Abstract
Many features of posttraumatic stress disorder (PTSD) can be linked to exaggerated and dysregulated emotional responses. Central to the neurocircuitry regulating emotion are functional interactions between the amygdala and the ventromedial prefrontal cortex (vmPFC). Findings from human and animal studies suggest that disruption of this circuit predicts individual differences in emotion regulation. However, only a few studies have examined amygdala-vmPFC connectivity in the context of emotional processing in PTSD. The aim of the present research was to investigate the hypothesis that PTSD is associated with disrupted functional connectivity of the amygdala and vmPFC in response to emotional stimuli, extending previous findings by demonstrating such links in an understudied, highly traumatized, civilian population. 40 African-American women with civilian trauma (20 with PTSD and 20 non-PTSD controls) were recruited from a large urban hospital. Participants viewed fearful and neutral face stimuli during functional magnetic resonance imaging (fMRI). Relative to controls, participants with PTSD showed an increased right amygdala response to fearful stimuli (pcorr<.05). Right amygdala activation correlated positively with the severity of hyperarousal symptoms in the PTSD group. Participants with PTSD showed decreased functional connectivity between the right amygdala and left vmPFC (pcorr <.05). The findings are consistent with previous findings showing PTSD is associated with an exaggerated response of amygdala-mediated emotional arousal systems. This is the first study to show that the amygdala response may be accompanied by disruption of an amygdala-vmPFC functional circuit that is hypothesized to be involved in prefrontal cortical regulation of amygdala responsivity.
Keywords: PTSD, amygdala, fMRI, functional connectivity, medial prefrontal cortex, emotion
Introduction
PTSD is a highly debilitating anxiety disorder that develops in some individuals after exposure to trauma. Among the general population, PTSD is estimated to affect approximately 7% (Kessler et al., 2005), and a critical question for the field is why this disorder develops in some, but not others, following severe trauma exposure. An increasing body of evidence shows that in addition to combat veterans, the risk for traumatic life experiences and PTSD is especially high among impoverished individuals living in urban settings with high violence exposure, with trauma rates at nearly 88% and PTSD prevalence at 46% (Alim et al., 2006; Gillespie et al., 2009; Liebschutz et al., 2007; Schwartz, Bradley, Sexton, Sherry, & Ressler, 2005).
After a traumatic event, symptoms may develop that interfere with everyday function, including increased emotional arousal and hypervigilance, reexperiencing the traumatic event, and avoidance of trauma reminders. Because these symptoms only persist in a subset of people who experience trauma, one important goal of PTSD research is to identify characteristics that confer resilience or vulnerability for the disorder. Current neurobiological models of PTSD posit that neural circuits typically involved in healthy emotion regulation are disrupted in PTSD (Liberzon & Sripada, 2007; Shin, Rauch, & Pitman, 2006). The goal of the current research was to examine the neural circuits involved in emotion processing in a high-risk urban civilian sample of traumatized African American women.
A wealth of functional neuroimaging evidence has shown that PTSD symptoms are associated with increased activation of the amygdala and insula, brain regions that are involved in initiating and coordinating emotional arousal responses (e.g., Fonzo et al., 2010; Rauch et al., 2000; Shin et al., 2005; Simmons et al., 2011). In addition, PTSD has been associated with decreased activation of prefrontal regions involved in emotion regulation such as the ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (ACC; Jovanovic et al., 2012; Milad et al., 2009; Rougemont-Bucking et al., 2011; Shin et al., 2001; Shin et al., 2005; Williams et al., 2006). A meta-analysis of neuroimaging studies of anxiety disorders found that reduced vmPFC activation is specific to PTSD, and is not consistently observed in studies of other anxiety disorders (Etkin & Wager, 2007). Evidence from studies of overt emotion regulation and fear extinction in healthy participants suggests that the vmPFC and rostral ACC are involved in decreasing arousal responses to negative emotional stimuli (Etkin, Egner, & Kalisch, 2011; Kim & Hamann, 2007; Milad, Wright, et al., 2007; Ochsner, Bunge, Gross, & Gabrieli, 2002).
Examining connectivity between the amygdala and vmPFC can provide a more direct test of the hypothesis that PTSD involves a disruption of medial prefrontal regulation of amygdala responses to emotional stimuli. Recent diffusion tensor imaging (DTI) evidence suggests that white matter integrity is compromised in the cingulum bundle, a major pathway between the amygdala and vmPFC/ACC (Fani, King, et al., 2012). Similarly, examination of resting state functional connectivity, which often parallels structural connectivity, has shown decreased functional coupling between the amygdala and vmPFC in PTSD (Sripada et al., 2012).
Only a small number of studies have examined amygdala functional connectivity in response to emotionally arousing stimuli in PTSD, with mixed findings relating to the strength of connectivity between the amygdala and vmPFC. In response to angry face stimuli, PTSD participants showed decreased connectivity in a circuit involving the amygdala, insula, and dorsal ACC (Fonzo et al., 2010). In contrast, PTSD participants recalling traumatic or emotionally negative autobiographical memories showed increased amygdala functional connectivity with the vmPFC relative to controls (Gilboa et al., 2004; St Jacques, Botzung, Miles, & Rubin, 2011). The variability in findings may relate to differences in the experimental tasks used, or in the populations examined. Previous studies examined PTSD related to recent intimate-partner violence, accidental injury, and among a general civilian population, respectively. In addition, these studies did not include traumatized controls and thus did not differentiate the effects of trauma versus PTSD on amygdala connectivity (Fonzo et al., 2010; St Jacques et al., 2011), or included participants who were taking psychotropic medications at the time of the scan (Gilboa et al., 2004).
In the present study, we investigated the effects of PTSD on amygdala responses to threat cues (fearful facial expressions) and on the functional coupling of the amygdala with other brain areas, in a highly traumatized sample of African-American women. The primary comparison was between unmedicated participants with or without current PTSD, matched for degree of trauma exposure. Little previous work has examined the neural processing of emotional stimuli in an urban civilian population with very high levels of trauma. We hypothesized that PTSD would be associated with an increased amygdala response, and with decreased functional connectivity between the amygdala and vmPFC/ACC, in response to fearful stimuli.
Materials and Methods
Participants
Forty African-American women ages 18 – 59 were recruited through an ongoing study of risk factors for PTSD. Participants were approached in the general medical clinics of Grady Memorial Hospital, a publicly funded hospital that serves economically disadvantaged individuals in Atlanta, Georgia. High rates of trauma and posttraumatic symptoms have been previously observed within this patient population (e.g., Binder et al., 2008). The hospital population is >85% African-American, and therefore we only included subjects with self-reported African-American race/ethnicity to enhance data homogeneity, in addition to the fact that this is an under-represented group in psychiatric imaging research studies. All participants were screened and met the following inclusion criteria: no neurological disorder, psychosis, current psychotropic medication, or metal clips or implants. Individuals who endorsed a history of bipolar disorder, schizophrenia or any other psychotic disorder were also excluded. Due to the high co-morbidity of PTSD and depression, participants with depression were not excluded. Participants had normal or corrected-to-normal vision. Urine tests for pregnancy and illegal drug use (cocaine, marijuana, opiates, amphetamines, methamphetamines) were conducted 24 hours prior to the MRI scan, and individuals who showed positive results for pregnancy or drugs were excluded. Men were not included in the current study, as significant sex differences have been observed in the neural processing of emotional stimuli (Stevens & Hamann, 2012). All participants provided written informed consent prior to participating. Participants received monetary compensation for their time. The institutional review board of Emory University approved the study procedures, and testing took place at Grady Memorial Hospital and the Biomedical Imaging Technology Center at Emory University Hospital.
Psychological assessment
The Modified PTSD Symptom Scale (PSS; Foa & Tolin, 2000) was used to assess PTSD symptoms, and the Traumatic Events Inventory (TEI) was used to assess types and severity of trauma experience. Anxiety levels were assessed using the State Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, & Lushene, 1970). Childhood trauma was assessed using the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994; Scher, Stein, Asmundson, McCreary, & Forde, 2001). These measures have been used in our previous studies with this population (Binder et al., 2008; Fani, Jovanovic, et al., 2012; Schwartz et al., 2005). Traumatic experiences were assessed using the TEI and CTQ during recruitment, and the additional psychological measures were administered during a laboratory visit one day prior to the MRI scan. PTSD diagnosis was based on DSM-IV-TR criteria (presence of trauma; presence of at least one reexperiencing symptom; presence of at least 3 avoidant/numbing symptoms; presence of at least 2 hyperarousal symptoms; occurrence for at least one month), as assessed by the PSS. All participants had experienced at least one trauma, and PTSD diagnosis was used to classify participants into PTSD and traumatized control (TC) groups. PTSD diagnoses were verified for a subset of participants (55%) using the CAPS. Diagnoses using CAPS and PSS were positively correlated, Spearman’s rho = .57, p = .006. Table 1 lists clinical and demographic characteristics of each group.
Table 1.
Group characteristics.
| Demographic Variable | Trauma Control (n = 20) | PTSD (n = 20) | t |
|---|---|---|---|
| M (SD) | M (SD) | ||
|
| |||
| Age | 41.1 (10.7) | 35.7 (12.5) | 1.5 |
| PTSD symptoms (PSS) | 6.7 (6.0) | 27.1 (7.9) | 9.2** |
| Intrusive | 1.6 (2.0) | 7.0 (2.7) | 7.1** |
| Avoidance/Numbing | 2.4 (2.5) | 11.3 (3.7) | 9.0** |
| Hyper-arousal | 2.7 (3.0) | 8.9 (3.1) | 6.5** |
| # traumas, different types (TEI) | 5.7 (2.3) | 5.7 (2.6) | 0.1 |
| Childhood trauma (CTQ) | 43.9 (19.3) | 45.9 (16.7) | 0.3 |
| Emotional abuse | 9.0 (3.7) | 9.7 (4.3) | 0.5 |
| Physical abuse | 8.5 (3.5) | 7.5 (3.8) | 0.9 |
| Sexual abuse | 9.5 (6.3) | 11.5 (6.6) | 1.0 |
| State anxiety (STAI) | 30.9 (9.7) | 35.0 (11.0) | 1.2 |
| Trait anxiety (STAI) | 33.2 (8.6) | 42.1 (9.6) | 3.1** |
| Years Education | % | % | Mann-Whitney U |
|
|
|||
| <12th grade | 5.0 | 5.3 | 2.1* |
| 12th grade/high school graduate | 25.0 | 47.4 | |
| GED | 0.0 | 5.3 | |
| Some college/technical school | 35.0 | 36.8 | |
| College/tech school graduate | 35.0 | 5.3 | |
| Monthly income | |||
| $0–249 | 20.0 | 22.2 | 0.3 |
| $250–499 | 15.0 | 11.1 | |
| $500–999 | 30.0 | 38.9 | |
| $1000–1999 | 20.0 | 16.7 | |
| $2000+ | 15.0 | 11.1 | |
p < .05
p < .01
Procedure
Eight fearful and eight neutral (4 male and 4 female) faces were selected from the stimulus set of Ekman and Friesen (1976). Stimuli were projected onto a 24-inch screen at a resolution of 1280 × 1024 using EPrime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Blocks of fearful and neutral stimuli (15 blocks each) were presented in a pseudorandom order. Each block was composed of all eight faces presented in a random order. Each face stimulus was presented for 500 ms, followed by a 500 ms presentation of a fixation cross. After every 10th block, a 10000 ms rest period with the instruction “relax and look at the screen” was presented. Face stimuli were presented at a size of 4.3 × 6.7″ on a black background, and the fixation cross and instructions were presented in white 18-point Courier New font on a black background. Participants were instructed to pay attention to the faces, but did not make any behavioral response, in order to minimize motion artifacts and neural activation unrelated to processing the visual stimulus.
Brain imaging acquisition and analysis
Brain imaging data were acquired on a Siemens 3.0 Tesla Magnetom Trio TIM whole-body MR scanner (Siemens, Malvern, PA) using a 12-channel head coil. Functional images were acquired using the Z-SAGA pulse sequence (Heberlein & Hu, 2004) to minimize signal loss due to susceptibility artifacts. Each scan volume contained 30 axially acquired 4mm thick images with an in-plane resolution of 3.44 × 3.44 mm2 utilizing the parameters: pulse repetition time 3000 ms, echo time 1= 30 ms, echo time 2 = 67 ms, at a flip angle of 90 deg. Structural images were acquired using a gradient-echo, T1-weighted pulse sequence (TR=2600ms, TE=3.02ms; 1mm×1mm×1mm voxel size). The echo-planar imaging (EPI) data were slice-timed and realigned using AFNI software and the matrix to coregister the EPI images to the anatomical image was calculated using FMRIB Software Library (FSL). The anatomical image was registered and normalized into standard Montreal Neurological Institute (MNI) space using FSL and the resulting matrix was combined with the coregistration matrix and applied to the EPI images. The functional images were then smoothed with an 8mm Gaussian kernel.
Individual participants’ imaging data were analyzed using first level general linear models implemented using statistical parametric mapping software (SPM5; Holmes & Friston, 1998). For each participant, the evoked hemodynamic responses for blocks of fearful and neutral stimuli were modeled with a boxcar function representing the onset and 8000 ms duration of the block, convolved with a canonical hemodynamic response function, as implemented in SPM5. Participant-specific motion parameters were included as regressors of non-interest. Statistical contrasts between conditions (e.g., fearful vs. neutral) were assessed using linear contrasts. Contrast images representing the linear comparison of beta values for the fearful versus neutral conditions were constructed for each participant, and were entered into group-level random effects analysis to identify clusters of significant activation. To address the a priori prediction that the PTSD and TC groups would differ in amygdala activation, we additionally examined group differences using small-volume correction within a bilateral amygdala region of interest (ROI), defined anatomically using the SPM Anatomy Toolbox (Eickhoff et al., 2005).
Task-based functional connectivity analyses were conducted using the CONN toolbox (http://web.mit.edu/swg/software.htm), a toolbox that allows for flexible analyses of connectivity that are conceptually similar to psychophysiological interaction analysis. Seed regions were defined anatomically using the mean time course across voxels within the right and left amygdala ROIs. For each voxel within the whole-brain mask, covariance with amygdala activation during responses to fearful face stimuli was contrasted with covariance with amygdala activation during responses to neutral faces. Individual participants’ motion parameters and main effects of task condition were modeled as nuisance covariates. Non-task-specific covariance between regions was controlled by examining statistical contrasts of connectivity for fearful relative to neutral face stimuli, such that results included only regions that showed significantly increased connectivity with the amygdala for fearful relative to neutral faces. The resulting contrast images for individual participants then entered group-level analyses comparing PTSD and TC participants.
Analyses of regional activation and functional connectivity were conducted using a combined height-extent threshold to correct for multiple comparisons. Monte Carlo simulation was implemented using AlphaSim within the REST toolbox for SPM5 (Song et al., 2011). This method for establishing a height-extent threshold represents a “principled” approach to controlling the family-wise error rate, allowing for similar control of type 1 error across studies (Bennett, Wolford, & Miller, 2009). The corrected height-extent threshold was calculated for voxels within a gray matter mask based on the ICBM 152-subject atlas. For whole-brain analyses, a cluster-forming threshold of p < .01 was used, and when combined with a cluster size of k = 19 resulted in a corrected probability of p < .043, (voxel-wise probability p < .0006). The a priori threshold for the ROI analysis of amygdala activation was determined for voxels within the bilateral anatomical amygdala ROI. A cluster-forming threshold of p < .05 was used, and when combined with a cluster size of k = 11 resulted in a corrected probability of p < .049 (voxel-wise probability p < .005).
Results
Group characteristics
Table 1 shows demographic and clinical characteristics for each group. The PTSD and TC groups did not differ in the number of different types of traumas experienced, the amount of childhood trauma experienced, age, or monthly income. PTSD and TC did not differ in state anxiety just prior to entering the MRI scanner. Relative to TC, PTSD participants had greater PTSD symptoms, and greater trait anxiety. PTSD participants also had lower levels of education than TC.
FMRI activation in response to fearful facial expressions
Participants in the PTSD group showed bilateral activation within the amygdala ROI in response to fearful relative to neutral faces (left: k = 65, Z = 3.13, x,y,z = −32, −4, −20, pcorr < .05; right: k = 70, Z = 2.69, x,y,z = 40, −4, −28, pcorr < .05), overlapping the majority of the left and right anatomical regions. Participants in the TC group also showed bilateral amygdala activation (left: k = 44, Z = 2.65, x,y,z = −28, −12, −12, pcorr < .05; right: k = 15, Z = 2.31, x,y,z = 24, −4, −28, pcorr < .05). Relative to TC, the PTSD group showed an increased response to fearful faces in the right amygdala (k = 11, Z = 2.5, x,y,z = 20, 0, −16, pcorr < .05), as shown in Figure 1.
Figure 1. Increased amygdala response to fearful stimuli in PTSD subjects.
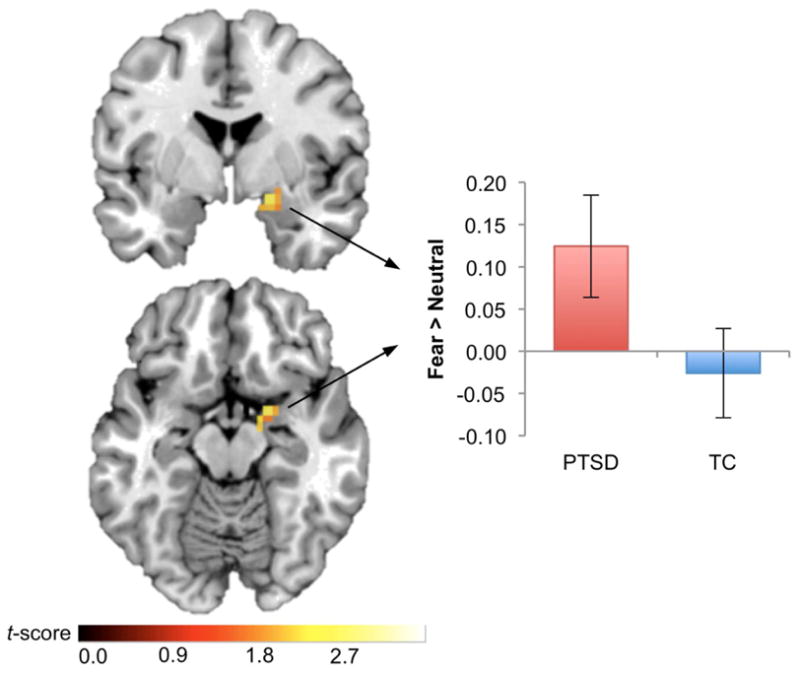
Increased right amygdala response to fearful stimuli in the posttraumatic stress disorder (PTSD) group, relative to the traumatized control (TC) group, pcorr < .05. Results are displayed in neurological orientation on a representative single-subject template brain in MNI space. Bar graph shows the mean contrast estimate across voxels in the right amygdala cluster, for the Fear > Neutral contrast, and error bars show standard error of the mean.
In the analysis of whole-brain regional activation in response to fearful relative to neutral faces (Table 2 and Figure S1), both PTSD and TC groups showed activation of visual areas such as the fusiform gyrus and inferior occipital gyrus, and activation of the temporal cortex, and middle cingulate gyrus. PTSD participants showed greater responses than TC in a cluster containing peaks in the right temporal pole, right nucleus accumbens, and right entorhinal cortex. This cluster overlapped the right amygdala. The PTSD group also showed greater responses to fearful faces than the TC group in the superior frontal gyrus, bilaterally. The PTSD group showed decreased responses relative to TC in the right postcentral gyrus.
Table 2.
Regional activation for fearful relative to neutral faces
| Region | HEM | MNI Coordinates | Z* | k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| PTSD | ||||||
| Amygdala | L | −32 | 0 | −16 | 3.23 | 207 |
| Putamen | L | −20 | 12 | −8 | 3.05 | (LM) |
| Inf. Temporal G. | L | −40 | −16 | −28 | 2.69 | (LM) |
| Mid. Temporal G. | R | 48 | −48 | 8 | 4.17 | 236 |
| Sup. Temporal G. | R | 68 | −44 | 16 | 3.64 | (LM) |
| Calcarine Fissure | R | 28 | −56 | 8 | 2.72 | (LM) |
| Mid. Temporal G. | L | −52 | −56 | 4 | 3.49 | 160 |
| Fusiform G. | L | −40 | −56 | −20 | 3.04 | (LM) |
| Supramarginal G. | L | −64 | −52 | 24 | 2.79 | (LM) |
| Temporal Pole | R | 36 | 12 | −24 | 3.34 | 263 |
| Globus Pallidus | R | 16 | 4 | −8 | 3.16 | (LM) |
| Mid. Temporal G. | R | 52 | −12 | −24 | 2.87 | (LM) |
| Orbitofrontal Cortex | R | 52 | 36 | −8 | 3.83 | 40 |
| Orbitofrontal Cortex | L | −40 | 32 | −12 | 3.11 | 69 |
| Orbitofrontal Cortex | L | −32 | 28 | −20 | 2.79 | (LM) |
| Sup. Frontal G. | R | 16 | 60 | 24 | 3.59 | 113 |
| Sup. Frontal G. | L | −4 | 64 | 16 | 3.31 | (LM) |
| Mid. Frontal G. | L | −32 | 52 | 20 | 2.86 | (LM) |
| Mid. Cingulate G. | R | 12 | −12 | 32 | 2.96 | 27 |
| Fusiform G. | R | 28 | −72 | −12 | 2.87 | 34 |
| Lingual G. | R | 24 | −80 | 0 | 2.54 | (LM) |
| TC | ||||||
| Mid. Cingulate G. | R | 20 | 8 | 32 | 3.43 | 49 |
| Inf. Frontal G. | R | 28 | 0 | 28 | 3.08 | (LM) |
| Inf. Frontal G. | R | 36 | 12 | 20 | 2.65 | (LM) |
| Thalamus | R | 24 | −28 | 16 | 3.40 | 125 |
| Mid. Temporal G. | R | 32 | −44 | 20 | 2.98 | (LM) |
| Heschl G. | R | 32 | −36 | 16 | 2.96 | (LM) |
| Putamen | R | 36 | −16 | −4 | 2.84 | 37 |
| Putamen | R | 36 | 4 | 4 | 2.83 | (LM) |
| Insula | R | 40 | −8 | −8 | 2.75 | (LM) |
| Cerebellum | L | −40 | −80 | −20 | 4.27 | 1430 |
| Inf. Occipital G. | L | −36 | −80 | −12 | 4.21 | (LM) |
| Inf. Occipital G. | R | 36 | −80 | −12 | 4.08 | (LM) |
| PTSD > TC | ||||||
| Temporal Pole | R | 36 | 12 | −24 | 3.25 | 57 |
| Nucleus Accumbens | R | 16 | 4 | −12 | 2.84 | (LM) |
| Entorhinal Cortex | R | 12 | −12 | −20 | 2.63 | (LM) |
| Sup. Frontal G. | R | 12 | 64 | 12 | 4.34 | 169 |
| Sup. Frontal G. | L | −8 | 64 | 8 | 4.23 | (LM) |
| Sup. Frontal G. | L | −20 | 60 | 20 | 2.89 | (LM) |
| Cerebellum | R | 24 | −76 | −44 | 3.21 | 20 |
| PTSD < TC | ||||||
| Postcentral G. | R | 60 | −24 | 52 | 3.34 | 26 |
Significant clusters reported for a whole brain height-extent-corrected threshold of p < .05
Correlation between fMRI activation and PTSD symptom severity
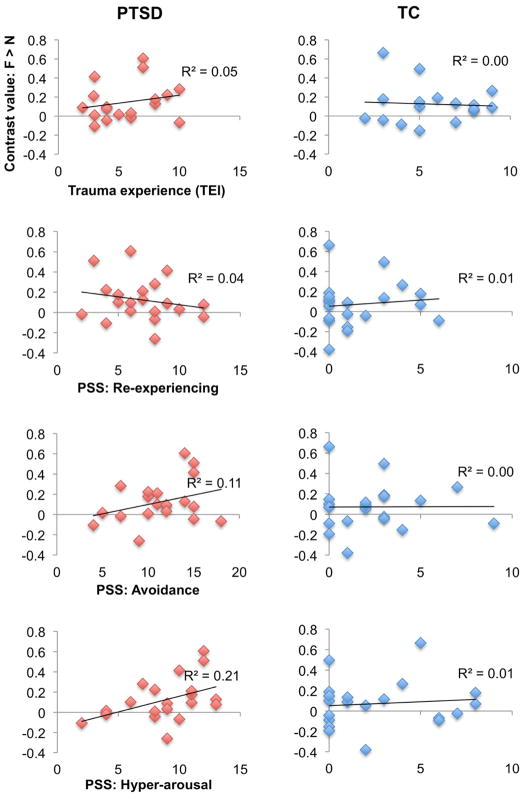
To further characterize the relationship between PTSD and the increased right amygdala response to fearful faces, we examined linear correlations between the right amygdala response, trauma experience, and PTSD symptoms (Figure 2). Taking the mean contrast value for fearful relative to neutral faces across all voxels of the anatomical right amygdala ROI, correlations were performed with the sum of different types of trauma experiences on the TEI (TEI total), and with symptom severity (PSS total) and PSS subscales for reexperiencing, avoidance, and hyperarousal symptoms, respectively. Neither trauma experience nor total symptom severity correlated with right amygdala responses for the PTSD or TC groups, ps > .10. For the PTSD group, right amygdala responses correlated positively with hyperarousal symptoms, R2 = .21, p = .04, but not with reexperiencing or avoidance symptoms, ps > .10. For the TC group, no significant correlation was observed for reexperiencing, avoidance, or hyperarousal symptoms, ps > .10.
Figure 2. Relationship between amygdala response and trauma or PTSD symptoms.
Correlations between the right amygdala response to fearful stimuli, trauma experience, and posttraumatic stress disorder (PTSD) symptoms are shown. The right amygdala response was extracted as the mean of all voxels within the anatomically-defined amygdala ROI, for the fearful > neutral faces contrast. The right amygdala response correlated only with hyperarousal symptoms in the PTSD group.
To examine regions outside the amygdala, correlations were performed between symptom severity (PSS total score), and the contrast of fearful relative to neutral faces for each voxel within the whole brain. Results are shown in Figure S2. When the PTSD and TC groups were examined separately, activation for fearful relative to neutral faces did not correlate with symptom severity in any region. When all participants were examined irrespective of PTSD diagnosis, symptom severity correlated positively with activation in a cluster in anterior medial PFC overlapping the right and left superior frontal gyrus (k = 29, Z = 3.02, x,y,z = 12, 64, 16, pcorr < .05), and a cluster overlapping the left inferior frontal gyrus and the left anterior insula (k = 28, Z = 2.80, x,y,z = −24, 16, −12, pcorr < .05). No region displayed a significant negative correlation with symptom severity.
Functional connectivity with the amygdala
For fearful relative to neutral faces, within-groups analysis of the PTSD group (Table 3) showed increased functional connectivity between the left amygdala seed and the anterior cingulate cortex and left caudate. The PTSD group also showed increased functional connectivity between the right amygdala seed and bilateral temporal lobe regions, the left postcentral gyrus, and right cerebellum. Within-groups analysis of the TC group (Table 3) showed increased functional connectivity between the left amygdala seed and left subgenual cingulate cortex and orbitofrontal cortex, left superior parietal gyrus, and left pre- and postcentral gyri. TC participants also showed increased functional connectivity between the right amygdala seed and bilateral orbitofrontal cortex, the left superior parietal gyrus, and right cerebellum.
Table 3.
Functional connectivity with the amygdala for fearful relative to neutral faces
| Seed | Region | HEM | MNI Coordinates
|
Z* | k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| PTSD | |||||||
| Left amygdala | Ant. Cingulate | L | −12 | 28 | −4 | 3.14 | 32 |
| Caudate | L | −8 | 20 | −4 | 2.77 | (LM) | |
| Right amygdala | Inf. Temporal G. | R | 68 | −32 | −24 | 3.74 | 26 |
| Mid. Temporal G. | R | 44 | 0 | −28 | 3.57 | 20 | |
| Mid. Temporal G. | L | −12 | −8 | −32 | 3.67 | 19 | |
| Sup. Frontal G. | L | −20 | −4 | 68 | 3.59 | 19 | |
| Postcentral G. | L | −36 | −20 | 40 | 2.71 | 21 | |
| Postcentral G. | L | −44 | −20 | 28 | 2.58 | (LM) | |
| Cerebellum | R | 28 | −40 | −28 | 2.69 | 20 | |
| Cerebellum | R | 24 | −28 | −28 | 2.63 | (LM) | |
| TC | |||||||
| Left amygdala | Subgenual Cingulate | L | −4 | 28 | −20 | 3.34 | 54 |
| Orbitofrontal Cortex | L | −8 | 28 | −12 | 3.24 | (LM) | |
| Orbitofrontal Cortex | L | −12 | 20 | −28 | 2.5 | (LM) | |
| Sup. Parietal G. | L | −36 | −56 | 64 | 4.33 | 55 | |
| Sup. Parietal G. | L | −28 | −60 | 68 | 4.23 | (LM) | |
| Postcentral G. | L | −40 | −40 | 68 | 3.09 | (LM) | |
| Postcentral G. | L | −28 | −40 | 44 | 2.94 | 45 | |
| Inf. Parietal G. | L | −48 | −28 | 48 | 2.47 | (LM) | |
| Precentral G. | L | −28 | −20 | 56 | 3.08 | 51 | |
| Precentral G. | L | −40 | −16 | 68 | 3.05 | (LM) | |
| Med. Frontal G. | L | −12 | −12 | 56 | 2.63 | (LM) | |
| Right Amygdala | G. Rectus | L | −4 | 32 | −20 | 3.42 | 72 |
| G. Rectus | L | −4 | 48 | −20 | 2.81 | (LM) | |
| Orbitofrontal Cortex | R | 16 | 48 | −4 | 3.26 | 82 | |
| Orbitofrontal Cortex | R | 12 | 68 | −12 | 3.21 | (LM) | |
| Orbitofrontal Cortex | R | 16 | 60 | −8 | 3.19 | (LM) | |
| Sup. Parietal G. | L | −28 | −60 | 64 | 3.6 | 28 | |
| Cerebellum | R | 16 | −52 | −36 | 3.24 | 36 | |
| PTSD > TC | |||||||
| Left amygdala | Inf. Frontal G. | R | 48 | 28 | 20 | 3.65 | 35 |
| Right amygdala | Globus Pallidus | R | 20 | −8 | 4 | 3.34 | 32 |
| PTSD < TC | |||||||
| Left amygdala | * No significant clusters | ||||||
| Right amygdala | G. Rectus | -- | 0 | 36 | −20 | 2.72 | 22 |
| Subgenual Cingulate | L | −4 | −24 | 20 | 2.71 | (LM) | |
Significant clusters reported for a whole brain height-extent-corrected threshold of p < .05
In the between-group comparisons (Table 3, Figure 3), PTSD showed less connectivity than TC between the right amygdala and a cluster in the subgenual cingulate cortex. This cluster overlapped with the area for which the TC group showed a significant within-groups effect for right amygdala connectivity. PTSD also showed greater connectivity than TC between the left amygdala and a cluster in right dorsolateral prefrontal cortex with a peak in the inferior frontal gyrus, and between the right amygdala and a cluster in the right globus pallidus. However, these clusters were not observed to show significant connectivity with the left amygdala in the within-groups analysis of PTSD participants. To examine whether connectivity between the amygdala and subgenual cingulate cortex was related to PTSD symptom severity, we examined correlations between PSS total score and the contrast value representing connectivity for fearful relative to neutral faces, extracted from the peak of the subgenual cingulate cluster. Although the PTSD group showed less connectivity than TC between the amygdala and subgenual cingulate, individuals with PTSD showed a significant positive correlation between symptom severity and amygdala – subgenual cingulate connectivity, R2 = .37, p = .004. TC individuals showed a non-significant negative correlation between symptom severity and amygdala-subgenual cingulate connectivity, R2 = .15, p = .10. When all participants were examined irrespective of diagnosis, symptom severity as a predictor of functional connectivity was best described by a quadratic, inverted u-shaped function. Curve fit regression analysis showed that a linear function was not significant, R2 = .04, p = .21, the quadratic term accounted for significant variance, R2 = .33, p = .001.
Figure 3. Differential functional connectivity with amygdala in PTSD vs. Trauma Control.
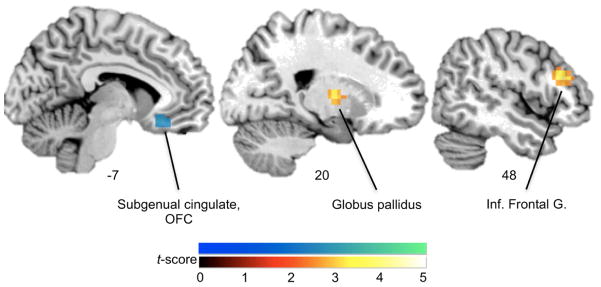
Group differences in functional connectivity with the amygdala, for fearful relative to neutral images are shown. Clusters showing differences in connectivity with either the left or right amygdala seed are overlaid on a representative single-subject template brain, pcorr < .05. Blue-green color scale: significantly decreased connectivity in posttraumatic stress disorder (PTSD) relative to traumatized control (TC) participants. Red-yellow color scale: significantly increased connectivity in PTSD relative to TC participants.
Discussion
In the present study, we investigated amygdala activation and functional connectivity with the vmPFC in response to fearful stimuli, in traumatized participants with and without PTSD. The findings were consistent with the hypotheses that PTSD would be associated with enhanced amygdala responses to fearful emotional stimuli and changes in the networks that include the amygdala. Right amygdala responses were greater in the PTSD group, accompanied by decreased task-related functional connectivity between the right amygdala and vmPFC (subgenual cingulate cortex). Further, the PTSD group showed a significant correlation between right amygdala responses and hyperarousal symptoms, but not trauma experience, reexperiencing, or avoidance symptoms. This finding suggests that exaggerated amygdala reactivity in PTSD is specifically related to the hyperarousal component of the disorder. Taken together, the findings are consistent with current neurocircuitry theories of emotion dysregulation PTSD (e.g., Liberzon & Sripada, 2007; Shin et al., 2006), showing differences in an amygdala-vmPFC circuit involved in processing emotional stimuli.
The finding that participants with PTSD showed increased amygdala responses to fearful stimuli is consistent with previous observations in PTSD (e.g., Bryant et al., 2008; Fonzo et al., 2010; Shin et al., 2005). Meta-analytic evidence indicates that this pattern is observed reliably across neuroimaging studies (Etkin & Wager, 2007; Patel, Spreng, Shin, & Girard, 2012). A strength of the current investigation was that group differences in amygdala reactivity and functional connectivity were not confounded by trauma experience. The PTSD and control groups did not differ in the number of traumas experienced, but only in their PTSD status. A major outstanding question regarding the role of the amygdala in PTSD has been whether exaggerated amygdala reactivity reflects a trait which pre-disposes individuals to develop PTSD symptoms in response to a trauma, or whether it reflects a maladaptive reaction which develops after the trauma has occurred. The current findings cannot address this issue directly, but provide initial evidence to suggest that increased amygdala activation is not a product of trauma exposure.
Because the current sample was drawn from a relatively understudied population experiencing high levels of trauma, we had the opportunity to further investigate links between amygdala activation and trauma exposure. In contrast with the sample typically examined in studies of PTSD after acute trauma, the majority of these participants experienced more than one traumatic event (M = 5.6) that would meet PTSD criterion A. It is therefore notable that amygdala reactivity was not related to the number of traumas experienced. Instead, we observed that the amygdala response to fearful stimuli was related to hyperarousal symptoms in PTSD, such that individuals who reported more severe hyperarousal symptoms showed greater amygdala reactivity. Similarly, total PTSD symptom severity correlated with activation in anterior medial PFC in BA 10, and left inferior frontal gyrus/insula. The medial PFC region was located in frontopolar cortex bilaterally, rostral to vmPFC and anterior cingulate cortex, an area that has been shown to correlate positively with symptoms of anhedonia and anxiety in major depressive disorder (Keedwell, Andrew, Williams, Brammer, & Phillips, 2005). Further clarification of the relationship between amygdala activation, trauma exposure, and PTSD risk will be critical to understanding PTSD psychopathology. This question would be best addressed by longitudinal investigation examining whether amygdala reactivity before a traumatic event is predictive of PTSD development after trauma.
Several lines of evidence suggest that amygdala function is modulated by the vmPFC (which includes the orbitofrontal cortex and anterior cingulate cortex). Neuroanatomical research in macaques indicates that the amygdala and vmPFC are densely and bi-directionally interconnected (Amaral & Price, 1984; Barbas & de Olmos, 1990; Ghashghaei & Barbas, 2002), and rodent research indicates that the medial prefrontal cortex exerts an inhibitory influence over amygdala activity (Quirk, Likhtik, Pelletier, & Paré, 2003; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006). Prefrontal inhibition of the amygdala has also been found using structural equation modeling of limbic circuits in humans (Stein et al., 2007). This circuit appears to play a central role in emotion regulation. For example, human neuroimaging studies show that medial prefrontal activation correlates positively and amygdala activation correlates negatively with cognitive emotion regulation (Etkin et al., 2011; Ochsner et al., 2002) and fear extinction (Milad, Wright, et al., 2007). Deficits in the ability to regulate emotion and extinguish learned fear are key phenotypic elements of PTSD (Bradley et al., 2011). Previous neuroimaging studies have found that PTSD is associated with changes in functional networks centered around the ACC and vmPFC, but these studies have not probed interactions with the amygdala (Fonzo et al., 2010; Lanius et al., 2010; Lanius et al., 2004; Milad, Wright, et al., 2007), or found increased functional coupling with the amygdala (Gilboa et al., 2004; St Jacques et al., 2011). In contrast, we observed for the first time a decrease in the functional coupling of the amygdala and vmPFC during the processing of emotional stimuli, a finding that is consistent with non-human research and the phenotypic pattern of emotion dysregulation in PTSD.
Several factors may explain the discrepancy between the current findings and those of previous studies. The current study addressed limitations of previous research by including only non-medicated individuals, and by balancing the PTSD and non-PTSD groups for level of trauma. Perhaps more importantly, however, the vmPFC area showing decreased connectivity with the amygdala in PTSD in the current study differed slightly in location from areas showing increased connectivity in previous studies. We observed that PTSD was associated with decreased connectivity between the amygdala and a specific subregion of the vmPFC: the subgenual cingulate cortex, BA 25. Two previous studies observed that PTSD was associated with increased functional connectivity between the amygdala and more rostral regions of the vmPFC, in BA 32 (St Jacques et al., 2011) and BA 24 (Gilboa et al., 2004). Findings from fear conditioning in rodents point to heterogeneity of function among different subregions of the vmPFC. The infralimbic subregion of rodent vmPFC seems to underlie fear extinction and exerts an inhibitory influence over the amygdala, whereas the prelimbic subregion underlies fear expression and exerts an excitatory influence over the amygdala (Milad & Quirk, 2002; Vidal-Gonzalez et al., 2006). The subgenual cingulate (BA25) is hypothesized to be homologous with rodent infralimbic cortex, whereas BA 32 and 24 are hypothesized to be homologous with rodent prelimbic cortex (Milad, Quirk, et al., 2007; Myers-Schulz & Koenigs, 2012; Quirk & Beer, 2006; Slattery, Neumann, & Cryan, 2011). The current findings may therefore be compatible with previous findings, such that emotional dysregulation features of PTSD may involve disrupted connectivity between the amygdala and BA 25, as well as enhanced connectivity between the amygdala and BA 32 and 24.
Interestingly, functional connectivity and PTSD symptom severity were associated in a quadratic, U-shaped curve, such that individuals with the lowest and highest symptom severity scores showed greatest connectivity between amygdala and subgenual cingulate. This pattern resulted in an overall group difference with the TC group showing greater connectivity than PTSD, but within the PTSD group, symptoms and connectivity were positively correlated. Several interpretations are possible. Highly symptomatic individuals may recruit greater PFC engagement in order to inhibit overactive amygdala activity. Alternatively, the direction of connectivity may differ between the two extremes in symptoms, i.e., among highly symptomatic individuals the amygdala may drive connectivity, whereas among asymptomatic individuals the PFC may drive connectivity. It is important to note that the current analyses of functional connectivity indicate only connectivity strength, indexing the correlation between regional time courses, and do not allow conclusions regarding directional or causal relations between regions. Future investigations that include indices of structural connectivity, or the time-course of neural activation would more clearly elucidate the direction of amygdala – PFC interactions, and relationships with PTSD symptoms.
The finding of decreased amygdala-subgenual cingulate connectivity may be of relevance to PTSD treatment approaches. Among vmPFC subregions, the subgenual cingulate cortex (BA 25) shares the highest density of projections with the amygdala, and sends more projections to the amygdala than it receives (Ghashghaei, Hilgetag, & Barbas, 2007). This region has served as a target for deep-brain stimulation in severe refractory depression, producing a marked reduction in symptoms and long-term remission (Holtzheimer et al., 2012; Mayberg et al., 2005). Treatment studies of PTSD indicate symptom improvement correlated with increased activation of the anterior cingulate, across several treatment strategies including psychotherapy (Felmingham et al., 2007; Peres et al., 2007), and pharmacological intervention using SSRIs (Fani et al., 2011; Fernandez et al., 2001; Seedat et al., 2004). Such findings suggest that plasticity in this region provides a neural substrate for PTSD symptom improvement. Relatively little research has addressed PTSD treatment effects on the circuit between the amygdala and vmPFC. Because of the centrality of this circuit to healthy emotion regulation, therapeutic or pharmacological treatments that increase structural or functional connectivity between these regions may provide a fruitful target for intervention.
It is notable that we did not observe the decreased medial prefrontal activation in response to fearful stimuli that is often observed in studies of PTSD (e.g., Jovanovic et al., 2012). It appeared that the task did not strongly engage this region. Neither the PTSD nor control group showed vmPFC activation in response to the fearful relative to neutral stimuli. In contrast with previous studies that have identified impairments in medial prefrontal activation (Fani, Jovanovic, et al., 2012; Jovanovic et al., 2012), the current study employed a passive viewing task, and did not require participants to engage in a behavioral response to the fearful or neutral stimuli. Further replication is needed, testing the same hypotheses using a task that more strongly engages the vmPFC.
This study included several limitations that must be acknowledged. First, future research would benefit from the inclusion of a non-traumatized control group, allowing group comparisons that would account for independent effects of trauma (i.e., PTSD & TC > non-traumatized control), PTSD (PTSD > TC), and resilience (TC > non-traumatized control & PTSD). In the current study, we were only able to account for effects of PTSD independent of trauma. Second, PTSD diagnoses were made using the PSS, rather than through a clinician-administered interview. Future studies would benefit from PTSD diagnosis through such interviews, and by defining diagnosis criteria and PTSD symptom severity using independent measures. In addition, although the participants were screened for psychiatric co-morbidities, only a subset of participants completed a clinician-administered interview for psychiatric diagnoses. Therefore, potentially co-morbid disorders may have been present. Third, this study included only female participants; the findings may not generalize to men. Fourth, the participants viewed stimuli that depicted Caucasian faces, raising the possibility that social out-group biases may influence neural responses. The primary reason for selecting this task was that it has been well validated in the literature (e.g., Breiter et al., 1996; Shin et al., 2005), however, future work should examine neural responses to African-American faces. Fifth, the findings may be influenced by the fact that the PTSD group was less educated than the traumatized control group. This is notable given that lower education level may be a risk factor for PTSD (Brewin, Andrews, & Valentine, 2000).
In summary, the current findings demonstrate that PTSD is associated with enhanced amygdala activation and reduced functional connectivity between the amygdala and vmPFC among civilian women drawn from an all-traumatized population at high risk for PTSD. This study expands on previous research in that it shows that amygdala reactivity is positively correlated with hyperarousal symptoms of PTSD on a continuum in addition to diagnostic categorization. These findings provide support for a model of PTSD in which the neural substrates supporting healthy emotion regulation are disrupted. Future research should include investigation of whether amygdala-vmPFC connectivity before a trauma is predictive of PTSD development, and the effects of current treatment approaches on this circuit.
Supplementary Material
Figure S1. Whole-brain analysis of the response to fearful stimuli, relative to neutral stimuli. Significant clusters of activation are shown for each group, and the comparison of the post-traumatic stress disorder (PTSD) group, relative to the traumatized control (TC) group, pcorr < .05. Results are displayed in neurological orientation on a representative single-subject template brain in MNI space.
Figure S2. Regions showing a significant positive correlation with post-traumatic stress disorder (PTSD) symptom severity, pcorr < .05. For all participants irrespective of PTSD diagnosis, symptom severity scores were correlated with the activation of each voxel within the whole brain, for the fearful > neutral faces contrast. Results are displayed in neurological orientation on a representative single-subject template brain.
Acknowledgments
We would like to thank Allan Graham, Angelo Brown, Julia Merlin, and Rahim Dhanani, as well as the Grady Trauma Project staff for help with participant recruitment, and Robert Smith III, at the Biomedical Imaging Technology Center for his assistance with imaging.
Role of funding source
This work was primarily supported by the National Institutes of Mental Health (MH071537 to K.J.R. and MH098212 to T.J.). Support was also received from Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039), National Center for Advancing Translational Sciences of the NIH (UL1TR000454), and Howard Hughes Medical Institute (K.J.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Contributors
Kerry Ressler, Tanja Jovanovic, Negar Fani, Timothy Ely, and Bekh Bradley designed the study and wrote the protocol. Ebony Glover, Timothy Ely, and Jennifer Stevens collected the data. Jennifer Stevens and Timothy Ely conducted the analyses. Jennifer Stevens wrote the first draft of the paper. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alim TN, Graves E, Mellman TA, Aigbogun N, Gray E, Lawson W, Charney DS. Trauma exposure, posttraumatic stress disorder and depression in an African-American primary care population. J Natl Med Assoc. 2006;98(10):1630–1636. [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of Comparative Neurology. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Barbas H, de Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. The Journal of Comparative Neurology. 1990;300(4):549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Social Cognitive and Affective Neuroscience. 2009;4(4):417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, DeFife JA, Guarnaccia C, Phifer J, Fani N, Ressler KJ, Westen D. Emotion dysregulation and negative affect: Association with psychiatric symptoms. J Clin Psychiatry. 2011;72(5):685–691. doi: 10.4088/JCP.10m06409blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68(5):748–766. doi: 10.1037/0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Human Brain Mapping. 2008;29(5):517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Ashraf A, Afzal N, Jawed F, Kitayama N, Reed L, Bremner JD. Increased neural response to trauma scripts in posttraumatic stress disorder following paroxetine treatment: A pilot study. Neurosci Lett. 2011;491(3):196–201. doi: 10.1016/j.neulet.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB, Ressler KJ. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol. 2012;90(2):134–142. doi: 10.1016/j.biopsycho.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(12):2740–2746. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychological Science. 2007;18(2):127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Pissiota A, Frans Ö, von Knorring L, Fischer H, Fredrikson M. Brain function in a patient with torture related post-traumatic stress disorder before and after fluoxetine treatment: A positron emission tomography provocation study. Neuroscience Letters. 2001;297(2):101–104. doi: 10.1016/S0304-3940(00)01674-8. [DOI] [PubMed] [Google Scholar]
- Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. J Trauma Stress. 2000;13(2):181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68(5):433–441. doi: 10.1016/J.Biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/S0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55(3):263–272. doi: 10.1016/J.Biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magnetic Resonance in Medicine. 2004;51(1):212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, Ressler KJ. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2012 Sep 5; doi: 10.1016/j.cortex.2012.08.011. 2012, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Neufeld RWJ, Williamson PC, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandinavica. 2010;121(1):33–40. doi: 10.1111/J.1600-0447.2009.01391.X. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. The nature of traumatic memories: A 4-T fMRI functional connectivity analysis. Am J Psychiatry. 2004;161(1):36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: A critical review. Stress Hormones and Post Traumatic Stress Disorder: Basic Studies and Clinical Perspectives. 2007;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Liebschutz J, Saitz R, Brower V, Keane T, Lloyd-Travaglini C, Averbuch T, Samet J. PTSD in urban primary care: High prevalence and low physician recognition. Journal of General Internal Medicine. 2007;22(6):719–726. doi: 10.1007/s11606-007-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/J.Biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry. 2012;17(2):132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Peres JFP, Newberg AB, Mercante JP, Simao M, Albuquerque VE, Peres MJP, Nasello AG. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: A SPECT study. Psychol Med. 2007;37(10):1481–1491. doi: 10.1017/S003329170700997X. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Curr Opin Neurobiol. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. The Journal of Neuroscience. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neurosci Ther. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJG, McCreary DR, Forde DR. The Childhood Trauma Questionnaire in a community sample: Psychometric properties and normative data. J Trauma Stress. 2001;14(4):843–857. doi: 10.1023/a:1013058625719. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56(2):212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Seedat S, Warwick J, van Heerden B, Hugo C, Zungu-Dirwayi N, Van Kradenburg J, Stein DJ. Single photon emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. J Affect Disord. 2004;80(1):45–53. doi: 10.1016/S0165-0327(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Matthews SC, Strigo IA, Baker DG, Donovan HK, Motezadi A, Stein MB, Paulus MP. Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biol Mood Anxiety Disord. 2011;1(1):6. doi: 10.1186/2045-5380-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID, Cryan JF. Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. Journal of Psychopharmacology. 2011;25(10):1295–1303. doi: 10.1177/0269881110368873. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory: Test manual. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry & Neuroscience. 2012;37(4):241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45(5):630–637. doi: 10.1016/J.Jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & Memory. 2006;13(6):728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29(2):347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Whole-brain analysis of the response to fearful stimuli, relative to neutral stimuli. Significant clusters of activation are shown for each group, and the comparison of the post-traumatic stress disorder (PTSD) group, relative to the traumatized control (TC) group, pcorr < .05. Results are displayed in neurological orientation on a representative single-subject template brain in MNI space.
Figure S2. Regions showing a significant positive correlation with post-traumatic stress disorder (PTSD) symptom severity, pcorr < .05. For all participants irrespective of PTSD diagnosis, symptom severity scores were correlated with the activation of each voxel within the whole brain, for the fearful > neutral faces contrast. Results are displayed in neurological orientation on a representative single-subject template brain.