Abstract
This review summarizes the major advances that had been reported since the outstanding contributions that Professor Benet and his group had made in the 1980’s and 1990’s concerning the metabolism and pharmacologic action of organic nitrates (ORN). Several pivotal studies have now enhanced our understanding of the metabolism and the bioactivation of ORN, resulting in the identification of a host of cysteine-containing enzymes that can carry out this function. Three isoforms of aldehyde dehydrogenase, all of which with active catalytic cysteine sites, are now known to metabolize, somewhat selectively, various members of the ORN family. The existence of a long-proposed but unstable thionitrate intermediate from organic nitrate metabolism has now been experimentally observed. ORN-induced thiol oxidation in multiple proteins, called the “Thionitrate Oxidation Hypothesis”, can be used not only to explain the phenomenon of nitrate tolerance, but also the various consequences of chronic nitrate therapy, viz., rebound vasoconstriction, and increased morbidity and mortality. Thus, a unifying biochemical hypothesis can account for the myriad of pharmacological events resulting from nitrate therapy. Optimization of future uses of ORN in cardiology and other diseases could benefit from further elaboration of this unifying hypothesis.
Keywords: Aldehyde Dehydrogenase, Bioactivation, Metabolism, Nitrate Tolerance, Organic nitrates, Redox Signaling, Thiol Oxidation, Thionitrate
Contributions of the Benet Group to the Understanding of Organic Nitrate Action
From the mid-1980’s to the late 1990’s, Professor Benet and his associates carried out a number of pivotal studies that greatly enhanced our understanding of the pharmacological properties of nitroglycerin (NTG) and its dinitrate metabolites, viz., 1,2-glyceryl dinitrate (1,2-GDN), and 1,3-glyceryl dinitrate (1,3-GDN). Expanding on the assay originally described by Miyazaki et al.1, Benet’s group utilized electron capture detection to determine the plasma concentrations of these organic nitrates (ORN),2–4 which allowed them to characterize the metabolism and pharmacokinetics of NTG and its dinitrate metabolites after different routes of administration,5 including the intravenous/vascular route,6–8 cutaneous/transdermal application,9–14 sublingual 8 and oral dosing.15–17
Benet and his group then followed with several studies which delineated the pharmacokinetic/pharmacodynamic relationships of NTG and its dinitrate metabolites in dogs,18,19 healthy volunteers,20,21 and in elderly conscious patients.22 NTG metabolism was first examined in rabbit hepatic tissue,23,24 which identified the involvement of glutathione-S-transferases (GST). The relative roles of GST isozymes in mediating NTG-induced vascular relaxation were further examined in bovine coronary artery25 and rabbit aorta,26–29 resulting in the finding that the cytosolic mu isozyme of GST may be substantially involved in these preparations. Additionally, cytochrome P450 3A (CYP 3A) activity was found to correlate with in vivo bioactivation in rats, but its contribution to the overall NTG bioactivation was limited.30 Finally, the role of thiol content in mediating NTG action and tolerance was investigated.30–32
Benet and his group therefore had provided the most comprehensive information on the pharmacokinetic/pharmacodynamic behavior of nitroglycerin and (uniquely) its dinitrate metabolites in humans after clinical doses. Their work had contributed to the understanding of the relationship between enzyme bioactivation and the vascular relaxant activity of NTG, and of the complexity in identifying the critical enzyme(s) involved in this process. These studies have led to subsequent efforts by other investigators to delineate the complex relationships among metabolism, action and tolerance of ORN.
Where are We Now Concerning ORN Metabolism and Bioactivation?
Besides GST and CYP 3A mentioned above, several enzymes have been implicated in the vascular metabolism of NTG and other ORN, including other cytochrome P450 isoforms,30,33 xanthine oxidoreductase (XOR),34 and various isoforms of aldehyde dehydrogenase (ALDH)35–37 (Table 1). Complicating the investigations and interpretation of ORN metabolism and bioactivation are two factors. First, not all members of this drug class are metabolized by the same enzyme(s). ORN can be loosely classified into two groups: (a) the high-potency ORN containing three or four nitrate groups, as exemplified by NTG and pentaerythritol tetranitrate (PETN), (b) the low-potency ORN which contain only one or two nitro groups, as exemplified by isosorbide dinitrate (ISDN), isosorbide-5-mononitrate (IS-5-MN), nicorandil, and the novel nitro-conjugated drugs such as NO-aspirin.38 As discussed below in relation to each enzyme, several investigative groups have found that different enzymes display selectivity toward one group of ORN vs. the other.36,39,40
Table 1.
Summary of enzymes known to metabolize organic nitrates
| Class | Isoforms | ORN Metabolized | ORN NOT Metabolized | Predominant NTG metabolite | References |
|---|---|---|---|---|---|
| GST | GSTmu | NTG, ISDN | nicorandil | 1,2-GDN | 24,45 |
| MGST1 | NTG | 1,3-GDN | 24,47 | ||
| Xanthine Oxidase | NTG, ISDN, IS-5-MN, IS-2-MN | 1,2-GDN | 34,50,51 | ||
| Cytochrome P450 | 3A4 | NTG, ISDN, NO-aspirin | N.D. | 54–56,59,60 | |
| 2B11 | NTG | N.D. | 54–56 | ||
| 2J2, 1A2, 2A6, 2C9, 2E1 | ISDN, NO- aspirin | N.D. | 59,60 | ||
| Aldehyde Dehydrogenase | ALDH1a1 | NTG, ISDN, IS-5-MN, IS-2-MN, nicorandil | 1,2-GDN | 42,43 | |
| ALDH2 | PETN, NTG, ISDN, nicorandil | IS-2-MN, IS-5-MN | 1,2-GDN | 35,64,42,138 | |
| ALDH3a1 | NTG, ISDN, IS-2-MN, nicorandil | IS-5-MN | 1,2-GDN | 69 |
(N.D. = no available data)
Second, there are two major chemical pathways of denitration, viz: mechanism-based vs. clearance-based.41 The mechanism-based pathway (i.e., bioactivation) is associated with the generation of vasoactive nitric oxide (NO), with 1,2-GDN being the predominant metabolite from NTG denitration. On the other hand, the clearance-based pathway generates the weakly vasoactive nitrite ion (NO2−) and 1,3-GDN from NTG metabolism. While an enzyme may preferentially produce a specific dinitrate metabolite at low substrate concentrations, this selectivity may be lost at higher concentrations, as well as under different reaction conditions.42,43 Thus, while an enzyme can be shown to metabolize an ORN at a certain substrate concentration, it is possible that the reaction results primarily in metabolic clearance, with an insignificant role, if at all, in bioactivation.
More extensive information are available regarding ORN metabolism by several enzymes/enzyme families, viz; GST, XOR, CYP450 and ALDH, and these findings are discussed in more detail below.
ORN Metabolism by Glutathione-S-Transferase
GST represents a large superfamily of ubiquitously expressed enzymes containing at least 9 separate classes comprised of multiple isoforms. GST, found in various subcellular fractions, is responsible for the conjugation of reduced glutathione to an electrophilic center of various endogenous compounds and xenobiotics as a mechanism for detoxification.44 Although cytosolic GST was found to generate the 1,2-GDN metabolite preferentially, thus suggesting their likely role as bioactivating enzymes,24,45 its metabolism of NTG was not associated with NO production in the microsomes of bovine coronary aorta smooth muscle cells.46 It has also been demonstrated that microsomal associated GST (MGST1) possesses the ability to denitrate NTG24,47 but it is associated with a notable lack of cyclic guanylyl cyclase activation (a marker of NO generation), and the generation of 1,3-GDN as the predominant metabolite, suggesting that this enzyme may be involved in the clearance, rather than the bioactivation of NTG.47 The role of GST in the metabolism of ORN other than NTG is very poorly understood. While cytosolic GST possesses the ability to denitrate ISDN,45 it is not known whether NO is concomitantly produced.
The mechanism-based inactivation of GST by NTG was extensively studied by Lee and Fung48 who found thiol redox reactions to be critically involved. Exogenous glutathione inhibited this inactivation, which was characterized by GST dimerization, and not by tyrosine nitration. Since the spontaneous NO donor S-nitroso N-acetylpenicillamine did not inactivate GST at equal molar concentration to NTG, the mechanism of inactivation was unlikely to be mediated via NO exposure as such.
ORN metabolism by Xanthine Oxidoreductase
XOR is a complex homodimeric flavoenzyme which contains one molybdenum center, two ferredoxin iron-sulfur clusters and one flavin adenine dinucleotide (FAD) cofactor within each dimer.49 It is responsible for purine degradation, and oxidizes hypoxanthine to xanthine and then to uric acid using NAD+ and/or molecular oxygen as cofactors. When first discovered, XOR was thought to be two different enzymes, i.e., xanthine oxidase (XO) and xanthine dehydrogenase (XDH) based on different cofactor requirements and differing expression patterns in various animals. However, it is now clear that XO and XDH represent the same enzyme which however exists in differing post-translational cysteine modifications, resulting in the alterations of its conformational structure.49 XO contains the intact disulfide and utilizes molecular oxygen as the preferred electron acceptor while XDH contains the reduced disulfide and prefers NAD+ as the terminal electron acceptor.49
Direct measurement of NO showed that, under hypoxic conditions, purified XO was able to reduce inorganic nitrite, nitrate, and NTG to vasoactive NO in the presence of the cofactor NADH. This reduction was inhibited by oxygen and specific XOR inhibitors.50 The ability of XOR to reduce NTG was later confirmed using a platelet aggregation inhibition bioassay which is highly sensitive for detecting NO generation 34 It is currently unknown which dinitrate metabolite of NTG is predominantly formed by XOR. In addition to NTG, purified XOR (both XO and XDH forms) can also bioactivate the lower potency ORN, viz., ISDN, IS-5-MN, and isosorbide-2-mononitrate (IS-2-MN) in vitro using either xanthine or NADH as a cofactor.51 Despite these encouraging in vitro results, the role of XOR in vivo has not been established, although a couple of factors might argue for its useful involvement in maintaining vascular function, i.e., XOR is highly expressed in the vascular endothelium,52 thus its ability to liberate NO from ORN under hypoxic conditions would be highly beneficial to correct the existing ischemia.
ORN metabolism by cytochrome P450
Servent et al.53 first demonstrated that isolated rat hepatic microsomes were able to denitrate NTG and this reaction was prevented by CYP inhibition. Subsequent studies utilizing CYP enzyme inhibitors and inducing agents identified the principal isozymes involved, viz., CYP 3A and/or CYP 2B11.54–56 However, the bioactivating ability of CYP enzymes to release the vasoactive NO is less clear. Both positive30 and negative57,58 findings regarding the in vivo role of the CYP enzymes in NTG bioactivation have been reported.
Only sparse data exist concerning CYP-mediated metabolism of ORN other than NTG. Using a microsomal preparation containing transfected human CYP, it was demonstrated that CYP 1A2, 2A6, 2C9, 2E1, 3A4 and 2J2 were able to bioactivate ISDN to liberate NO in vitro. 59,60 In addition, it has been noted that CYP 1A2, 2E1, 3A4 and 2J2 are localized in human cardiac vascular tissue.59,60 The involvement of these CYP isozymes in the in vivo bioactivation of ORN is however still unexplored.
ORN metabolism by Aldehyde Dehydrogenase
ALDH is a super family of at least 17 ubiquitously expressed NAD/P+ dependent enzymes responsible for the oxidation of various endogenous and exogenous aldehydes to their corresponding carboxylic acids. They possess a range of functions including the detoxification of reactive aldehydes, biogenic amine synthesis, and maintenance of proper retinal (vitamin A) signaling.61–63
Mitochondrial ALDH (ALDH2) was identified in 2002 as a major enzyme responsible for the bioactivation of NTG,35 producing 1,2-GDN as the predominant metabolite, thus consistent with mechanism-based metabolism.42 This finding was confirmed via follow-up studies utilizing specific ALDH2 inhibitors, an ALHD2 null mouse model,64–66 and in clinical investigations.67,68 This enzyme is also critical to the bioactivation and in vivo activity of PETN. However although purified ALDH2 can bioactivate several lower potency ORN (e.g., ISDN and nicorandil) in vitro, it plays an insignificant role in the pharmacological activity of these agents.36,39,64
Two cytosolic forms of ALDH, ALDH1a1 and ALDH3a1, have been demonstrated to bioactivate NTG in vitro producing the 1,2-GDN metabolite predominantly.43,69 In addition, these isoforms can metabolize several lower-potency ORN such as ISDN, IS-5-MN, and nicorandil, although the substrate selectivity varies somewhat between the isoforms.69 While purified ALDH1a1 can bioactivate ORN in vitro, studies with ALDH1a1 null mice suggest that the isoform is not critical to the in vivo activity of ORN, most likely due to a lack of expression in the vascular tissue.70 However, ALDH1a1 is heavily expressed in the liver where it may serve as a clearance enzyme for these drugs. The in vivo role of ALDH3a1 as well as the role of other ALDH isoforms in ORN metabolism is still unclear at present.
ORN Metabolism: A Tower of Babel? Or can all the findings be unified?
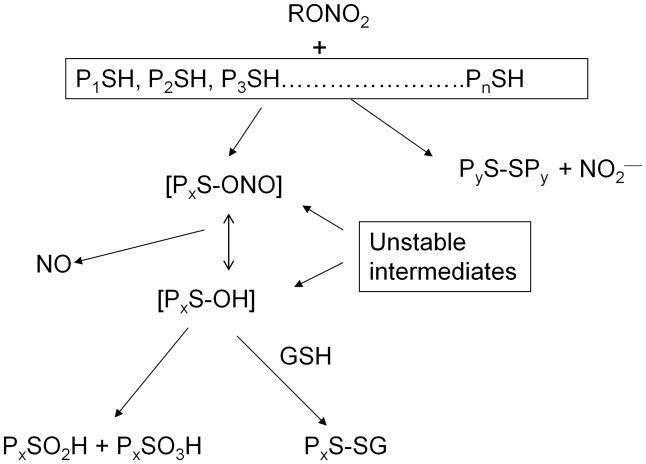
It is now clear that ORN metabolism and bioactivation are mediated by multiple enzymes, some perhaps even unidentified at this point. Why is there such a lack of enzyme specificity for this class of compounds? In 2004, we proposed an overall biochemical scheme, for all involved enzymes, that could account for ORN bioactivation and its subsequent mechanism-based inactivation. Because this mechanism proceeds through a unifying intermediate, viz., a thionitrate, we have termed it the “Thionitrate Oxidation Hypothesis”.71 While this unstable intermediate had long been proposed for the biochemical action of ORN, it avoided direct detection until we documented its existence recently.72
We proposed that the locus of enzyme reaction with ORN, for most if not all metabolizing enzymes, involves one or more critical cysteine residue(s) to produce a thionitrate initially (Fig. 1). This highly facile intermediate can then interact with excess free thiol(s) to liberate inorganic nitrite ion while forming a disulfide between the two involved thiols, leading to clearance-based metabolism. On the other hand, the thionitrate may also liberate NO via a mechanism-based metabolic pathway, producing a sulfenyl intermediate [PxS-OH] in the protein (Px denotes the reactive protein, x = 1, 2……n). Subsequent oxidation of these unstable thiol intermediates would lead to formation of further oxidation modifications of the proteins (PxS-SPx, PxSO2H, PxSO3H and PxS-SG, where G represents a gluthathione residue).
Fig. 1.
The Thionitrate Oxidation Hypothesis of organic nitrate metabolism, bioactivation, and enzyme inactivation. PS = cysteine-containing proteins; SG = glutathione residue,-SO2H = sulfinic acid protein modification and -SO3H = sulfonic acid modification. Adapted from Fung71
The promiscuity of reaction between the nitrate moiety in ORN with protein cysteine residues is not surprising because it has long been known (see for example73) that ORN react avidly even with non-protein thiols to produce NO. Site mutagenesis studies have also identified critical cysteine groups in ALDH2 (cysteine 319) and ALDH1a1 (cysteine 303) as essential for metabolizing ORN.36 It was also recently demonstrated in an animal model that NTG-induced vasodilation was mediated by oxidation of critical cysteine residues in protein kinase G 1 alpha, the oxidation of which is associated with nitrate tolerance.74 The sequential nature of the thiol oxidation scheme shown in Fig. 1 also provides an explanation why ORN-mediated enzyme inactivation can sometimes be reversible (e.g., with sulfenyl and S-glutathionylated proteins), while not so at other times because irreversible products are formed (e.g., sulfinic and sulfonic acids). This partially reversible nature of cysteine oxidation by NTG was demonstrated for GST48 and ALDH275. All these reaction products with NTG have been observed recently by us using LCMS/MS.72
How does the Thionitrate Oxidation Hypothesis explain pharmacological tolerance?
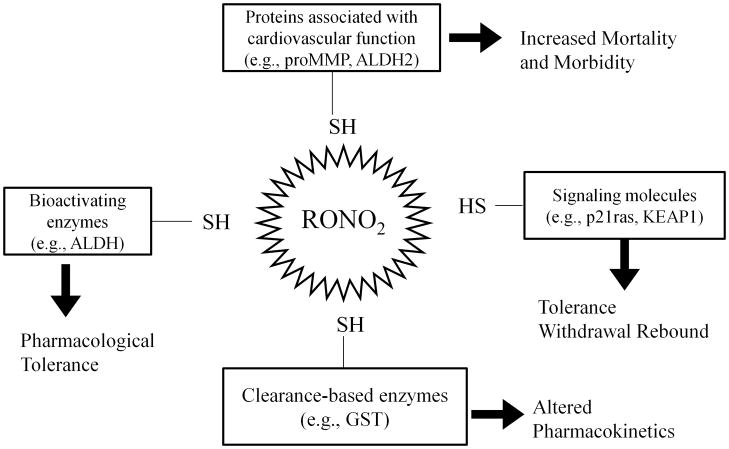
Another characteristic of repeated ORN therapy is the rapid development of pharmacological tolerance This effect has been observed with all ORN via all routes of administration, and is accompanied by multiple consequences, including the inactivation of bioactivating enzymes,68 depletion/oxidation of free thiol,76 counter-regulatory responses such as rebound vasoconstriction,77,78 and increased oxidative stress in the vasculature leading to endothelial dysfunction.79,80 The multiplicity of these effects, some apparently unconnected, is not surprising when viewed in the context of the biochemical reaction scheme shown in Fig. 1. Because multiple proteins are affected by ORN-mediated oxidation, whether NO is produced or not as a result, their oxidation can lead to a wide array of pharmacological consequences, as determined by the specific proteins involved. In addition, abrupt discontinuation of short acting ORN leads to the phenomenon of withdrawal rebound.
Thus, as shown in Fig. 2, the oxidation of active site cysteine residues in bioactivating enzymes such as ALDH2 will result in reduced bioactivation of NO, leading to pharmacological tolerance observed in animal models65 and in clinical studies68. It has also been demonstrated that NTG can inactivate ALDH1a1,36 ALDH3a1,69 and several GST48 and CYP isoforms.81 ISDN and IS-2-MN can inactive ALDH1a1, ALDH2 and ALDH3a1 while IS-5-MN can inactivate ALDH1a1 in vitro.36
Fig. 2.
Schematic showing how thiol oxidation in different proteins by organic nitrates (RONO2) can lead to a wide array of pharmacological consequences. Adapted from Page and Fung143
Similarly, inactivation of clearance-based enzymes such as GST will lead to drug accumulation (concomitant with tolerance induction), as shown in patients for both NTG82 and ISDN.83 The induction of counter-regulatory responses may potentially be mediated through the S-oxidation of signaling proteins, such as the small GTPase secondary messenger p21ras, and Kelch-like ECH-associated protein 1 (KEAP1), e.g., through the formation of sulfenyl and S-glutathionylated modifications. The actual roles of signaling proteins or pathways in mediating nitrate tolerance remain undefined currently, as involvement of other thiol proteins is being discovered.74 Although our hypothesis of thiol oxidation indeed produces a state of oxidative stress in the vasculature, it differs from the superoxide oxidative stress hypothesis that was quite prevalently accepted in the last decade.38 We have shown recently that increased superoxide formation was not a cause of nitrate tolerance, but rather as a consequence of ORN-mediated S-oxidation.84 Our hypothesis also differs from suggestion that endothelial nitric oxide synthase (eNOS) is critically involved in nitrate tolerance38 since we have shown that eNOS knockout mice exhibits a similar degree of vascular tolerance toward NTG as the wild-type mice.85
Viewing Tolerance Avoidance Findings through our Unifying Hypothesis
Tolerance avoidance strategies toward ORN can also be interpreted through Fig. 2. The use of nitrate-free period to regenerate nitrate sensitivity is consistent with the regeneration of the appropriate vascular redox state through, e.g., the thioredoxin or glutaredoxin enzyme system. Reversal or prevention of protein cysteine oxidation, and thereby nitrate tolerance, can be understandably accomplished by co-administration of antioxidants or reduced thiols. These agents include N-acetylcysteine,86 vitamin C,87,88 vitamin E,89 folic acid,90,91 lipoic acid,92 and possibly carvedilol,93 hydralazine,94 and statins.95 Recently, ALDA-1, a small molecule inducer of ALDH2 expression and activity has been demonstrated to prevent tolerance formation through increased availability of this enzyme.96
Other tolerance avoidance agents may act to overcome the counter-regulatory responses produced by ORN. In the rat aorta, NTG exposure is associated with non-specific S-glutathionylation of proteins (a consequence of thiol oxidation), concomitant with decreased bioactivation and action of NTG.84 Oxidation of proteins responsible for signal transduction may also be partly responsible for the observed changes in vascular gene expression.97 In cultured endothelial cells, it was demonstrated that NTG exposure increased S-glutathionylation and activity of p21ras, which is integral to the signaling of the AKT and extracellular signal-regulated kinase 1/2 (ERK 1/2) pathways which play a part in the regulation of smooth muscle cell proliferation.98 NTG incubation led to a significant increase in the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB p50/p65) activity in differentiated human monocytic leukemia cells,99 possibly through nuclear factor kappa-B kinase subunit alpha (IKK-α) activation via its cysteine-179 residue.
To counter the vasoconstrictive effects of nitrate tolerance, diuretics100 and anti-vasoconstrictive agent such as captopril and losartan have been used.101 Interestingly, PETN has been reported to induce less tolerance formation than the other ORN, likely through its unique ability to upregulate hemeoxygenase-1 (HO-1).102 In addition, this induction of HO-1 can prevent and reverse the oxidative stress-mediated endothelial dysfunction.103 HO-1 is one of several antioxidant response elements (ARE) that are unregulated in response to increased oxidative stress as a protective mechanism. ARE expression is controlled via the NRF2 signaling pathway, and its upregulation is mediated by cysteine oxidation of NRF2’s inhibitor KEAP1. Maintained as an inactive NRF2/KEAP1 dimer in the cystosol, oxidation of KEAP1 allows NRF2 dissociation and translocation into the nucleus to upregulate ARE.104
Cyclic guanosine monophosphate, the secondary messenger responsible for NO mediated vasorelaxation, is degraded in the vasculature by various phosphodiesterase (PDE) isoforms and specific inhibition of PDE5 by 4-[[3,4-(Methylenedioxy)benzyl]amino]-6-chloroquinazoline (MBCQ) has been demonstrated to prevent tolerance formation presumably through increased persistence of this messenger.105 This approach addresses the down-stream effects of ORN, and it does not prevent the initial tolerance-inducing biochemical reactions of thionitrate formation and protein thiol oxidation. Thus, while the net potency of ORN is increased by PDE inhibitors, the root cause of nitrate tolerance is not abrogated by these agents.
Table 2 summarizes the various agents that have been used for the in vivo prevention of tolerance and the experimental systems employed, and their likely mechanisms of action. However, it is likely that some agents may exert multiple mechanism that have not yet been fully characterized.
Table 2.
Agents demonstrated to prevent or avoid organic nitrate tolerance in vivo
| Agent | ORN Examined | Likely Mechanism | Experimental Model | References |
|---|---|---|---|---|
| ALDA-1 | NTG | ALDH2 upregulation | Normal Rats | 96 |
| Atorvastatin | NTG | Anti-oxidant | Health Volunteers | 95 |
| Captopril | ISDN | RAS pathway modulation and anti- oxidant | CAD Patients | 101 |
| Carvedilol | NTG | Anti-oxidant | HTN Patients | 93 |
| Folic Acid | NTG | Anti-oxidant | Health Volunteers | 90,91 |
| Hydralazine | NTG | Anti-oxidant and vasodilation | CHF-induced Rats | 94 |
| Normal Rabbit | 139 | |||
| Hydrochlorothiazide with amiloride | ISDN | Diuresis | CAD Patients | 100 |
| L-arginine | NTG | eNOS mediated | CAD patients | 140 |
| Lipoic acid | NTG | Anti-oxidant | Normal Rats | 92 |
| Losartan | ISDN | RAS pathway modulation | CAD Patients | 101 |
| MBCQ | NTG | PDE inhibition | Normal Rats | 105 |
| N-acetylcysteine | NTG | Anti-oxidant and thiol supplementation | CHF Patients | 86 |
| Pravastatin/atorvastatin | NTG | Anti-oxidant | Normal Rats | 141 |
| Vitamin C | IS-5-MN | Anti-oxidant | Health Volunteers | 80 |
| Vitamin C | NTG | Anti-oxidant | CHF Patients | 87 |
| Vitamin E | NTG | Anti-oxidant | Obese Zucker Rat | 142 |
| Health Volunteers and CAD patients | 89 |
CHF=congestive heart failure, HTN=hypertension, CAD=coronary artery disease, MBCQ=4-[[3,4-(Methylenedioxy)benzyl]amino]-6-chloroquinazoline
Organic Nitrate Toxicity: Can it also be explained by thiol oxidation?
In the last decade, pharmacoepidemiological studies have revealed the existence of possible deleterious effects, in terms of increased mortality and morbidity, for patients who are on chronic nitrate therapy.106,107 Although prospective studies will need to be carried out to confirm these analyses, there are two potential mechanistic explanations for this phenomenon, based on the reaction scheme shown in Fig. 2.
In the heart, ALDH2 is responsible for the detoxification oxidation of reactive toxic aldehydes (e.g., 4-hydroxy-2-nonenal) that are generated via lipid peroxidation during periods of ischemia and reperfusion.108,109 As discussed earlier, NTG has the ability to reduce cardiac ALDH2 activity via mechanism-based inactivation of this enzyme, and this effect has been demonstrated, in an ex vivo heart model, to increase cardiac damage after ischemia/reperfusion injury.108 Mechanisms to restore cardiac ALDH2 activity, such as administration of the small molecule inducer of ALDH2, ALDA-1, have been proposed as a therapy to minimize damage after cardiac infarction.109
In addition, ORN have the ability to modulate proteins responsible for maintaining the fibrous cap of atherosclerotic plaques which is comprised of extracellular matrix components such as collagen. This cap can degrade and weaken, ultimately leading to rupture of the plaque, platelet adhesion and thrombus, and ultimately resulting in infarction. We have shown that NTG exposure, both in vitro and in vivo, can activate matrix metalloproteinases (MMP) which degrade components of the extracellular matrix, leading to weakening of the fibrous cap.99,110 The mechanism of activation of MMP by NTG involves the oxidation of the “cysteine switch” 111 which converts the inactive Pro-MMP to its active protein.
Thus, based on the analysis of the available findings, a potential unifying hypothesis (Fig. 2) that focuses on the oxidation of protein cysteine residues in multiple proteins can be used to rationalize on the multi-faceted actions of organic nitrates, including its metabolism, bioactivation, enzyme inactivation, vascular tolerance, and long-term toxicity.
Looking Forward: Clinical Uses of Organic Nitrates beyond Cardiology
The involvement of NO in many physiological and pathological conditions is now well documented. Because ORN can serve as clinical NO donors, their pharmacological use in humans have been extended beyond cardiology. Below we summarize some of the more mature areas of studies that suggest the potential extension of ORN use in other diseases.
Treatment of osteopenia/osteoporosis
Bone turnover is a tightly controlled process that relies on a variety of humoral and micro-environmental signals that ultimately alter the function of the catabolic osteoclasts and the anabolic osteoblasts. One of these signal molecules is NO which is endogenously generated by both osteoblasts and osteoclasts, with a net inhibitory effect on osteoclast activity preventing bone resorption. In a variety of animal models, it has been demonstrated that modulating NO exposure via stimulation of endogenous NOS activity112,113 or administration of topical ORN114,115 can attenuate the reduction of bone mineral density due to osteoporosis. A prospective case-controlled study in Denmark had examined the administration of ORN and the incidence of fraction in over 100,000 subjects. Use of ORN (IS-5-MN, ISDN, or NTG) was associated with an approximately 11% reduction in any fracture in both men and women and a 15% reduction in hip fractures in women. The risk reduction was dose- and duration-dependent.116 While these studies have demonstrated promising results, randomized controlled studies have yielded mixed conclusions regarding the therapeutic benefits of ORN. A small study randomized 186 female post-menopausal subjects to receive topical NTG or placebo. At the end of their 3-year study, topical ORN administration was associated with no change in bone mineral density compared to baseline although the results were confounded by compliance problems due to adverse events such as headaches.117 A second randomized study examined 205 post-menopausal women who received topical NTG or placebo for 2 years. At the end of the study period, there was a small but significant improvement in bone density as well as markers in bone turnover in the subjects receiving NTG.118 Neither study was powered sufficiently to examine fractures. Because of these conflicting results, more studies are required to define the role of ORN in the treatment of osteoporosis.
Protection of the Gastric Mucosa
NO exerts a protective effect on the gastric mucosa via several mechanisms, including promotion of angiogenesis around gastric ulcers, promotion of adequate blood flow, modulation of the local inflammatory response and the promotion of mucus secretion.119,120 Inhibition of endogenous NO production via inhibition of nitric oxide synthase (NOS) slows ulcer healing in animal models of gastric ulceration121,122 while supplementation of exogenous NO prevented ischemia-induced gastrointestinal damage.123
The ability of NO to prevent gastric ulcers has garnered the most interest in the prevention of ulcers induced by non-steroidal anti-inflammatory drugs (NSAID) including aspirin. These agents act through cyclooxygenase (COX) 1 and 2 inhibition, leading to altered prostaglandin signaling in the gastrointestinal tract, direct irritation of the mucosal lining and, for aspirin, irreversible inhibition of platelet aggregation.124 This has led to a new class of drug, the NO-releasing drug hybrids called NO-NSAIDs including NO-aspirin. These drugs contain a nitro group attached to the parent drug via a labile linker (generally an ester linkage) which is hydrolyzed in vivo to release the parent drug and the linker containing the nitro moiety which is further metabolized to liberate NO.120 Through these hybrid drugs, the severity and incidence of the gastric adverse effects of NSAIDs may be reduced without compromising their beneficial effects. Thus, NO-aspirin has been demonstrated to prevent and reverse animal models of gastric damage125 Two small proof-of-concept studies using healthy volunteers showed that after 7 days and 14 days of administration, NO-aspirin was associated with less gastric damage (determined via endoscopic study) compared to aspirin.126,127 Both NO-aspirin and NO-naproxen are currently in clinical trial.128,129
Use of NTG to normalize ALDH expression in overexpressing malignancies
The over-expression of ALDH1a1 and subsequent alteration of retinal (vitamin A) signaling is a potential factor of malignancy aggressiveness that has been noted in a variety of cancers, including lung,130 prostrate,131 and breast cancer.132 It has been demonstrated that downregulation of over expressed ALDH1a1 in vitro via siRNA has the potential to inhibit tumor growth.130 In addition, the increased expression of ALDH1a1 serves as a chemotherapeutic resistance mechanism because this enzyme has the ability to detoxify the active metabolites of cyclophosphamide-based alkylating agents.
The ability of NTG to inactivate various ALDH isoforms, via irreversible oxidation of the enzyme’s catalytic cysteine residues,36,40 could potentially be exploited to reverse this over-expression of this enzyme’s activity, with the potential to reduce tumor virulence while sensitizing the tumor toward alkylating agents. In contrast to the more complex (and therapeutically difficult) approach of employing siRNA, NTG has a proven track record of clinical safety with minimal and easily managed adverse effects (headaches and hypotension). We believe, therefore, that ORN use in modulating cancer growth deserves further investigation.
Finally, several non-cardiovascular uses of ORN rely in its vasodilatory action to restore normalized blood flow in pathological tissue/organs. These applications include the treatment of erectile dysfunction,133,134 anal fissures,135,136 and the management of esophageal varices due to portal hypertension.137
Conclusion
From the early groundwork by Benet and his associates who provided critical information on the pharmacokinetics and pharmacodynamics of NTG and its dinitrate metabolites, significant progress has been made to gain understanding of this well-established class of drugs. We believe that ORN are metabolized by a variety of enzymes because of the promiscuous reaction between ORN and the many cysteine residues available in proteins. However, not all of these reactions produce the necessary vasoactive NO. The promiscuous cysteine-nitrate reaction also explains the multiplicity of pharmacologic effects that have been observed after chronic nitrate use, including pharmacological tolerance, reduced ORN systemic clearance, induction of counter-regulatory vasoconstriction and increased morbidity/mortality. Most of the findings relating to tolerance avoidance strategies can also been explained by this mechanism. Thus, a unifying hypothesis linking the metabolism and action of organic nitrates is available, and it may find utility as a biochemical basis to further optimize and extend the use of this group of useful pharmacological agents.
Acknowledgments
This work was supported in part by a National Institutes of Health grant, HL081580, awarded to HLF.
References
- 1.Miyazaki H, Ishibashi M, Hashimoto Y, Idzu G, Furuta Y. Simultaneous determination of glyceryl trinitrate and its principal metabolites, 1,2- and 1,3-glyceryl dinitrate, in plasma by gas chromatography-negative ion chemical ionization-selected ion monitoring. Journal of chromatography. 1982;239:277–286. doi: 10.1016/s0021-9673(00)81988-3. [DOI] [PubMed] [Google Scholar]
- 2.Han C, Gumbleton M, Lau DT, Benet LZ. Improved gas chromatographic assay for the simultaneous determination of nitroglycerin and its mono- and dinitrate metabolites. Journal of chromatography. 1992;579(2):237–245. doi: 10.1016/0378-4347(92)80387-6. [DOI] [PubMed] [Google Scholar]
- 3.Lee FW, Watari N, Rigod J, Benet LZ. Simultaneous determination of nitroglycerin and its dinitrate metabolites by capillary gas chromatography with electron-capture detection. Journal of chromatography. 1988;426(2):259–266. doi: 10.1016/s0378-4347(00)81954-4. [DOI] [PubMed] [Google Scholar]
- 4.Noonan PK, Kanfer I, Riegelman S, Benet LZ. Determination of picogram nitroglycerin plasma concentrations using capillary gas chromatography with on-column injection. Journal of pharmaceutical sciences. 1984;73(7):923–927. doi: 10.1002/jps.2600730715. [DOI] [PubMed] [Google Scholar]
- 5.Noonan PK, Benet LZ. Variable glyceryl dinitrate formation as a function of route of nitroglycerin administration. Clinical pharmacology and therapeutics. 1987;42(3):273–277. doi: 10.1038/clpt.1987.146. [DOI] [PubMed] [Google Scholar]
- 6.Lau DT, Gumbleton M, Labisch C, Benet LZ. Pharmacokinetic studies of the nitroglycerin metabolites, 1,2- and 1,3- glyceryl dinitrates, in the rat. Biopharmaceutics & drug disposition. 1991;12(3):215–222. doi: 10.1002/bdd.2510120306. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima E, Lau DT, Benet LZ. Variable glyceryl dinitrate formation following infusions of glyceryl trinitrate at different vascular sites in the rat. Pharmaceutical research. 1991;8(7):877–882. doi: 10.1023/a:1015851412175. [DOI] [PubMed] [Google Scholar]
- 8.Noonan PK, Benet LZ. Incomplete and delayed bioavailability of sublingual nitroglycerin. The American journal of cardiology. 1985;55(1):184–187. doi: 10.1016/0002-9149(85)90325-x. [DOI] [PubMed] [Google Scholar]
- 9.Higo N, Hinz RS, Lau DT, Benet LZ, Guy RH. Cutaneous metabolism of nitroglycerin in vitro. II. Effects of skin condition and penetration enhancement. Pharmaceutical research. 1992;9(3):303–306. doi: 10.1023/a:1015822431178. [DOI] [PubMed] [Google Scholar]
- 10.Higo N, Hinz RS, Lau DT, Benet LZ, Guy RH. Cutaneous metabolism of nitroglycerin in vitro. I. Homogenized versus intact skin. Pharmaceutical research. 1992;9(2):187–190. doi: 10.1023/a:1018925004345. [DOI] [PubMed] [Google Scholar]
- 11.Kikkoji T, Gumbleton M, Higo N, Guy RH, Benet LZ. Percutaneous penetration kinetics of nitroglycerin and its dinitrate metabolites across hairless mouse skin in vitro. Pharmaceutical research. 1991;8(10):1231–1237. doi: 10.1023/a:1015887309391. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima E, Noonan PK, Benet LZ. Transdermal bioavailability and first-pass skin metabolism: a preliminary evaluation with nitroglycerin. Journal of pharmacokinetics and biopharmaceutics. 1987;15(4):423–437. doi: 10.1007/BF01066522. [DOI] [PubMed] [Google Scholar]
- 13.Williams RL, Thakker KM, John V, Lin ET, Liang-Gee W, Benet LZ. Nitroglycerin absorption from transdermal systems: formulation effects and metabolite concentrations. Pharmaceutical research. 1991;8(6):744–749. doi: 10.1023/a:1015802101272. [DOI] [PubMed] [Google Scholar]
- 14.Han C, Jung P, Sanders SW, Lin ET, Benet LZ. Pharmacokinetics of nitroglycerin and its four metabolites during nitroglycerin transdermal administration. Biopharmaceutics & drug disposition. 1994;15(2):179–183. doi: 10.1002/bdd.2510150210. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima E, Rigod JF, Lin ET, Benet LZ. Pharmacokinetics of nitroglycerin and its dinitrate metabolites over a thirtyfold range of oral doses. Clinical pharmacology and therapeutics. 1990;47(5):592–598. doi: 10.1038/clpt.1990.80. [DOI] [PubMed] [Google Scholar]
- 16.Noonan PK, Benet LZ. The bioavailability of oral nitroglycerin. Journal of pharmaceutical sciences. 1986;75(3):241–243. doi: 10.1002/jps.2600750306. [DOI] [PubMed] [Google Scholar]
- 17.Yu DK, Williams RL, Benet LZ, Lin ET, Giesing DH. Pharmacokinetics of nitroglycerin and metabolites in humans following oral dosing. Biopharmaceutics & drug disposition. 1988;9(6):557–565. doi: 10.1002/bod.2510090606. [DOI] [PubMed] [Google Scholar]
- 18.Lee FW, Salmonson T, Metzler CH, Benet LZ. Pharmacokinetics and pharmacodynamics of glyceryl trinitrate and its two dinitrate metabolites in conscious dogs. The Journal of pharmacology and experimental therapeutics. 1990;255(3):1222–1229. [PubMed] [Google Scholar]
- 19.Lee FW, Hu J, Metzler CH, Benet LZ. Nitroglycerin dinitrate metabolites do not affect the pharmacokinetics and pharmacodynamics of nitroglycerin in the dog: a preliminary report. Journal of pharmacokinetics and biopharmaceutics. 1993;21(2):163–173. doi: 10.1007/BF01059768. [DOI] [PubMed] [Google Scholar]
- 20.Gumbleton M, Benet LZ. Pharmacological activity of the dinitrate metabolites of nitroglycerin following their oral administration to healthy volunteers. British journal of clinical pharmacology. 1991;31(2):211–213. doi: 10.1111/j.1365-2125.1991.tb05521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumbleton M, Benet LZ. Simultaneous pharmacodynamic modeling of the non-steady-state effects of three oral doses of 1,3-glyceryl dinitrate upon blood pressure in healthy volunteers. Journal of pharmacokinetics and biopharmaceutics. 1993;21(5):515–532. doi: 10.1007/BF01059112. [DOI] [PubMed] [Google Scholar]
- 22.Cahalan MK, Hashimoto Y, Aizawa K, Verotta D, Ionescu P, Balea M, Eger EI, 2nd, Benet LZ, Ehrenfeld WK, Goldstone J, et al. Elderly, conscious patients have an accentuated hypotensive response to nitroglycerin. Anesthesiology. 1992;77(4):646–655. doi: 10.1097/00000542-199210000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lau DT, Benet LZ. Differential formation of dinitrate metabolites from glyceryl trinitrate in subcellular fractions of rabbit liver. Biochemical pharmacology. 1989;38(3):543–546. doi: 10.1016/0006-2952(89)90398-5. [DOI] [PubMed] [Google Scholar]
- 24.Lau DT, Benet LZ. Nitroglycerin metabolism in subcellular fractions of rabbit liver. Dose dependency of glyceryl dinitrate formation and possible involvement of multiple isozymes of glutathione S-transferases. Drug metabolism and disposition: the biological fate of chemicals. 1990;18(3):292–297. [PubMed] [Google Scholar]
- 25.Lau DT, Chan EK, Benet LZ. Glutathione S-transferase-mediated metabolism of glyceryl trinitrate in subcellular fractions of bovine coronary arteries. Pharmaceutical research. 1992;9(11):1460–1464. doi: 10.1023/a:1015867031004. [DOI] [PubMed] [Google Scholar]
- 26.Kenkare SR, Benet LZ. Effect of ethacrynic acid, a glutathione-S-transferase inhibitor, on nitroglycerin-mediated cGMP elevation and vasorelaxation of rabbit aortic strips. Biochemical pharmacology. 1993;46(2):279–284. doi: 10.1016/0006-2952(93)90415-s. [DOI] [PubMed] [Google Scholar]
- 27.Kenkare SR, Benet LZ. Tolerance to nitroglycerin in rabbit aorta. Investigating the involvement of the mu isozyme of glutathione S-transferases. Biochemical pharmacology. 1996;51(10):1357–1363. doi: 10.1016/0006-2952(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 28.Kenkare SR, Han C, Benet LZ. Correlation of the response to nitroglycerin in rabbit aorta with the activity of the mu class glutathione S-transferase. Biochemical pharmacology. 1994;48(12):2231–2235. doi: 10.1016/0006-2952(94)00415-3. [DOI] [PubMed] [Google Scholar]
- 29.Lau DT, Benet LZ. Effects of sulfobromophthalein and ethacrynic acid on glyceryl trinitrate relaxation. Biochemical pharmacology. 1992;43(10):2247–2254. doi: 10.1016/0006-2952(92)90184-k. [DOI] [PubMed] [Google Scholar]
- 30.Yuan R, Sumi M, Benet LZ. Investigation of aortic CYP3A bioactivation of nitroglycerin in vivo. The Journal of pharmacology and experimental therapeutics. 1997;281(3):1499–1505. [PubMed] [Google Scholar]
- 31.Haj-Yehia AI, Benet LZ. Dissociation of tissue thiols content from nitroglycerin-induced cyclic-3′,5′-guanosine monophosphate and the state of tolerance: in vivo experiments in rats. The Journal of pharmacology and experimental therapeutics. 1995;273(1):94–100. [PubMed] [Google Scholar]
- 32.Haj-Yehia AI, Benet LZ. In vivo depletion of free thiols does not account for nitroglycerin- induced tolerance: a thiol-nitrate interaction hypothesis as an alternative explanation for nitroglycerin activity and tolerance. The Journal of pharmacology and experimental therapeutics. 1996;278(3):1296–1305. [PubMed] [Google Scholar]
- 33.Minamiyama Y, Imaoka S, Takemura S, Okada S, Inoue M, Funae Y. Escape from tolerance of organic nitrate by induction of cytochrome P450. Free Radic Biol Med. 2001;31(11):1498–1508. doi: 10.1016/s0891-5849(01)00733-x. [DOI] [PubMed] [Google Scholar]
- 34.O’Byrne S, Shirodaria C, Millar T, Stevens C, Blake D, Benjamin N. Inhibition of platelet aggregation with glyceryl trinitrate and xanthine oxidoreductase. The Journal of pharmacology and experimental therapeutics. 2000;292(1):326–330. [PubMed] [Google Scholar]
- 35.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99(12):8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsou PS, Page NA, Lee SG, Fung SM, Keung WM, Fung HL. Differential metabolism of organic nitrates by aldehyde dehydrogenase 1a1 and 2: substrate selectivity, enzyme inactivation, and active cysteine sites. The AAPS journal. 2011;13(4):548–555. doi: 10.1208/s12248-011-9295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer B, Beretta M. The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles. Br J Pharmacol. 2008;155(2):170–184. doi: 10.1038/bjp.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97(7):618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 39.Daiber A, Oelze M, Coldewey M, Bachschmid M, Wenzel P, Sydow K, Wendt M, Kleschyov AL, Stalleicken D, Ullrich V, Mulsch A, Munzel T. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol. 2004;66(6):1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- 40.Pietruszko R, Mukerjee N, Blatter EE, Lehmann T. Nitrate esters as inhibitors and substrates of aldehyde dehydrogenase. Adv Exp Med Biol. 1995;372:25–34. doi: 10.1007/978-1-4615-1965-2_4. [DOI] [PubMed] [Google Scholar]
- 41.Bennett BM, McDonald BJ, Nigam R, Simon WC. Biotransformation of organic nitrates and vascular smooth muscle cell function. Trends Pharmacol Sci. 1994;15(7):245–249. doi: 10.1016/0165-6147(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 42.Beretta M, Gruber K, Kollau A, Russwurm M, Koesling D, Goessler W, Keung WM, Schmidt K, Mayer B. Bioactivation of nitroglycerin by purified mitochondrial and cytosolic aldehyde dehydrogenases. J Biol Chem. 2008;283(26):17873–17880. doi: 10.1074/jbc.M801182200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page NA, Tsou PS, Beretta M, Mayer B, Fung HL. Selective activation of organic nitrates by, and inactivation of, ALDH isoforms. FASEB J. 2009;23(1_MeetingAbstracts):LB374. [Google Scholar]
- 44.Dourado DF, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Current protein & peptide science. 2008;9(4):325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa N, Hirose T, Fukushima K, Suwa T, Satoh T. Metabolism of a nitrate ester, dihydropyridine derivative in rabbit hepatic microsomes and cytosol. Xenobiotica; the fate of foreign compounds in biological systems. 1995;25(3):283–290. doi: 10.3109/00498259509061852. [DOI] [PubMed] [Google Scholar]
- 46.Chung SJ, Chong S, Seth P, Jung CY, Fung HL. Conversion of nitroglycerin to nitric oxide in microsomes of the bovine coronary artery smooth muscle is not primarily mediated by glutathione-S-transferases. The Journal of pharmacology and experimental therapeutics. 1992;260(2):652–659. [PubMed] [Google Scholar]
- 47.Ji Y, Bennett BM. Biotransformation of Glyceryl Trinitrate by Rat Hepatic Microsomal Glutathione S-Transferase 1. The Journal of pharmacology and experimental therapeutics. 2006;318(3):1050–1056. doi: 10.1124/jpet.106.103713. [DOI] [PubMed] [Google Scholar]
- 48.Lee WI, Fung HL. Mechanism-based partial inactivation of glutathione S-transferases by nitroglycerin: tyrosine nitration vs sulfhydryl oxidation. Nitric Oxide. 2003;8(2):103–110. doi: 10.1016/s1089-8603(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 49.Nishino T, Okamoto K, Eger BT, Pai EF. Mammalian xanthine oxidoreductase -mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275(13):3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 50.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS letters. 1998;427(2):225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 51.Doel JJ, Godber BL, Eisenthal R, Harrison R. Reduction of organic nitrates catalysed by xanthine oxidoreductase under anaerobic conditions. Biochim Biophys Acta. 2001;1527(1–2):81–87. doi: 10.1016/s0304-4165(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 52.Jarasch ED, Bruder G, Heid HW. Significance of xanthine oxidase in capillary endothelial cells. Acta physiologica Scandinavica Supplementum. 1986;548:39–46. [PubMed] [Google Scholar]
- 53.Servent D, Delaforge M, Ducrocq C, Mansuy D, Lenfant M. Nitric oxide formation during microsomal hepatic denitration of glyceryl trinitrate: involvement of cytochrome P-450. Biochemical and biophysical research communications. 1989;163(3):1210–1216. doi: 10.1016/0006-291x(89)91106-6. [DOI] [PubMed] [Google Scholar]
- 54.McDonald BJ, Bennett BM. Cytochrome P-450 mediated biotransformation of organic nitrates. Canadian journal of physiology and pharmacology. 1990;68(12):1552–1557. doi: 10.1139/y90-236. [DOI] [PubMed] [Google Scholar]
- 55.McDonald BJ, Monkewich GJ, Long PG, Anderson DJ, Thomas PE, Bennett BM. Effect of dexamethasone treatment on the biotransformation of glyceryl trinitrate: cytochrome P450 3A1 mediated activation of rat aortic guanylyl cyclase by glyceryl trinitrate. Canadian journal of physiology and pharmacology. 1994;72(12):1513–1520. doi: 10.1139/y94-217. [DOI] [PubMed] [Google Scholar]
- 56.Bennett BM, McDonald BJ, St James MJ. Hepatic cytochrome P-450-mediated activation of rat aortic guanylyl cyclase by glyceryl trinitrate. The Journal of pharmacology and experimental therapeutics. 1992;261(2):716–723. [PubMed] [Google Scholar]
- 57.Braun M, Grosser T, Schror K. Bioactivation of nitroglycerin in vascular smooth muscle cells is different from that in non-vascular tissue. Eur J Pharmacol. 1995;276(3):239–245. doi: 10.1016/0014-2999(95)00031-f. [DOI] [PubMed] [Google Scholar]
- 58.Liu Z, Brien JF, Marks GS, McLaughlin BE, Nakatsu K. Lack of evidence for the involvement of cytochrome P-450 or other hemoproteins in metabolic activation of glyceryl trinitrate in rabbit aorta. The Journal of pharmacology and experimental therapeutics. 1993;264(3):1432–1439. [PubMed] [Google Scholar]
- 59.Minamiyama Y, Takemura S, Akiyama T, Imaoka S, Inoue M, Funae Y, Okada S. Isoforms of cytochrome P450 on organic nitrate-derived nitric oxide release in human heart vessels. FEBS letters. 1999;452(3):165–169. doi: 10.1016/s0014-5793(99)00612-2. [DOI] [PubMed] [Google Scholar]
- 60.Minamiyama Y, Takemura S, Imaoka S, Funae Y, Okada S. Cytochrome P450 is responsible for nitric oxide generation from NO-aspirin and other organic nitrates. Drug metabolism and pharmacokinetics. 2007;22(1):15–19. doi: 10.2133/dmpk.22.15. [DOI] [PubMed] [Google Scholar]
- 61.Moon KH, Abdelmegeed MA, Song BJ. Inactivation of cytosolic aldehyde dehydrogenase via S-nitrosylation in ethanol-exposed rat liver. FEBS letters. 2007;581(21):3967–3972. doi: 10.1016/j.febslet.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida A, Rzhetsky A, Hsu LC, Chang C. Human aldehyde dehydrogenase gene family. Eur J Biochem. 1998;251(3):549–557. doi: 10.1046/j.1432-1327.1998.2510549.x. [DOI] [PubMed] [Google Scholar]
- 63.Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004;36(2):279–299. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- 64.Wenzel P, Hink U, Oelze M, Seeling A, Isse T, Bruns K, Steinhoff L, Brandt M, Kleschyov AL, Schulz E, Lange K, Weiner H, Lehmann J, Lackner KJ, Kawamoto T, Munzel T, Daiber A. Number of nitrate groups determines reactivity and potency of organic nitrates: a proof of concept study in ALDH-2−/− mice. Br J Pharmacol. 2007;150(4):526–533. doi: 10.1038/sj.bjp.0707116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DiFabio J, Ji Y, Vasiliou V, Thatcher GR, Bennett BM. Role of mitochondrial aldehyde dehydrogenase in nitrate tolerance. Mol Pharmacol. 2003;64(5):1109–1116. doi: 10.1124/mol.64.5.1109. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, Kitagawa K, Nakayama KI, Hess DT, Stamler JS. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2005;102(34):12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackenzie IS, Maki-Petaja KM, McEniery CM, Bao YP, Wallace SM, Cheriyan J, Monteith S, Brown MJ, Wilkinson IB. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler Thromb Vasc Biol. 2005;25(9):1891–1895. doi: 10.1161/01.ATV.0000179599.71086.89. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel P, Schulz E, Gori T, Ostad MA, Mathner F, Schildknecht S, Gobel S, Oelze M, Stalleicken D, Warnholtz A, Munzel T, Daiber A. Monitoring white blood cell mitochondrial aldehyde dehydrogenase activity: implications for nitrate therapy in humans. The Journal of pharmacology and experimental therapeutics. 2009;330(1):63–71. doi: 10.1124/jpet.108.149716. [DOI] [PubMed] [Google Scholar]
- 69.Page NA. Doctoral dissertation. State University of New York at Buffalo. Department of Pharmaceutical Sciences; Ann Arbor: ProQuest/UMI; 2011. Role of aldehyde dehydrogenase in the metabolism and action of organic nitrate vasodilators. (Publication No. AAT 3495429) [Google Scholar]
- 70.Page NA, Lee SG, Fung SM, Duester G, Fung HL. Role of Aldehyde Dehydrogenase 1a1 in the Metabolism and Vasodilation of Organic Nitrates (abstract). AAPS/FIP Pharm Sci Annual Meeting; New Orleans, La. November 13–18, 2010; 2010. p. W4300. [Google Scholar]
- 71.Fung HL. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annual review of pharmacology and toxicology. 2004;44:67–85. doi: 10.1146/annurev.pharmtox.44.101802.121646. [DOI] [PubMed] [Google Scholar]
- 72.Krishnatry AS, Kamei T, Wang H, Qu J, Fung H-L. Identification of nitroglycerin-induced cysteine modifications of pro-matrix metalloproteinase-9. Rapid Communications in Mass Spectrometry. 2011;25(16):2291–2298. doi: 10.1002/rcm.5118. [DOI] [PubMed] [Google Scholar]
- 73.Chong S, Fung HL. Biochemical and pharmacological interactions between nitroglycerin and thiols. Effects of thiol structure on nitric oxide generation and tolerance reversal. Biochemical pharmacology. 1991;42(7):1433–1439. doi: 10.1016/0006-2952(91)90456-f. [DOI] [PubMed] [Google Scholar]
- 74.Rudyk O, Prysyazhna O, Burgoyne JR, Eaton P. Nitroglycerin Fails to Lower Blood Pressure in Redox-Dead Cys42Ser PKG1α Knock-In Mouse. Circulation. 2012;126(3):287–295. doi: 10.1161/CIRCULATIONAHA.112.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beretta M, Sottler A, Schmidt K, Mayer B, Gorren AC. Partially irreversible inactivation of mitochondrial aldehyde dehydrogenase by nitroglycerin. J Biol Chem. 2008;283(45):30735–30744. doi: 10.1074/jbc.M804001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Needleman P, Lang S, Johnson EM., Jr Organic nitrates: relationship between biotransformation and rational angina pectoris therapy. The Journal of pharmacology and experimental therapeutics. 1972;181(3):489–497. [PubMed] [Google Scholar]
- 77.Olivari MT, Carlyle PF, Levine TB, Cohn JN. Hemodynamic and hormonal response to transdermal nitroglycerin in normal subjects and in patients with congestive heart failure. J Am Coll Cardiol. 1983;2(5):872–878. doi: 10.1016/s0735-1097(83)80234-4. [DOI] [PubMed] [Google Scholar]
- 78.Bauer JA, Fung HL. Effect of apparent elimination half-life on nitroglycerin-induced hemodynamic rebound in experimental heart failure. Pharmaceutical research. 1993;10(9):1341–1345. doi: 10.1023/a:1018930032203. [DOI] [PubMed] [Google Scholar]
- 79.Munzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95(1):187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49(12):1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 81.Minamiyama Y, Takemura S, Nishino Y, Okada S. Organic nitrate tolerance is induced by degradation of some cytochrome P450 isoforms. Redox Rep. 2002;7(5):339–342. doi: 10.1179/135100002125000947. [DOI] [PubMed] [Google Scholar]
- 82.Zimrin D, Reichek N, Bogin KT, Aurigemma G, Douglas P, Berko B, Fung HL. Antianginal effects of intravenous nitroglycerin over 24 hours. Circulation. 1988;77(6):1376–1384. doi: 10.1161/01.cir.77.6.1376. [DOI] [PubMed] [Google Scholar]
- 83.Fung HL, McNiff EF, Ruggirello D, Darke A, Thadani U, Parker JO. Kinetics of isosorbide dinitrate and relationships to pharmacological effects. British journal of clinical pharmacology. 1981;11(6):579–590. doi: 10.1111/j.1365-2125.1981.tb01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsou PS, Addanki V, Haas JA, Page NA, Fung HL. Role of glutaredoxin-mediated protein S-glutathionylation in cellular nitroglycerin tolerance. The Journal of pharmacology and experimental therapeutics. 2009;329(2):649–656. doi: 10.1124/jpet.108.149997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang EQ, Lee WI, Fung HL. Lack of critical involvement of endothelial nitric oxide synthase in vascular nitrate tolerance in mice. Br J Pharmacol. 2002;135(2):299–302. doi: 10.1038/sj.bjp.0704532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Packer M, Lee WH, Kessler PD, Gottlieb SS, Medina N, Yushak M. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med. 1987;317(13):799–804. doi: 10.1056/NEJM198709243171304. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y. Randomized, double-blind, placebo-controlled study of ascorbate on the preventive effect of nitrate tolerance in patients with congestive heart failure. Circulation. 1998;97(9):886–891. doi: 10.1161/01.cir.97.9.886. [DOI] [PubMed] [Google Scholar]
- 88.Bassenge E, Fink N, Skatchkov M, Fink B. Dietary supplement with vitamin C prevents nitrate tolerance. J Clin Invest. 1998;102(1):67–71. doi: 10.1172/JCI977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y. Randomized, double-blind, placebo-controlled study of supplemental vitamin E on attenuation of the development of nitrate tolerance. J Cardiol. 1998;31(3):173–181. [PubMed] [Google Scholar]
- 90.Gori T, Burstein JM, Ahmed S, Miner SE, Al-Hesayen A, Kelly S, Parker JD. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation. 2001;104(10):1119–1123. doi: 10.1161/hc3501.095358. [DOI] [PubMed] [Google Scholar]
- 91.Gori T, Saunders L, Ahmed S, Parker JD. Effect of folic acid on nitrate tolerance in healthy volunteers: differences between arterial and venous circulation. J Cardiovasc Pharmacol. 2003;41(2):185–190. doi: 10.1097/00005344-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Dudek M, Bednarski M, Bilska A, Iciek M, Sokołowska-Jeżewicz M, Filipek B, Włodek L. The role of lipoic acid in prevention of nitroglycerin tolerance. European Journal of Pharmacology. 2008;591(1–3):203–210. doi: 10.1016/j.ejphar.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y. Preventive effects of carvedilol on nitrate tolerance--a randomized, double-blind, placebo-controlled comparative study between carvedilol and arotinolol. J Am Coll Cardiol. 1998;32(5):1201–1206. doi: 10.1016/s0735-1097(98)00398-2. [DOI] [PubMed] [Google Scholar]
- 94.Bauer JA, Fung HL. Concurrent hydralazine administration prevents nitroglycerin-induced hemodynamic tolerance in experimental heart failure. Circulation. 1991;84(1):35–39. doi: 10.1161/01.cir.84.1.35. [DOI] [PubMed] [Google Scholar]
- 95.Liuni A, Luca MC, Di Stolfo G, Uxa A, Mariani JA, Gori T, Parker JD. Coadministration of Atorvastatin Prevents Nitroglycerin-Induced Endothelial Dysfunction and Nitrate Tolerance in Healthy Humans. Journal of the American College of Cardiology. 2011;57(1):93–98. doi: 10.1016/j.jacc.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 96.Sun L, Ferreira JC, Mochly-Rosen D. ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance. Science translational medicine. 2011;3(107):107ra111. doi: 10.1126/scitranslmed.3002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang EQ, Lee WI, Brazeau D, Fung HL. cDNA microarray analysis of vascular gene expression after nitric oxide donor infusions in rats: implications for nitrate tolerance mechanisms. AAPS pharmSci. 2002;4(2):E10. doi: 10.1208/ps040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clavreul N, Bachschmid MM, Hou X, Shi C, Idrizovic A, Ido Y, Pimentel D, Cohen RA. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26(11):2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 99.Krishnatry AS, Fung SM, Brazeau DA, Soda D, Fung HL. Nitroglycerin alters matrix remodeling proteins in THP-1 human macrophages and plasma metalloproteinase activity in rats. Nitric Oxide. 2011;24(2):66–76. doi: 10.1016/j.niox.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sussex BA, Campbell NR, Raju MK, McKay DW. The antianginal efficacy of isosorbide dinitrate therapy is maintained during diuretic treatment. Clinical pharmacology and therapeutics. 1994;56(2):229–234. doi: 10.1038/clpt.1994.128. [DOI] [PubMed] [Google Scholar]
- 101.Cotter G, Metzkor-Cotter E, Kaluski E, Blatt A, Litinsky I, Baumohl Y, Moshkovitz Y, Vered Z, Zaidenstein R, Golik A. Usefulness of losartan, captopril, and furosemide in preventing nitrate tolerance and improving control of unstable angina pectoris. The American journal of cardiology. 1998;82(9):1024–1029. doi: 10.1016/s0002-9149(98)00548-7. [DOI] [PubMed] [Google Scholar]
- 102.Daiber A, Oelze M, Wenzel P, Bollmann F, Pautz A, Kleinert H. Heme oxygenase-1 induction and organic nitrate therapy: beneficial effects on endothelial dysfunction, nitrate tolerance, and vascular oxidative stress. International journal of hypertension. 2012;2012:842632. doi: 10.1155/2012/842632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schuhmacher S, Wenzel P, Schulz E, Oelze M, Mang C, Kamuf J, Gori T, Jansen T, Knorr M, Karbach S, Hortmann M, Mathner F, Bhatnagar A, Forstermann U, Li H, Munzel T, Daiber A. Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension. 2010;55(4):897–904. doi: 10.1161/HYPERTENSIONAHA.109.149542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 2010;285(11):8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.MacPherson JD, Gillespie TD, Dunkerley HA, Maurice DH, Bennett BM. Inhibition of Phosphodiesterase 5 Selectively Reverses Nitrate Tolerance in the Venous Circulation. Journal of Pharmacology and Experimental Therapeutics. 2006;317(1):188–195. doi: 10.1124/jpet.105.094763. [DOI] [PubMed] [Google Scholar]
- 106.Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C. Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. American Heart Journal. 1999;138(3):577–585. doi: 10.1016/s0002-8703(99)70163-8. [DOI] [PubMed] [Google Scholar]
- 107.Kanamasa K, Hayashi T, Kimura A, Ikeda A, Ishikawa K secondary prevention group. Long-term, Continuous Treatment with Both Oral and Transdermal Nitrates Increases Cardiac Events in Healed Myocardial Infarction Patients. Angiology. 2002;53(4):399–408. doi: 10.1177/000331970205300405. [DOI] [PubMed] [Google Scholar]
- 108.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321(5895):1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen C-H, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovascular Research. 2010;88(1):51–57. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnatry AS, Brazeau DA, Fung HL. Broad regulation of matrix and adhesion molecules in THP-1 human macrophages by nitroglycerin. Nitric Oxide. 2010;22(1):11–17. doi: 10.1016/j.niox.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci U S A. 1990;87(1):364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brandi ML, Hukkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, Polak JM, MacIntyre I. Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Natl Acad Sci U S A. 1995;92(7):2954–2958. doi: 10.1073/pnas.92.7.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kasten TP, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko TP, Settle SL, Currie MG, Nickols GA. Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc Natl Acad Sci U S A. 1994;91(9):3569–3573. doi: 10.1073/pnas.91.9.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hukkanen M, Platts LA, Lawes T, Girgis SI, Konttinen YT, Goodship AE, MacIntyre I, Polak JM. Effect of nitric oxide donor nitroglycerin on bone mineral density in a rat model of estrogen deficiency-induced osteopenia. Bone. 2003;32(2):142–149. doi: 10.1016/s8756-3282(02)00955-9. [DOI] [PubMed] [Google Scholar]
- 115.Hao YJ, Tang Y, Chen FB, Pei FX. Different doses of nitric oxide donor prevent osteoporosis in ovariectomized rats. Clinical orthopaedics and related research. 2005;(435):226–231. doi: 10.1097/01.blo.0000153990.74837.73. [DOI] [PubMed] [Google Scholar]
- 116.Rejnmark L, Vestergaard P, Mosekilde L. Decreased Fracture Risk in Users of Organic Nitrates: A Nationwide Case-Control Study. Journal of Bone and Mineral Research. 2006;21(11):1811–1817. doi: 10.1359/jbmr.060804. [DOI] [PubMed] [Google Scholar]
- 117.Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal Nitroglycerin Therapy May Not Prevent Early Postmenopausal Bone Loss. Journal of Clinical Endocrinology & Metabolism. 2009;94(9):3356–3364. doi: 10.1210/jc.2008-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA: the journal of the American Medical Association. 2011;305(8):800–807. doi: 10.1001/jama.2011.176. [DOI] [PubMed] [Google Scholar]
- 119.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 120.Martelli A, Rapposelli S, Calderone V. NO-releasing hybrids of cardiovascular drugs. Curr Med Chem. 2006;13(6):609–625. doi: 10.2174/092986706776055634. [DOI] [PubMed] [Google Scholar]
- 121.Konturek SJ, Brzozowski T, Majka J, Pytko-Polonczyk J, Stachura J. Inhibition of nitric oxide synthase delays healing of chronic gastric ulcers. European Journal of Pharmacology. 1993;239(1–3):215–217. doi: 10.1016/0014-2999(93)90997-v. [DOI] [PubMed] [Google Scholar]
- 122.Whittle BJ, Lopez-Belmonte J, Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990;99(3):607–611. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wallace JL, Cirino G, De Nucci G, McKnight W, MacNaughton WK. Endothelin has potent ulcerogenic and vasoconstrictor actions in the stomach. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1989;256(4):G661–G666. doi: 10.1152/ajpgi.1989.256.4.G661. [DOI] [PubMed] [Google Scholar]
- 124.Perini R, Fiorucci S, Wallace JL. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal injury and repair: a window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2004;18(4):229–236. doi: 10.1155/2004/890585. [DOI] [PubMed] [Google Scholar]
- 125.Konturek PC, Brzozowski T, Kania J, Konturek SJ, Hahn EG. Nitric oxide-releasing aspirin protects gastric mucosa against ethanol damage in rats with functional ablation of sensory nerves. Inflammation research: official journal of the European Histamine Research Society [et al] 2003;52(9):359–365. doi: 10.1007/s00011-003-1183-7. [DOI] [PubMed] [Google Scholar]
- 126.Fiorucci S, Santucci L, Wallace JL, Sardina M, Romano M, del Soldato P, Morelli A. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc Natl Acad Sci U S A. 2003;100(19):10937–10941. doi: 10.1073/pnas.1933204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fiorucci S, Santucci L, Gresele P, Faccino RM, del Soldato P, Morelli A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: A proof of concept endoscopic study. Gastroenterology. 2003;124(3):600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- 128.Di Napoli M, Papa F. NCX-4016 NicOx. Curr Opin Investig Drugs. 2003;4(9):1126–1139. [PubMed] [Google Scholar]
- 129.Geusens P. Naproxcinod, a new cyclooxygenase-inhibiting nitric oxide donator (CINOD) Expert opinion on biological therapy. 2009;9(5):649–657. doi: 10.1517/14712590902926071. [DOI] [PubMed] [Google Scholar]
- 130.Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer. 2008;59(3):340–349. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 131.Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, Stass SA, Jiang F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Laboratory investigation; a journal of technical methods and pathology. 2010;90(2):234–244. doi: 10.1038/labinvest.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sladek NE, Kollander R, Sreerama L, Kiang DT. Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: a retrospective study. Rational individualization of oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer chemotherapy and pharmacology. 2002;49(4):309–321. doi: 10.1007/s00280-001-0412-4. [DOI] [PubMed] [Google Scholar]
- 133.Cavallini G. Minoxidil versus nitroglycerin: a prospective double-blind controlled trial in transcutaneous erection facilitation for organic impotence. J Urol. 1991;146(1):50–53. doi: 10.1016/s0022-5347(17)37712-1. [DOI] [PubMed] [Google Scholar]
- 134.Meyhoff HH, Rosenkilde P, Bodker A. Non-invasive management of impotence with transcutaneous nitroglycerin. Br J Urol. 1992;69(1):88–90. doi: 10.1111/j.1464-410x.1992.tb15466.x. [DOI] [PubMed] [Google Scholar]
- 135.Yoncheva K, Doytchinova I, Tankova L. Preparation and Evaluation of Isosorbide Mononitrate Hydrogels for Topical Fissure Treatment. Curr Drug Deliv. doi: 10.2174/156720112802650635. [DOI] [PubMed] [Google Scholar]
- 136.Tjandra JJ, Tan JJ, Lim JF, Murray-Green C, Kennedy ML, Lubowski DZ. Rectogesic (glyceryl trinitrate 0.2%) ointment relieves symptoms of haemorrhoids associated with high resting anal canal pressures. Colorectal Dis. 2007;9(5):457–463. doi: 10.1111/j.1463-1318.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- 137.Lo GH, Chen WC, Chan HH, Tsai WL, Hsu PI, Lin CK, Chen TA, Lai KH. A randomized, controlled trial of banding ligation plus drug therapy versus drug therapy alone in the prevention of esophageal variceal rebleeding. J Gastroenterol Hepatol. 2009;24(6):982–987. doi: 10.1111/j.1440-1746.2009.05792.x. [DOI] [PubMed] [Google Scholar]
- 138.Beretta M, Wölkart G, Schernthaner M, Griesberger M, Neubauer R, Schmidt K, Sacherer M, Heinzel FR, Kohlwein SD, Mayer B. Vascular Bioactivation of Nitroglycerin Is Catalyzed by Cytosolic Aldehyde Dehydrogenase-2/Novelty and Significance. Circulation Research. 2012;110(3):385–393. doi: 10.1161/CIRCRESAHA.111.245837. [DOI] [PubMed] [Google Scholar]
- 139.Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, Freeman BA, Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98(6):1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parker JO, Parker JD, Caldwell RW, Farrell B, Kaesemeyer WH. The effect of supplemental L-arginine on tolerance development during continuous transdermal nitroglycerin therapy. J Am Coll Cardiol. 2002;39(7):1199–1203. doi: 10.1016/s0735-1097(02)01729-1. [DOI] [PubMed] [Google Scholar]
- 141.Fontaine D, Otto A, Fontaine J, Berkenboom G. Prevention of nitrate tolerance by long-term treatment with statins. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy. 2003;17(2):123–128. doi: 10.1023/a:1025383601304. [DOI] [PubMed] [Google Scholar]
- 142.Laight DW, Kengatharan KM, Gopaul NK, Anggard EE, Carrier MJ. Investigation of oxidant stress and vasodepression to glyceryl trinitrate in the obese Zucker rat in vivo. Br J Pharmacol. 1998;125(4):895–901. doi: 10.1038/sj.bjp.0702132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Page NA, Fung HL. Pharmacology of Nitrovasodilators. In: Bryan NS, Loscalzo J, editors. Nitrite and Nitrate in Human Health and Disease. Humana Press; 2011. pp. 207–224. [Google Scholar]