated that indivduals vaccined with a tick-borne encephalitis virus (TBEV) vaccine produced antibodies that were capable of neutralized Omsk hemmoragic fever virus (OHFV). This suggests that vaccination with the TBEV vaccine, already approved for use in humans, may provide some protection against OHFV. [DOI] [PubMed] [Google Scholar]
- 67*.Terekhina L, Pripuzova N, Vorovitch M, Rogova Y, Romanova L, Tereshkina N, Timofeev A, Karganova G. Inactivated Vaccine Against Tick-Borne Encephalitis Virus as Surrogate Vaccine Against Omsk Hemorrhagic Fever Virus. Antiviral Res. 2011;90:A45. Demonstrated that an approved tick-borne encephalitis virus vaccine provides some protection and reduced virus dissemination following challenge with Omsk hemorrhagic fever virus (OHFV) in mouse and African green monkey models. This suggests that a vaccine already approved for use in humans could provide some protection against OHFV. [Google Scholar]
- 68.Pollabauer EM, Pavlova BG, Low-Baselli A, Fritsch S, Prymula R, Angermayr R, Draxler W, Firth C, Bosman J, Valenta B, et al. Comparison of immunogenicity and safety between two paediatric TBE vaccines. Vaccine. 2010;28:4680–4685. doi: 10.1016/j.vaccine.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 69.Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, Lewis RF, Staples JE, Tomori O, Wilder-Smith A, Monath TP. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- 70.WHO. Yellow Fever. Edited by; 2011. http://www.who.int/mediacentre/factsheets/fs100/en/
- 71.Khromava AY, Eidex RB, Weld LH, Kohl KS, Bradshaw RD, Chen RT, Cetron MS. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events. Vaccine. 2005;23:3256–3263. doi: 10.1016/j.vaccine.2005.01.089. [DOI] [PubMed] [Google Scholar]
- 72*.Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. 2011;29:6008–6016. doi: 10.1016/j.vaccine.2011.06.034. Determined that following vaccination with an inactived vaccine, neutralizing antibody titers were responsible for protection in the yellow fever virus hamster model. This helps define the corrleates of protection for future clinical trials in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schafer B, Holzer GW, Joachimsthaler A, Coulibaly S, Schwendinger M, Crowe BA, Kreil TR, Barrett PN, Falkner FG. Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever. PLoS One. 2011;6:e24505. doi: 10.1371/journal.pone.0024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, Trent DW. An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. Describes a phase 1 clinical trial of an inactivated yellow fever virus vaccine demonstrating that it is immunogenic and well-tolerated. The current live atteunated yellow fever virus vaccine can cause serious disease in immunocomprised individuals so an effective alternative is highly desired. [DOI] [PubMed] [Google Scholar]
- 75.Monath TP, Lee CK, Julander JG, Brown A, Beasley DW, Watts DM, Hayman E, Guertin P, Makowiecki J, Crowell J, et al. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]