Abstract
The ability to precisely coordinate motor control to regularly-paced sensory stimuli requires an ability often called ‘mental timekeeping’, a distinct form of cognitive function. A consistent feature among conceptual models of the internal clock mechanism is an element of ‘top-down’ cognitive control. Although lesion and fMRI studies have provided indirect evidence supporting the role of the prefrontal cortex in exerting top-down influence over lower-level sensory and motor regions, little direct evidence exists. We investigated changes in Dynamic Causal Modeling (DCM)-measured top-down control of sensorimotor timing during different phases of a unimanual, auditory-paced finger-tapping task in a cohort of healthy adults and adolescents. The brain regions examined were organized into a network of excitatory connections between bilateral dorso- and ventrolateral prefrontal cortices and motor and auditory cortices. This baseline connectivity changed depending on whether participants listened passively to the pacing cue, synchronized their regular interval finger tapping with the cue, or continued tapping in absence of the cue. Subjects who performed better at maintaining the prescribed tapping pace in the absence of the auditory cue relied more on top-down control of the motor and sensory regions, while those with less accurate performance relied more on sensory driven, bottom-up control of the motor cortex. No significant maturational effects were observed in either the behavioral or DCM path weight data. Both right and left prefrontal cortex were found exert control over timing behavioral accuracy, but there were distinctly lateralized roles with respect to optimal performance.
Keywords: fMRI, dynamic causal modeling, development, mental timekeeping, sensorimotor timing, connectivity
INTRODUCTION
The ability to precisely coordinate motor control to regularly-paced sensory stimuli requires an ability often called ‘mental timekeeping’, thought to be a distinct form of cognitive function (Wiener et al. 2010). A consistent feature among numerous conceptual models of the internal clock mechanism (e.g., Gibbon 1977; Matell and Meck 2004; Meck and Benson 2002; A. M. Wing 2002), such as pacemaker-accumulator models, is an element of ‘top-down’ cognitive control. Specifically, in pacemaker-accumulator models (e.g., Gibbon 1977; Matell and Meck 2004; Meck 1996; Meck and Benson 2002; A. M. Wing 2002) information about the passage of time is believed represented within an ‘accumulator’ (i.e., working memory component), which then must be integrated within an executive ‘comparator’ mechanism that evaluates this information against goal-directed plans for responding to the environment. Such models exemplify commonly held cognitive definitions of top-down control, wherein putatively lower-level sensorimotor functions or operations are supervised and influenced by higher-level processes important for flexible, adaptive behavior (e.g., Desimone and Duncan 1995; Duncan et al. 1996; Knudsen 2007; Miller and Cohen 2001). Following the work of a number of studies investigating the neural underpinnings of timekeeping, a synchronization-continuation task (c.f., Wing and Kristofferson 1973) was chosen as a convenient paradigm with which to interrogate top-down control in conceptual models of the internal clock mechanism. While such sensorimotor timing tasks have also been frequently used to test theoretical models of sensorimotor synchronization (e.g., Molinari et al. 2007; Pollok et al. 2005; Repp 2005), this study focuses almost exclusively on the mental timekeeping processing underlying these tasks (e.g., Collier and Ogden 2004; A.M. Wing and Kristofferson 1973), rather than on the theoretical underpinnings of implementation of the motor response processes (Repp 2005).
A number of neurobiological models of prefrontal cortex function and connectivity (e.g., Miller and Cohen 2001) are consistent with the general organizational framework of top-down control in mental timekeeping and highlight the role of prefrontal cortex as the apex of this hierarchy. Lesions in the prefrontal cortex have been shown to cause general deficits in executive abilities, such as working memory, with few if any discernable deficits in more basic sensory discrimination and motor performance (e.g., Diamond and Goldman-Rakic 1989; Duncan et al. 1996; Vendrell et al. 1995). Results of these lesion studies suggest that executive abilities controlled by the prefrontal cortex would need to be integrated with these more basic sensory discrimination and motor performance abilities to produce successful sensorimotor timing performance. Prefrontal driven top-down control can also be inferred from fMRI studies that link individual differences in prefrontal hemodynamic activity levels with executive-type abilities (e.g., Garavan et al. 2003; Rypma et al. 2002). While these findings indirectly support both the concept of top-down influence over planning and performance of complex behavior and the role of the prefrontal cortex in exerting such influence, such experiments do not formally test causal relationships among activity levels within prefrontal and lower-level brain regions. Direct tests would provide clearer, more meaningful evidence of top-down control. Data analytic techniques such as Dynamic Causal Modeling (DCM) recently were developed to use fMRI and similar forms of functional neuroimaging data so that neuroimaging researchers could formally test predictions about hierarchical top-down control. DCM and similar techniques presume that statistical ‘causal’ relationships can be quantified wherein brain activity within one region can be shown to influence that in another (Guye et al. 2008). Communication between putatively higher-level cognitive (e.g., prefrontal) and lower-level sensory and motor areas can be conceptualized as generative models, which propose the existence of forward and backward connections among brain regions (Friston and Price 2001). Forward connections are driving in nature and thought largely to promulgate sensory information via bottom-up signaling. In contrast, backward connections from cognitive regions to sensory and association regions are thought to instantiate top-down control, influencing cellular assemblies within cognitively-modularized systems that bias brain activation in sensory and association cortex regions (Friston and Price 2001).
None of the growing number of studies using DCM to investigate connectivity among brain regions has examined synchronization-continuation tasks directly, but some understanding about regional interactions relevant to mental timekeeping can be gleaned piecemeal through studies of different cognitive functions that use conceptually related tasks. For instance, several reports have described the underlying connectivity of the motor effector system itself (e.g., Rehme et al. 2011), or characterized top-down control of the motor system during performance of cognitive tasks (e.g., Cieslik et al. 2011; Hare et al. 2011). These latter two studies showed top-down influence over the primary motor system, both direct and indirect, by the prefrontal cortex in the cases of simple choice (Hare et al. 2011) and stimulus-response compatibility (Cieslik et al. 2011) tasks. As informative as these previous DCM-based studies are in setting the stage for predictions about how top-down control over motor behavior – both cognitively and neurally – may manifest during mental timekeeping, they fail to capture a number of phenomena that underpin most sensorimotor timing experiments, such as the competing influences of top-down and bottom-up control engaged specifically to guide motor performance. Additional study is needed to clearly understand how prefrontal brain regions interact with other neural structures known to be engaged during synchronized, measured motor output indicative of the successful ability to guide behavior by a cognitive representation of the passage of time.
We investigated changes in DCM-measured top-down control of sensorimotor timing during different phases of an auditory-paced, regular interval finger-tapping task. Our task was a variant of the synchronization-continuation task (Wing and Kristofferson 1973) used successfully in other studies of mental timekeeping (e.g., Jantzen et al. 2007; Jantzen et al. 2004, 2005; Lewis et al. 2004; Rao et al. 1997), in which participants first passively listened to a regularly paced auditory cue, then synchronized index-finger tapping responses to the cue, and finally continued to tap at the same rate in the absence of the auditory cue. Previous meta-analyses and fMRI studies using this type of task have identified a diverse collection of active brain regions including primary and secondary motor areas, basal ganglia, prefrontal cortices, inferior parietal regions, and primary auditory cortices (Jantzen et al. 2007; Jantzen et al. 2004, 2005; Lewis et al. 2004; Rao et al. 1997; Rubia and Smith 2004; Smith et al. 2011; Wencil et al. 2010; Wiener et al. 2010; Witt et al. 2008). As these synchronization-continuation paradigms readily engage both frontal and sensorimotor regions, they are ideal tasks with which to measure top-down control during mental timekeeping. Due to the limitations of fMRI hemodynamic measurement, studies specifically examining the underlying timing processes of sensorimotor timing tasks (Jantzen et al. 2007; Jantzen et al. 2004, 2005; Lewis et al. 2004; Rao et al. 1997; Rubia and Smith 2004; Smith et al. 2011; Wencil et al. 2010; Wiener et al. 2010) often unavoidably conflate conceptually-distinct clock, accumulator, and comparator functions into a single profile of timekeeping-related brain activation, with no easy way of disentangling which of these functions is the source of observed top-down control. One study employing a parametric temporal discrimination task was able to dissociate brain activity likely related to the accumulator to that of the comparator, noting that activity in different regions within bilateral ventral prefrontal cortices (BA 44/45/46) corresponded to accumulator and comparator functioning (Wencil et al. 2010). However, the authors observed that they could not be absolutely certain whether their results represented temporal processing specifically or that of a more general attentional control system. The best evidence to date for top-down control during sensorimotor timing (as opposed to temporal discrimination) comes from Jantzen et al. (2007), who showed greater bilateral dorsal and ventral lateral prefrontal cortex activation during a task requiring participants to continue tapping at a rate learned to a pacing tone, but in the absence of auditory cues (Jantzen et al. 2007). Such a continuation task phase ideally represents cognitive control because regular motor output must be maintained solely based on cognitive presentations of the timing interval in the absence of pacing cues. However, although that study supports the importance of prefrontal cortex to mental timekeeping top-down control, it relied on a conceptual argument for top-down control, as it did not examine causal interactions among timekeeping brain regions.
The primary study aim was to characterize organization of top-down and bottom-up influences among bilateral prefrontal cortices, bilateral primary auditory cortices, and the left primary motor cortex during all task phases in a cohort of 45 healthy, right-handed adolescents and adults. We anticipated we would find DCM evidence for top-down neural control of the primary auditory cortices and left motor cortex by prefrontal regions during all phases of an auditory-paced finger-tapping task, but that the target region of this control would change with various task demands that theoretically differed in the degree of top-down control expected. We specifically hypothesized that the auditory cortices would be the target of top-down control during the passive listening condition of the task (i.e., this connectivity should serve to establish and maintain neural representation of the interval needed for motor synchronization). Frontal lobe regions, in particular, have been shown to exhibit task condition-dependent levels of activity (e.g., Chadick and Gazzaley 2011; Jantzen et al. 2007). Specifically, because previous a previous fMRI study has shown that the prefrontal cortex exhibited increased activity during the continuation phase of a sensorimotor timing task (Jantzen et al. 2007), we also predicted that the degree of top-down control over the motor cortex would be most apparent during the continuation phase, relative to either passive listening or synchronized tapping. This prediction is consistent with assumptions of current models of mental timekeeping that the accumulator and comparator have a frontal lobe origin and are the source of top-down control during sensorimotor synchronization. We additionally hypothesized that top-down control would be relatively distributed among all prefrontal regions to be examined (i.e., bilaterally), consistent with current distributed network theories of working memory (e.g., Wager and Smith 2003) and temporal processing (e.g., Wiener et al. 2010), with no single prefrontal region demonstrating overall control.
A secondary study aim was to describe the developmental trajectory of this control from early adolescence through adulthood. Several behavioral studies find developmental gains when comparing younger children with adults, either on motor interval timing tasks as used in the current study (McAuley et al. 2006) or other timekeeping paradigms (e.g., Greene and Williams 1993). Some evidence suggests that temporal processing behavioral performance on synchronize-continue tasks is largely at adult performance levels by adolescence, with moderate developmental gains throughout adolescence into early adulthood (McAuley et al. 2006). However, it is not known at what age brain activation required for adult-like performance matures. For instance, one functional neuroimaging study found evidence for age-related hemodynamic signal increases in left dorsolateral prefrontal cortex and left inferior frontal gyrus during temporal perception challenge across adolescence (Smith et al. 2011), but these changes occurred in the absence of age-related differences in task performance itself. Because the latter study also observed decreased functional connectivity between right frontal and parietal regions and right and left frontal regions in adolescents compared with adults, it is plausible that immature connectivity patterns might be related to immature behavioral performance profiles in adolescents. Consistent with this idea, we hypothesized that the degree of top-down control between prefrontal and primary auditory and motor cortices would exhibit age-related changes in the form of increased path weight coefficient values, particularly for the presumably most demanding continue condition. We also predicted age-related decreases in how much adolescents relied on bottom-up influences during the continue condition, measured in the form of decreased DCM path weight coefficient values for pathways originating in the auditory cortices.
METHODS
Participants
A total of 45 right-handed adolescent and adult participants (28 females) were recruited for several studies at the Olin Neuropsychiatry Research Center through community advertisements. Participants ranged in age from 12 to 43 years (mean (SD) = 20.3 (6.7) years) and were screened to ensure that they were medically healthy and had no past head injury, neurologic condition, learning disability, or other neurodevelopmental conditions. The absence of current or lifetime psychiatric and substance abuse disorders was determined using the screening module of the Structured Clinical Interview for Diagnosis (SCID-IV; First et al. 1994) or the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL; Kaufman et al. 1997). All participants underwent written informed consent using procedures approved by the Hartford Hospital IRB.
FMRI Stimulus Delivery/Response Recording
The paced finger-tapping task was implemented using custom software (VAPP; http://www.nrc-iol.org/vapp/) for presentation. Visual stimuli were presented using a projection system (5000 ANSI lumens) and displayed on a high-resolution screen located just behind the participant’s head. The participant viewed the screen using a mirror attached to the head coil. Corrective lenses were provided as needed. MR-compatible headphones were used to deliver the auditory stimulus. An MR-compatible fiber optic response device (Lightwave Medical, Inc., Vancouver, B.C.) was used to acquire behavioral responses.
Paced Finger-Tapping Paradigm
Our study employed a simple auditory-paced finger-tapping task. The finger-tapping paradigm was designed as a block design fMRI task. Each run consisted of six blocks of 14 seconds of passive listening to the metronomic audio cue (listen), 12 seconds of tapping in synchrony with the audio cue (tap), and 12 seconds of continuing to tap at the same pace in the absence of the audio cue (continue). Blocks were separated by a 12 second rest period. Instructions to change task phase (i.e., ‘listen’, ‘tap’, ‘continue’) were visually presented throughout the duration of the respective task phase. The audio cue was a 500Hz tone of 100msec duration and presented at a pacing rate of 0.75Hz and an intensity of 100dB. All participants reported being able to both hear the pacing tone and discriminate it from the background scanner noise. During the task, participants tapped with their right index finger. Two runs, each approximately 7 minutes and 40 seconds in duration, were collected for each participant.
Imaging Parameters
MR images were acquired on a 3T Siemens Allegra (Siemens Medical Solutions, Erlangen, Germany) located at the Olin Neuropsychiatry Research Center at the Institute of Living/Hartford Hospital in Hartford, CT. The functional imaging volumes were collected in axial orientation to the anterior commissure-posterior commissure line using a single-shot-gradient-echo echo-planar sequence (TR/TE = 1,500/28 msec; flip angle = 65°; FOV = 24 cm; matrix = 64; 3.4×3.4 mm in-plane resolution; slice thickness = 5 mm; 30 slices) with whole brain coverage. The two runs each consisted of 309 time points, with an initial 9 second rest session to allow for T1 effects to stabilize. These initial six images were not included in subsequent analyses.
High-resolution T1- and T2-weighted anatomical images were also acquired on all participants to ensure that all were free from obvious vascular injury that might otherwise influence both task performance results and interpretation of functional imaging results.
Image Processing
Functional images were reconstructed offline. Each run was corrected for slice-timing acquisition differences and separately realigned using INRIalign (Freire and Mangin 2001; Freire et al. 2002) as implemented in SPM8 (Wellcome Department of Cognitive Neurology, London, UK). A mean functional image volume was constructed for each participant for each run from the realigned image volumes and used to determine the parameters for spatial normalization into standardized Montreal Neurological Institute (MNI) space. These normalization parameters were then applied to the corresponding functional image volumes, and the normalized images were smoothed with an 8 mm FWHM Gaussian kernel. All participants’ data were individually inspected to ensure that no participant had translational or rotational head motion greater than the acquired voxel size.
FMRI Statistics and Region of Interest Extraction
The regressors from each participant’s fMRI model were defined as the onsets of each of trial block and modeled using a synthetic hemodynamic response function. The functional imaging data of each participant were modeled individually in SPM8 and included three separate regressors for each of the three conditions (listen, tap, continue). The six motion-corrected parameter estimates (x, y, and z displacements and pitch, roll, and yaw rotations) were included as covariates of no interest to statistically control signal change related to head motion. A high-pass filter (cutoff period = 128 s) was incorporated into the model to remove low-frequency signals.
In order to assess the role of top-down modulation in paced finger tapping, eight, 6mm spherical regions of interest (ROIs) were defined corresponding to the dorsolateral prefrontal, ventrolateral prefrontal, motor, and primary auditory cortices in both the left and right hemispheres (Table 1). The positioning of the ROIs was determined from a map of the main effects of the task across all subjects (Figure 1, Supplemental Table 1). This map of the overall main effects of the task agreed well with what has been published by us (Stevens et al. 2007) for this same task and others using similar tasks (Jantzen et al. 2007; Jantzen et al. 2004, 2005; Lewis et al. 2004; Rao et al. 1997). The current instantiation of dynamic causal modeling in SPM8 limits models to eight or fewer regions, making it impossible to include all potential regions (i.e., bilateral striatum) discussed in the introduction as being important for sensorimotor timing. Individual subject time series data were extracted for each 6mm spherical region of interest centered at that subject’s nearest local maximum to the coordinate of peak functional activity listed in Table 1. The time series data extracted for each participant at each region was of zero mean and unit standard deviation. To ensure that time series data could be extracted for each region for all participants, no statistical thresholding was applied to the individual subject’s data.
Table 1.
Regions-of-interest extracted from the fMRI data for use as input to DCM analyses. ROIs were extracted as 6mm spheres centered at the MNI coordinates listed for all subjects. LDLPFC: left dorsolateral prefrontal cortex. LVLPFC: left ventrolateral prefrontal cortex. RDLPFC: right dorsolateral prefrontal cortex. RVLPFC: right ventrolateral prefrontal cortex. LM1: left primary motor cortex. LAUD: left primary auditory cortex. RAUD: right primary auditory cortex.
| Region of Interest | X | Y | Z |
|---|---|---|---|
| LM1 | −36 | −21 | 54 |
|
|
|||
| RM1 | 36 | −21 | 54 |
|
|
|||
| LAud | −51 | −21 | 0 |
|
|
|||
| RAud | 54 | −18 | 0 |
|
|
|||
| LDLPFC | −39 | 39 | 27 |
|
|
|||
| RDLPFC | 45 | 45 | 15 |
|
|
|||
| LVLPFC | −60 | 6 | 21 |
|
|
|||
| RVLPFC | 55 | 12 | 12 |
|
|
|||
Figure 1.
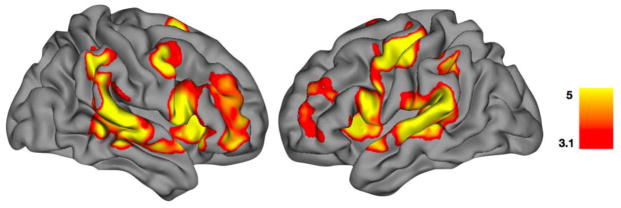
FMRI results for the main effects of task performance across all subjects. This map of the main effects of the task shows positive activation only for all three task conditions (listen, tap, continue) averaged across all 45 subjects. These activation results were used to define the peak location of the eight regions-of-interest for the primary DCM analyses. The activation results have been corrected for multiple comparisons across the whole brain at p < 0.01 using False Discovery Rate. Color bar units are given in terms of t-score.
Dynamic Causal Modeling
Dynamic causal modeling (DCM; Friston et al. 2003) was used to assess the role of top-down influence in a sensorimotor synchronization task. DCM utilizes a bilinear model, formulated to include both neurodynamics and a version of the extended Balloon model (Buxton et al. 1998; Friston 2002). Changes in neuronal states over time are described by a multivariate differential equation, where x represents the state vector, A the intrinsic connectivity matrix, B the task-dependent modulations driven by the input function u, and C the influence of direct inputs into the system (driving input).
| Equation 1 |
To model the underlying intrinsic connections, all three task conditions (listen, tap, continue) were included as the driving input (C in Equation 1). These three conditions were also considered separately, as modulatory variables (B in Equation 1), to assess changes to the baseline connectivity during each task condition. Practically, this driving and modulatory input took the form of a standard SPM-type GLM design with four separate regressors corresponding to the (1) onset timings for all experimental conditions, (2) the onset timings for the listen condition, (3) the onset timings for the tap condition, and (4) the onset timings for the continue condition. To capture the broadest picture of neural dynamics, we chose a model that included all possible connections among the eight brain regions of interest.
Causal models were estimated for each subject and session individually using DCM10 (Wellcome Department of Cognitive Neurology, London, UK), and Bayesian Parameter Averaging (BPA; e.g., Stephan et al. 2010) was used to calculate the within-subject average of the parameter estimates across the two sessions. Bayesian Parameter Averaging can be used to compute a joint posterior density within- or between-subjects by combining the individual posterior densities across multiple scanning sessions, treating the posterior from one scanning session as the prior for the next (Garrido et al. 2007; Neumann and Lohmann 2003). However, as random effects statistics have been shown to provide robust estimates of between-subject averages of simulated DCM data in comparison to fixed effects methods (Kasess et al. 2010), random effects statistics were employed to assess group averages of both the intrinsic connectivity matrices (A) and task-dependent modulations (B).
While our model included all possible connections, we made a priori predictions only for the 12 top-down paths from the left and right dorso- and ventrolateral prefrontal cortices to the left and right auditory cortices and left motor cortex, as well as the two bottom-up paths between the left and right auditory cortices and the left motor cortex. Building on the concept of generative models (Friston and Price 2001), we considered top-down paths to be those paths that originate in higher-level brain areas and terminate in lower-level sensory and motor regions and modulate the response of these lower-level regions to sensory input. Bottom-up paths, in contrast, were considered to be those paths that originate in lower-level sensory regions and serve as driving input to elicit a pre-specified response for a given pattern of input. A series of 1-sample t-tests were used to assess the overall path significance at the group level. Only path evidence with statistical significance surviving Bonferoni correction at p < 0.05 for comparing across all fourteen (12 top-down and 2 bottom-up) paths of interest was reported. Post-hoc, repeated measures ANOVAs were used to assess relative changes in a given path across the three task-dependent modulations (listen, tap, continue). Readers may note that our model included the right primary motor cortex, even though we made no specific hypotheses concerning either top-down or bottom-up connections to this region. It was primarily included as a “control” region to assess whether the results of the DCM analysis were neurobiologically reasonable. As the motor output of the task consisted solely of right index-finger tapping, we did not anticipate any significant involvement of the primary right motor cortex vis-à-vis our main theoretical interests concerning top-down control.
Behavioral Data and Developmental Effects
We considered two different measures to assess the relationship between behavioral performance and DCM path weight data. First, inter-tap interval was calculated for each subject as the time interval between any two successive taps, considering the tap and continue conditions separately. Inter-tap interval can be considered to be a self-correcting measure of the ability to maintain the prescribed tapping rate (i.e., a measure of internal clock functioning). Second, reaction time data were used to calculate the average deviation from the pacing stimulus for the tapping and continue conditions for each subject individually. Deviation was defined as how early or late a participant tapped compared to the prescribed pacing rate (e.g., tap-tone asynchrony). For the tap condition, deviation was calculated as the absolute value of the time between each auditory cue and corresponding button press occurring within a 500 msec window around the cue presentation. A similar calculation was made for the continue condition, recognizing that participants had already established the 0.75 Hz pacing rate in previous listen and tap conditions and guided their motor output using a working memory representation of the interval. As our task employed 12 second blocks for both the tap and continue conditions, measures of deviation may more readily capture a greater degree of performance abnormality and better represent an absolute performance index of accuracy (i.e., a measure of working memory functioning) than measures of inter-tap interval. Additional, more complex, measures of behavioral performance for isochronous tapping data also exist (e.g., estimates of clock variance, motor variance, drift, etc.) using either the Collier-Ogden or Wing-Kristofferson models (Collier and Ogden 2004; Wing and Kristofferson 1973), however, we chose to adopt inter-tap interval and absolute average deviation from the pacing rate as more basic measures of overall behavioral performance. Using these arguably simpler measures has the advantage of making our results more applicable to future studies of mental timekeeping employing different types of timing paradigms. We first confirmed performance differences between the tap and continue conditions, as well as evaluated possible age effects using repeated-measures ANOVAs for both behavioral measures. Then, separate multivariate ANOVAs (SPSS; IBM Corporation, Somers, NY) were used to determine the relationship between the 14 top-down and bottom-up paths and the two behavioral measures for the tap and continue conditions. Post hoc analyses were performed to determine which, if any, individual paths had statistically significant relationships with the behavioral data. Those paths identified as having a significant relationship to the behavioral data were subjected to further linear regression analyses to determine the exact nature of the relationship. For our secondary aim to determine developmental effects, repeated-measures multivariate ANOVAs were used to assess age-related changes in these two behavioral measures for the tap and continue conditions. For the DCM path weight data, separate multivariate ANOVAs were used to identify any age-related dependences in any of the 14 paths across for both DCM-estimated intrinsic connectivity (matrix A), plus path strength modulation (matrix B) during all three task conditions (listen, tap, and continue). For all age-related analyses, participants were binned into eight separated age bins (12–13yrs, 14–15yrs, 16–17yrs, 18–19yrs, 20–22yrs, 23yrs, 24–25yrs, 26+yrs) to be used as a between-subjects factor. This somewhat irregular binning was employed to ensure that each age bin had approximately the same number of subjects (each bin had on average six subjects) in an effort to preserve overall stability. These supplemental analyses examining the relationships between DCM coefficients and behavioral data were performed to provide additional insight into the cognitive relevance of the fMRI modeling results.
RESULTS
Intrinsic Connectivity
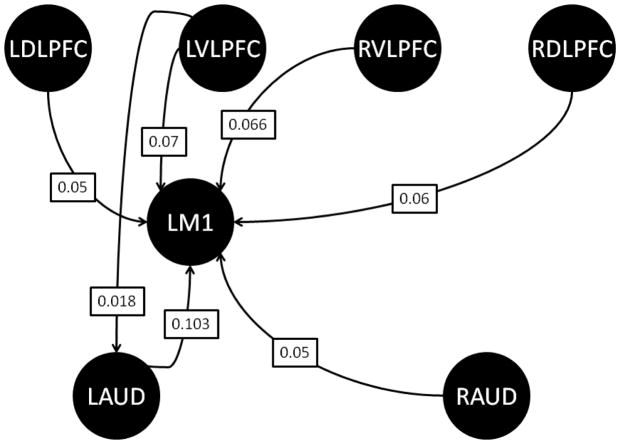
Analyses of intrinsic connectivity are valuable because they provide evidence as to the basic configuration of relationships among brain regions during task performance. The average intrinsic connectivity results summarized across all subjects (Figure 2; Table 2) showed that task performance, regardless of task phase, resulted in a network of excitatory connections between all the prefrontal cortical regions and the left motor cortex. The only significant top-down influence to either of the auditory cortices was an excitatory connection between the left ventrolateral prefrontal cortex and the left primary auditory cortex. Additional significant excitatory connections also were observed between the left primary auditory cortex and the left primary motor cortex, and the right auditory cortex and left motor cortex. While no formal hypotheses were made concerning the top-down and bottom-up connections into the right primary motor cortex, our model did show that this region displayed similar intrinsic connectivity to that of the left primary motor cortex. However, as will be discussed more fully below for the left primary motor cortex, no statistically significant task-dependent modulations were observed for either any of the top-down connections from the four prefrontal regions of interest to the right primary motor cortex or the two bottom-up connections from the left and right primary auditory cortices.
Figure 2.
Diagram of baseline, intrinsic connectivity results summarized across all subjects. Only those paths surviving Bonferoni correction across all 14 paths under consideration are shown. Path weight values are given in the boxes superimposed over the path arrows. LDLPFC: left dorsolateral prefrontal cortex. LVLPFC: left ventrolateral prefrontal cortex. RDLPFC: right dorsolateral prefrontal cortex. RVLPFC: right ventrolateral prefrontal cortex. LM1: left primary motor cortex. LAUD: left primary auditory cortex. RAUD: right primary auditory cortex.
Table 2.
Intrinsic, baseline connectivity path weight values. Mean path weights (and standard deviations) are listed for each path with statistical significance surviving Bonferoni correction at p < 0.05 across all 14 paths under consideration. The associated t-score and p-value represent the results from the 1-sample t-tests conducted across all subjects. Paths are labeled with the originating region first and the terminating region second (i.e., LAud – LM1 indicates the path from the left primary auditory cortex to the left primary motor cortex). Paths not surviving correction for multiple comparisons are indicated by “ --“ in the p-value column.
| Path | mean | std | T | p |
|---|---|---|---|---|
|
| ||||
|
Intrinsic
|
||||
| LAud – LM1 | 0.1028 | 0.1096 | 6.293 | 1.25e-7 |
| RAud – LM1 | 0.0531 | 0.1023 | 3.481 | 0.0011 |
| LDLFPC - LAud | -- | |||
| LDLPFC – RAud | -- | |||
| LDLFPC – LM1 | 0.0528 | 0.0527 | 6.717 | 2.98e-8 |
| RDLPFC – LAud | -- | |||
| RDLPFC – RAud | -- | |||
| RDLPFC – LM1 | 0.0587 | 0.0628 | 6.277 | 1.32e-7 |
| LVLPFC – LAud | 0.0175 | 0.0346 | 3.380 | 0.0015 |
| LVLPFC – RAud | -- | |||
| LVLPFC – LM1 | 0.0674 | 0.0622 | 7.269 | 4.62e-9 |
| RVLPFC - LAud | -- | |||
| RVLPFC – RAud | -- | |||
| RVLPFC – LM1 | 0.0657 | 0.0565 | 7.804 | 7.72e-10 |
Task Dependent Modulations
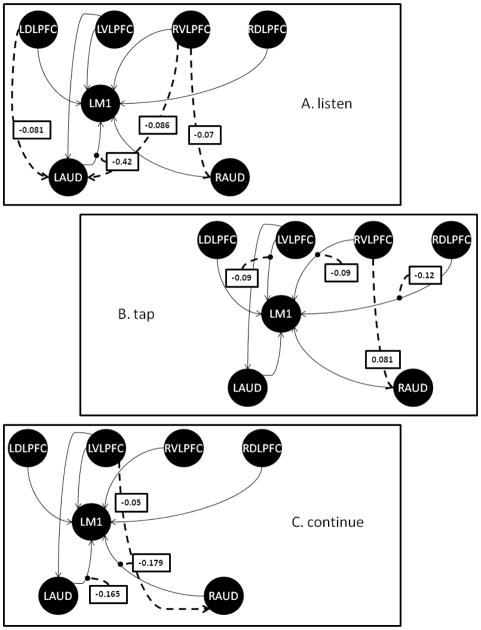
The primary study aim hypotheses were evaluated by testing whether presumed top-down or bottom-up intrinsic connections involving the prefrontal cortex were modulated by different task conditions. DCM results for these task-dependent modulations are summarized in Figures 3A–C and Table 3, noting that in cases where there was no significant task-dependent modulation of a path, the baseline, intrinsic path weight of that path (Figure 2, Table 2) still applies.
Figure 3.
Path diagrams for the three modulatory task conditions: A. Listen, B. Tap, C. Continue. Baseline connectivity that remained unmodulated by the respective task phase is indicated by the thin solid arrows, while task-dependent modulations are shown by thick, dashed arrows. The values given in the boxes represent the modulatory value and NOT the overall net path weight. While only modulatory values for significantly modulated paths are indicated, we note that the path weights summarized in Figure 1 for the baseline, intrinsic connectivity still apply for any unmodulated paths (thin solid arrows).
Table 3.
Modulatory path values for the three task phases. The mean modulatory path values listed are those exhibiting statistical significance surviving Bonferoni correction a p < 0.05 across all 14 paths. The associated t-score and p-value represent the results from the 1-sample t-tests conducted across all subjects. Paths are labeled with the originating region first and the terminating region second (i.e., LAud – LM1 indicates the path from the left primary auditory cortex to the left primary motor cortex). Paths not surviving correction for multiple comparisons are indicated by “ --“ in the p-value column.
| Path | mean | std | T | p | mean | std | T | p | mean | std | T | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Listen | Tap | Continue | ||||||||||
|
|
||||||||||||
| LAud – LM1 | −0.4202 | 0.5648 | −4.991 | 9.95e-6 | -- | −0.1647 | 0.2294 | −4.816 | 1.77E-5 | |||
| RAud – LM1 | -- | -- | −0.1789 | 0.2262 | −5.306 | 3.49E-6 | ||||||
| LDLFPC - LAud | −0.0807 | 0.1701 | −3.185 | 0.0027 | -- | -- | ||||||
| LDLPFC – RAud | -- | -- | -- | |||||||||
| LDLFPC – LM1 | -- | -- | -- | |||||||||
| RDLPFC – LAud | -- | -- | -- | |||||||||
| RDLPFC – RAud | -- | -- | -- | |||||||||
| RDLPFC – LM1 | -- | −0.1181 | 0.2315 | −3.422 | 0.0014 | -- | ||||||
| LVLPFC – LAud | -- | -- | -- | |||||||||
| LVLPFC – RAud | -- | -- | −0.0528 | 0.1060 | −3.341 | 0.0017 | ||||||
| LVLPFC – LM1 | -- | −0.0924 | 0.1526 | −4.063 | 0.0002 | -- | ||||||
| RVLPFC - LAud | −0.0855 | 0.1468 | −3.908 | 0.0003 | -- | -- | ||||||
| RVLPFC – RAud | −0.0703 | 0.1501 | −3.141 | 0.003 | −0.0806 | 0.1687 | 3.206 | 0.0025 | -- | |||
| RVLPFC – LM1 | -- | −0.0942 | 0.1209 | −5.229 | 4.50e-6 | -- | ||||||
Listen
During the listen condition, inhibitory connections (i.e., negative DCM coefficients) were newly established between the right ventrolateral prefrontal cortex and both left and right auditory cortices, as well as between the left dorsolateral prefrontal cortex and the left auditory cortex, that were otherwise not present in the baseline intrinsic connectivity. Significant negative modulation of the connection between the left auditory cortex and left motor cortex also was noted. No other paths exhibited significant modulation by the listen condition.
Tap
During the tap condition, negative modulation of the connections between the left ventrolateral, right ventrolateral, and right dorsolateral prefrontal cortices and the left primary motor cortex was observed, effectively extinguishing these three top-down paths. A significant excitatory connection between right ventrolateral prefrontal cortex and right auditory cortex regions (not found in the intrinsic baseline connectivity) also was observed. None of the other paths exhibited significant modulation from its baseline state during the tap condition.
Continue
During the continue condition, we observed an inhibitory connection between the left ventrolateral prefrontal cortex and the right auditory cortex that was not otherwise present in the baseline intrinsic connectivity. Additionally, significant negative modulation of connections between the left auditory cortex and left motor cortex, and right auditory cortex and left motor cortex was noted. No other path was significantly modulated during the continue condition.
Behavioral Data
The average inter-tap interval across all subjects was found to be mean (SD) = 1.38 (0.04) seconds for the tap condition and mean (SD) = 1.45 (0.07) for the continue condition. We noted a significant between-condition effect (F(1,43) = 39.99; p < 1.234 × 10−7; ηp2 = 0.482), indicating that performance during the continue task was significantly worse than during the tap condition. No significant overall multivariate effect for the relationship of inter-tap interval to DCM coefficients was observed for either the tap or continue condition. The average deviation across all subjects from the pacing stimulus during the tap condition was found to be mean (SD) = 0.112 (0.057) seconds. For the continue condition, the average deviation from the theoretical pacing stimulus was found to be mean (SD) = 0.358 (0.061) seconds. Again we noted a significant between-condition effect indicating that continue performance was worse than tap performance (F(1,43) = 311.0; p < 2.66 × 10−21; ηp2 = 0.879) and suggesting that our supposition that deviation would be a more sensitive measure of absolute performance accuracy was with merit. We identified a significant overall multivariate effect of average deviation from the pacing rate during the continue condition on path weight (F(14,29) = 2.541; p < 0.017). The results of post hoc tests revealed that several paths exhibited significant relationships with average deviation during the continue condition, including left auditory cortex to left primary motor cortex (LAud-LM1: F(1,42) = 5.19; p < 0.03), left dorsolateral prefrontal cortex to left motor cortex (LDLPFC-LM1: F(1,42) = 4.50; p < 0.04), left ventrolateral prefrontal cortex to left auditory cortex (LVLPFC-LAud: F(1,42) = 5.64; p < 0.02), and left ventrolateral prefrontal cortex to left motor cortex (LVLPFC-LM1: F(1,42) = 3.54; p < 0.06). In support of our hypothesis that the continue condition would rely more on top-down control, results from the regression analysis on these four paths showed increasing average deviation (i.e., worse performance) was paralleled by decreasing path weights between LDLPFC-LM1 (β = −0.311), LVLPFC-Laud (β = −0.344), and LVLPFC-LM1 (β = −0.28), while the path between LAud-LM1 (β = 0.331) exhibited an increasing path weight in relation to increasing average deviation from the pacing rate. No overall multivariate effect was observed for the tapping condition.
Developmental Effects
Contrary to our hypotheses, we did not observe any significant developmental effects across either the behavioral data or DCM path weight data, regardless of behavioral measure or task condition.
DISCUSSION
In a sample of 45 healthy adolescents and adults, we have demonstrated that sensorimotor timing, as measured using a simple paced auditory finger-tapping task, elicited DCM-measured evidence for top-down neural control of both the primary auditory and motor cortices by prefrontal cortex regions. Additionally, the target regions of this top-down control changed depending on the exact task requirements in ways that have important implications for our theoretical understanding of the neural basis of mental timekeeping. The main findings of this study were four-fold. First as expected, the roles of specific brain regions and exactly how they showed causal profiles consistent with top-down and bottom-up control depended on the task condition (listen, tap, continue). Second, top-down control for this form of mental timekeeping appeared to be relatively lateralized to the right hemisphere, while poorer behavioral performance during the continue phase was linked specifically to presumably less effective reliance on bottom-up control in the left hemisphere. Third, in relation to existing theoretical models of internal clock systems and temporal perception, our results suggest that brain regions most consistent with the proposed function of both accumulator and comparator mechanisms (e.g., the dorsal and ventral prefrontal cortices) were the brain regions that exerted top-down control. Finally, no overall age-related effects were observed, either in the behavior data or in the DCM-measured network connectivity data. Each of these findings will be discussed in turn.
Task performance configured the brain regions we examined into a functional network that had countervailing top-down and bottom-up influences over primary motor cortex behavior, whose exact configuration depended greatly on the dominant cognitive demands of each task condition. Passive listening (listen condition) elicited additional top-down control not observed in the intrinsic connectivity measurements. Specifically, right ventrolateral prefrontal cortex over both left and right auditory cortices and left dorsolateral prefrontal cortex over left auditory cortex pathways were modulated for passive listening to a regularly-paced auditory cue. Presumably, this additional top-down control of the auditory cortices served to bias these regions for processing the pacing stimulus, consistent with theories of attention (Knudsen 2007). We interpreted this pattern as reflecting simple attention to regularly paced auditory cues, possibly including the anticipation of and preparation for using this information to guide future behavior. However, the pattern of connectivity did not indicate any overt integration of this auditory information with the motor system via top-down mechanisms engaged for directed listening. During the synchronized tapping phase (tap condition), any top-down control of either the primary auditory or motor cortices was virtually extinguished in favor of a bottom-up, sensory driven network configuration. In other words, the causal influences within the system were consistent with facilitating the ability of the auditory information to more directly guide motor output in the absence of prefrontal excitatory connections over motor cortex. Finally, the continuation phase of the task (continue condition) represented an almost reversal of the synchronized tapping condition, with significant task-related prefrontal inhibition of the bottom-up influences of auditory cortex to the primary motor cortex. The network configuration during the continuation phase was largely consistent with current theories of cognitive control, relying more on top-down control of motor function by prefrontal regions than on the bottom-up control observed during the tapping phase. However, given the narrow focus of our model, we can only speculate that these top-down causal influences over the motor cortex were dominant compared with other, unmodeled influences over the motor cortex. Post hoc tests to examine potential magnitude differences in path weights between task conditions – arguably an important concept for making inferences about the relative importance of each task-related modulation – were largely uninformative, as only one path (from left primary auditory cortex to left primary motor cortex) exhibited similar inhibitory modulation across task conditions. No specific conclusions could be drawn as to whether any single path played a more important role in one task condition compared to another (e.g., was the connection between two regions significantly more inhibitory in the tap condition versus the continue condition).
Contrary to our hypothesis that top-down control would equally involve all four prefrontal regions included in our model, we instead found evidence for a predominant influence of one prefrontal region in timekeeping task performance. Right ventrolateral prefrontal cortex was most often significantly engaged in exerting top-down control over both auditory and motor cortices, showing task condition-specific modulations for both listen and tap. Previous fMRI studies suggest that supra-second timing tasks, such as that used in this study, recruit right prefrontal cortex regions more than sub-second (Smith et al. 2011; Wiener et al. 2010). A recent meta-analysis also found this right prefrontal hemispheric preference between sub- and supra-second timing, linking both right inferior frontal gyrus and right middle frontal gyrus to supra-second temporal perception and sensorimotor timing tasks (Wiener et al. 2010). Our study extended these results by showing that the greater activation in these right frontal regions found in previous studies likely did in part represent top-down control we observed in right ventrolateral prefrontal cortex, in terms of its influence over both the auditory and motor cortices during the listen and tap conditions, respectively. It may be that this top-down control serves to bias the auditory cortices to pacing stimulus during the listen condition and the motor cortex during the tapping condition, akin to models of auditory attention proposed by (Fritz et al. 2007) and visual attention proposed by (Knudsen 2007). While other theoretical interpretations most certainly exist (e.g., Corbetta and Shulman 2002) proposed a dual network system), most theoretical models of cognitive top-down control are grounded in this idea of the biasing of lower-level brain regions by higher-level regions. The results of our study seem to be consistent with this theoretical model of cognitive top-down control, whereby the causal influence exerted by the right ventrolateral prefrontal cortex could be interpreted as biasing both auditory cortices to attend the pacing stimulus during the listen condition and biasing the right auditory cortex to attend the pacing stimulus during the tap condition.
While our results echo those of previous studies in suggesting a key role for the right ventrolateral prefrontal cortex in the successful performance of sensorimotor timing tasks, we also found left dorsolateral prefrontal cortex and left ventrolateral prefrontal cortex played important roles in top-down control for the listen and continue condition as well. This suggests, at least in terms of DCM-measured effective connectivity, this hemispheric/temporal scale distinction proposed previously (Smith et al. 2011; Wiener et al. 2010) may not be so clear-cut. Of note was that behavioral performance measure considering average deviation from the pacing stimulus correlated more with these top-down paths in the left hemisphere. Results from the multivariate analysis showed that participants who had trouble maintaining a high degree of accuracy during the continue condition relied more on (i.e., had stronger connectivity) the bottom-up connection from the left auditory cortex than the top-down connections from the left dorsolateral and ventrolateral prefrontal cortices. In contrast, there were no significant multivariate effects between inter-tap interval (e.g., the ability to maintain the prescribed tapping rate) and the connectivity results. The association of performance accuracy with the connectivity results suggests that top-down control in sensorimotor timing may be related more to working memory functioning and less to the integrity of the internal clock mechanism. The significant relationships between behavioral performance and changes in relative top-down and bottom-up path strengths in regions in the left hemisphere also indicate that, while right prefrontal regions may be key to the successful execution of these sorts of temporal processing tasks, top-down control from both hemispheres is necessary for optimal performance. Prior working-memory studies have noted a dissociation between activity in the left and right prefrontal cortices depending on task context or stimulus type (e.g., Cabeza et al. 2003; Opitz et al. 2000), suggesting that both left and right prefrontal cortices can be simultaneously engaged in different aspects of the same task. A similar dissociation may also exist for performance of sensorimotor timing tasks, such that the connectivity between the right prefrontal cortex and the motor and auditory cortices overtly controls performance, while top-down control between the left prefrontal cortex and motor and auditory cortices appears to determine how successfully one will perform the task. So, while top-down control originating in the right prefrontal cortex might be sufficient to achieve basic sensorimotor timing, top-down control originating in the left prefrontal cortex appears necessary to ensure optimal performance. Accordingly, insufficient top-down control from the left prefrontal cortex appears to result in difficulty in maintaining the prescribed tapping pace. Future studies should confirm and further describe the nature of this apparent dissociation, as well as examine whether sensorimotor timing performance correlates with connectivity among brain regions not considered in our model (e.g., striatum). Overall, the results from the multivariate behavioral analyses suggest that top-down control from both left and right prefrontal cortices is vital to temporal processing, and that bottom-up connectivity between primary sensory and motor regions is inadequate in performing paced finger-tapping in the absence of an external cue.
Mental timekeeping conceptual models take into account frontal, top-down contributions in the guise of the accumulator and comparator (e.g., Matell and Meck 2004; Meck and Benson 2002; Wing 2002), and our results appear to support this notion. The accumulator is often framed in terms of working memory and the comparator in terms of decision making, both of which are known to engage the lateral prefrontal cortex regions examined in this study (e.g., Tanji and Hoshi 2008; Wager and Smith 2003). Working memory and top-down control have been shown to share what can be best described as a highly integrated relationship, where top-down control regulates what information gains access to working memory stores and information held in working memory regulates how top-down control biases lower-level sensory and association regions to relevant aspects of incoming information (Knudsen 2007). While it may be tempting to characterize the top-down control observed during the listen condition as that exerted by the accumulator and that observed during the continue condition as the comparator, our fMRI methods, like many others (e.g., Jantzen et al. 2007; Smith et al. 2011), did not allow us to dissociate the working memory contributions of the accumulator and the executive decision making of the comparator in these lateral prefrontal regions. While other temporal discrimination studies have proposed a role for the lateral prefrontal cortex in facilitating the encoding of the pacing of rhythmic sequences into working memory (Buhusi and Meck 2005; Teki et al. 2011) only a single previous fMRI study on temporal perception has provided some direct evidence for engagement of the left and right inferior frontal gyri in accumulator functions and the left inferior frontal gyrus in comparator functions (Wencil et al. 2010). This, to a certain extent, parallels what we observed with our sensorimotor timing task. Given that the continue phase of the fMRI task was the most cognitively demanding, it is probable that both working memory (in the guise of ongoing access to information held in short term storage) and executive decision making functions of the lateral prefrontal cortices were engaged during the continue task condition. As it was beyond the scope of this study, we chose not to consider the role of the theoretical clock mechanism in this study of the neural network correlates of top-down control. Furthermore, due to the limitation of the number of estimable regions allowed by DCM, it was not feasible to include striatal regions in our bi-hemispherical model of prefrontal and primary motor and sensory regions, which could be hypothesized as the most likely candidate for a regionally-specialized central clock neural mechanism based upon previous work (e.g., Meck and Benson 2002; Wiener et al. 2010). It might be informative for future studies to build upon specific aspects of effective connectivity relationships we describe by extending the models tested here to new ones that purposefully test how such striatal “clock” regions influence or otherwise alter our understanding of top-down prefrontal control over motor and auditory function during sensorimotor timing.
There were no age-related effects in our results, either in behavioral performance or causal interactions among brain regions assessed. Our behavioral results were in line what others have previously shown, with behavioral data in older children and adults performing a similar sensorimotor timing task to ours (McAuley et al. 2006) and behavioral data in older children/adolescents and adults performing a temporal discrimination task (Smith et al. 2011). However, in at least one previous report age-related increases in BOLD signal were found in left dorsolateral prefrontal cortex and left inferior frontal gyrus (Smith et al. 2011). Additional studies have noted age-related performance differences in younger children (e.g., Greene and Williams 1993), but an extensive literature search yielded very few “timekeeping” studies that specifically included adolescents. The lack of observed further age-related increases in performance metrics beyond late childhood suggests that the cognitive abilities underlying mental timekeeping develop to near-adult levels before adolescence, making it perhaps one of the earlier cognitive abilities engaging prefrontal regions to mature. The DCM results presented here appear to confirm this, and add further evidence that while connectivity among more functionally disparate regions is still maturing during adolescence in other paradigmatic contexts (Fair et al. 2009; Fair et al. 2007), connectivity between frontal and more lower-level, primary sensory and motor regions appears to be largely in place by adolescence. A single functional connectivity study that examined age-related gains found adults exhibited increased connectivity between a large cluster comprising the right ventrolateral prefrontal cortex/insula/caudate/thalamus and both a cluster in the right inferior parietal lobe and a cluster comprising the left inferior frontal cortex/SMA/caudate compared with adolescents (Smith et al. 2011), suggesting that any age-related developmental gains in connectivity may be occurring between frontal and more higher-level regions, such as the parietal lobe and secondary motor regions. These regions were outside the model used in this study. As Dynamic Causal Modeling is limited to models with eight or fewer regions, replicating whether these findings of altered connectivity between frontal and parietal regions observed for a temporal discrimination task could be generalized to other mental timekeeping tasks (such as the sensorimotor timing task employed in this study) was outside of our purview of considering top-down control of primary sensory and motor regions. Alternatively, it could be that our smaller sample size meant that we were only sensitive to medium to large effects, whereas the developmental effects we hypothesized might be more moderate. Beyond confirming our results in terms of the near-adult levels of top-down control during sensorimotor timing, future studies should also look to examine these effects in younger participants to better capture the developmental trajectory of top-down control over lower-level sensory and motor regions.
CONCLUSIONS
We have demonstrated that brain activation profiles consistent with top-down control exist in sensorimotor timing, adding to our theoretical understanding of how mental timekeeping is instantiated in the brain. The cognitive top-down control proposed in these theoretical models involves varying excitatory and inhibitory influences in both left and right prefrontal cortices, with an apparent hemispherical dissociation in how the prefrontal cortex engages in top-down control during sensorimotor timing that relates to performance individual differences. In line with previous studies, we found that the right prefrontal cortex was specifically engaged for neural control over overt task performance, as both right dorso- and ventrolateral prefrontal cortices exhibited task condition-dependent modulatory changes in connectivity. However, we also found that top-down connectivity originating in the left prefrontal cortex was also key in guiding successful task performance, as reduced top-down connectivity between left prefrontal regions and lower-level sensory and motor regions correlated with worse behavioral performance accuracy during the continue condition. While we did not observe any age-related developmental changes either in the behavioral or connectivity data, future studies should both confirm our absence of adolescent developmental effects, as well as examine younger children to determine what, if any, changes in neural top-down control occurs during early life development.
Supplementary Material
Contributor Information
Suzanne T. Witt, Email: stwitt@harthosp.org.
Michael C. Stevens, Email: msteven@harthosp.org.
References
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. nrn1764 [pii] [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39(6):855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J Cogn Neurosci. 2003;15(2):249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat Neurosci. 2011;14(7):830–832. doi: 10.1038/nn.2823. nn.2823 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Grefkes C, Eickhoff SB. Dynamic interactions in the fronto-parietal network during a manual stimulus-response compatibility task. Neuroimage. 2011;58(3):860–869. doi: 10.1016/j.neuroimage.2011.05.089. S1053-8119(11)00636-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier GL, Ogden RT. Adding drift to the decomposition of simple isochronous tapping: an extension of the Wing-Kristofferson model. J Exp Psychol Hum Percept Perform. 2004;30(5):853–872. doi: 10.1037/0096-1523.30.5.853. 2004-18791-006 [pii] [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. nrn755 [pii] [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget’s AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res. 1989;74(1):24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cogn Psychol. 1996;30(3):257–303. doi: 10.1006/cogp.1996.0008. S0010-0285(96)90008-0 [pii] [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. 0705843104 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for Axis I DSM-IV disorders. New York: Biometrics Research; 1994. [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14(3):709–722. doi: 10.1006/nimg.2001.0869. S1053-8119(01)90869-9 [pii] [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21(5):470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Bayesian estimation of dynamical systems: an application to fMRI. Neuroimage. 2002;16(2):513–530. doi: 10.1006/nimg.2001.1044. S1053811901910444 [pii] [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [pii] [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ. Generative models, brain function and neuroimaging. Scand J Psychol. 2001;42(3):167–177. doi: 10.1111/1467-9450.00228. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention--focusing the searchlight on sound. Curr Opin Neurobiol. 2007;17(4):437–455. doi: 10.1016/j.conb.2007.07.011. S0959-4388(07)00094-3 [pii] [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20(2):1132–1139. doi: 10.1016/S1053-8119(03)00334-3. S1053811903003343 [pii] [DOI] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Friston KJ. Dynamic causal modelling of evoked potentials: a reproducibility study. Neuroimage. 2007;36(3):571–580. doi: 10.1016/j.neuroimage.2007.03.014. S1053-8119(07)00227-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychological Review. 1977;84(3):279. [Google Scholar]
- Greene LS, Williams HG. Age-related differences in timing control of repetitive movement: application of the Wing-Kristofferson model. Res Q Exerc Sport. 1993;64(1):32–38. doi: 10.1080/02701367.1993.10608776. [DOI] [PubMed] [Google Scholar]
- Guye M, Bartolomei F, Ranjeva JP. Imaging structural and functional connectivity: towards a unified definition of human brain organization? Curr Opin Neurol. 2008;21(4):393–403. doi: 10.1097/WCO.0b013e3283065cfb. 00019052-200808000-00003 [pii] [DOI] [PubMed] [Google Scholar]
- Hare TA, Schultz W, Camerer CF, O’Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proc Natl Acad Sci U S A. 2011;108(44):18120–18125. doi: 10.1073/pnas.1109322108. 1109322108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Oullier O, Marshall M, Steinberg FL, Kelso JA. A parametric fMRI investigation of context effects in sensorimotor timing and coordination. Neuropsychologia. 2007;45(4):673–684. doi: 10.1016/j.neuropsychologia.2006.07.020. S0028-3932(06)00317-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JA. Brain networks underlying human timing behavior are influenced by prior context. Proc Natl Acad Sci U S A. 2004;101(17):6815–6820. doi: 10.1073/pnas.0401300101. 0401300101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JA. Functional MRI reveals the existence of modality and coordination-dependent timing networks. Neuroimage. 2005;25(4):1031–1042. doi: 10.1016/j.neuroimage.2004.12.029. S1053-8119(04)00787-6 [pii] [DOI] [PubMed] [Google Scholar]
- Kasess CH, Stephan KE, Weissenbacher A, Pezawas L, Moser E, Windischberger C. Multi-subject analyses with dynamic causal modeling. Neuroimage. 2010;49(4):3065–3074. doi: 10.1016/j.neuroimage.2009.11.037. S1053-8119(09)01221-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. S0890-8567(09)62555-7 [pii] [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Wing AM, Pope PA, Praamstra P, Miall RC. Brain activity correlates differentially with increasing temporal complexity of rhythms during initialisation, synchronisation, and continuation phases of paced finger tapping. Neuropsychologia. 2004;42(10):1301–1312. doi: 10.1016/j.neuropsychologia.2004.03.001. S0028393204000624 [pii] [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21(2):139–170. doi: 10.1016/j.cogbrainres.2004.06.012. S0926-6410(04)00169-7 [pii] [DOI] [PubMed] [Google Scholar]
- McAuley JD, Jones MR, Holub S, Johnston HM, Miller NS. The time of our lives: life span development of timing and event tracking. J Exp Psychol Gen. 2006;135(3):348–367. doi: 10.1037/0096-3445.135.3.348. 2006-09007-002 [pii] [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3(3–4):227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. S0278262601913132 [pii] [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. 24/1/167 [pii] [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Thaut MH. The cerebellum and neural networks for rhythmic sensorimotor synchronization in the human brain. Cerebellum. 2007;6(1):18–23. doi: 10.1080/14734220601142886. 772876729 [pii] [DOI] [PubMed] [Google Scholar]
- Neumann J, Lohmann G. Bayesian second-level analysis of functional magnetic resonance images. Neuroimage. 2003;20(2):1346–1355. doi: 10.1016/S1053-8119(03)00443-9. S1053811903004439 [pii] [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, Friederici AD. Functional asymmetry of human prefrontal cortex: encoding and retrieval of verbally and nonverbally coded information. Learn Mem. 2000;7(2):85–96. doi: 10.1101/lm.7.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok B, Gross J, Muller K, Aschersleben G, Schnitzler A. The cerebral oscillatory network associated with auditorily paced finger movements. Neuroimage. 2005;24(3):646–655. doi: 10.1016/j.neuroimage.2004.10.009. S1053-8119(04)00611-1 [pii] [DOI] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17(14):5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011;55(3):1147–1158. doi: 10.1016/j.neuroimage.2011.01.014. S1053-8119(11)00032-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repp BH. Sensorimotor synchronization: a review of the tapping literature. Psychon Bull Rev. 2005;12(6):969–992. doi: 10.3758/bf03206433. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp (Wars) 2004;64(3):329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14(5):721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Smith AB, Giampietro V, Brammer M, Halari R, Simmons A, Rubia K. Functional development of fronto-striato-parietal networks associated with time perception. Front Hum Neurosci. 2011;5:136. doi: 10.3389/fnhum.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. Neuroimage. 2010;49(4):3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. S1053-8119(09)01199-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Hum Brain Mapp. 2007;28(5):394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88(1):37–57. doi: 10.1152/physrev.00014.2007. 88/1/37 [pii] [DOI] [PubMed] [Google Scholar]
- Teki S, Grube M, Kumar S, Griffiths TD. Distinct neural substrates of duration-based and beat-based auditory timing. J Neurosci. 2011;31(10):3805–3812. doi: 10.1523/JNEUROSCI.5561-10.2011. 31/10/3805 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell P, Junque C, Pujol J, Jurado MA, Molet J, Grafman J. The role of prefrontal regions in the Stroop task. Neuropsychologia. 1995;33(3):341–352. doi: 10.1016/0028-3932(94)00116-7. [pii] [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wencil EB, Coslett HB, Aguirre GK, Chatterjee A. Carving the clock at its component joints: neural bases for interval timing. J Neurophysiol. 2010;104(1):160–168. doi: 10.1152/jn.00029.2009. jn.00029.2009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub P, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49(2):1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. S1053-8119(09)01063-5 [pii] [DOI] [PubMed] [Google Scholar]
- Wing AM. Voluntary timing and brain function: an information processing approach. Brain Cogn. 2002;48(1):7–30. doi: 10.1006/brcg.2001.1301. S0278262601913016 [pii] [DOI] [PubMed] [Google Scholar]
- Wing AM, Kristofferson A. The timing of interresponse intervals. Attention, Perception, & Psychophysics. 1973;13(3):455–460. [Google Scholar]
- Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42(1):343–356. doi: 10.1016/j.neuroimage.2008.04.025. S1053-8119(08)00323-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.