Abstract
Objective
To assess effects of vitamin D and Calcium (Ca) on hormonal and metabolic milieu of polycystic ovary syndrome (PCOS)
Design
Single arm open label trial
Methods
Twelve overweight and vitamin D deficient women with PCOS underwent a 2 hour oral glucose tolerance testing at baseline and following 3 month supplementation with vitamin D (daily dose of 3533 IU, increased to 8533 IU after the first 5 participants) and 530mg elemental Ca daily.
Main Outcome Measures
Blood pressure (BP), plasma glucose, insulin, total testosterone (T) androstenedione (A), sex hormone binding globulin, lifestyle parameters were assessed at baseline and following 3 months intervention. Insulin resistance and AUC for glucose and insulin were computed; paired analyses were conducted.
Results
Improved serum 25OHD (p<0.001) and reductions in total T (p=0.036) and A (p=0.090) levels were noted following 3 month supplementation, compared to baseline. Significant lowering in BP parameters was seen in participants with baseline BP ≥ 120/80 mmHg (n=8) and in those with baseline serum 25OHD ≤ 20ng/ml (n=9). Parameters of glucose homeostasis and insulin resistance remained unchanged (p>0.05).
Conclusions
Androgen and BP profiles improved followed three month intervention, suggesting therapeutic implications of vitamin D and Ca in overweight and vitamin D deficient women with PCOS.
Keywords: Polycystic ovary syndrome, testosterone, androgen, vitamin D, calcium, insulin resistance
Introduction
Polycystic Ovarian Syndrome (PCOS) is a recognized risk for cardiovascular disease and diabetes [1–4]. Limited data suggest pathophysiological relevance of vitamin D deficiency for PCOS [5–13]. Data from a pilot study of vitamin D and calcium (Ca) supplementation in a community sample of overweight and vitamin D deficient women with PCOS are presented.
Methods
The study was posted on clinicaltrials.gov (NCT00743574) [14]; institutional approval and written consent were obtained. Oligomenorrhea (menstrual cycles >35 days) and hyperandrogenism defined PCOS [15]. Serum 25OHD <25ng/ml and body mass index (BMI) ≥ 27 kg/m2 were eligibility criteria; thyroid dysfunction, hyperprolactinemia, late onset congenital adrenal hyperplasia, use of insulin sensitizers, oral contraceptives, anti-epileptics, vitamin D and Ca supplements and known systemic disorders (e.g. hypertension, diabetes, urolithiasis, inflammatory bowel disease) were exclusion criteria.
Blood draw and anthropometric measurements were undertaken at baseline and after 3 month supplementation with study drugs. Serum 25OHD was tested after 1 month to ensure against toxicity (level>150ng/ml). Oral glucose tolerance testing (OGTT) was scheduled within 5 days of onset of bleeding (spontaneous or provoked following a 10 day course of oral progesterone) after overnight fast. BMI, waist circumference (WC, cm) and blood pressure (BP) were recorded. Serial blood samples were collected (fasting, 30, 60, 90 and 120 minutes after 75 gram oral glucose load [Trutol, Thermo Fisher Scientific Inc. RI]). Plasma glucose (G) levels were analyzed by glucose oxidase method and sample aliquots were stored at −80°C for assessment of insulin (I, Millipore RIA, St. Charles MO, sensitivity: 2uU/ml), total testosterone (T, IRA DSL Webster TX. sensitivity: 5 ng/dl), Androstenedione (A, ELISA, ALPCO, Salem, NH, sensitivity: 4ng/dl) and sex hormone binding globulin (SHBG, EIA, ALPCO, sensitivity: 0.1nmol/L). Free androgen index (FAI) was calculated. 25OHD was assessed by RIA (Diasorin Stillwater, MN, sensitivity 5ng/ml).
The study aimed to provide daily vitamin D3 (2000IU) and monthly vitamin D2 (50,000IU); D2 regimen was modified to 50,000IU weekly after observation of a suboptimal rise in serum 25OHD in the first 5 participants. All received 530mg of elemental Ca/day. Compliance was verified by pill count and tolerance was assessed.
Physical activity was determined by BDDS Adult PA Screener (www.nutritionquest.com) [16]; analyses provided estimates of total calories “expended” per day, and “MET minutes” per day at moderate and vigorous activity levels.
Statistics
Correlation analyses (Pearson’s or Spearman) assessed relationships between continuous data. Systolic BP (SBP) ≥ 140mmHg and/or diastolic BP (DBP) ≥ 90mmHg defined hypertension. Fasting G:I ratio, HOMA (Homeostatic model assessment), QUICKI (Quantitative insulin sensitivity check index), and area under the curve (AUC) for G and I were computed to assess insulin resistance (IR) [17]. Paired analyses compared baseline and completion data utilizing STATA 10 (College Station, TX); two tailed p ≤0.05 reflects statistically significance.
Results
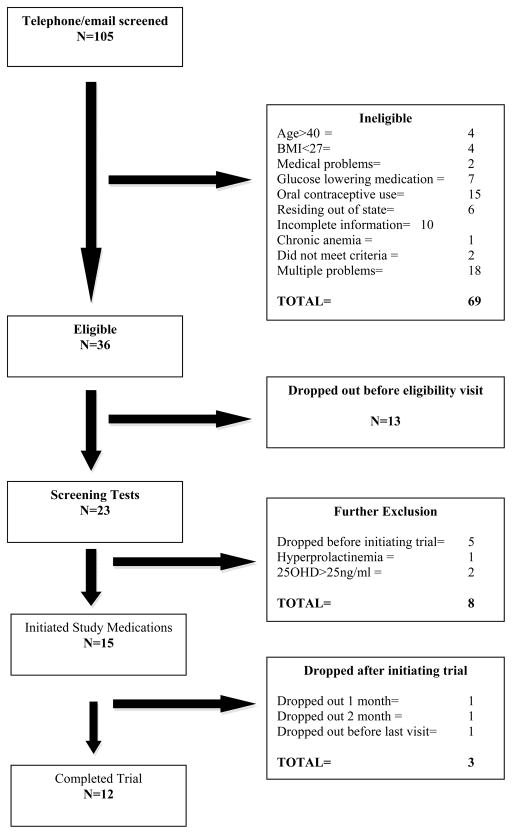
Figure 1 provides enrollment overview (8-2008 to 3-2010); 80% of those initiating study drugs completed the trial (12/15). Compliance was 100% for vitamin D2, and between 80 and 90% for D3 and Ca. No adverse effects were noted.
Figure 1.
Flow diagram outlines enrollment details for the clinical trial, registration number NCT00743574, (clinicaltrials.gov).
Participant characteristics and data from baseline and following 3- month intervention are presented in Table I (cohort data) and II (individual participant data).
Table I.
Participant characteristics and biochemical data at baseline (B) and following completion (C) of 3 month supplementation with vitamin D and calcium.
| Parameter | Baseline (n=12) | Completion (n=12) | P value |
|---|---|---|---|
|
| |||
| Age (years) | 26.42 ± 6.11 | - | |
|
| |||
| Waist Circumference (cm) | 114.61 ± 20.90 | 114.55± 21.47 | 0.984a |
|
| |||
| BMI (kg/m2) | 39.84 ±10.86 | 39.83 ±10.53 | 0.982a |
|
| |||
| Blood Pressure (BP, mmHg) | |||
| Systolic BP | 120.67±16.11 | 113.5 ± 9.74 | 0.104a |
| Diastolic BP | 76.17 ±10.65 | 70.83 ± 7.32 | 0.147a |
| Mean Arterial BP | 99.71 ± 13.58 | 90.87 ± 10.18 | 0.101a |
|
| |||
| 25OHD (ng/ml) | 17.58 ± 4.56 | 28.758 ± 6.30 | <0.001a |
|
| |||
| IFGd n(%) | 5 (42) | 7(58) | 0.157b |
|
| |||
| IGTe n(%) | 4 (33) | 3 (25) | 0.655b |
|
| |||
| Fasting Insulin (μIU/ml) | 24.92 ± 15.42 | 25.17 ± 13.67 | 0.591a |
|
| |||
| Fasting Glucose (mg/dl) | 101.67 ± 17.90 | 103.79 ± 22.83 | 0.386a |
|
| |||
| Fasting Glucose:Insulin Ratiof | 5.57 ± 3.17 | 5. 50 ± 3.58 | 0.860a |
|
| |||
| HOMAg | 6.68 ± 5.83 | 6.90 ± 5.74 | 0.736a |
|
| |||
| QUICKIh | 0.13 ± 0.01 | 0.13 ±0.01 | 0.597a |
|
| |||
| AUCi Glucose (mg/min/120min) | 16648.41± 3949.98 | 16726.56 ± 3941.80 | 0.915a |
|
| |||
| AUCi Insulin (μIU/ml/120min) | 16136.50 ± 10086.29 | 16715.57 ± 10371.76 | 0.622a |
|
| |||
| Total Testosterone (ng/dl) | 82 ± 44 | 72± 39 | 0.036a |
|
| |||
| SHBG (nmol/L) | 23.58 ±3.30 | 22.25± 2.20 | 0.718a |
|
| |||
| Androstenedione (ng/dl) | 361 ± 204 | 299 ± 126 | 0.090a |
|
| |||
| FAIj | 13.72 (4.68–26.96) | 8.42 (4.86–25.88) | 0.814c |
Continuous data are presented as mean ± standard deviation if Gaussian in distribution, and as median (interquartile range if skewed); categorical data are presented as percentage.
Student T test;
McNemar test;
Wilcoxon Sign Rank Test
Fasting glucose ≥ 100 mg/dl
2 hour glucose>=140 & <200mg/dl
G:I ratio<4.5 reflects insulin resistance 26
fasting glucose (mg/dl) x fasting insulin (mIU/ml)/405
1/log (fasting glucose (mg/dl) x fasting insulin (mIU/ml)
AUC calculated by trapezoidal rule
Free androgen index: total testosterone in nmol/L/SHBGin nmol/L X 100
Serum 25OHD levels following 1 and 3 months of supplementation were significantly higher compared to baseline (26.17±4.82 and 28.58±6.30 ng/ml respectively compared to 17.58 ±4.56ng/ml at baseline, p<0.001); the observed changes in 25OHD were comparable between vitamin D dosing regimens (data not shown).
Lowering in SBP, DBP, and mean arterial BP (MAP) was observed compared to baseline (Table 1). Significant improvements in BP parameters were observed in those with SBP ≥120mmHg and/or DBP ≥80mmHg at baseline (n= 8, SBP 129.37 ± 5.75 versus 116.5 ±8.57 mmHg, p= 0.010; DBP 81.75 ± 7.57 versus 71.87 ±5.94 mmHg, p= 0.014); significant lowering in SBP was seen in those with baseline serum 25OHD ≤20ng/ml (n= 9, 122.22 ± 11.19 versus 112.56 ±10.42 mmHg, p= 0.033). At baseline, 17% (2/12) of the previously undiagnosed met criteria for hypertension compared to 0% at trial completion (p< 0.001, Fisher’s exact test).
Significant reduction in total T and lowering in serum A were observed compared to baseline values (Table I). Indices of G homeostasis, BMI and WC were unaltered (Table I), as was physical activity (p> 0.05, data not shown).
Discussion
Vitamin D insufficiency is a modifiable risk factor for atherogenesis and hypertensive disorders [18–19] and our pilot data support this impression. Given that as little as 2mmHg decrease in SBP has been suggested to reduce CVD related morbidity and morbidity by almost 6% and all cause mortality by 3% [20], the observed mean reduction in SBP by 7mm is clinically meaningful. A lowering in SBP was previously described with 800IU of vitamin D and Ca in a cohort of elderly women [21], and we observed similar effects, albeit in a younger population.
The observed decline in androgens following 3 month supplementation with vitamin D and Ca is of interest. Reduction in total T and A (by 12 and 17% respectively) is comparable in magnitude to effect described with metformin use [22]. Since IR parameters were unaltered in our population, direct effects of vitamin D and Ca supplementation on the steroidogenesis pathway (ovarian and/or adrenal) can be hypothesized to explain the observed reduction in circulating androgens.
Although literature supports facilitatory influences of vitamin D on IR [23–25], our data fail to demonstrate any such effect. Selimoglu et al. [13], in contrast showed improvement in HOMA indices within 3 weeks of a single oral mega dose of 300,000 IU D3 in a pilot study of 11 women with PCOS, but did not observe any effect on androgens. A differential in the prevalence of morbid obesity, in the employed doses, and variations in the drug formulations may explain these contrasting findings; power constraint remains a plausible explanation. While the indices of IR were unaltered in our population, improvements in G homeostasis are suggested (participant #3, 4, 5 and 11) implying that perhaps the benefit of vitamin D supplementation may lie in mitigating progression of overt diabetes or in abnormalities of G homeostasis; a need for cautious interpretation however is underscored by evidence of deterioration in G homeostasis in participant#2 (Table II).
Table II.
Individual participant data at baseline (B) and following completion (C) of 3 month supplementation with vitamin D and calcium.
| Subject | Age (Yrs) | Racea | Vitamin D Doseb | BMI (Kg/m2) | 25OHD (ng/dl) | MAPc (mmHg) | Glucosed (mg/dl) | Insulin d (μIU/ml) | OGTT | Total Th (ng/dl) | Ai (ng/dl) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | C | B | C | B | C | B | C | B | C | B | C | B | C | B | C | ||||
| 1 | 39 | NHW | Low | 56.6 | 55.1 | 18 | 19 | 91 | 100 | 104 | 116 | 30 | 40 | IGTe | IGTe | 27 | 25 | 90 | 78 |
| 2 | 28 | H | Low | 30.5 | 30.4 | 20 | 27 | 98 | 102 | 93 | 94 | 12 | 14 | NLf | IGTe | 90 | 55 | 516 | 288 |
| 3 | 23 | H | Low | 32.6 | 32.9 | 19 | 29 | 109 | 97 | 98 | 83 | 37 | 22 | DMg | IGTe | 138 | 111 | 307 | 252 |
| 4 | 29 | NHW | Low | 40.2 | 38.2 | 14 | 37 | 111 | 95 | 101 | 106 | 15 | 28 | IGTe | NLf | 36 | 37 | 236 | 290 |
| 5 | 23 | NHW | Low | 38.1 | 38.2 | 13 | 23 | 103 | 83 | 105 | 102 | 21 | 23 | IGTe | NLf | 45 | 39 | 230 | 229 |
| 6 | 23 | H | High | 43.2 | 43.9 | 23 | 25 | 97 | 97 | 94 | 95 | 36 | 26 | NLf | NLf | 132 | 105 | 269 | 362 |
| 7 | 23 | NHW | High | 62.1 | 60.7 | 22 | 43 | 72 | 90 | 155 | 170 | 61 | 55 | DMg | DMg | 40 | 40 | 182 | 243 |
| 8 | 37 | NHW | High | 41.1 | 43.1 | 16 | 30 | 117 | 70 | 95 | 100 | 8 | 7 | NLf | NLf | 31 | 28 | 142 | 165 |
| 9 | 21 | NHW | High | 28.2 | 27.5 | 12 | 30 | 90 | 76 | 92 | 104 | 11 | 12 | NLf | NLf | 82 | 84 | 547 | 408 |
| 10 | 26 | H | High | 45.5 | 46.7 | 10 | 25 | 112 | 99 | 90 | 88 | 33 | 34 | NLf | NLf | 114 | 104 | 501 | 283 |
| 11 | 26 | NHW | High | 28.5 | 28.1 | 20 | 28 | 83 | 85 | 102 | 101 | 11 | 11 | IGTg | NLf | 132 | 134 | 732 | 538 |
| 12 | 19 | NHW | High | 31.5 | 33.2 | 24 | 27 | 110 | 92 | 87 | 85 | 24 | 30 | NLf | NLf | 114 | 111 | 577 | 455 |
NHW: Non Hispanic White; H: Hispanic
Vitamin D2 dosing regimen: 50,000IU monthly (Low); 50,000IU weekly (High)
Mean arterial blood pressure
Fasting
Impaired glucose tolerance evident during OGTT: 2 hour glucose>=140 & <200mg/d
Normal glucose tolerance test: fasting glucose<100 and 2 hour glucose <140mg/dl
Met OGTT criteria for diabetes based on 2 hour glucose level >=200mg/d
Total testosterone
Androstenedione
The small sample size, non-randomized design and variable dosing are limitations of this study; stratified analyses however failed to identify a dose related differential in studied outcomes (data not shown). Our study design precludes from assessing effects of supplementation on hirsutism and acne; longer trial duration may have allowed study of effects of intervention on signs of hyperandrogenism.
In conclusion, our pilot data suggest potential therapeutic benefits of vitamin D and Ca supplementation in ameliorating the hormonal milieu and PCOS related sequelae in women deficient in vitamin D. Appropriately designed future studies are needed to better explore the proposed benefits of vitamin D and Ca for clinical stigmata of PCOS. Given that millions are affected by PCOS worldwide [26], our observations, if substantiated by randomized controlled studies, suggest far reaching public health implications.
Acknowledgments
The authors extend their sincere appreciation to Ralph Jacob, Aida Groszmann and Christine Simpson of the YCCI core laboratory for their invaluable contributions towards completion of the study assays, to Dr’s Karl Insogna and Robert Sherwin (Endocrinology, Internal Medicine, Yale University School of Medicine) for their insightful comments and guidance, and to the YCCI and HRU staff for their expertise and help with the OGTT tests.
Footnotes
Disclosure statement: The authors have no conflicts to disclose
Declaration of Interest: This research was made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- 1.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS) arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–1899. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 2.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–6. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 3.Lakhani K, Constantinovici N, Purcell WM, Fernando R, Hardiman P. Internal carotid artery haemodynamics in women with polycystic ovaries. Clin Sci (Lond) 2000;98:661–5. doi: 10.1042/cs0980661. [DOI] [PubMed] [Google Scholar]
- 4.Shroff R, Kirschner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A. Young Obese Women with Polycystic Ovary Syndrome have Evidence of Early Coronary Atherosclerosis. J Clin Endocrinol Metab. 2007;92:4609–14. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 5.Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64:430–435. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 6.Panidis D, Balaris C, Farmakiotis D, Rousso D, Kourtis A, Balaris V, Katsikis I, Zournatzi V, Diamanti-Kandarakis E. Serum parathyroid hormone concentrations are increased in women with polycystic ovary syndrome. Clin Chem. 2005;51:1691–7. doi: 10.1373/clinchem.2005.052761. [DOI] [PubMed] [Google Scholar]
- 7.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen OE. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–83. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 8.Kotsa K, Yavropoulou MP, Anastasiou O, Yovos JG. Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril. 2009;92:1053–8. doi: 10.1016/j.fertnstert.2008.07.1757. [DOI] [PubMed] [Google Scholar]
- 9.Rashidi B, Haghollahi F, Shariat M, Zayerii F. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol. 2009;48:142–7. doi: 10.1016/S1028-4559(09)60275-8. [DOI] [PubMed] [Google Scholar]
- 10.Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, Obermayer-Pietsch B. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575–82. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- 11.Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, Kamaci M. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280:559–63. doi: 10.1007/s00404-009-0958-7. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z. Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril. 2010;93:1208–14. doi: 10.1016/j.fertnstert.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Selimoglu H, Duran C, Kiyici S, Ersoy C, Guclu M, Ozkaya G, Tuncel E, Erturk E, Imamoglu S. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest. 2010;33:234–8. doi: 10.1007/BF03345785. [DOI] [PubMed] [Google Scholar]
- 14. [last accessed 11/20/11];Health Benefits of Vitamin D and Calcium in Women with PCOS (Polycystic Ovarian Syndrome) NCT00743574; http://clinicaltrials.gov/ct2/show/NCT00743574.
- 15.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 16.NutritionQuest. [last accessed 11/20/11]; http://www.nutritionquest.com/
- 17.Borai A, Livingstone C, Ferns GA. The biochemical assessment of insulin resistance. Ann Clin Biochem. 2007;44:324–42. doi: 10.1258/000456307780945778. [DOI] [PubMed] [Google Scholar]
- 18.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–32. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6:621–30. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 20.Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65:626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 21.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 22.Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Comparison of simvastatin and metformin in treatment of polycystic ovary syndrome: prospective randomized trial. J Clin Endocrinol Metab. 2009;94:4938–45. doi: 10.1210/jc.2009-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, Jacques PF. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr. 2010;91:1627–33. doi: 10.3945/ajcn.2009.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarty MF. Poor vitamin D status may contribute to high risk for insulin resistance, obesity, and cardiovascular disease in Asian Indians. Med Hypotheses. 2009;72:647–51. doi: 10.1016/j.mehy.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle- aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 26.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–8. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]