Abstract
Objectives
To determine if a higher blood transfusion threshold would prevent new or worsening delirium symptoms in the hospital after hip fracture surgery.
Design
Ancillary study to a randomized clinical trial.
Setting
Thirteen hospitals in United States and Canada.
Participants
One-hundred-thirty-nine hospitalized hip fracture patients, age ≥50, with cardiovascular disease or risk factors, and hemoglobin<10 g/dL within 3 days of surgery, recruited in an ancillary study of “Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) trial.”
Intervention
Treatment groups: 1) Liberal: received one unit of packed red blood cells and as much blood as needed to maintain hemoglobin >10 g/dL; 2) Restrictive: received transfusions if developed symptoms of anemia or hemoglobin fell below 8 g/dL.
Measurements
Delirium assessments performed pre-randomization and up to three times post-randomization. Primary outcome: Severity of delirium using Memorial Delirium Assessment Scale (MDAS) scale. Secondary outcome: presence or absence of delirium defined by Confusion Assessment Method Diagnostic Algorithm (CAM).
Results
Mean age was 81.5 (SD=9.1). Liberal group received a median 2 units and Restrictive group 0 units of blood. Hemoglobin concentration on day 1 post randomization was 1.4 g/dL higher in the Liberal group. Treatment groups did not significantly differ at any time point or over time on either MDAS delirium severity (p=0.28) or CAM delirium presence (p=0.83).
Conclusion
Blood transfusion to maintain hemoglobin >10 g/dL alone is unlikely to influence delirium severity or rate in postoperative hip fracture patients with hemoglobin concentration <10 g/dL.
Trial Registration
ClinicalTrials.gov identifier: NCT00071032 http://clinicaltrials.gov/ct2/show/NCT00071032
Keywords: Delirium, Hip Fracture, Blood Transfusion
INTRODUCTION
Delirium is a serious illness of disrupted brain physiology that results in symptoms of acute confusion, reduced attention, and/or reduced consciousness.1, 2 Delirium is identified in 10–62% of all hospitalizations1, 3–5 and is more prevalent in elderly patients.1, 6 It is especially common in hip fracture patients (35–62%),5–10 in whom delirium is associated with longer hospital length of stay, greater risk of death, more nursing home placements, and poorer functional and cognitive recovery.1, 6, 7, 9, 11–13
Hip fracture patients are frequently anemic (about 75% have postoperative hemoglobin <10 g/dL14–16) and commonly receive blood transfusion.17 Observational studies have shown an association between postoperative hemoglobin<10g/dL and subsequent incidence of delirium.18 Transfusion was one component of two multi-factorial geriatric consultation interventions shown to reduce delirium; blood was administered to maintain hematocrit at 30% or greater (equivalent to hemoglobin of 10 g/dL). 19, 20 However, it is unknown if transfusion contributed to the improved outcome.
The Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) was a randomized clinical trial of 2,016 hip fracture patients designed to test whether a higher blood transfusion threshold improved functional recovery, morbidity and mortality.21 Patients were randomly allocated to receive blood transfusion to keep the hemoglobin concentration >10 g/dL (Liberal strategy) versus transfusion only if hemoglobin concentration was <8 g/dL or when symptoms of anemia developed (Restrictive strategy). We report results from the FOCUS Cognitive Ancillary Study that assessed the presence and severity of delirium during hospitalization in 139 FOCUS participants. We hypothesized that the Liberal transfusion strategy would prevent new or worsening delirium symptoms.
METHODS
FOCUS
Patients were eligible for FOCUS if they were 50 years of age or older, undergoing surgical repair of hip fracture, had a hemoglobin <10 g/dL within three days after surgery, and had clinical evidence for cardiovascular disease or cardiovascular disease risk factors. 17, 21 Patients were excluded if they were unable to walk without human assistance prior to hip fracture; declined blood transfusions; suffered multiple trauma; had pathologic hip fracture, clinically recognized acute myocardial infarction within 30 days prior to randomization, previously participated in the trial, had symptoms associated with anemia (e.g., ischemic chest pain); or, were actively bleeding at the time of potential randomization.14,20
Subjects were randomized using an automated central telephone randomization system to the Liberal transfusion arm or Restrictive arm. The Liberal group received one unit of packed red blood cells and as much blood as needed to maintain hemoglobin >10 g/dL. The Restrictive group received transfusion if they developed symptoms of anemia or if, at study physician’s discretion, hemoglobin was below 8 g/dL. Symptoms of anemia that were indications for transfusion were: 1) chest pain thought to be cardiac in origin; 2) congestive heart failure; 3) unexplained tachycardia or hypotension unresponsive to fluid replacement. Blood was administered one unit at a time and the presence of symptoms was reassessed after each unit. Subjects with dementia were transfused when their hemoglobin concentrations fell below 8 g/dL because they might not be able to report their symptoms. Delirium or altered mental status was not considered an indication for transfusion.
Delirium was initially considered as an outcome for the larger study, but it was recognized that recorded delirium in the medical records alone would miss many cases of unrecognized delirium. The resources required to study this outcome adequately, including daily interviews, were not available to the main study. Thus, the Cognitive Ancillary Study was proposed (and subsequently funded).
Both the FOCUS and Cognitive Ancillary Study protocols were approved by the Institutional Review Boards or Ethics Committees at participating institutions. There was an independent Data and Safety Monitoring Board. Informed consent was obtained from study participants or proxies. FOCUS methods and results were previously reported17, 19, 21, 22
FOCUS Cognitive Ancillary Study
The enrollment period for this ancillary study was April 2008 to February 2009. Subjects were recruited from 13 clinical sites. One additional exclusion criterion for this study was non-English speaking due to the lack of equivalent non-English versions of many cognitive measures. All eligible FOCUS subjects at each participating site were approached for the ancillary study during this time frame.
Delirium assessments were performed pre-randomization (at the time of consent, some done pre-surgery) and multiple times within five days following randomization or up to hospital discharge (if hospital stay was shorter). All post-surgical assessments were done at least 12 hours after surgery in order to avoid the effects of anesthesia. Research staff members conducting the delirium assessments were not blinded to treatment status except at one site.
Delirium presence and severity were determined using a battery of assessments from prior delirium studies23, 24 including the Mini-Mental State Examination,25 Digit Span,26 and Delirium Symptom Interview,27 which were then used to score the following:
Memorial Delirium Assessment Scale (MDAS)28 was the primary outcome. This 10-item scale rates the severity of delirium.23, 29 Each item is rated from 0 (not present) to 3 (severe), to generate a 0–30 scale (30 is most severe). MDAS scores of 0–4 are indicative of no delirium, 5–9 mild delirium, 10–14 moderate delirium, and ≥15 severe delirium severity.23, 29
Confusion Assessment Method Diagnostic Algorithm (CAM) was the secondary outcome. This short 4-item algorithm operationalizes Diagnostic and Statistical Manual of Mental Disorder criteria of delirium30 including presence of acute onset/fluctuating course and inattention, with either disorganized thinking or altered level of consciousness.
This combination of measures, administered by trained research assistants, has been found to have high inter-rater agreement (Kappa>0.87 for all components of the assessment; Kappa=0.94 for MDAS, Kappa=0.95 for CAM) and validity.24 All delirium assessors underwent both in-person and web-based training, and their competence was tested.
In addition to the clinical characteristics and transfusion variables collected in the main study22, we recorded the number of years of formal education, marital status, and history of dementia as documented in the medical record on admission. We determined pre-fracture cognition from proxies using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE).31, 32 This 16-item measure was collected in-person or by telephone, and correlates well with direct cognitive assessments to evaluate the presence of dementia.33 The family member or significant other most knowledgeable about the subject rated the items reporting change over the 10 years prior to hip fracture. Using a cut-off point of >3.44, the IQCODE questionnaire has a sensitivity of 100% and specificity of 86% for diagnosing dementia in a hospitalized sample.34 Proxies also reported whether the subject had a previous diagnosis of dementia.
Use of psychoactive medications was abstracted from the medical chart using American Hospital Formulary Service (AHFS)35 coding for class 28:XXX.XX, including subgroups of antipsychotics (28:16.08), antidepressants (28:16.04), opiates (28:08.08), other analgesics (28:08.04 and 28:08.92), and sedative-hypnotics (28:24). Medications were coded as use of any medication within the class during the pre-randomization time frame (excluding the day of randomization).
Statistical Analyses
Analyses examined differences in the severity of delirium (MDAS) over time by treatment groups. There was 1 pre-randomization measure and up to 3 post-randomization assessments. Generalized Estimating Equations (GEE)36 were used to evaluate the longitudinal patterns comparing the two groups of hip fracture patients using all measurement time points. There were 2–4 measurement points available for the MDAS measure [pre-randomization and inhospital measures up to 3 times post-randomization (day 1 to day 5 post-randomization)]. The Stata 9 procedure XTGEE was used, which allows for robust standard error estimates, explicit modeling of covariance matrices, and is relatively tolerant of missing data.36 An independent covariance structure was specified in order to avoid problems resulting from non-random patterns of missing data. Robust standard error estimates were obtained using a technique described by Huber.37
The independent variables included a main effect term for the transfusion intervention. Binary indicator (dummy) variables were used to indicate the time points with pre-randomization serving as the reference to allow for non-linear trajectories over time. Interactions between these dummy variables and the intervention term were included as fixed effects in the longitudinal model. This model was used to estimate the mean and standard error of the outcome measure at each time point for each of the two treatment groups. A global p-value for the differences in longitudinal trajectories between the two groups was obtained from a test of the null hypothesis that all the treatment by time interaction coefficients in the model were simultaneously zero. Time-specific between-group contrasts were tested at the 5% level using Wald statistics derived from the linear combination of model coefficients used to estimate the difference in means and its standard error.
The FOCUS Cognitive Ancillary Study was powered at 90% to detect a 2.6 point difference between groups on the MDAS with n=100 per group, assuming 2 time points (1 pre, 1 post), a correlation of r=0.5 over time, and α=0.01. Previous work38 has found a clinically meaningful difference of 2.5 points on the MDAS and a medium effect size39 difference (0.5 SD) of 2.7 (previous data showing SD=5.5). With a sample size n=139 and over-time correlation (r=0.62), we had 80% power to detect a difference of 0.46 SD (2.5 MDAS points) and 90% power to detect a difference of 0.53 S.D. (2.9 MDAS points).
RESULTS
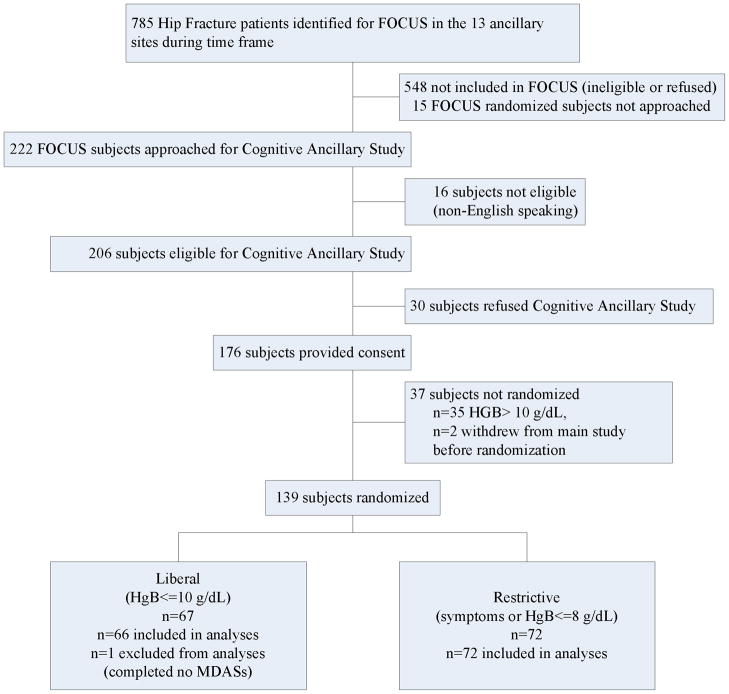
Of the 222 FOCUS subjects approached, informed consent was obtained from 176 (79%) subjects and 139 were randomized (Figure 1). Failure to randomize was due to hemoglobin concentration not falling below 10 g/dL (n=35) or the subject withdrawing consent (n=2). Eleven of the 13 participating sites enrolled subjects; the remaining two sites consented one subject each but neither was randomized. There was one subject in the Liberal group not included in the analyses because delirium assessment was not performed in the hospital.
Figure 1.
Flow of Participants Through the Trial. Legend: MDAS Memorial Delirium Assessment Scale (primary outcome); HGB Hemoglobin
The groups did not differ in presence of pre-randomization assessment (88% in each group, missing due to unavailability of staff) or number of post-randomization assessments [Liberal group mean=2.4 (SD=1.4), Restrictive group mean=2.5 (SD=1.2)]. Most pre-randomization assessments were done before surgery (62%) with an average 1.4 days between surgery and randomization and did not differ by group.
The two treatment arms’ characteristics were similar (Table 1), except that the Liberal group had more females (81%) compared to Restrictive (65%; p=0.03). Pre-randomization use of two classes of psychoactive medications was greater in the Liberal group vs. the Restrictive group: sedative hypnotics: 38% vs. 24%, p=.07, and antidepressants: 33% vs. 19%, p=.06. Dementia was present in over 25% of the sample based on medical record review, with an additional 14–15% in each group having dementia detected from the proxy informant interview. The groups did not differ on hemoglobin levels pre-surgery (mean=11.9, SD=1.5) nor pre-randomization (mean=8.9, SD=0.9). The hemoglobin concentration on post-randomization day 1 was on average 1.4 g/dL higher (p<0.001) in the Liberal group (mean=10.2, SD=1.1) compared to the Restrictive group (mean=8.8, SD=0.9). The median number of units transfused was 2 in the Liberal group and 0 in the Restrictive group; 54.2% of Restrictive patients did not receive any transfusion after randomization.
Table 1.
Sample Description by Treatment Group
| Baseline Variable | Liberal (n=66) | Restrictive (n=72) |
|---|---|---|
|
| ||
| Age (years), mean (std. dev.) | 82.4 (7.4) | 80.6 (10.4) |
|
| ||
| Sex | ||
| Female | 54 (81.8%) | 47 (65.3%) |
| Male | 12 (18.2%) | 25 (34.7%) |
|
| ||
| Race | ||
| White | 59 (89.4%) | 66 (91.7%) |
| Black | 7 (10.6%) | 5 (6.9%) |
| Unspecified | 0 (0.0%) | 1 (1.4%) |
|
| ||
| Education (years), mean (std. dev.) | 12.3 (3.4) | 12.4 (3.1) |
|
| ||
| Marital Status | ||
| Married | 23 (36.5%) | 25 (34.7%) |
| Widowed | 30 (47.6%) | 30 (41.7%) |
| Divorced/Separated | 3 (4.8%) | 8 (11.1%) |
| Never Married | 7 (11.1%) | 8 (11.1%) |
| Unspecified | 0 (0.0%) | 1 (1.4%) |
|
| ||
| Pre-admission residence | ||
| Home | 59(89.4%) | 56 (77.8%) |
| Retirement home | 4 (6.1%) | 8 (11.1%) |
| Nursing home | 3 (4.6%) | 7 (9.7%) |
| Unspecified | 0 (0.0%) | 1 (1.4%) |
|
| ||
| History of dementia | ||
| any | 18 (27.3%) | 26 (36.1%) |
| from chart | 8 (12.1%) | 16 (22.2%) |
| from significant other but not chart | 1 (1.5%) | 4 (5.6%) |
| per Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE>3.44) but not chart or significant other | 9 (13.6%) | 6 (8.3%) |
|
| ||
| Comorbidities (History from chart): | ||
| Stroke or Transient Ischemic Accident (TIA) | 5 (7.6%) | 12 (16.7%) |
| Chronic lung disease | 16 (24.4%) | 13 (18.1%) |
| Cancer | 10 (15.2%) | 12 (16.7%) |
| Diabetes | 14 (21.2%) | 14 (19.4%) |
| Atrial fibrillation | 21 (31.8%) | 23 (31.9%) |
| Parkinson’s disease | 2 (3.0%) | 2 (2.8%) |
| Hearing problems/deaf | 10 (15.2%) | 15 (20.8%) |
| Visual problems/blind | 7 (10.6%) | 9 (12.5%) |
| Alcohol abuse or withdrawal | 2 (3.0%) | 5 (6.9%) |
|
| ||
| Malnourished or cachectic at admission | 2 (3.0%) | 3 (4.2%) |
|
| ||
| Labs | ||
| White Blood Count, mean (std. dev.) | 10.8 (4.6) | 10.1 (3.7) |
| Sodium, mean (std. dev.) | 137.1 (4.1) | 137.0 (4.3) |
| Blood Urea Nitrogen (BUN), mean (std. dev.) | 22.1 (13.8) | 23.3 (13.7) |
| Glucose, mean (std. dev.) | 124.7 (48.5) | 127.9 (36.3) |
| Albumin, mean (std. dev.) | 3.7 (0.5) | 3.7 (0.5) |
| Creatinine, mean (std. dev.) | 1.1 (0.5) | 1.1 (0.8) |
| BUN/creatinine ratio ≥18 | 40 (61.5%) | 43 (59.7%) |
|
| ||
| Type of hip fracture | ||
| Femoral neck | 33 (50.0%) | 30 (41.7%) |
| Intertrochanteric/Subtrochanteric | 33 (50.0%) | 42 (58.3%) |
|
| ||
| Anesthesia Type | ||
| General or Combined General/Regional/Spinal | 38 (57.6%) | 42 (58.3%) |
| Regional/Spinal only | 28 (42.4%) | 30 (41.7%) |
|
| ||
| American Society of Anesthesiologists Physical Status score, mean (std. dev.) | 2.8 (0.5) | 3.0 (0.5) |
|
| ||
| Length of surgery (minutes), mean (std. dev.) | 131.3 (55.2) | 140.0 (44.7) |
|
| ||
| Hospital Length of Stay, mean (std. dev.) | 6.6 (3.9) | 6.7 (3.6) |
|
| ||
| Prerandomization Assessment Timea | ||
| Before surgery | 35 (61.4%) | 38 (60.3%) |
| After surgery | 22 (38.6%) | 25 (39.7%) |
|
| ||
| Days between surgery and randomization, mean (std.) | 1.4 (0.7) | 1.4 (0.8) |
|
| ||
| Hemoglobin value, mean (std. dev.) | ||
| Pre-surgery | 11.9 (1.3) | 11.9 (1.7) |
| Pre-randomization | 8.9 (0.8) | 8.9 (0.7) |
| 1 day post-randomization | 10.2 (1.1) | 8.8 (0.9) |
| 2 days post-randomizationab | 10.4 (0.9) | 8.7 (0.9) |
| 3 days post-randomizationc | 10.8 (0.8) | 8.7 (0.9) |
| 4 days post-randomizationd | 10.8 (1.0) | 9.3 (0.8) |
| 5 days post-randomizatione | 10.9 (1.1) | 9.3 (1.0) |
|
| ||
| Number of units of blood transfused post-randomization | ||
| None | 3 (4.5%) | 39 (54.2%) |
| 1 unit | 27 (40.9%) | 22 (30.6%) |
| 2 units | 24 (36.4%) | 9 (1.4%) |
| 3 units | 8 (12.1%) | 0 (0.0%) |
| 4+ units | 4 (6.1%) | 2 (2.8%) |
|
| ||
| Total units of blood transfused post-randomizationf | 115 | 53 |
|
| ||
| Pre-transfusion Hemoglobin (if transfused post-randomization), mean(g/dL) (std. dev.) g | 8.9 (0.8) | 7.7 (0.4) |
|
| ||
| Medications given pre-randomization | ||
| Any psychoactive | 57 (86.4%) | 63 (87.5%) |
| Antipsychotic medications | 6 (9.1%) | 6 (8.3%) |
| Antidepressants | 22 (33.3%) | 14 (19.4%) |
| Opiates | 52 (78.8%) | 54 (75.0%) |
| Other Analgesics | 45 (68.2%) | 44 (61.1%) |
| Sedative-hypnotics | 25 (37.9%) | 17 (23.6%) |
|
| ||
| Post-randomization Complications | ||
| Infections | 3 (4.6%) | 3 (4.2%) |
| Pulmonary Embolism | 2 (3.0%) | 0 (0.0%) |
| Congestive Heart Failure | 1 (1.5%) | 2 (2.8%) |
| Hemorrhaging (>100cc) | 6 (9.1%) | 4 (5.6%) |
Footnotes:
Only if MDAS administered, numbers will not add up to all subjects in group.
Total n includes only those still in hospital 2 days post-randomization (L n=48, R n=58),
Total n includes only those still in hospital 3 days post-randomization (L n=34, R n=45),
Total n includes only those still in hospital 4 days post-randomization (L n=21, R n=28),
Total n includes only those still in hospital 5 days post-randomization (L n=12, R n=15),
Raw number of units across all subjects within group,
Values per transfusion and only if transfused (L n=63, R n=33 transfusions).
Although the two groups did not differ on timing of randomization after surgery, there was a significant association of days from surgery with MDAS delirium severity scores over time (p=0.04). The MDAS averaged below 5 points before surgery and peaked at 8–10 points on the day after surgery.
For the primary outcome (MDAS score), neither the unadjusted means (Table 2), nor the results from the GEE models (Figure 2) showed statistically significant differences between the two treatment arms over time or at any time point. Before randomization, the Restrictive transfusion group had a similar MDAS delirium severity score to the Liberal group (difference=−0.66, 95% Confidence Interval: −2.50 to 1.18). On post-randomization day 1, there was virtually no difference between the two groups (difference=−0.05, 95% Confidence Interval: −1.67 to 1.58). Thereafter, differences remained small (post-randomization day 2 difference=0.98, 95% Confidence Interval: −1.11 to 3.07; post-randomization day 3 difference=0.88, 95% Confidence Interval: −1.24 to 2.99; post-randomization day 4/5 difference =1.20, 95% Confidence Interval: −0.93 to 3.32). Notably, all of the observed MDAS differences were smaller than the 2.5 points shown to be clinically meaningful, but the confidence intervals for post-randomization days 2–5 do include 2.5 in the upper boundary.
Table 2.
Means (standard deviations) or Number (percentages) for FOCUS CAS Outcomes by Measurement Timepoint
| Measures | Pre-randomization | Post-rand day 1 | Post-rand day 2 | Post-rand day 3 | Post-rand day 4 | Post-rand day 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liberal (n=57) |
Restr. (n=63) |
Liberal (n=53) |
Restr. (n=55) |
Liberal (n=36) |
Restr. (n=46) |
Liberal (n=23) |
Restr. (n=31) |
Liberal (n=9) |
Restr. (n=9) |
Liberal (n=5) |
Restr. (n=3) |
|
| Primary Outcome | ||||||||||||
| Memorial Delirium Assessment Scale, Mean (SD) | 6.7 (5.3) | 6.4 (5.2) | 6.8 (4.4) | 6.9 (4.6) | 6.9 (5.5) | 7.4 (4.9) | 5.7 (4.8) | 6.0 (5.0) | 3.0 (1.9) | 5.0 (4.3) | 5.2 (4.3) | 2.7 (1.5) |
| (time-specific p from t-tests) | p=0.72 | p=0.93 | p=0.69 | p=0.87 | p=0.23 | p=0.28 | ||||||
| Secondary Outcome | ||||||||||||
| Confusion Assessment Method, n (% delirium) | 14 (24.6%) | 15 (23.8%) | 16 (30.2%) | 22 (40.0%) | 12 (33.3%) | 12 (26.7%) | 6 (26.1%) | 5 (16.1%) | 1 (11.1%) | 2 (22.2%) | 1 (20.0%) | 0 (0.0%) |
| (time-specific p from chi-squares) | p=0.92 | p=0.29 | p=0.51 | p=0.37 | p=0.53 | p=0.41 | ||||||
Figure 2.
Primary Outcome: MDAS Delirium Severity Score (Estimated Mean and 95% CI From GEE) by Days Relative to Randomization by Treatment Group. Footnote: Time by Treatment Interaction p=0.23
There were also no significant differences for the presence of delirium as defined by CAM (secondary outcome) between the groups at any time point or in the trend over time. The largest difference in magnitude was seen at post-randomization day 1 (unadjusted percentage Restrictive=40% delirium vs. Liberal=31%; Relative Risk=1.26, 95% Confidence Interval=0.76–2.08 in GEE models, Figure 3).
Figure 3.
Secondary Outcome CAM Delirium (Estimated Probability and 95% CI From GEE) by Days Relative to Randomization by Treatment Group. Footnote: Time by Treatment Interaction p=0.83
Sensitivity analyses
We adjusted for baseline differences in gender, use of sedative hypnotics and anti-depressants, and the effect of days from surgery on delirium over time. The estimated effects and statistical significance did not change substantially when any or all of these variables were included in the models. For example, the p-value for the post-randomization between-treatment differences in MDAS delirium severity scores reported in Figure 2 is p=0.23; in the sensitivity analyses these p values ranged from 0.26 to 0.31.
Also, since dementia is a known risk factor for delirium, and there was an absolute difference of 9% in dementia prevalence between the groups, models adjusting for dementia were also tested. These did not affect the overall results, but did decrease the magnitude of the difference in CAM delirium at the first randomization day (Relative Risk=1.13, 95% Confidence Interval=0.64–1.86).
CONCLUSION
Administration of blood transfusion to maintain hemoglobin concentration greater than 10 g/dL did not significantly reduce the severity or frequency of in-hospital delirium compared to a blood transfusion threshold of 8 g/dL. There was a clinically significant difference in amount of blood transfused between the treatment arms. These results suggest that liberal transfusion alone does not reduce the risk of postoperative delirium among hip fracture patients with hemoglobin concentrations less than 10 g/dL. This finding supports the overall conclusions of the main FOCUS trial which found that the liberal transfusion strategy, as compared with a restrictive strategy, did not improve functional recovery or reduce mortality or in-hospital morbidity, in elderly patients with cardiovascular disease or risk factors. 21
Consistent with other studies, we observed a peak in delirium severity one day after surgery.40 The naturally occurring peak of delirium severity on postoperative day one and subsequent decline highlights the importance of including an appropriate control group in all delirium intervention trials. Because of concern about residual effects of anesthesia (one potential explanation for a peak in delirium after surgery), we waited at least 12 hours after surgery ended to begin our delirium assessments; however, this amount of time may not have been sufficient to allow for anesthesia effects to fully clear. Time from surgery to randomization or assessment did not differ between the two groups and thus we do not believe it influenced our overall conclusions.
Interventions to prevent delirium may differ from those to treat delirium.41 Other studies have shown that geriatric consultation reduced the incidence and severity (prevention) but not duration of delirium (treatment).19, 20 A trial of low dose haloperidol (given as prophylaxis) in patients with hip fracture and elective hip replacement found a reduction in delirium duration and severity but not in the incidence of delirium.42 In contrast, a study evaluating olanzapine (given as prophylaxis perioperatively) in elective total hip and knee replacement patients showed decreased incidence of delirium but increased severity in the patients who got delirious.43 Our study did not find an effect of transfusion in preventing delirium or improving delirium symptoms.
It is also possible that a single intervention strategy such as transfusion may be ineffective for a multi-factorial geriatric syndrome such as delirium. Previous work showed that a multi-faceted delirium intervention, which included transfusion for hemoglobin < 10 g/dl, did prevent delirium incidence19 and a geriatrics intervention in Sweden, which also included transfusion, found improvement in symptoms among patients with delirium. 20 The Swedish study20 had a different threshold for those already delirious (11 g/dL) than to prevent delirium (10 g/dL) as part of the multi-factorial intervention. Our threshold did not differentiate between prevalent and incident delirium and was lower than their higher threshold. It is possible that a higher threshold would have been beneficial or it may be that that transfusion does not make any difference in the multi-component interventions. The Hospital Elder Life Program44 did not include transfusions, but is another multi-component intervention that has been shown to prevent delirium in general medical and surgical patients.44, 45
The frequency of delirium was lower (not significant) day 1 post-randomization in the Liberal group but higher on days 2 and 3 compared to the Restrictive transfusion group. These observed differences were smaller (largest 30% Liberal vs. 40% Restrictive) than seen in many other successful interventions, including geriatrics consultation (32% in intervention vs. 50% usual care),19 anesthesia sedation reduction (19% light vs. 40% deep sedation),46 and melatonin treatment (12% melatonin vs. 31% placebo). 47
There were more subjects with dementia, a known risk factor for delirium,3–5 (9% point difference, not statistically significant) in the Restrictive group such that the slightly higher delirium rates in this group were not surprising. Models adjusting for dementia attenuated the small, non-significant effect of transfusion seen on post-randomization day 1.
There were some potential limitations to our study. Even though we were not able to achieve the target sample size, we still had good power (>80%) for detecting moderate-sized differences in the primary outcome.39 We chose the MDAS severity measure as the primary outcome for this trial because it predicts the long-term outcomes of delirium3, 21, and a large proportion of hip fracture patients have symptoms of delirium (including subsyndromal delirium) in the absence of full diagnostic criteria.3, 23 We had pre-specified 2.5 points on the MDAS as a clinically meaningful difference38, and this difference was not observed between treatment groups at any time point, although confidence intervals on some days did include this value. We did not have three full days of post-randomization assessments for many of the subjects, which could also limit the power for many of these comparisons, although 61.6% had at least 2 post-randomization assessments. There was an imbalance between the two arms of age, sedative-hypnotic, and antidepressant use, although our sensitivity analyses did not find that it substantially altered our findings.
Another limitation of the study was the delirium evaluators were not blind to treatment (although the investigators were). To overcome this, we utilized objective delirium assessment measures and, more importantly, did not have the interviewers calculate any summary scores or the final CAM determinations. We had only one site that was blinded (n=24 subjects), so we were unable to test the impact of blinding on the results. It would have been ideal if we could have had blinded assessments, but this was not feasible in this trial because of inadequate staffing. We also could not fully blind our evaluators since they might be present when blood was being given.
This study did have a number of strengths. Our study was conducted in the context of a rigorous, multi-site randomized trial. This ancillary trial showed substantial differences in post-randomization hemoglobin concentrations and the quantity of blood administered in the two arms. 21 Pre-surgery (baseline) hemoglobin values (11.9 g/dL in both groups) suggest that most participants had primarily acute blood loss and not severe chronic anemia. The results are consistent with the larger FOCUS trial that liberal transfusion did not improve function, mortality, or morbidity 21, with previous literature48, and with recently published transfusion guidelines49. In addition, the FOCUS Cognitive Ancillary Study utilized rigorous, state-of-the-art delirium measures (MDAS and CAM), including extensive training and oversight of all delirium assessments. Finally, only 1 subject was excluded from analyses because there was no inhospital assessments.
In conclusion, transfusion of hip fracture patients after surgery to maintain hemoglobin above 10 g/dL does not appear to prevent or reduce the severity of delirium. These results suggest it is reasonable to withhold blood transfusion in post-surgical patients unless the patient develops symptoms from anemia, or if hemoglobin concentration falls below 8 g/dL.
Acknowledgments
The FOCUS CAS ancillary study was funded as a separate grant (R01 HL085706) from the primary FOCUS study (grants U01 HL073958 and U01 HL074815 from the National Heart, Lung, & Blood Institute). The Cognitive Ancillary Study funding began February 2008; FOCUS began in 2003. Research was also supported, in part by a National Institute on Aging training grant: T32 AG00262, and by funds from the Claude D. Pepper Older Americans Independence Center, National Institute on Aging, P30 AG028747. Dr. Marcantonio is a recipient of a Mid-Career Investigator Award in Patient-Oriented Research (K24 AG035075) from the National Institute on Aging.
FOCUS Steering Committee and Ancillary Study and Publications Committee: Jeffrey L. Carson, (Principal Investigator and Study Chairman) UMDNJ-Robert Wood Johnson Medical School; Michael L. Terrin, (Principal Investigator Data Coordinating Center) School of Medicine, University of Maryland; Lauren Beaupre, University of Alberta Hospital, University of Alberta; Bernard R. Chaitman, (Principal Investigator Core ECG Laboratory) Saint Louis University School of Medicine; Gwendolyn Dobbin, Queen Elizabeth II Health Sciences Center; Lee A. Fleisher, University of Pennsylvania School of Medicine; A. Gerson Greenburg, Brown University School of Medicine, (until 2007); Paul C. Hébert, Ottawa Health Research Institute and the University of Ottawa; David A. Heck, Baylor Health Care System (until 2007); Kevin A. Hildebrand, University of Calgary; Harold Kaplan, New York- Mount Sinai School of Medicine; Courtland Lewis, Hartford Hospital; Jay Magaziner, School of Medicine, University of Maryland; William Macaulay, New York-Presbyterian Hospital at Columbia University; George G. Rhoads, UMDNJ-School of Public Health; David Sanders, The University of Western Ontario; Ex-Officio: Helaine Noveck, UMDNJ-Robert Wood Johnson Medical School; Karen Dragert, UMDNJ-Robert Wood Johnson Medical School; Sandra Forman, University of Maryland.
Management Committee: Jeffrey L. Carson, Michael L. Terrin, Jay Magaziner, David Sanders, George Rhoads, Sandy Forman, Helaine Noveck, Karen Dragert
National Heart, Lung, and Blood Institute: Simone Glynn, George Nemo, Traci Heath Mondoro, Luiz H. Barbosa, Nancy L. Geller, Jungnam Joo.
Data and Safety Monitoring Board: Merlyn Sayers, (Chair), Clifford Colwell, Marion Danis, Jeanne Lusher, Bruce McLeod, Angelique Reitsma (until 2008), Paula Roberson, Amy Shapiro, Peter Stone, Carolyn Whitsett.
Clinical Coordinating Center: University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, Division of General Internal Medicine: Jeffrey L. Carson (Principal Investigator and Director), Helaine Noveck, (Deputy Director), Karen Dragert (Lead Research Nurse), Diana Abad, Pat Pane, Maricar Raymundo, Judy Kenny, Carolina Torres, Pat Affrunti.
Data Coordinating Center: University of Maryland Baltimore, Division of Gerontology: Department of Epidemiology and Public Health. Michael Terrin, (Principal Investigator and Director), Richard Hebel, Cindy Geppi, Andrea Lefever, Elizabeth Casher, Verita Custis Buie, William Hawkes, Michelle Werner-Bronzert, Justine Golden, Sue Miller, Tamara McNair, Tiffany Smith, Yvonne Aro, Sandra Forman (consultant). University of Maryland Baltimore, Bioinformatics Group: Mark Pohl, Tamar Pair, Teresa Yates, Kristin Frey. VAMC, Perry Point, Cooperative Studies Program Coordinating Center: Rebecca (Anne) Horney, Elizabeth Spence, Christina Carty, Connie Glassman, Maxine Rhoads, Sandra Pritt, Barbara Yndo, Heather Buckland, Karen Lawson, Christine Dalzell, Howard (Norman) Oales, Jennifer Smith.
Sponsor’s Role: The FOCUS CAS ancillary study was funded as a separate grant (R01 HL085706) from the primary FOCUS study (grants U01 HL073958 and U01 HL074815 from the National Heart, Lung, & Blood Institute). The Cognitive Ancillary Study funding began February 2008; FOCUS began in 2003. Research was also supported, in part by a National Institute on Aging training grant: T32 AG00262, and by funds from the Claude D. Pepper Older Americans Independence Center, National Institute on Aging, P30 AG028747. Dr. Marcantonio is a recipient of a Mid-Career Investigator Award in Patient-Oriented Research (K24 AG035075) from the National Institute on Aging.
The NHLBI conducted the independent DSMB but had no direct role in the design, methods, subject recruitment, data collections, analysis and preparation of paper. No other sponsors had a direct role in design, methods, subject recruitment, data collections, analysis and preparation of paper.
FOCUS CAS Clinical Sites
Allen Pavilion at Columbia Univerity Medical Center
Clinical Site Director: Eugene Wong, MD
Research Coordinator: Todd Morrison
Audie L. Murphy Veteran’s Hospital
Clinical Site Directors: Paul Chang, DO, Thomas M. Brown, MD
Research Coordinator: Rudy Balli, PA-C
Cleveland Clinic Florida
Clinical Site Director: Jerry O. Ciocon, MD
Research Coordinator: Milagros Formoso
Hartford Hospital
Clinical Site Directors: Courtland Lewis, MD, Mary King, MD
Research Coordinators: Arben Ademi, Chris Waszynski, APRN
The Johns Hopkins Bayview Medical Center
Clinical Site Director: Khwaja Zakriya, MD
Research Coordinator: Mary-Rita Blute, RN
Maimonides Medical Center
Clinical Site Director: Barbara Paris, MD
Research Coordinator: Aleksandra Zagorin, NP, Michele Irwin, MA, GNP-BC
Ottawa Hospital, Civic Campus
Clinical Site Director: Eugene K. Wai, MD
Research Coordinator: Darren M. Roffey, PhD
Robert Wood Johnson University Hospital
Clinical Site Director: Jeffrey Carson, MD
Research Coordinator: Karen Dragert, RN
Wake Forest University Health Sciences
Clinical Site Director: Franklin Watkins, MD
Research Coordinators: Rose Fries, Michelle Gordon
Wayne Memorial Hospital
Clinical Site Director: David Rockwell, MD
Research Coordinators: Beverly Pedraza, Cheryl Dobson
William Beaumont Hospital
Clinical Site Director: Donald Knapke, MD
Research Coordinators: Melissa Lurie, RN, BSN, Claudia Westbrook, RN, BSN, Gloria Kopper, RN, BSN, Beth Mitchell, RN
Conflict of Interest
Dr. Magaziner received support from the following companies to conduct research through his institution, provide academic consultation, or serve on an advisory board: Amgen, Eli Lilly, Glaxo SmithKline, Merck, Novartis, and Sanofi Aventis. Dr. Roffey reports working as a consultant for Palladian Health. Dr. Carson reports receiving grant support to his institution from Amgen.
Author Contributions
A. L Gruber-Baldini, E. Marcantonio, D. Orwig, J. Magaziner, M. Terrin, E. Barr, Hebel, J. L. Carson: Study concept and design.
A. L Gruber-Baldini, E. Marcantonio, D. Orwig, J. Magaziner, M. Terrin, E. Barr, J.P. Brown, B. Paris, A. Zagorin, DM Roffey, K Zakriya, M. Blute, J. L. Carson: Acquisition of subjects and/or data
A. L Gruber-Baldini, E. Marcantonio, D. Orwig, J. Magaziner, M. Terrin, E. Barr, J.P. Brown, B. Paris, A. Zagorin, DM Roffey, K Zakriya, M. Blute, J. R. Hebel, J. L. Carson: Analysis and interpretation of data
A. L Gruber-Baldini, E. Marcantonio, J. Magaziner, J.P. Brown, B. Paris, A. Zagorin, DM Roffey, K Zakriya, M. Blute, J. R. Hebel, J. L. Carson: Preparation of manuscript
Contributor Information
Ann L Gruber-Baldini, University of Maryland School of Medicine, Baltimore, MD.
Edward Marcantonio, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA.
Denise Orwig, University of Maryland School of Medicine, Baltimore, MD.
Jay Magaziner, University of Maryland School of Medicine, Baltimore, MD.
Michael Terrin, University of Maryland School of Medicine, Baltimore, MD.
Erik Barr, University of Maryland School of Medicine, Baltimore, MD.
Jessica Pelletier Brown, University of Maryland School of Medicine, Baltimore, MD.
Barbara Paris, Maimonides Medical Center, Brooklyn, NY.
Aleksandra Zagorin, Maimonides Medical Center, Brooklyn, NY.
Darren M. Roffey, Ottawa Hospital Research Institute, Ottawa ON.
Khwaja Zakriya, Total Health Care, Baltimore, MD.
Mary-Rita Blute, Johns Hopkins Bayview, Baltimore, MD.
J. Richard Hebel, University of Maryland School of Medicine, Baltimore, MD.
Jeffrey L. Carson, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ.
References
- 1.Lipowski ZJ. Delirium (acute confusional states) In: Hazzard W, Bierman EL, Blass JP, Ettinger WH Jr, Halter JH, editors. Principles of geriatric medicine and gerontology. New York: McGraw-Hill; 1994. pp. 1021–1026. [Google Scholar]
- 2.American psychiatric associations, committee on nomenclature and statistics. 4. 1995. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 3.Levkoff SE, Evans DA, Liptzin B, et al. Delirium: The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152:334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 4.Pompei P, Foreman M, Rudberg MA, et al. Delirium in hospitalized older persons: Outcomes and predictors. J Am Geriatr Soc. 1994;42:809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 5.Bitsch M, Foss N, Kristensen B, et al. Pathogenesis of and management strategies for postoperative delirium after hip fracture: A review. Acta Orthop Scand. 2004;75:378–389. doi: 10.1080/00016470410001123. [DOI] [PubMed] [Google Scholar]
- 6.Gruber-Baldini AL, Zimmerman S, Morrison RS, et al. Cognitive impairment in hip fracture patients: Timing of detection and longitudinal follow-up. J Amer Geriatr Soc. 2003;51:1227–1236. doi: 10.1046/j.1532-5415.2003.51406.x. [DOI] [PubMed] [Google Scholar]
- 7.Magaziner J, Simonsick EM, Kashner TM, et al. Predictors of functional recovery one year following hospital discharge for hip fracture: A prospective study. J Gerontol: Med Sci. 1990;45:M101–M107. doi: 10.1093/geronj/45.3.m101. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson Y, Berggren D, Brannstrom B, et al. Acute confusional states in elderly patients treated for femoral neck fracture. J Am Geriatr Soc. 1988;36:525–530. doi: 10.1111/j.1532-5415.1988.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 9.Magaziner J, Simonsick EM, Kashner M, et al. Survival experience of aged hip fracture patients. Am J Public Health. 1989;79:274–278. doi: 10.2105/ajph.79.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray AM, Levkoff SE, Wetle TT, et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48:M181–M186. doi: 10.1093/geronj/48.5.m181. [DOI] [PubMed] [Google Scholar]
- 11.Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 12.Steiner JF, Kramer AM, Eilertsen TB, et al. Development and validation of a clinical prediction rule for prolonged nursing home residence after hip fracture. J Am Geriatr Soc. 1997;45:1510–1514. doi: 10.1111/j.1532-5415.1997.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 13.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halm EA, Wang JJ, Boockvar K, et al. Effects of blood transfusion on clinical and functional outcomes in patients with hip fracture. Transfusion. 2003;43:1358–1365. doi: 10.1046/j.1537-2995.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 15.Carson JL, Terrin ML, Barton FB, et al. A pilot randomized trial comparing symptomatic versus hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion. 1998;38:522–529. doi: 10.1046/j.1537-2995.1998.38698326331.x. [DOI] [PubMed] [Google Scholar]
- 16.Carson JL, Terrin ML, Magaziner J. Anemia and postoperative rehabilitation. Can J Anesth. 2004;50:S60–S64. [PubMed] [Google Scholar]
- 17.Carson JL, Duff A, Berlin JA, et al. Perioperative blood transfusion and postoperative mortality. JAMA. 1998;279:199–205. doi: 10.1001/jama.279.3.199. [DOI] [PubMed] [Google Scholar]
- 18.Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105:380–384. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 19.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 20.Lundstrom M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: A randomized intervention study. Aging Clin Exp Res. 2007;19:178–186. doi: 10.1007/BF03324687. [DOI] [PubMed] [Google Scholar]
- 21.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carson JL, Terrin ML, Magaziner J, et al. Transfusion trigger trial for functional outcomes in cardiovascular patients undergoing surgical hip fracture repair (focus) Transfusion. 2006;46:2192–2206. doi: 10.1111/j.1537-2995.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 23.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: Their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 24.Simon S, Bergmann M, Marcantonio E. Reliability of a comprehensive delirium assessment utilizing four instruments. Gerontologist. 2001;41:365. [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” - a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler adult intelligence scale-revised manual. New York: Psychological Corporation-A Harcourt Assessment Company; 1989. [Google Scholar]
- 27.Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: An interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5:14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- 28.Breitbart W, Rosenfeld B, Roth A, et al. The memorial delirium assessment scale. J Pain Symptom Manage. 1997;13:128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 29.Schuurmans MJ, Deschamps PI, Markham SW, et al. The measurement of delirium: Review of scales. Res Theory Nurs Pract. 2003;17:207–224. doi: 10.1891/rtnp.17.3.207.53186. [DOI] [PubMed] [Google Scholar]
- 30.Inouye SK, van Dyck CH, Alessi CA. Clarifying confusion: The confusion assessment method. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 31.Jorm AF, Scott R, Cullen JS, et al. Performance of the informant questionnaire on cognitive decline in the elderly (iqcode) as a screening test for dementia. Psychol Med. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 32.Jorm AF, Christensen H, Henderson AS, et al. Informant ratings of cognitive decline of elderly people: Relationship to longitudinal change on cognitive tests. Age Ageing. 1996;25:125–129. [PubMed] [Google Scholar]
- 33.Pisani MA, Redlich C, McNicoll L, et al. Underrecognition of preexisting cognitive impairment by physicians in older icu patients. Chest. 2003;124:2267–2274. doi: 10.1378/chest.124.6.2267. [DOI] [PubMed] [Google Scholar]
- 34.Harwood DM, Hope T, Jacoby R. Cognitive impairment in medical inpatients. I: Screening for dementia--is history better than mental state? Age Ageing. 1997;26:31–35. doi: 10.1093/ageing/26.1.31. [DOI] [PubMed] [Google Scholar]
- 35.McEvoy GK, Snow EK, Miller J, et al. Ahfs drug information 2009. Feb, 2009. [Google Scholar]
- 36.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 37.Huber PJ. The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. 1967;1:221–223. [Google Scholar]
- 38.Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: Their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1977. [Google Scholar]
- 40.Juliebo V, Bjoro K, Krogseth M, et al. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354–1361. doi: 10.1111/j.1532-5415.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 41.Pitkala KH, Strandberg TE, Tilvis RS, et al. Effective treatment of delirium is difficult but not impossible. J Am Geriatr Soc. 2011;59:167–168. doi: 10.1111/j.1532-5415.2010.03204.x. author reply 168-169. [DOI] [PubMed] [Google Scholar]
- 42.Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: A randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658–1666. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 43.Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: A randomized, controlled trial. Psychosomatics. 2010;51:409–418. doi: 10.1176/appi.psy.51.5.409. [DOI] [PubMed] [Google Scholar]
- 44.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 45.Vidan MT, Sanchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57:2029–2036. doi: 10.1111/j.1532-5415.2009.02485.x. [DOI] [PubMed] [Google Scholar]
- 46.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: A randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26:687–694. doi: 10.1002/gps.2582. [DOI] [PubMed] [Google Scholar]
- 48.Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20:255–268. doi: 10.1016/j.ccc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: A clinical practice guideline from the aabb*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]