Abstract
Liver fibrosis results from extracellular matrix accumulation during the wound healing process when the liver is insulted with chronic viral infection, inflammation, or alcoholic diseases. The current diagnosis of liver fibrosis is mainly dependent on biopsy, which is an invasive approach. Identification of serological biomarkers has been considered as the most promising way for early detection of the disease. Although several biomarkers in liver fibrosis have been identified, the problem is that these markers can be also detected in fibrogenesis which occurred in other organs. In this study, we have identified and characterized some cellular proteins which can be recognized by autoantibodies in the sera from patients with pre-cirrhotic stage of liver fibrosis. Among 180 sera from patients with liver fibrosis, 14.4% (26/180) of sera contained autoantibody against a protein migrating around 47-kDa on SDS-PAGE gel. Indirect immunofluorescence assay using purified autoantibody against the 47-kDa protein showed that this protein mainly localized in the cytoplasm. Using immunoproteomic approach, the 47-kDa protein was identified as alpha-enolase. In further study, the frequency of anti-alpha-enolase antibody in sera from patients with pre-cirrhotic stage of liver fibrosis (21.6%, 27/125) was significantly higher than that in sera from patients with cirrhosis (9.1%, 5/55) and liver cancer (14.3%, 12/84), as well as in sera from healthy individuals (4.1%, 3/74). Therefore, alpha-enolase is an autoantigen that elicits autoimmune response in liver fibrosis and can be a potential prognostic factor for liver fibrosis diagnosis.
Keywords: liver fibrosis, autoantigen, autoimmune response, immunoproteomics, alpha-enolase
INTRODUCTION
Liver fibrosis is typified by the excessive extracellular matrix protein such as collagen accumulation in liver 1. For example, when the liver is infected by a virus, such as hepatitis C, the hepatic stellate cells (HSCs), or Ito cells, a type of liver residing cells that normally remain quiescent, may proliferate and be transformed into collagen-producing.1 Therefore, the fibrogenesis of liver represents the impaired balance between extracellular matrix synthesis and degradation during wound healing process. Based on the severity of fibrosis in the liver, this disease can be generally divided into two different stages: early stage (pre-cirrhotic stage, stages 1–3) and end stage (cirrhosis, stage 4). The pre-cirrhotic stage of fibrosis is considered as a reversible stage, since with appropriate treatment the liver is able to improve its function to normal or near normal condition.2 Unfortunately, cirrhosis is irreversible because the cross-linked extracellular matrix (ECM) becomes resistant to proteolytic degradation.3
At the current time, the “gold standard” diagnosis of liver fibrosis is liver biopsy, which is an invasive procedure.4 In addition, when a small biopsy sample is analyzed, the accuracy of diagnosis may be also questionable. The histological examination of the biopsy specimens may also vary among different observers, making the clinical prediction unreliable.4 Therefore, the development of a non-invasive method for diagnosis of liver fibrosis is necessary.
Many studies have demonstrated that antigenic changes in cells can be recognized by the immune system of patients. This is manifested in several ways, one of which is the appearance of circulating autoantibodies.5 The presence of autoantibodies in human sera has been observed not only in systematic autoimmune diseases such as diabetes,6 rheumatoid arthritis (RA),7 and systemic lupus erythematosus (SLE)8 but also in non-autoimmune diseases such as cancer.5,9–11 Although the underlying mechanism of the generation of such autoantibodies remains poorly understood, the identification and characterization of the autoantigens that are recognized by host immune system provides invaluable tools for the clinical diagnosis in some diseases.5 Autoantibodies can target molecules such as double-strand DNAs, histones and chromatins in nucleus, known as anti-nuclear antibodies (ANAs), which is exemplified in autoimmune diseases.
Studies on the change of the expression level of blood proteins during liver fibrogenesis have been proved to be a valuable way in the identification of biomarkers in liver fibrosis. For example, several serum molecules such as hyaluronic acid (HA), TIMP metallopeptidase inhibitor 1 (TIMP-1), and α-2-macroglobulin have been identified.1 Alteration in the levels of cytokines like transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) has also been implicated in this process.12 However, these molecules are not liver-specific and their expression level tends to change in other conditions such as lung fibrosis.12 So far, only a few studies have been performed in the field of using autoantibodies as diagnostic markers in liver fibrosis.
Enolase is a family of cytoplasmic proteins involved in glycolytic metabolism and energy regulation in both of prokaryotes and eukaryotes.13 This family of proteins is constituted of three isoenzymes, named alpha-, beta-, and gamma-enolase. The expression of these isoenzymes is controlled by both of the cell type and development. Alpha-enolase is expressed ubiquitously at the early stage of embryonic development, whereas beta-enolase is only expressed in adult cardiac and skeletal muscles. Gamma-enolase, on the other hand, is neuron-specific and is expressed in mature neuron and neuroendocrine tissues.13 Besides its major biological function of catalyzing the dehydration of 2-phosphoglycerate to phosphoenolpyruvate in the cytoplasm, alpha-enolase located on the cell surface in some pathogens can promote their pathogenesis.14 Alpha-enolase is also located in the nucleus and helps regulate cell growth and differentiation by downregulating the activity of the proto-oncogene c-myc.14 Autoantibodies against alpha-enolase have been found in systematic autoimmune diseases such as SLE, RA, Crohn’s disease.15–18 Although anti-alpha-enolase autoantibodies could be detected in liver disease like autoimmune hepatitis, their titers were relatively low.18 At the present study, we defined and determined the prevalence and specificities of autoantibodies in sera from patients with liver fibrosis, and further demonstrated that autoantibodies against alpha-enolase have relatively high frequency in patients with early stage of liver fibrosis but not in advanced stage of liver fibrosis or liver cancer, which implied alpha-enolase could be a possible indicator of benign liver diseases.
MATERIALS AND METHODS
Serum sample collection
In this study, sera from 125 patients with pre-cirrhotic stage (stage 0–3) of liver fibrosis and 25 patients with liver cirrhosis were provided by collaborators at The University of Texas Medical Branch (Galveston, Texas), and another group of sera from 30 patients with liver cirrhosis were derived from the US–Mexico border region (El Paso, Texas). Sera from 84 HCC patients and 74 normal human sera were obtained from the serum bank in the investigator’s (Zhang) laboratory at The University of Texas at El Paso (UTEP), which were originally provided by a collaborator in Sun Yat-sen University, Guangzhou, China. Normal human sera were collected from adult individuals who had no obvious evidence of hepatic diseases during annual health examinations. Histopathological staging of liver fibrosis was defined using METAVIR scoring system. All HCC patients were diagnosed according to the criteria described in a previous study.19 This study was approved by the Institutional Review Board of The University of Texas at El Paso and collaborating institutions.
Cell culture and cell extracts
The hepatocellular carcinoma cell line (HepG2) was purchased from American type Culture Collection (ATCC, Manassas, VA), and cultured in Modified Eagle Medium (MEM) media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 units/ml streptomycin. Cells grown in 75-cm2 Falcon tissue culture flasks were allowed to reach 90% confluence. Then, cells were rinsed once with MEM without fetal bovine serum (FBS) and removed from the flask by incubating them with a solution containing trypsin-EDTA (Gibco, Carlsbad, CA), and harvested in a 15 mL centrifuge tube for further study.
Two-dimensional gel electrophoresis (2DE) analysis
HepG2 cells were directly lysed in rehydration sample buffer (8 M Urea, 50 mM dithiothreitol (DTT), 4% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 0.2% carrier ampholytes) as provided by Bio-Rad Laboratories (Hercules, CA) and were vortexed vigorously for 1 hr at room temperature (RT). Insoluble substances were removed by centrifuge at 16,000 × g for 30 min at 4°C. Supernatant was collected and protein concentration was measured by the Bradford assay (Bio-Rad). For the first dimensional gel electrophoresis (1DE) analysis, a total of 200 μg of protein was mixed with rehydration buffer containing a trace bromophenol blue prepared in proteomics-grade water and applied on a pH 3–10, 11-cm isoelectric focusing (IEF) strip (Bio-Rad, Hercules, CA). IEF was performed at a current of 50 mA per gel, 300 V for 30 min, followed by 8,000 V for 2.5 h, and additional 8,000 V for 5 h. Strips were immediately stored at −80 °C for the second dimensional gel electrophoresis (2DE) analysis. For the second dimensional electrophoresis, 12% SDS-polyacrylamide gels (SDS-PAGE) were used. Proteins were transferred onto nitrocellulose membrane (Osmonics Inc., MA) for Western blotting analysis or stained with 0.1% Coomassie blue R-250 prepared in 40% methanol/10% acetic acid. The spots were visualized using PDQuest 2-DE analysis software as described in the manufacturer’s manual (Bio-Rad) and also in our previous study.20
One- and two-dimensional Western blotting analysis
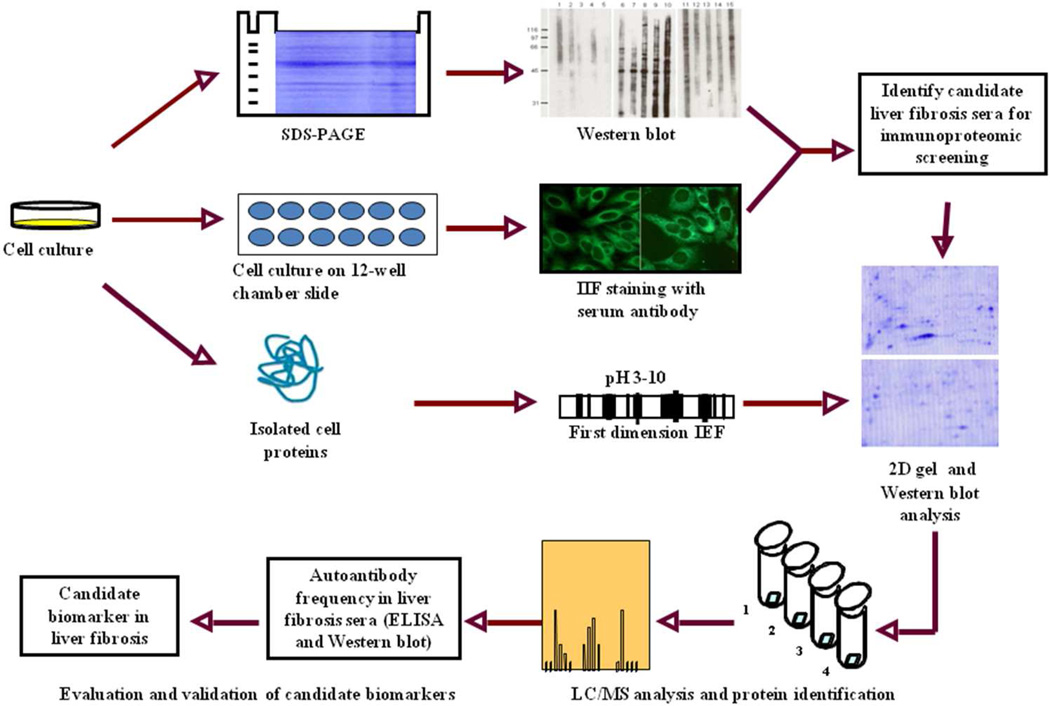
To screen the autoantibody-positive sera, HepG2 cells are lysed directly in Laemmli’s sample buffer and boiled for 10 mins. After the removing of the insoluble fraction by centrifuge, samples were loaded onto 12% SDS-PAGE gel, which is then transferred onto nitrocellulose membrane (Osmonics Inc., MA) for Western blotting. The membrane was stained with Ponceau S to confirm the transfer efficacy of the proteins and then the membrane is cut into 2-cm wide stripes. After blocking with 5% nonfat milk prepared in Tris-buffered saline (TBS), containing 0.05% Tween-20 (TBST), for 1 hr at RT, the nitrocellulose membrane was incubated with sera at a dilution of 1:200. Horseradish peroxidase-conjugated goat anti-human IgG (Caltag Laboratories, San Francisco, CA) was used as secondary antibody with a dilution of 1:5,000 for 1 hr at RT. The positive bands were detected with Enhanced Chemiluminescence (ECL) kit (Amersham, Arlington Heights, IL). For 2DE-western blotting, the proteins on 2DE gel are directly transferred into nitrocellulose membrane and following the same protocol as described above. A schematic representation of protein biomarker identification in liver fibrosis is summarized in Figure 1.
Figure 1. Schematic representation of potential protein biomarker identification in liver fibrosis.
In brief, the sera from liver fibrosis patients and controls were initially examined using extracts of tissue culture cells as source of antigens in Western blot and by indirect immunofluorescence (IIF) on whole cells. With these two techniques, we identify sera which have high-titer fluorescent staining or strong signals to cell extracts on Western blot and subsequently use the antibodies in these sera as probes in immunoproteomic screening. Cell extract of cultured human cells was also applied onto the first dimension gel (isoelectrofocusing gel), and subsequently loaded onto the second-dimension gel (2D-SDS-PAGE). The proteins were transferred to the nitrocellulose membrane or visualized by silver staining or Coomassie brilliant blue staining. After immunoblotting with liver fibrosis sera and control sera, a number of protein spots of interest were excised from the 2-D gels, digested by trypsin, and subsequently analyzed by liquid chromatography-tandem mass spectrometry (LC/MS). In subsequent studies, we will characterize the identified cellular proteins that are potential biomarkers in liver fibrosis.
Antibody absorption
To purify the enolase-specific autoantibody from human sera, HepG2 cell lysates were separated on 12% SDS-PAGE gel and then transferred onto nitrocellulose membrane. The membrane around 47-kDa was cut off from the whole membrane, and then further cut into small pieces. Those pieces of membrane were incubated with individual serum containing autoantibody against 47-kDa autoantigen for 2 hr at RT. The membranes were subsequently washed three times with TBST (10 min per washing cycle). Autoantibodies bound on the membrane were eluted with 1 M glycine solution, pH 3.0, which was immediately neutralized with 1 M Tris-HCl, pH 8.0.
Indirect immunofluorescence assay
Indirect immunofluorescence assay was performed on HEp-2 anti-nuclear antigen tissue slides (Bion Enterprises, Des Plaines, IL). The sera were diluted at 1:40 in phosphate-buffered saline (PBS), pH 7.4 and incubated with the slides for 30 min at RT. After extensive washing, the slides were incubated with fluorescein isothiocyanate (FITC) conjugated goat-anti-human IgG secondary antibody (Santa Cruz Biotechnology, CA) as secondary antibody diluted 1:1,000 in PBS for 1 hr at RT. The slides were washed three times with PBS before adding a drop of mounting media containing 1.5 μg/ml 4’, 6’-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc. Burlingame, CA) to prevent photobleaching. The slides were then examined under fluorescence microscopy, Leica DM1000 (Leica Microsystems, Houston, United States), at 400X magnification. Images were acquired using the software QCapture 290.1 (Qimaging, Burnaby, BC, Canada).
Proteomic analysis
Gel spots were excised and digested as previously described,21 and the resulting peptides were desalted in a reversed phase microcolumn and dried in an Eppendorf vacuum centrifuge.22 Peptides were injected into a trap column (1 cm × 75 μm, 5 μm 100 Å, C18, Luna, Phenomenex) and the separation was performed C18 capillary column (20 cm × 75 μm, 5 μm 100 Å, C18, Luna, Phenomenex) connected to a nanoHPLC system (nano-LC 1D plus, Eksigent). The elution was carried out in a linear gradient from 5 to 40% solvent A to B (Solvent A: 5% acetonitrile/0.1% formic acid, Solvent B: 80% acetonitrile/0.1% formic acid) over 60 min at a flow rate of 300 nL/min. Eluting peptides were directly subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, using nanoelectrospray ionization (TriVersa NanoMate system, Advion) coupled to a linear ion trap-mass spectrometer (LTQ XL, Thermo-Fisher Scientific). MS spectra were collected in centroid mode at the 400–1,700 m/z range, and the ten most intense ions were subjected twice to collision-induced dissociation (35% normalized collision energy) before being dynamically excluded for 120 sec.
MS/MS spectra derived from peptides having a mass range of 800-3,500 Da, and at least 15 fragments were submitted for database search using TurboSequest (available in Bioworks version 3.3.1) against the human IPI database (v3.48), in both correct and reverse orientations (142804 sequences total) to enable false-discovery rate (FDR) calculation. The database search parameters included: (i) trypsin cleavage in both peptide termini, allowing for one missed cleavage site; (ii) carbamidomethylation of cysteine residues as a fixed modification; (iii) oxidation of methionine residues as a variable modification; and (iv) 2.0 Da and 1.0 Da mass tolerance for peptides and fragments, respectively. The following filters were applied in Bioworks: DCn ≥ 0.85; consensus score ≥ 10.0; protein probability ≤ 1 × 10−3; and Xcorr ≥ 1.5, 2.0, and 2.5, for singly-, doubly- and triply-charged peptides, respectively.
Enzyme-linked immunosorbent assay (ELISA)
Purified yeast alpha-enolase protein was commercially purchased from Sigma-Aldrich (St. Louis MO). The protein purity was >95% by SDS-PAGE. Proteins were diluted in PBS to a final concentration of 0.5 μg/mL for coating polystyrene 96-well microtiter plates (Dynatech Laboratories, Alexandria, VA). A volume of 200 μL of each human serum samples at 1:200 dilutions was added to the antigen-coated wells and incubated for 1.5 h at RT. Horseradish peroxidase-conjugated goat antihuman IgG (Caltag Laboratories, San Francisco, CA) at 1:5,000 dilution and the substrate 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (Boehringer Mannheim GmbH, Mannheim, Germany) were used as detecting reagents. Each sample was tested in duplicate, and the average OD at 405 nm was used for data analysis. The cutoff value designating positive reaction was the mean optical density (OD) of 30 normal human sera plus 3 standard deviations (SD). The detailed protocol of ELISA was used as described by Rubin23.
Statistical analysis
To determine whether the frequency of autoantibodies in each cohort of patients’ sera was significantly higher than that in sera from healthy individuals, the data were analyzed using the χ2 test with Yates’ correction. Two significant levels (0.05 and 0.01) were used.
RESULTS
Prevalence of autoantibodies in patients with early stage of liver fibrosis and liver cirrhosis
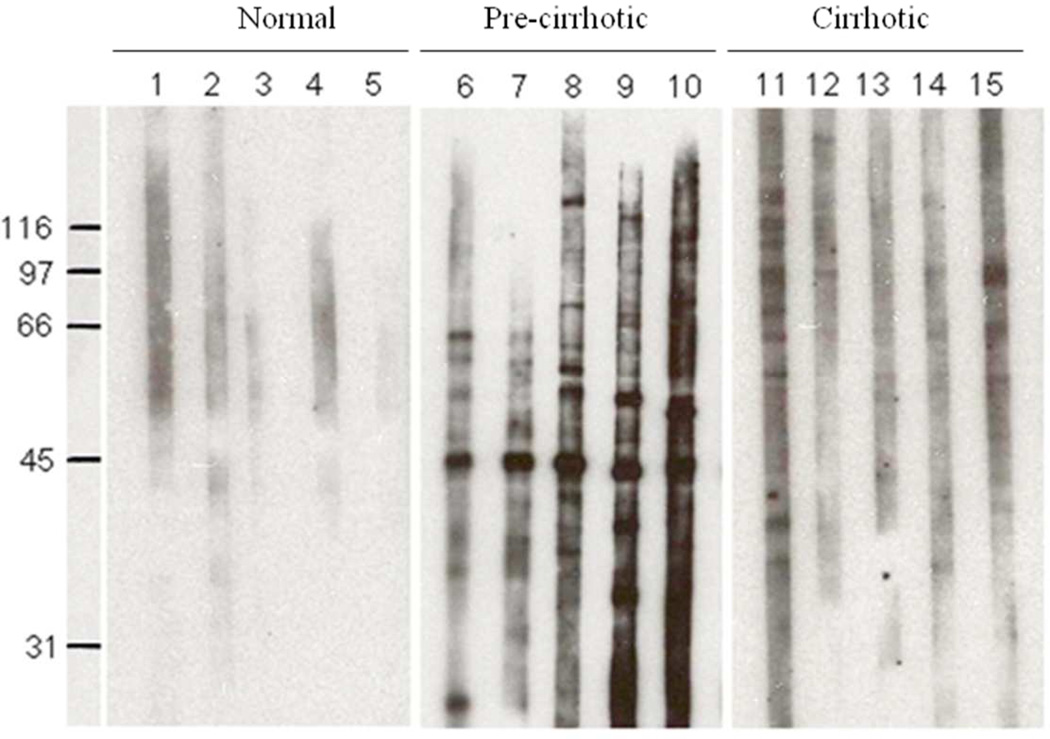
Autoantibodies against intracellular antigens are commonly found in a number of systemic autoimmune diseases. Their utility in providing insights into molecular and cellular biology and in the differential diagnosis of autoimmune diseases is well documented. Autoimmune phenomena manifested as autoantibodies to cellular components have been described in many types of liver diseases. The objective of this study was to analyze the frequency and specificity of autoantibodies in sera from patients with liver fibrosis, and further identify and evaluate the autoantibodies and targeted antigens as biomarkers in liver fibrosis. In this study, 180 sera from patients with liver fibrosis and 30 sera from normal human individuals were examined for autoantibodies using indirect immunofluorescence assay and western blotting. We found that some of the patients’ sera contained autoantibodies reacting with one or more cellular antigens (Figure 2). As shown in Table 1, autoantibodies were detected in 90 of 180 (50%) sera from patients with liver fibrosis, which were significantly higher than that in normal human sera (6.7%, 2/30). Significant difference of autoantibody positivity was also detected between pre-cirrhotic stage of liver fibrosis (stage 1–3) and cirrhotic stage of liver fibrosis (stage 4), which is consistent with the previous finding that production of autoantibodies were increased along with the progression of diseases.1, 5 Interestingly, 26 of 180 (14.4%) liver fibrosis sera were identified by western blotting analysis containing antibodies against an unknown 47-kDa cellular protein. As shown in Table 2, no reactivity with the 47-kDa protein was detected in 30 normal human sera. However, further analysis of the autoantibody positivity to this 47-kDa autoantigen between pre-cirrhotic stage of liver fibrosis and cirrhotic stage of liver fibrosis showed that pre-cirrhotic stage of liver fibrosis contained higher frequency of autoantibody to this antigen (17.6%, 22/125) than that in liver cirrhotic stage (7.2%, 4/55). Western blotting analysis of five representative sera from each of the cohort groups is shown in Figure 2. Subcellular fractionation of HepG2 cells showed the 47-kDa proteins were predominantly in the cytoplasm, and were not found in the nuclear fraction (data not shown). The analysis using immunohistochemistry on HEp-2 cells confirmed that the sera with antibodies to the 47-kDa antigen appeared to have the cytoplasmic and perinuclear staining patterns (Figure 3).
Figure 2. Detection of autoantibodies against a 47-kDa cellular protein in sera from patients with liver fibrosis.
Western blot shows that only sera from patients with pre-cirrhotic stage of liver fibrosis (lanes 6–10) rather than normal human sera (lanes 1–5) or sera from patients at the cirrhotic stage of liver fibrosis (lanes 11–15) contain antibodies against a 47-kDa cellular protein.
Table 1.
Frequency of autoantibodies in sera from patients with liver fibrosis
| Type of diseases | No. tested | Frequency (Patients with autoantibody/ total patients with disease) |
|---|---|---|
| Liver fibrosis: | 180 | 50.0% (90/180)**a |
| Pre-cirrhotic stage | 125 | 46.4% (58/125)*a |
| Cirrhotic stage | 55 | 58.1% (32/55) |
| Normal human sera (NHS) | 30 | 6.7% (2/30) |
P value relative to NHS: * p < 0.05; ** p < 0.01
Table 2.
Frequency of autoantibodies against the 47 kD autoantigen in sera from patients with pre-cirrhotic stage and cirrhotic stage of liver fibrosis and sera from normal human individuals
| Type of diseases | No. tested | Frequency (Patients with autoantibody/ total patients with disease) |
|---|---|---|
| Liver fibrosis: | 180 | 14.4% (26/180)**a |
| Pre-cirrhotic stage | 125 | 17.6% (22/125)*a,b |
| Cirrhotic stage | 55 | 7.2% (4/55) |
| Normal human sera (NHS) | 30 | 0% (0/30) |
P value relative to NHS: * p < 0.05; ** p < 0.01
P value relative to cirrhotic stage: * p < 0.05; ** p < 0.01
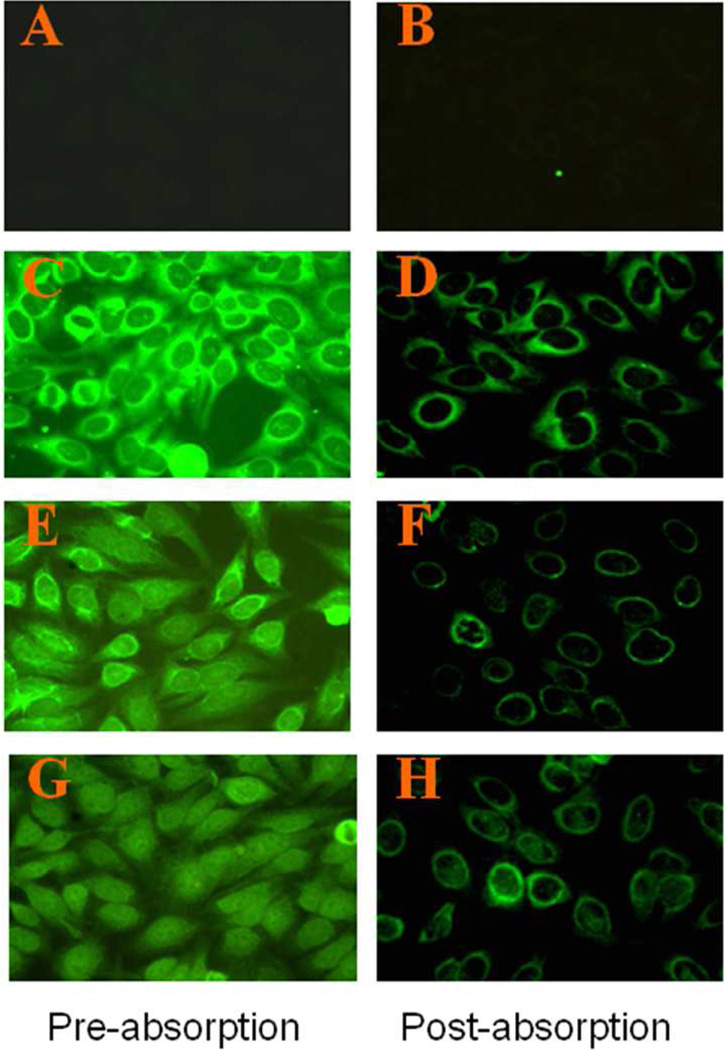
Figure 3. The 47-kDa protein is a cytoplasmic or perinuclear protein, as revealed by immunofluorescence analysis of HEp-2 cell substrate using human liver fibrosis sera.
Immunofluorescence was done using patients’ sera or sera purified with 47-kDa antigen (see Materials and Methods). Left panel (A, C, E, G): pre-absorption sera; right panel (B, D, F, H): post-absorption sera. Three representative liver fibrosis sera (D, F and H) were used, and one normal human serum (A) was used as control.
Identification by immunoproteomic approach of the 47-kDa protein as alpha-enolase
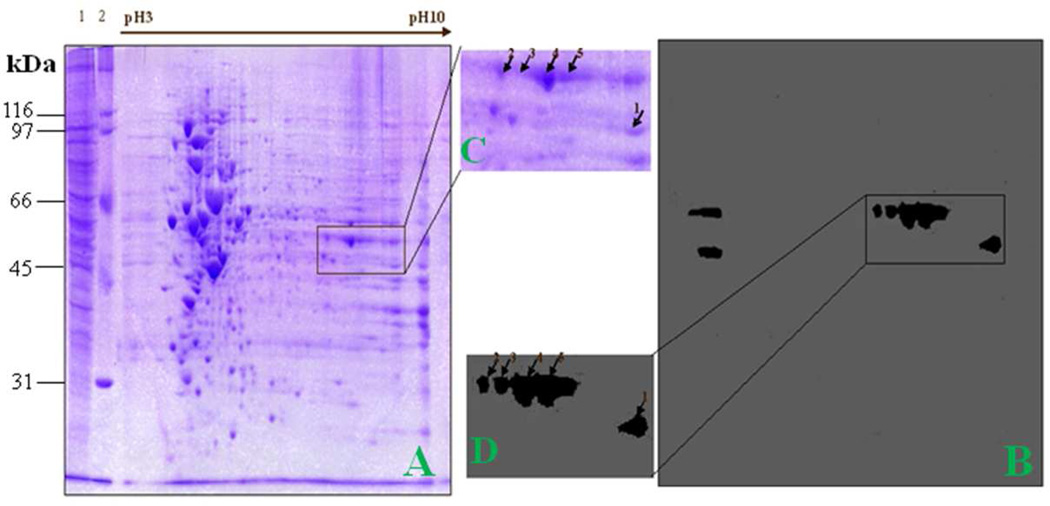
An immunoproteomic approach has been previously used in our lab in the identification of tumor-associated antigens as biomarkers in cancer.25 A brief description of this approach is shown in Figure 1. The advantage of this method is based on the accurate identification of protein by tandem mass spectrometry analysis. In the present study, an immunoproteomic approach was also applied to identify potential protein biomarkers in liver fibrosis. To obtain a global view of the proteins in liver cells, proteins extracted from HepG2 cells were separated by 2DE (Figure 4A and 4C). After electrophoresis, the gels were either stained with Coomassie blue or transferred onto nitrocellulose membrane. In this current study, we have used two serum samples that contain high titer of autoantibody to the 47-kDa protein as probes in 2DE Western blot analysis for identification of this protein. Figure 4 represents results from one of these two sera. Interestingly, four positive protein spots around 47-kDa and one around 45-kDa were shown in the Western blot. The upper four protein spots (2, 3, 4, and 5) and lower spot (1) were cut out and analyzed by LC-MS/MS analysis. As shown in Table 3, proteomic analysis demonstrated that these upper four reactive protein spots matched with alpha-enolase, and the lower spot with phosphoglycerate kinase 1 (PGK1). Another serum reacting with alpha-enolase showed the similar pattern in 2DE Western blot but reactivity to PGK1 in this serum was not detected (data not shown). Therefore, we can conclude that the 47-kDa unknown protein corresponds to alpha-enolase, but not to PGK1.
Figure 4. Identification of the 47-kDa liver fibrosis-associated protein by immunoproteomics.
(A) The 2DE protein profile of HepG2 cells. Lane 1, HepG2 cell lysate; lane 2, protein molecular marker. (B) Western blotting analysis of 2DE gel was probed with a high-titer liver fibrosis serum which contains antibodies to the 47-kDa protein. (C) and (D) The corresponding area (rectangle) of A and B was enlarged. Positive spots were picked up by using PDQUEST software.
Table 3.
Summary of identified protein spots by mass spectrometry
| Spot no. |
Accession no. | Identified protein | Molecular mass (Da) |
Xcorr sum |
%of matched peptides |
Protein functions |
|---|---|---|---|---|---|---|
| 1 | IPI00169383.3 | Phosphoglycerate kinase 1 |
44586.2 | 187.63 | 51 | Key enzyme in glycolysis and gluconeogenesis, converting glucose to pyruvate to produce ATP. |
| 2 | IPI00465248.5 | ENOl isoform alpha enolase |
47139.4 | 59.21 | 18 | glycolytic enzyme expressed in most human tissues. |
| 3 | IPI00465248.5 | ENOl isoform alpha enolase |
47139.4 | 68.33 | 17 | glycolytic enzyme expressed in most human tissues. |
| 4 | IPI00465248.5 | ENOl isoform alpha enolase |
47139.4 | 54.47 | 14 | glycolytic enzyme expressed in most human tissues. |
| 5 | IPI00465248.5 | ENO1 isoform alpha enolase |
47139.4 | 103.36 | 23 | glycolytic enzyme expressed in most human tissues. |
Validation of alpha-enolase as biomarker in liver fibrosis
Alpha-enolase is an evolutionarily conserved protein that plays a critical role in glycolytic metabolism. Previous studies had indicated that this protein can elicit immune response in different types of autoimmune diseases and infection caused by S. pneumonia and C. albicans.4 Autoantibodies to alpha-enolase in liver fibrosis has not been reported so far. At the present study, commercially available yeast alpha-enolase, which shares 62% homology with human alpha-enolase,4 was used as coating antigen in ELISA for the detection of autoantibodies against this protein in sera from patients with pre-cirrhotic stage of liver fibrosis, cirrhotic stage of liver cirrhosis, and liver cancer as well as normal human sera. Table 4 shows the frequency of antibodies to alpha-enolase in sera from different type of diseases. The cutoff value designating positive reaction was established as the mean OD of 74 normal human sera (NHS) plus 3 standard deviations (SD). Of 180 sera with liver fibrosis analyzed, 32 (17.8.0%) were reactive with alpha-enolase, only 3 (4.1%) was positive in 74 normal human sera. Statistical analysis indicated that there was a significant difference (p<0.01) of anti-alpha-enolase antibody frequency between liver fibrosis and normal human sera. Among these 32 alpha-enolase ELISA positive sera, 24 (75.0%) sera were Western-blot positive in our initial screening while another two Western-blot positive sera in the initial screening were ELISA negative. The correlation between ELISA and Western blotting analysis demonstrates the consistence between these two methods. Sera with anti-alpha-enolase positive in ELISA were also confirmed by Western blotting analysis with alpha-enolase recombinant protein. Figure 5 shows three representative liver fibrosis sera that have positive reaction to alpha-enolase in ELISA, and also have strong reactivity by Western blotting analysis.
Table 4.
Frequency of autoantibody to alpha-enolase in sera from patients with liver diseases
| Type of diseases | No. tested | Frequency (Patients with anti-alpha-enolase/ total patients with disease)a |
|---|---|---|
| Liver fibrosis: | 180 | 17.8% (32/180)**b |
| Pre-cirrhotic stage | 125 | 21.6% (27/125)*b,c |
| Cirrhotic stage | 55 | 9.1% (5/55) |
| Liver cancer | 84 | 14.3% (12/84)**b |
| Normal human sera (NHS) | 74 | 4.1% (3/74) |
Cutoff value: Mean + 3 SD of NHS
P value relative to NHS: * p < 0.05; ** p < 0.01
P value relative to cirrhotic stage: * p < 0.05; ** p < 0.01
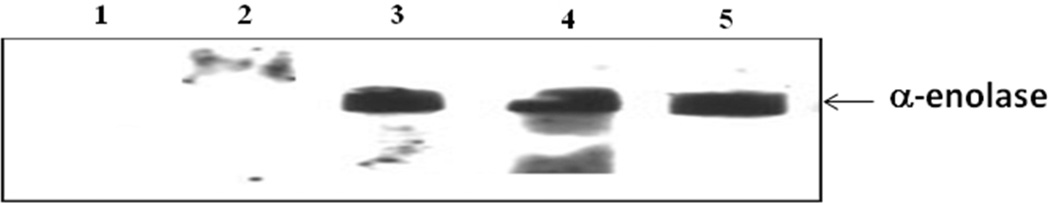
Figure 5. Validation of ELISA results by Western blotting analysis.
Two pre-cirrhotic liver fibrosis sera with anti-alpha-enolase antibody negative (lane 1 and 2) and three pre-cirrhotic liver fibrosis sera with anti-alpha-enolase antibody positive (lanes 3, 4, and 5) were randomly chosen for Western blotting analysis with alpha-enolase recombinant protein.
DISCUSSION
Liver fibrosis, which progresses to cirrhosis, has been considered to be a pre-malignant stage or a precursor lesion of liver cancer in hepatitis C patients.24–26 In order to identify specific liver fibrosis biomarkers, we have screened sera from 180 patients with liver fibrosis. In the present study, we found that 50% of the sera from patients with liver fibrosis contained autoantibodies against a variety of cellular antigens. Among these autoantibody positive sera, 14.4% sera contained autoantibodies against the 47-kDa protein seen on the Western blotting analysis. Indirect immunofluorescence using purified autoantibodies against this 47-kDa protein showed that it was mainly localized in the cytoplasm. Therefore, an immunoproteomic approach was used to identify this protein and found that four 2DE-western blot-positive spots corresponded to alpha-enolase. Interestingly, a previous study described a similar finding of the alpha-enolase in eliciting autoimmune response in patients suffered from Hashimoto’s encephalopathy (HE). They have found that autoantibodies can recognize different oxidized forms of alpha-enolase (migrating in a similar pattern as shown in our results) in HE patients by 2-DE gel.27 Thus, the proteome-based approach has been extensively used, and it was considered as a valuable tool in the identification of autoantigens in many types of disease. The combination of 2DE gel, tandem mass spectrometry and immunoblotting provides convenient way for protein identification when compared with the traditional approach. Although there are technical limitations of this method, for instance the insolubility of membrane proteins, we were still successful in identifying this protein as alpha-enolase.
The autoimmune responses to alpha-enolase have been previously described in patients with autoimmune diseases and bacterial or fungal infection.14 To validate our proteomic results, an ELISA was used to test the autoantibodies against alpha-enolase in additional sera from patients with more advanced hepatic disease such as liver cancer. The results indicated that frequencies of anti-alpha-enolase antibody in sera from patients with liver fibrosis and liver cancer are significant higher than that in sera from healthy individuals. Although studies had shown that autoantibodies against alpha-enolase have been detected in benign liver diseases, there are no data to show the relationship between anti-alpha-enolase autoantibodies and liver fibrosis. In addition, an interesting observation in our study is the significantly different positivity of alpha-enolase autoantibody between patients with pre-cirrhotic stage of liver fibrosis and patients with liver cirrhosis. Noticeably, a previous publication reported the presence of higher frequency of autoantibodies against alpha-enolase in sera from patients with autoimmune hepatitis ranging from 56%–60% than that in primary biliary cirrhosis (30%).14, 28 The tendency of the decreased titer of autoantibodies against alpha-enolase with the progression of liver diseases was consistent with our findings. Even though there is still lack of a comprehensive study to specifically address the titer change of autoantibodies against alpha-enolase during the progression of benign liver diseases to advanced diseases, by examining the titer of alpha-enolase autoantibodies from pre-cirrhotic liver fibrosis to liver cancer our study has extended the previous finding and implied that the presence of autoantibody against alpha-enolase may be a predictive marker for better prognosis of liver diseases. However, further elucidation of the role of alpha-enolase could come from serial studies of patients with liver fibrosis, comparing those who recover with those who progress to cirrhosis and HCC. In one way, we can observe the serological change of autoantibody to alpha-enolase in these patients, and in other way, we can also investigate the expression of alpha-enolase in their tissue specimens to clarify the role of alpha-enolase and its antibody as biomarkers for pre-cirrhotic liver fibrosis.
Although whether alpha-enolase play active role during the formation liver fibrosis have not been addressed yet, it was shown that this enzyme was able to bind pasminogen on the cell surface and thus promote the migration of monocytes to the inflamed area during acute lung injury.29 Furthermore, it is also reported that alpha-enolase is transcriptionally regulated by c-Jun NH2-terminal kinase (JNK) signaling pathway, which is frequently activated in liver fibrosis. 30, 31 Therefore, the ability of alpha-enolase to recruit the immune cells to the site of inflammation indicated that alpha-enolase may also participate in the inflammation at early stage of liver fibrosis. However, with the progression to the irreversible late stage of liver fibrosis, more factors are engaged in the pathogenesis like the accumulation of certain types of ECM like Type I, Type III, Type IV, and Type V collagen.32 The cirrhotic liver can have approximately six times of ECM than that in the normal liver and much higher level of Type III and Type V collagen and fibronectin than those accumulated at early stage of liver fibrosis.33 Therefore, it is possible that alpha-enolase plays important role at the early stage of liver fibrosis by serving as a proinflammatory factor and gradually became less active with the progression to the more advanced stage when ECMs become more important, which may lead to the reduced immune response to alpha-enolase by the host. Indirect evidence also showed that alpha-enolase could also elicit autoimmune response in biliary atresia patients which can rapidly progress to liver fibrosis affecting liver.34
CONCLUSIONS
Early detection of liver fibrosis will allow the patients to receive treatment while still in stage where it is still possible to prevent the progression to cirrhosis. The combinatorial utilization of serum biomarkers for this purpose is one of the promising approaches which is non-invasive and is of high sensitivity and specificity. Thus, the identification of novel serum biomarkers is of great importance. In the current study, we have used an immunoproteomic approach to identify and characterize alpha-enolase as an autoantigen in liver fibrosis. Further study demonstrated that frequency of anti-alpha-enolase autoantibody in sera from patients with pre-cirrhotic stage of liver fibrosis was significantly higher than that in sera from patients with other liver diseases such as cirrhosis and liver cancer, as well as in sera from normal individuals. These data suggest that alpha-enolase can elicit humoral immune response in liver fibrosis, and thus it can be a potential prognostic factor. Therefore, the subsequent studies like the expression level and enzymatic activity of alpha-enolase need to be carried out in different stages of liver disease to further validate whether it can be used as a biomarker in detection of liver fibrosis.
ACKNOWLEDGEMENTS
This work was supported by grants from NIH (SC1CA166016 and 5G12MD007592). We thank the Biomolecule Analysis Core Facility and Analytical Cytology Core Facility of Border Biological Research Center (BBRC) at The University of Texas at El Paso (UTEP), funded by NIH grants 5G12RR008124-16A1, 5G12RR008124-16A1S1, and 5G12MD007592, for their support.
REFERENCES
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Ann Hepatol. 2009;8(4):283–291. [PubMed] [Google Scholar]
- 3.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-sadler H, Gaca MD. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2006;126(7):1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99(6):1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 5.Tan EM, Zhang JY. Autoantibodies to tumor-associated antigens: reporter from the immune system. Immunol Rev. 2008;222:328–340. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54:S52–S61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 7.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 8.Smith PP, Gordon C. Systemic lupus erythematosus: clinical presentations. Autoimmun Rev. 2010;10(1):43–45. doi: 10.1016/j.autrev.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Zhang EM, Tan EM. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev Mol Diagn. 2010;10(3):321–328. doi: 10.1586/erm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himoto T, Kuriyama S, Zhang JY, Chan EK, Nishioka M, Tan EM. Significance of auto-antibodies against insulin-like growth factor II mRNA-binding proteins in patients with hepatocellular carcinoma. Int J Oncol. 2005;26(2):311–317. [PubMed] [Google Scholar]
- 11.Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann NY Acad Sci. 2000;910:121–137. doi: 10.1111/j.1749-6632.2000.tb06705.x. [DOI] [PubMed] [Google Scholar]
- 12.Bhogal RK, Bona CA. B cells: no longer bystanders in liver fibrosis. J Clin Invest. 2005;115(11):2962–2965. doi: 10.1172/JCI26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capello M, Ferri-Borgogno S, Cappello P, Novelli F. Alpha-enolase: a promising therapeutic and diagnostic tumor target. FEBS J. 2011;278(7):1064–1074. doi: 10.1111/j.1742-4658.2011.08025.x. [DOI] [PubMed] [Google Scholar]
- 14.Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L. Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmun Rev. 2007;6(3):176–182. doi: 10.1016/j.autrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Mosca M, Chimenti D, Pratesi F, Baldini C, Anzilotti C, Bombardieri S, Migoliorini P. Prevalence and clinic-serological correlations of anti-alpha-enolase, anti- Clq, and anti-dsDNA antibodies in patients with systemic lupus erythematosus. J Rheumatol. 2006;33(4):695–697. [PubMed] [Google Scholar]
- 16.Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, Moyes D, Taylor PC, Venables PJ. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7(6):1421–1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roozendaal C, Zhao MH, Horst G, Lockwood CM, Kleibeuker JH, Limburg PC, Kallenberg CG. Catalase and alpha-enolase: two novel granulocyte autoantigens in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112(1):10–16. doi: 10.1046/j.1365-2249.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P. Autoantibodies specific for alpha-enolase in systemic autoimmune disorders. J Rheumatol. 2000;27(1):109–115. [PubMed] [Google Scholar]
- 19.Johnson PJ, Leung N, Cheng P, Welby C, Leung WT, Lau WY, Yu S, Ho S. 'Hepatoma- specific' alphafetoprotein may permit preclinical diagnosis of malignant change in patients with chronic liver disease. Br J Cancer. 1997;75(2):236–240. doi: 10.1038/bjc.1997.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Looi KS, Nakayasu ES, Diaz RA, Tan EM, Almeida IC, Zhang JY. Using proteomic approach to identify tumor-associated antigens as markers in hepatocellular carcinoma. J Proteome Res. 2008;7(9):4004–4012. doi: 10.1021/pr800273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 22.Jurado JD, Rael ED, Lieb CS, Nakayasu E, Hayes WK, Bush SP, Ross JA. Complement inactivating proteins and intraspecies venom variation in Crotalus oreganus helleri. Toxicon. 2007;49(3):339–350. doi: 10.1016/j.toxicon.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Rubin RL. Enzyme-linked immunosorbent assays for antibodies to native DNA, histones, and (H2A-H2B)-DNA. In: Rose NR, de Macario EC, Folds JD, Lane HC, Nakamura RM, editors. Manual of Clinical Laboratory Immunology. 5th. Washington DC: American Society for Microbiology; 1997. pp. 935–941. [Google Scholar]
- 24.Xu R, Zhang Z, Wang FS. Liver fibrosis: mechanism of immune-mediated injury. Cell Mol Immunol. 2011;9:296–301. doi: 10.1038/cmi.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poynard T, Morra R, Ingiliz P, Imbert-Bismut F, Thabut D, Messous D, Benhamou Y, Ratziu V. Biomarker of liver fibrosis. Adv Clin Chem. 2008;46(5):131–160. doi: 10.1016/s0065-2423(08)00404-6. [DOI] [PubMed] [Google Scholar]
- 26.Seki E, De Minicis. S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochi H, Horiuchi I, Araki N, Toda T, Araki T, Sato K, Murai H, Oseoegawa M, Yamada T, Okamura K, Mizumoto K, Yamashita H, Saya H, Kira J. Proteomic analysis of human brain identifies alpha-enolase as a novel autoantigen in Hashimoto's encephalopathy. FEBS Lett. 2002;528(1–3):197–202. doi: 10.1016/s0014-5793(02)03307-0. [DOI] [PubMed] [Google Scholar]
- 28.Orth T, Kellner R, Diekmann O, Faust J, Meyer zum Buschenfelde KH, Mayet WJ. Identification and characterization of autoantibodies against catalase and alpha-enolase in patients with primary sclerosing cholangitis. Clin Exp Immunol. 1998;112(3):507–515. doi: 10.1046/j.1365-2249.1998.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wygrecka M, Marsh LM, Morty RE, Henneke I, Guenther A, Lohmeyer J, Markart P, Preissner KT. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;28(22):5588–5598. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- 30.Sousa LP, Brasil BS, Silva BdeM, Nogueira SV, Andrade AA, Ferreira PC, Teixeria SM, Gollob KJ, Kroon EG, Kato K, Bonjardim CA. Characterization of alpha-enolase as an interferon-alpha2/alpha1 regulated gene. Front Biosci. 2005;10:2534–2547. doi: 10.2741/1718. [DOI] [PubMed] [Google Scholar]
- 31.Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138(1):347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benyon R, Iredale J. Is liver fibrosis reversible? Gut. 2000;46(4):443–446. doi: 10.1136/gut.46.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burt AD, Griffiths MR, Schuppan D, Voss B, MacSween RN. Ultrastructural localization of extracellular matrix protein in liver biopsies using ultracryomicrotomy and immune-gold labeling. Histopathology. 1990;16(1):53–58. doi: 10.1111/j.1365-2559.1990.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 34.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. α-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology. 2010;139(5):1753–1761. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]