Abstract
Vertebrovertebral fistulae are rare vascular malformations that uncommonly can rupture to present clinically as intracranial subarachnoid hemorrhage. We present a 69-year-old man presenting following spontaneous apoplectic collapse. Initial workup revealed diffuse, intracranial subarachnoid hemorrhage, intraventricular hemorrhage, and hydrocephalus. However, the etiology was not apparent on CT angiography of the head. Catheter-based angiography was performed, demonstrating a single-hole, high-flow vertebrovertebral fistula, arising from the V2 segment and decompressing into both cervical and skull base venous structures. Definitive treatment consisted of endovascular fistula obliteration with a combination of coil and liquid embolic material. The patient made a full neurological recovery. High cervical and skull base fistulae are rare causes of intracranial hemorrhage; endovascular treatment is effective at disconnection of the arteriovenous shunt.
Keywords: Cervical, Endovascular, Hydrocephalus, Stroke, Subarachnoid, Vertebral artery, Vertebrovertebral fistula
1. Introduction
Arteriovenous fistulae (AVF) are vascular malformations typified by direct arterial-to-venous connections without an intervening nidus.1 Spinal AVF are classified by location of the arterio-venous shunt, and include perimedullary and dural types. Dural AVF (DAVF) comprise the majority of these lesions, and in the spine are most frequently found in the thoracolumbar region. DAVF have been reported in the cervical spine as a cause of congestive myelopathy and rarely subarachnoid hemorrhage (SAH).2,3 A distinct type of cervical spine AVF consists of a direct, single-hole fistula between the cervical segment of the vertebral artery and the adjacent vertebral venous plexus (also known as a vertebrovertebral fistula [VVF]). VVF are much less common than DAVF with causes ranging from non-traumatic (e.g., fibromuscular dysplasia, neurofibromatosis 1),4–6 to post-surgical,7 to post-traumatic.8,9 Variable presentations of VVF include cervical bruit, progressive myelopathy, cerebellar signs, and tinnitus.10–12 Here we describe an uncommon presentation of a rare entity, a VVF complicated by intracranial subarachnoid and intraventricular hemorrhage, as well as a novel multimodal treatment to disconnect the fistula.
2. Case report
2.1. History and examination
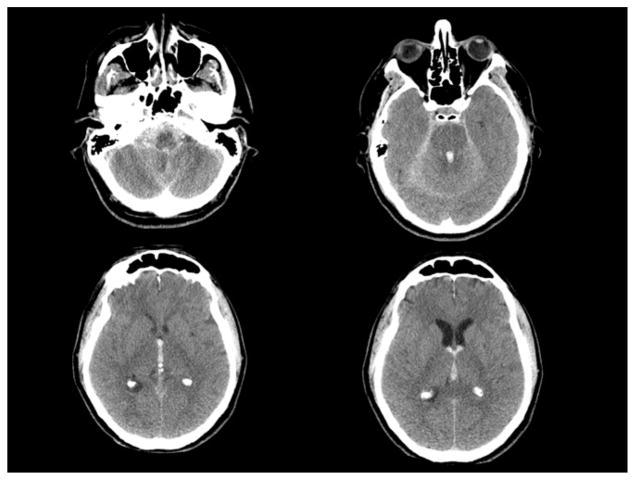
A 69-year-old man with past medical history significant only for mild hypertension presented with an abrupt loss of consciousness while playing tennis. An emergency department non-contrast head CT scan revealed diffuse SAH with intraventricular hemorrhage predominantly within the third and fourth ventricles. Prominence of the lateral and third ventricles was consistent with acute, obstructive hydrocephalus. CT angiography of the head revealed no apparent vascular abnormality to account for his hemorrhage (Fig. 1).
Fig. 1.
Axial CT scan of the head on presentation showing diffuse subarachnoid hemorrhage concentrated in the posterior fossa and foramen magnum. Intraventricular hemorrhage is most prominent in the fourth ventricle, third ventricle, extending into the bilateral lateral ventricles through the foramens of Monro.
On exam, he was comatose and intubated, with no eye opening to pain. He withdrew all four extremities to painful stimuli. A right frontal external ventriculostomy was placed to manage his intracranial hypertension. Given the suspicion for an occult vascular malformation, a catheter-based diagnostic cerebral angiogram was performed urgently.
2.2. Surgery
Diagnostic angiography revealed a single-hole, high-flow arteriovenous fistula involving the distal V2 segment of the vertebral artery and decompressing into the adjacent cervical venous plexus with further venous drainage intracranially into the skull base venous structures (Figs. 2 and 3). Informed consent for endovascular treatment of this lesion was then obtained from the patient’s healthcare proxy, including potential “off-label” use of liquid embolic agents. Using an arterial microcatheter, the fistula was selectively catheterized and the microcatheter advanced into the venous compartment. A total of 19 microcoils were then deployed sequentially. The vein draining superiorly into the skull base region was first occluded, with subsequent coiling more inferiorly to occlude the recipient venous pouch. Proximal venous outflow persisted, and therefore Onyx-34 (eV3 Neurovascular, Inc., Irvine, California, USA) liquid embolic agent was delivered using a thumb-tapping technique for a total volume of 0.6 mL. The Onyx-34 (eV3 Neurovascular, Inc.) penetrated into the coil construct. Repeat angiography at the conclusion of the procedure demonstrated no further filling of the fistulous connection.
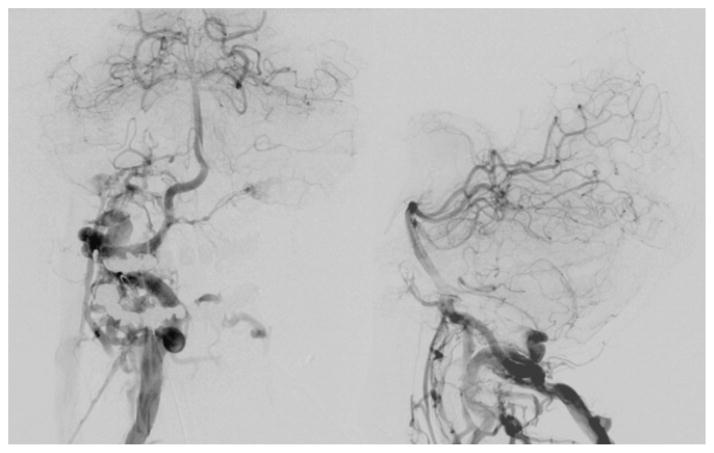
Fig. 2.
Initial angiogram (right vertebral injection) showing rapid arteriovenous shunting from the distal V-2 segment of the right vertebral artery through a single hole fistula draining into the high cervical venous structures. Late arterial phase images (left: anterioposterior projection, right: lateral projection) showing early and prominent opacification of the right suboccipital, paravertebral, clival (including contralateral), and epidural veins, as well as the marginal and condylar sinuses.
Fig. 3.
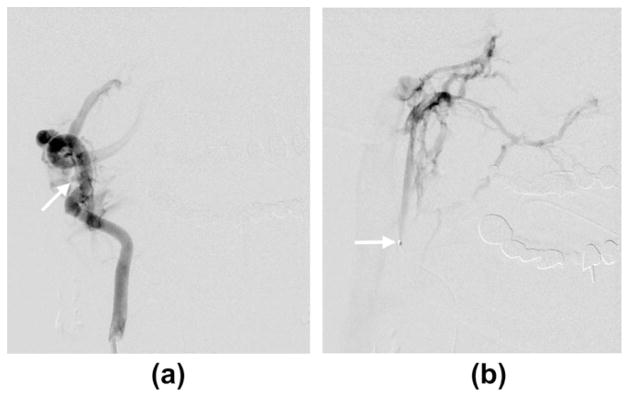
(a) Anteroposterior oblique view of right vertebral artery angiogram showing the fistulous connection (arrow). (b) The microcatheter traverses the fistula with its tip (arrow) in the venous compartment. Microcatheter injection showing ascending venous drainage into the skull base veins (magnified anterioposterior projection).
2.3. Postoperative course
Postoperatively, the patient recovered well, with successful external ventricular drain weaning over the course of several days. Upon discharge to inpatient rehabilitation examination was notable only for mild cognitive and executive-level deficits. Two weeks after discharge he developed communicating hydrocephalus and ventriculoperitoneal shunt placement was pursued, without complication. Follow-up clinic visits and repeat catheter-based angiography at 6 months demonstrated a return to baseline (normal) neurological function and a durable cure of his vertebral artery fistula (Figs. 4 and 5).
Fig. 4.
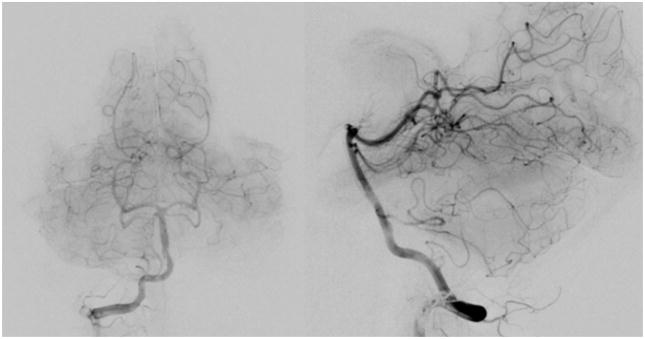
Final angiographic run following treatment showing complete obliteration of the fistula. Flow through the right vertebral artery was normal. (left: anterioposterior projection, right: lateral projection).
Fig. 5.
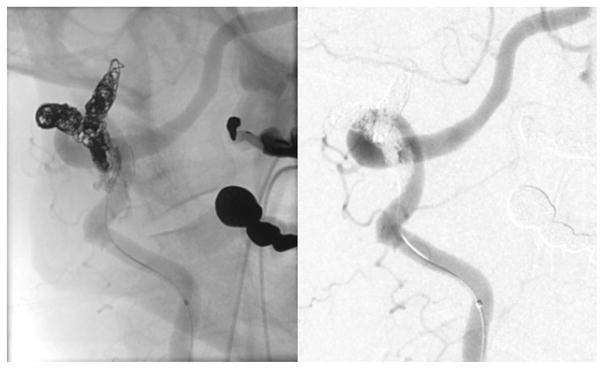
Magnified view of the right vertebral artery injection following treatment showing complete obliteration of the fistula after embolization with a combination of microcoils and liquid embolic material, appreciated best in the unsubtracted view (left: unsubtracted oblique projection, right: digital subtraction oblique projection).
3. Discussion
Vertebrovenous fistulae are rare single-hole fistulae between the cervical vertebral artery and the adjacent vertebral venous plexus. They most commonly produce a bruit, but are often asymptomatic. In the present case, there was no history of antecedent trauma, so it is probable that this fistula was spontaneous in origin and was asymptomatic for some time. Consistent with this idea is the location of the shunt at the C2 level, which is common for spontaneous VVF. More aggressive symptoms include vertebrobasilar insufficiency related to arterial steal in high-flow lesions, as well as radicular and spinal cord compression from dilated epidural veins. Less commonly, there may be reflux into the perimedullary veins resulting in congestive myelopathy. To our knowledge, this is the second report of intracranial hemorrhage associated with a vertebrovertebral fistula. The previous case was associated with trauma, and it is unclear whether the SAH was post-traumatic in nature or related to the VVF.13
The likely cause for intracranial bleeding in this case is the presence of ascending venous drainage from the VVF into the skull base veins. This mechanism has been noted in cases of SAH complicating cervicomedullary spinal AVF of the perimedullary and dural variety.14 Previously reported grading schema do not delineate a specific classification for lesions of the spine with intracranial drainage.15–19 Because of the rare nature of these lesions in the cervical region, it is unclear whether and to what degree this angiographic feature is a sign of elevated hemorrhage risk, but until more data are available, it is reasonable to consider this sign in deciding whether to treat.
Another important point is that SAH predominantly within the foramen magnum and the posterior fossa and without an identifiable cause on CT angiography should raise concern for a potential cervical etiology for the hemorrhage, and activate angiographic protocols appropriately. Specifically, angiography should include injections of both vertebral arteries (and possibly even bilateral subclavian artery injections if the clinical concern is high). As Do et al. have noted, the right vertebral artery is often not injected during angiographic workup of SAH if the left vertebral artery injection opacifies the origin of the right posterior inferior cerebellar artery and excludes an aneurysm in this location.2 This 3-vessel protocol ignores the possibility of a cervical AVF with supply from the right vertebral artery, as in our case.
Primary treatment of the fistula is aimed at the disconnection of the arteriovenous shunt with preservation of the parent vertebral artery if possible. This can be performed in most cases using an endovascular approach, and requires occlusion of the proximal venous pouch.20–22 A transarterial route is preferred, and occlusion may be achieved with detachable balloons (if available), coils and/or liquid embolic agent. The treatment approach that we employed (combination of coils and Onyx, eV3 Neurovascular, Inc.) has been described only recently in the setting of vertebrovertebral fistulae.11,23 In the three reported cases (including ours), successful obliteration of the VVF was achieved with preservation of the vertebral artery. There are several advantages of using Onyx (eV3 Neurovascular, Inc.), which is a non-adhesive liquid agent composed of ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide. It has a long working time, allowing for penetration and closure of the fistulous site. Moreover, guide catheter angiograms may be performed during injection to ensure that reflux of Onyx (eV3 Neurovascular, Inc.) does not threaten the parent artery. As in these three cases, the adjunctive use of coils is recommended because the coil construct serves as a scaffold around which the Onyx (eV3 Neurovascular, Inc.) may accumulate, allowing for focal accumulation of embolic material in the proximal venous pouch. In rare cases, open surgery is required.
Regardless of initial treatment modality, vigilance in angiographic follow-up is mandated. Even fistulae that are initially obliterated can demonstrate both short and long term recurrence. Artifact from clip or coil placement must be considered in anticipating the sensitivity of diagnostic imaging, and angiography remains the gold standard. In the case of surgical obliteration with clipping, angiography, including potentially intra-operative angiograms, may be useful to determine exact clip placement, fistula obliteration, and parent vessel architecture.24–27 Fistulae treated with embolization are of particular importance for close-interval follow-up given the phenomenon of recanalization through the coil mass. For this reason, we prefer to augment the coil construct with high-viscosity liquid embolic material. However, even with Onyx (eV3 Neurovascular, Inc.) treatment, recanalization can occur.28 With any treatment modality, recurrence can occur secondary to recruitment of alternate feeders.29
4. Conclusions
We report an unusual case of a vertebrovertebral fistula as a cause of subarachnoid and intraventricular hemorrhage. Cervical arteriovenous fistulae should be suspected in cases of SAH where the blood is predominantly in the region of the foramen magnum and there is no clear intracranial source on CT angiography. Complete diagnostic evaluation requires 4-vessel cervical and intracranial angiography, and treatment is best accomplished via endovascular embolization with surgery reserved for more difficult cases. Potential treatment for vasospasm and hydrocephalus are indicated with significant intracranial extension of hemorrhage. Post-treatment angiographic surveillance is necessary to confirm a durable cure.
Footnotes
Conflicts of interest/disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Rosenblum B, Oldfield EH, Doppman JL, et al. Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVM’s in 81 patients. J Neurosurg. 1987;67:795–802. doi: 10.3171/jns.1987.67.6.0795. [DOI] [PubMed] [Google Scholar]
- 2.Do HM, Jensen ME, Cloft HJ, et al. Dural arteriovenous fistula of the cervical spine presenting with subarachnoid hemorrhage. Am J Neuroradiol. 1999;20:348–50. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim MS, Han DH, Kwon OK, et al. Clinical characteristics of dural arteriovenous fistula. J Clin Neurosci. 2002;9:147–55. doi: 10.1054/jocn.2001.1029. [DOI] [PubMed] [Google Scholar]
- 4.Waga S, Otsubo K, Handa J, et al. Extracranial congenital arterio-venous malformations. Surg Neurol. 1974;2:241–5. [PubMed] [Google Scholar]
- 5.Markham JW. Spontaneous arteriovenous fistula of the vertebral artery and vein. Case report J Neurosurg. 1969;31:220–3. doi: 10.3171/jns.1969.31.2.0220. [DOI] [PubMed] [Google Scholar]
- 6.Sutton D, Pratt AE. Vertebral arterio-venous fistula. Clin Radiol. 1971;22:289–95. doi: 10.1016/s0009-9260(71)80075-2. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove GR, Theron J. Vertebral arteriovenous fistula following anterior cervical spine surgery. Report of two cases. J Neurosurg. 1987;66:297–9. doi: 10.3171/jns.1987.66.2.0297. [DOI] [PubMed] [Google Scholar]
- 8.Chou SN, French LA. Arteriovenous fistula of vertebral vessels in the neck. J Neurosurg. 1965;22:77–80. doi: 10.3171/jns.1965.22.1.0077. [DOI] [PubMed] [Google Scholar]
- 9.Elkin DC, Harris MH. Arteriovenous aneurysm of the vertebral vessels: report of ten cases. Ann Surg. 1946;124:934–49. [PMC free article] [PubMed] [Google Scholar]
- 10.Miralbes S, Cattin F, Andrea I, et al. Vertebral arteriovenous fistula: endovascular treatment with electrodetachable coils. Neuroradiology. 1998;40:761–2. doi: 10.1007/s002340050680. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Song D, Chen G. Endovascular treatment of high-flow cervical direct vertebro-vertebral arteriovenous fistula with detachable coils and Onyx liquid embolic agent. Acta Neurochir (Wien) 2011;153:347–52. doi: 10.1007/s00701-010-0850-z. [DOI] [PubMed] [Google Scholar]
- 12.Mortimer A, Stubbs E, Cookson D, et al. Delayed presentation of a vertebral arterio-venous fistula secondary to penetrating cervical trauma: endovascular management using coil embolisation – a case report. J Radiol Case Rep. 2009;3:9–15. doi: 10.3941/jrcr.v3i6.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kominami S, Liu Y, Alvarez H, et al. A case of vertebrovertebral arteriovenous fistula presenting with subarachnoid haemorrhage. A case report. Interv Neuroradiol. 1996;2:229–33. doi: 10.1177/159101999600200309. [DOI] [PubMed] [Google Scholar]
- 14.Kai Y, Hamada J, Morioka M, et al. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage: six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol. 2005;26:1949–54. [PMC free article] [PubMed] [Google Scholar]
- 15.Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006;59:S195–201. doi: 10.1227/01.NEU.0000237335.82234.CE. [discussion S193-113] [DOI] [PubMed] [Google Scholar]
- 16.Spetzler RF, Detwiler PW, Riina HA, et al. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96:145–56. doi: 10.3171/spi.2002.96.2.0145. [DOI] [PubMed] [Google Scholar]
- 17.Zozulya YP, Slin’ko EI, Al Q., II Spinal arteriovenous malformations: new classification and surgical treatment. Neurosurg Focus. 2006;20:E7. doi: 10.3171/foc.2006.20.5.8. [DOI] [PubMed] [Google Scholar]
- 18.Bao YH, Ling F. Classification and therapeutic modalities of spinal vascular malformations in 80 patients. Neurosurgery. 1997;40:75–81. doi: 10.1097/00006123-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Geibprasert S, Pereira V, Krings T, et al. Dural arteriovenous shunts: a new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke. 2008;39:2783–94. doi: 10.1161/STROKEAHA.108.516757. [DOI] [PubMed] [Google Scholar]
- 20.Walcott BP, Smith ER, Scott RM, et al. Pial arteriovenous fistulae in pediatric patients: associated syndromes and treatment outcome. J Neurointerv Surg. 2012 doi: 10.1136/neurintsurg-2011-010168. [DOI] [PubMed] [Google Scholar]
- 21.Goyal M, Willinsky R, Montanera W, et al. Spontaneous vertebrovertebral arteriovenous fistulae clinical features, angioarchitecture and management of twelve patients. Interv Neuroradiol. 1999;5:219–24. doi: 10.1177/159101999900500304. [DOI] [PubMed] [Google Scholar]
- 22.Madoz A, Desal H, Auffray-Calvier E, et al. Vertebrovertebral arteriovenous fistula diagnosis and treatment: report of 8 cases and review of the literature. J Neuroradiol. 2006;33:319–27. doi: 10.1016/s0150-9861(06)77289-3. [DOI] [PubMed] [Google Scholar]
- 23.Gordhan A. Onyx embolization of high-flow spontaneous cervical vertebral arteriovenous fistula. Vasc Endovascular Surg. 2012 doi: 10.1177/1538574412452156. [DOI] [PubMed] [Google Scholar]
- 24.Killory BD, Nakaji P, Gonzales LF, et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery. 2009;65:456. doi: 10.1227/01.NEU.0000346649.48114.3A. [DOI] [PubMed] [Google Scholar]
- 25.Klopfenstein JD, Spetzler RF, Kim LJ, et al. Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: a prospective assessment. J Neurosurg. 2004;100:230–5. doi: 10.3171/jns.2004.100.2.0230. [DOI] [PubMed] [Google Scholar]
- 26.Bruneau M, Sauvageau E, Nakaji P, et al. Preliminary personal experiences with the application of near-infrared indocyanine green videoangiography in extracranial vertebral artery surgery. Neurosurgery. 2010;66:305. doi: 10.1227/01.NEU.0000363596.52283.65. [DOI] [PubMed] [Google Scholar]
- 27.Hanel RA, Nakaji P, Spetzler RF. Use of microscope-integrated near-infrared indocyanine green videoangiography in the surgical treatment of spinal dural arteriovenous fistulae. Neurosurgery. 2010;66:978. doi: 10.1227/01.NEU.0000368108.94233.22. [DOI] [PubMed] [Google Scholar]
- 28.Adamczyk P, Amar AP, Mack WJ, et al. Recurrence of “cured” dural arteriovenous fistulas after Onyx embolization. Neurosurg Focus. 2012;32:12. doi: 10.3171/2012.2.FOCUS1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogueira R, Dabus G, Rabinov J, et al. Preliminary experience with Onyx embolization for the treatment of intracranial dural arteriovenous fistulas. Am J Neuroradiol. 2008;29:91–7. doi: 10.3174/ajnr.A0768. [DOI] [PMC free article] [PubMed] [Google Scholar]