Abstract
Background:
Chronic diseases impede the recovery trajectory of acutely injured persons with traumatic spinal cord injury (TSCI). This study compares the odds of prevalent heart disease, hypertension, diabetes mellitus, and obesity between persons with TSCI and persons with lower extremity fractures (LEF) who were discharged from acute care facilities.
Methods:
1,776 patients with acute TSCI (cases) and 1,780 randomly selected patients with LEF (controls) discharged from January 1, 1998, through December 31, 2009, from all nonfederal hospitals were identified. Data extracted from uniform billing files were compared between cases and controls in a multivariable logistic regression model controlling for sociodemographic and clinical covariables.
Results:
Thirty percent of patients with acute TSCI had at least 1 of 4 conditions compared with 18% of patients with LEF (P < .0001). Persons with acute TSCI were 4 times more likely (odds ratio [OR], 4.05; 95% CI, 1.65–9.97) to have obesity, 2.7 times more likely to have heart disease (P < .001), 2 times more likely to have hypertension (P < .001), and 1.7 times more likely to have diabetes (P = .044) at the onset of TSCI. Disproportionately more Blacks than Whites have TSCI and chronic diseases.
Conclusion:
This study suggests that there is an increased burden of cardiovascular and cardiometabolic diseases among persons with acute TSCI compared with LEF trauma controls. Unattended comorbid conditions will affect quality of life and the recovery process. This warrants continuous monitoring and management of chronic diseases during the rehabilitation process.
Key words: diabetes mellitus, heart disease, hypertension, obesity, spinal cord injury
Cardiovascular disease (CVD) results in the death of nearly a quarter of the population annually, greater than any other cause of death in the United States.1 Three precursors of CVD – diabetes, hypertension, and obesity – have a prevalence of 8.3%,2 29.9%,3 and 33.8%,4 respectively. Previous research5–7 has demonstrated that CVD is one of the leading causes of death in persons with traumatic spinal cord injury (TSCI). Hypertensive and ischemic heart disease accounts for 9.7% of deaths among persons with TSCI, which is only surpassed by respiratory infections.8 Studies5–7,9–14 have shown that persons with TSCI are at high risk for heart disease, hypertension, diabetes, and obesity. However, limited information exists on the prevalence of these comorbidities at the onset of TSCI. With the rise in the age of onset of TSCI,15 there has been a corresponding increase in the risk of heart disease and other comorbidities.13
The onset of heart disease is accelerated in the presence of comorbid conditions. It can be readily detected and treated prior to irreversible damage to the heart. Early detection and management of hypertension, diabetes, and obesity are crucial in preventing heart disease. Unfortunately, these conditions remain unnoticed and unattended when the main focus is on rehabilitation of the physically limiting spinal cord injury. A better understanding of the prevalence of these chronic conditions at the onset of TSCI is critical to avert serious health consequences and improve the quality of life.
The prevalence of less severe heart disease, hypertension, and diabetes in the general population is underestimated because a considerable portion of the population remains undiagnosed. For example, the Centers for Disease Control and Prevention (CDC) reports that approximately 2.2% of the US population remains undiagnosed for diabetes mellitus.2 The prevalence of these conditions is known among persons with newly acquired TSCI based on surveillance data,16 but the extent to which the rate compares with the general population is not known. Even if the prevalence of these conditions in persons with TSCI is presumed to be similar to the prevalence in the general population, it is highly probable that subclinical stages of these diseases may become overt with the onset of the SCI due to the stress associated with the trauma. Orakzai5 and Lee12 reported that CVD often remains undiagnosed in persons with TSCI due to their physical inactivity and impaired sensation, which increase the risk of progression of the disease and early mortality.
The purpose of this study is to assess the prevalence and quantify the odds ratios of heart disease, hypertension, diabetes, and obesity in persons with acute TSCI compared to persons with lower extremity fractures (LEF). We hypothesized that persons with TSCI are significantly more likely to have higher odds of heart disease, hypertension, diabetes, and obesity than persons with LEF after adjusting for various sociodemographic and clinical covariables. The increased baseline risk of these conditions among persons with TSCI is more likely to increase their risk of early mortality and compromise their quality of life unless continuous treatment and follow-up are implemented. To our knowledge, this is the first population-based study to compare the baseline risk of these conditions among spinal cord–injured patients with the risk in persons with LEF.
Methods
Data sources
This study relied on the South Carolina hospital discharge dataset for 1998–2009. State law mandates that all 62 nonfederal hospitals report uniform, abstracted billing data to the SC State Budget and Control Board. The contents of the data file are reported elsewhere.17 The CNS Registry System augments and validates the data through medical chart review and through interviewing a representative sample of patients. The Institutional Review Board of the Medical University of South Carolina approved this study.
Study setting and population
Patients who sustained a TSCI that resulted in hospital admission from January 1, 1998, through December 31, 2009, were eligible for the study. TSCI cases were defined as involving any primary or secondary diagnosis of an acute traumatic lesion of the spinal cord or cauda equina using ICD-9-CM18 diagnosis codes 806.0–806.9 and 952.0–952.9, fulfilling the requirements of case definition for TSCI provided by the CDC.19 We excluded patients who were coded as late effects of spinal cord injuries (907.2), persons who died while in acute care, and patients age 65 and older to minimize the influence of age-related chronic diseases. The final sample of cases was composed of 1,776 patients with newly diagnosed TSCI.
A control group of 1,780 individuals with LEF who were discharged from acute care hospitals from 2002 through 2004 was randomly selected from the statewide hospital discharge dataset. Random selection was based on a probability sample of 0.26 using SAS20 RANUNI function after restricting the sampling frame to the same eligibility criteria defined for TSCI cases. The specific ICD-9-CM codes used to identify LEF were 823–824. The selection of LEF discharges to serve as the control group emanated from the need to identify a hospital population that was relatively comparable to the general population regarding patterns of comorbidity. Persons with extremity fracture have been used as controls in other studies of central nervous system injuries.21,22
Definitions
The main independent variable – comorbidity – was comprised of 4 conditions that were selected from 31 conditions listed in the Elixhauser comorbidity scale for administrative data.23 These conditions included (1) heart disease, (2) hypertension, (3) diabetes mellitus, and (4) obesity, defined by body mass index (BMI) ≥30. Patients without any of the aforementioned conditions were defined as “none.” The ICD-9-CM codes used to identify these conditions are provided in Appendix 1. Furthermore, all diagnosis fields were searched for comorbid conditions, and the total count was categorized from 0 (none) to having 3 or more comorbidities.
Variables that were considered to be potential mitigating factors and were able to provide information about possible influence on TSCI (Table 1) were evaluated in the analysis. Injury severity was determined by translating ICD-9-CM diagnosis codes into abbreviated injury severity (AIS) using ICDMAP-90 software.24 The AIS provides an ordinal scale for the classification of injury severity according to threat to life.25 The Injury Severity Score (ISS) ranges from 1 to 75 and reflects the sum of the squares of the highest AIS score in each of the 3 most severely injured body regions to imitate the cumulative effect of multiple injuries.26 To account for this effect of multiple injuries, we dichotomized ISS as severe if it was 16 or greater and moderately severe otherwise. All 62 acute care nonfederal hospitals in South Carolina were categorized as level I, II, or III, representing medical facilities with best resources, good resources, and basic resources, respectively. These designations are based on the criteria of The Joint Commission on Accreditation of Healthcare Organizations and are granted by the Health Regulation Office of the SC Department of Health and Environmental Control. We grouped insurance into 4 major categories: (1) commercial, (2) Medicare, (3) Medicaid/indigent care, and (4) uninsured. County of residence was grouped as rural or urban based on the metropolitan statistical area designation. The remaining variables of interest were categorized as noted in Table 1.
Table 1. Clinical characteristics of persons by type of injury.
| Type of injury | ||||
|---|---|---|---|---|
| Characteristics | Total (N = 3,556) | TSCI (n = 1,776) | LEF (n = 1,780) | P |
| Comorbidity | <.0001 | |||
| Heart | 168(4.7) | 114 (6.4) | 54 (3.0) | |
| Hypertension | 486(13.7) | 302 (17.0) | 184 (10.3) | |
| Diabetes | 149( 4.2) | 80 (4.5) | 69 (3.9) | |
| Obesity | 57( 1.6) | 45 (2.5) | 12 (0.7) | |
| None | 2,696(75.8) | 1,235 (69.5) | 1,461 (82.1) | |
| Age group, years | <.0001 | |||
| 45–64 | 1,290 (36.3) | 706 (39.8) | 584 (32.8) | |
| 35–44 | 697 (19.6) | 355 (20.0) | 342 (19.2) | |
| 15–34 | 1,293 (36.4) | 653 (36.8) | 640 (36.0) | |
| 0–14 | 276 (7.8) | 62 (3.5) | 214 (12.0) | |
| Mean age (±SD) | 36.9 (±15.8) | 38.8(±15.0) | 35.1(±16.4) | <.0001 |
| Race and sex | <.0001 | |||
| Black male | 997 (28.0) | 657 (37.0) | 340 (19.1) | |
| White male | 1,509 (42.4) | 751 (42.3) | 758 (42.6) | |
| Black female | 307 ( 8.6) | 120 ( 6.8) | 187 (10.5) | |
| White female | 743 (20.9) | 248 (14.0) | 495 (27.8) | |
| Comorbidity count | <.0001 | |||
| 3 + | 201 ( 5.7) | 143 (8.05) | 58 (3.3) | |
| 2 | 273 (7.7) | 192 (10.8) | 81 (4.6) | |
| 1 | 411 (11.6) | 237 (13.3) | 174 (9.8) | |
| None | 2,671 (75.1) | 1,204 (67.8) | 1,467 (82.42) | |
| Injury Severity Score | <.0001 | |||
| Critical (ISS ≥25) | 419(11.8) | 358(20.2) | 61(3.4) | |
| Severe (ISS 16–24) | 703(19.8) | 610(34.4) | 93(5.2) | |
| Moderate (ISS 9–15) | 1,324(37.2) | 781(21.9) | 543(30.5) | |
| Mild (ISS 1–8) | 1,110(31.2) | 27(1.5) | 1,083(60.8) | |
| Residence | 0.3805 | |||
| Rural | 1,680 (47.2) | 826 (46.5) | 854 (48.0) | |
| Urban | 1,876 (52.8) | 950 (53.5) | 926 (52.0) | |
| Insurance status | <.0001 | |||
| Uninsured | 593 (16.7) | 295 (16.6) | 298 (16.7) | |
| Medicaid | 1248 (35.1) | 435 (24.5) | 813 (45.7) | |
| Medicare | 200 (5.6) | 134 (7.6) | 66 (3.7) | |
| Commercial | 1515 (42.6) | 912 (51.3) | 603 (33.9) | |
| Medical facility status | <.001 | |||
| Level I | 1782 (50.1) | 1106 (62.3) | 676 (38.0) | |
| Level II | 1391 (39.1) | 587 (33.1) | 804 (45.2) | |
| Level III | 383 (10.8) | 83 (4.7) | 300 (16.9) | |
Note: Values are given as n (%), unless otherwise indicated.
Statistical analysis
Data analyses were performed with the SAS software package.27 Descriptive statistics – t test for continuous variables and chi-square tests for proportions – were used to compare group characteristics. The associations of acute TSCI with the independent variables of interest (heart disease, hypertension, diabetes, and obesity) were assessed using univariate and multivariable logistic regression. Variables with bivariate association (P ≤ .20) were included in the multivariable model. Multicollinearity among covariables was evaluated by assessing the deviations of the regression coefficients and their standard errors in the fitted univariate and multivariate models.28 One of the variables – comorbidity count – was excluded because of its strong correlation with the main independent variable. Covariables were entered simultaneously into the model. Model fit was assessed using the Hosmer-Lemeshow goodness-of-fit test, and discriminatory capacity of the model was assessed using the area under the receiver operating characteristic (ROC) curve.29 The unadjusted and adjusted odds ratio and 95% CI are reported. Statistical significance was based on P < .05.
Results
During the 12-year study period, 1,776 TSCI cases and 1,780 LEF controls met study eligibility criteria. Mean age was 38.8 years for persons with TSCI and 35.1 years for persons with LEF. This age difference was statistically significant (P < .0001) (Table 1). A higher proportion of persons with TSCI were Black males, received care in level I hospitals, had commercial insurance, and had 1 or more comorbid conditions (all Ps < .0001). The 4 specific comorbid conditions of main interest (heart disease, hypertension, diabetes mellitus, and obesity) were significantly higher among persons with TSCI than with LEF. Thirty percent of persons with TSCI also had 1 of these 4 conditions compared with 18% of persons with LEF (P < .0001).
Table 2 shows the odds of the 4 comorbid conditions in persons with TSCI compared with LEF after adjusting for the covariables in the model. Persons with TSCI were significantly more likely to have heart disease, hypertension, diabetes, and obesity compared to controls (all Ps < .05). The strongest effect was noted between TSCI and obesity. Persons with TSCI were 4 times more likely to have obesity (OR, 4.05; 95% CI, 1.65–9.97), 2.7 times more likely to have heart diseases (P < .001), 2 times more likely to have hypertension (P < .001), and 1.7 times more likely to have diabetes (P = .044) than the controls. The result also showed that persons with TSCI were more likely to be either Black males (OR, 3.02; 95% CI, 2.28–4.02) or White males (OR, 1.53; 95% CI, 1.18–1.98). Compared to controls, persons with TSCI were 2.7 and 2.1 times more likely to have been treated and released from level I and level II facilities, respectively (both Ps < .001). In addition, persons with TSCI were more likely to have been older than 14 years of age, with the highest risk noted among persons 35 to 44 years of age (OR, 1.87; 95% CI, 1.20–2.93). However, the difference noted among age categories was not statistically different as is evident by the overlapping confidence intervals. In regard to insurance status, persons with TSCI were significantly less likely to be uninsured (OR, 0.66) or under Medicaid (OR, 0.35) compared to persons with LEF (P < .001).
Table 2. Severity adjusted association of risk characteristics with acute traumatic spinal cord injury.
| Characteristics | Adjusted odds ratio (95% confdence limit) | P |
|---|---|---|
| Comorbidity | ||
| Heart | 2.69 (1.66, 4.37) | <.001 |
| HTN | 1.97 (1.44, 2.70) | <.001 |
| Diabetes | 1.68 (1.01, 2.79) | .044 |
| Obesity | 4.05 (1.65, 9.97) | .002 |
| None | Referent | |
| Age, years | ||
| 45–64 | 1.68 (1.08, 2.61) | .021 |
| 35–44 | 1.87 (1.20, 2.93) | .006 |
| 15–34 | 1.74 (1.14, 2.66) | .01 |
| 0–14 | Referent | |
| Race and sex | ||
| Black male | 3.02 (2.28,4.02) | <.001 |
| White male | 1.53 (1.18, 1.98) | .001 |
| Black female | 1.18 (0.79, 1.75) | .426 |
| White female | Referent | |
| Residence | ||
| Urban | 1.18 (0.97, 1.43) | .104 |
| Rural | Referent | |
| Insurance status | ||
| Uninsured | 0.56 (0.43, 0.74) | <.001 |
| Medicaid | 0.27 (0.22, 0.34) | <.001 |
| Medicare | 0.72 (0.48, 1.10) | .125 |
| Commercial | Referent | |
| Medical facility status | ||
| Level I (Best) | 2.73 (1.91, 3.91) | <.001 |
| Level II (Good) | 2.14 (1.48, 3.08) | <.001 |
| Level III (Basic) | Referent | |
Significant differences existed between Blacks and Whites with TSCI regarding the distribution of comorbid conditions (Table 3). After adjusting for age, Blacks with TSCI were more than twice as likely as Whites with TSCI to have been with hypertension (OR, 2.05; 95% CI, 1.65–2.55), 1.7 times as likely to have diabetes (OR, 1.70; 95% CI, 1.20–2.40), and 1.63 times as likely to have heart diseases (OR, 1.63; 95% CI, 1.17–2.25).There was no significant difference between the racial groups regarding obesity.
Table 3. Distribution of comorbid conditions comparing Black and White persons with traumatic spinal cord injury.
| Race group |
||||
|---|---|---|---|---|
| Characteristics | Total (N = 1,776) | Black (n = 777) | White (n = 999) | Age-adjusted odds ratio (95% CI) |
| Comorbidity | ||||
| Heart | 114 ( 6.4) | 57 (7.4) | 54 (5.7) | 1.63 (1.17–2.25) |
| Hypertension | 302 (17.0) | 160 (20.6) | 142 (14.2) | 2.05 (1.65–2.55) |
| Diabetes | 80 ( 4.5) | 39 (5.0) | 41 (4.1) | 1.70 (1.20–2.40) |
| Obesity | 45 ( 2.5) | 15 (1.9) | 30 (3.0) | 0.85 (0.47–1.54) |
| None | 1,235 (69.5) | 506 (65.1) | 729 (73.0) | 1.00 |
Note: Values given as n (%).
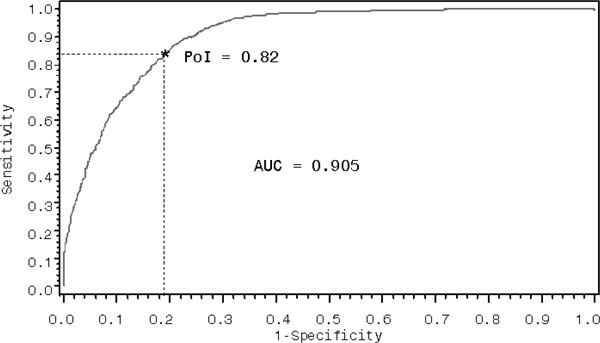
The multivariable model showed excellent discriminatory power (C = 0.91) as noted by the wider area under the curve in the ROC plot (Figure 1). ROC of 0.90 to 1.00 is the cutoff point that defines outstanding discriminatory power in a sensitivity analysis.28 The point of intersection, the ideal cutoff point that indicates the model’s ability to correctly identify true positives (sensitivity) and true negatives (specificity) with no tradeoffs,29 was 82%. Further, the model was found to have good fit (Hosmer-Lemeshow goodness-of-fit test, P = .527) and very good predictive power (max-rescaled, R2 = 0.63). Taken together, the analysis demonstrates statistically robust estimates of elevated baseline risk ratios of heart disease, hypertension, diabetes, and obesity among persons with TSCI compared with LEF after adjusting for potential confounding variables.
Figure 1. Sensitivity analysis of the logistic model.
Discussion
This large, population-based case-control study compared the prevalence of chronic comorbid conditions among persons hospitalized with acute TSCI and LEF. In support of our hypothesis, results showed that persons with TSCI have significantly higher odds of heart disease, hypertension, diabetes, and obesity than persons with LEF at the onset of SCI. The higher prevalence of chronic diseases is likely to affect the recovery trajectory, increase the risk of premature mortality, and compromise quality of life. Unlike previous studies that relied on the long-term documentation of comorbid illnesses following TSCI, this study provides important information on the prevalence of cardiovascular and cardiometabolic comorbidities at baseline. Physical limitation and psychosocial stressors—the salient predisposing factors that are common among persons with TSCI—aggravate these comorbid conditions. In addition to cardiovascular and cardiometabolic conditions, age, race, sex, insurance status, and the type of medical facility were found to be important characteristics associated with TSCI.
Higher baseline risks of cardiovascular and cardiometabolic illnesses suggest increased allostatic load—the cumulative biological burden exerted on the body through attempts to adapt to physiological demands—above and beyond the disablement of TSCI. Higher allostatic load cannot be maintained for long without dysregulation of multiple organs,30 placing persons with TSCI at risk for premature mortality. A prime example of this phenomenon is the increased release of cortisol and onset of type II diabetes and other stress-related disorders.31,32 Thus, there is a clarion call for thorough investigation and aggressive management of underlying chronic illnesses at the onset of SCI.
Although not included in the multivariable logistic analysis due to multicollinearity, the comorbidity counts among persons with TSCI were significantly higher than in persons with LEF. Consistent with epidemiological evidence of doseresponse and biological plausibility, our finding demonstrates that more comorbid conditions were firmly associated with TSCI than with LEF. Other studies have expressed similar increases in the number of comorbidities among persons with TSCI and also noted a corresponding increase in mortality rates.17,33 Specifically, the risk of mortality associated with preexisting heart disease has been documented in a recent large population-based trauma outcome study.34
There is an increased risk of early mortality due to heart disease, hypertension, diabetes, and obesity after onset of TSCI.5,7,8,12 It has been demonstrated that this risk increase is attributable to physical inactivity/sedentary living,5,10,12–14 depression,5 autonomic dysreflexia,5,9 changes in body composition,5,13 low levels of high-density lipoprotein cholesterol,10 and increased adipose tissue.10,12 In addition to the baseline risks noted in our study, other studies3,35–37 have shown that minorities, persons with disabilities (eg, TSCI), and persons with lower socioeconomic status (SES) are at substantial risk for developing heart disease, hypertension, and diabetes.
Obesity is a major precursor of heart disease, hypertension, and diabetes.1–3,9 The prevalence of obesity noted in our study (2.5%) is much lower than what is reported in the general adult US population (33.8%).4 This difference is mainly accounted for by the source of data used for our study. In the uniform billing system, a diagnosis code is assigned to a disease/condition only when it is severe enough to require clinical intervention and billable service. Therefore, the most severe forms of morbid obesity (BMI >34) are the predominant type of obesity noted in our study. Despite this gross underestimation of the prevalence of obesity in our study, the preponderance of obesity noted among person with TSCI is unlikely to be biased because hospital coding should not affect the TSCI group more than the LEF group. Persons with TSCI are at even greater risk for obesity and associated health conditions due to lack of physical activity and limited mobility.6,7,9–14,35,36
The disproportionate prevalence of TSCI among Blacks is a cause of concern. Whereas 30% of South Carolina’s population is Black, 44% of the persons with acute TSCI in the state are Black. This preponderance of TSCI among Blacks is associated with the higher prevalence of chronic diseases as noted in Table 3. Generally, there is wide disparity in overall health outcome between the races in South Carolina; the increased incidence of TSCI is yet another factor that widens the gap. Minorities are generally less healthy, less likely to receive adequate care, have lower income and employment rates, and have increased risk for early mortality.38–40 Employment translates to increased participation, and sufficient income translates to access to adequate health care and living conditions.39 Furthermore, employment can contribute to the reduction of complications like heart disease, hypertension, and diabetes, as well as raise the quality of life in persons with TSCI. Other factors that worsen the trajectory of Blacks with TSCI include the increased prevalence of hypertension41 and diabetes.13,30,37
Forty-one percent of the persons with TSCI can be classified as low income at baseline, because they are either uninsured (16.6%) or insured by Medicaid (24.5%). Persons with TSCI may be at even greater risk for serious health complications due to their typically lower SES.39,40 The cycle of poverty is perpetuated by low academic achievement, high unemployment rates,35 and lack of health insurance,40 which are aggravated by the disability following onset of TSCI.38 Earning $25,000 or less annually more than doubles the likelihood of early mortality compared with earning $75,000 or more in persons with TSCI.39 Conversely, having good financial and social support has been shown to increase the likelihood of good outcomes in persons with TSCI.39,40 Unfortunately, research35 has demonstrated that persons with TSCI rarely receive the support they need.
The primary focus during hospitalization after injury onset for persons with TSCI tends to be maximizing mobility and participation in activities. However, our findings suggest that screening for, detecting, and treating these cardiovascular and cardiometabolic conditions could be just as important. Detecting a heart condition as early as possible is especially important considering how therapeutic exercises and surgery place additional stress on the heart.5 Due to cost-containment strategies, acute care hospitals do not provide the rehabilitation care that patients need,42 and many patients cannot afford to obtain rehabilitation services elsewhere. In locations where health care personnel are unable to provide adequate preventive care to persons with acute TSCI, effort should be geared toward referral and health education.
Study strengths and limitations
Our study has several strengths. To our knowledge, it is the first study attempting to quantify the baseline prevalence of cardiovascular and cardiometabolic conditions and the impact these conditions pose in the recovery trajectory of persons with acute TSCI in a statewide population. Second, our study used standardized data collection protocol and uniform case ascertainment criteria consistent with the CDC guideline of SCI surveillance, which reduced the possibility of selection and ascertainment bias. Third, the inclusion of persons with LEF as hospital controls provided an estimation of the occurrence of the selected chronic diseases in persons with acute TSCI that approximates this pattern in the general population.
Despite these strengths, there are limitations worth noting. First, the analysis was based on administrative data primarily designed for billing purposes. As a result, the accuracy of the diagnosis might have been biased by maximization of reimbursement. Second, there is wide variability in skillset and diagnostic resources among the hospitals, and it is possible that the accuracy of the fourth and fifth digit of the diagnosis codes from underresourced hospitals might be unreliable. As a result of the incompleteness and/or inaccuracies of the fifth digit codes, we were unable to assess the effect of the completeness of lesion type on blood pressure regulation. It is known that complete cord lesions at the cervical level tend to increase hypotension whereas complete lesions at the thoracolumbar levels evoke a massive reflex sympathetic surge (autonomic dysreflexia) causing widespread vasoconstriction and hypertension. Nonetheless, a population-based study in Colorado confirmed 87% and 90% sensitivity for TSCI codes.43 Third, data did not include information on behavioral, clinical, and psychosocial risk factors that could determine the risk associated with the comorbid conditions of interest. Fourth, it is possible that there may be residual confounders, which we have not accounted for in our study. A salient example for this is prehypertension, a blood pressure range where systolic is 121 to 139 mm Hg and diastolic is 81 to 89 mm Hg. Prehypertension has a prevalence of 28% in the United States,44 and the rate of progression to hypertension is significantly accelerated among Blacks, with a median conversion time of 1.6 years versus 2.6 years for Whites.41 Failing to recognize prehypertension in a person with TSCI could be deleterious, because of the increased risk for hypertension and CVD associated with the condition. Fifth, measurement of obesity relied on gross cutoff points of BMI and not percent body fat; percent body fat is more relevant for risk factor assessment especially among persons with TSCI. Finally, the data collection year for LEF is shorter and begins midway that of TSCI. It is possible that this might introduce undercount bias in some chronic diseases that have been increasing overtime, specifically obesity and diabetes. Nonetheless, review of the prevalence of these conditions in South Carolina indicates that the median prevalence rate of 9% and 26% for diabetes and obesity, respectively, for the 12 years of observation is the average prevalence rate of 2003 and 2004 (http://www.cdc.gov/dibetes/statistics). Thus the bias due to undercount of these conditions in LEF control is minimal.
Conclusion
This study provides a comprehensive report of a comparison of the baseline risk of cardiovascular and cardiometabolic conditions between persons with TSCI and persons with LEF. Our results demonstrate that prevalence of these conditions is far greater in persons with acute TSCI than LEF. Initiation of early clinical intervention is critical to curtail the progression of these conditions and improve quality of life. Regular follow-ups and periodic monitoring of these conditions need to be integrated in the physical routines of persons with TSCI to prevent premature mortality. A prospective follow-up study of persons with acute TSCI with cardiovascular and cardiometabolic diseases is highly desirable to assess the long-term outcome of these conditions.
Acknowledgments
All authors have contributed significantly to the work submitted for consideration and declare no conflict of interest.
Financial disclosure/support: The contents of this publication were developed under grants from the Department of Education, NIDRR grant number H133B090005 (PI: James Krause, PhD) and bridge funding from the South Carolina Spinal Cord Injury Research Fund (SCIRF). However, those contents do not necessarily represent the policy of the Department of Education, and endorsement by the Federal Government should not be assumed. This publication was made possible by grant 5 R25 HL092611-04 from NHLBI/NIH. The South Carolina Department of Disabilities and Special Needs (DDSN) Head and Spinal Cord Injury Division of the South Carolina (Director Dr. Linda Veldheer) provided the core funding and data oversight for the surveillance program.
Additional contributions: The authors recognize Ms. Georgette Demian and Ms. Nicole Spivey from the South Carolina Department of Health and Environmental Control for their coordination of the abstraction process and for validating the accuracy of the medical record review report.
Appendix 1
| Disease category | ICD-9-CM codes |
|---|---|
| A. Heart diseases | |
| 1. Congestive heart failure | 398.91, 402.11, 402.91, 404.11, 404.13, 404.91, 404.93, 428.0x – 428.9x |
| 2. Cardiac arrhythmias | 426.10, 426.11, 426.13, 426.20 – 426.53, 426.60 – 426.89, 427.0x, 427.2x, 427.31, 427.60, 427.9x, 785.0x, V450, V533 |
| 3. Valvular lesions | 093.20 – 093.24, 394.0x – 397.1x, 424.0x – 424.91, 746.3x – 746.x, V422, V433 |
| 4. Cardiac COPD | 416.0x – 416.9x, 417.9x |
| 5. Pulmonary ventricular diseases | 440.0x– 440.9x, 441.2x, 441.4x, 441.7, 441.9x, 443.1x – 443.9x, 447.1x, 557.1x, 557.9x, V434 |
| B. Hypertension | |
| 1. Uncomplicated hypertension | 401.1x – 401.9x |
| 2. Complicated hypertension | 402.10, 402.90, 404.10, 404.90, 405.11, 405.19, 405.91, 405.99 |
| C Diabetes mellitus | |
| 1. Uncomplicated diabetes | 250.00 – 250.33 |
| 2. Complicated hypertension | 250.40 – 250.73, 250.90 – 250.93 |
| D. Obesity | |
| 1. Morbid obesity | 278.01 |
| 2. Obesity NOS | 278.00, V778 |
Note: COPD = chronic obstructive pulmonary disease; NOS = not otherwise specified.
References
- Centers for Disease Control and Prevention Heart disease fact sheet. 2010. http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/docs/fs_heart_disease.pdf Accessed June21, 2011
- Centers for Disease Control and Prevention National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf
- Centers for Disease Control and Prevention. Fact sheet: Health disparities in hypertension and hypertension control. http://www.cdc.gov/minorityhealth/reports/CHDIR11/FactSheets/Hypertension.pdf. [Accessed Jun 21;, 2011 ];2011
- Centers for Disease Control and Prevention Adult obesity. 2011. http://www.cdc.gov/obesity/data/adult.html Accessed July22, 2011
- Orakzai SH, Orakzai RH, Ahmadi N, et al. Measurement of coronary artery calcification by electron beam computerized tomography in persons with chronic spinal cord injury: Evidence for increased atherosclerotic burden. Spinal Cord. 2007;45(12):775–779 [DOI] [PubMed] [Google Scholar]
- Groah SL, Nash MS, Ljungberg IH, et al. Nutrient intake and body habitus after spinal cord injury: An analysis by sex and level of injury. J Spinal Cord Med. 2009;32(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen SA, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. The 2010 annual statistical report for the Spinal Cord Injury Model Systems. https://www.nscisc.uab.edu/public_content/pdf/2010%20NSCISC%20Annual%20Statistical%20Report%20-%20Complete%20Public%20Version.pdf. [Accessed Jun 28;, 2011 ];2010
- Lavela SL, Weaver FM, Goldstein B, et al. Diabetes mellitus in individuals with spinal cord injury or disorder. J Spinal Cord Med. 2006;29(4):387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirel S, Demirel G, Tukek T, Erk O, Yilmaz H. Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord. 2001;39(3):134–138 [DOI] [PubMed] [Google Scholar]
- Yekutiel M, Brooks ME, Ohry A, Yarom J, Carel R. The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia. 1989;27(1):58–62 [DOI] [PubMed] [Google Scholar]
- Lee CS, Lu YH, Lee ST, Lin CC, Ding HJ. Evaluating the prevalence of silent coronary artery disease in asymptomatic patients with spinal cord injury. Int Heart J. 2006;47(3):325–330 [DOI] [PubMed] [Google Scholar]
- Banerjea R, Sambamoorthi U, Weaver F, Maney M, Pogach LM, Findley T. Risk of stroke, heart attack, and diabetes complications among veterans with spinal cord injury. Arch Phys Med Rehabil. 2008;89(8):1448–1453 [DOI] [PubMed] [Google Scholar]
- Buchholz AC, McGillivray CF, Pencharz PB. Physical activity levels are low in free-living adults with chronic paraplegia. Obesity Res. 2003;11(4):563–570 [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. https://www.nscisc.uab.edu/public_content/pdf/Facts%202011%20Feb%20Final.pdf. [Accessed Jun 28;, 2011 ];2011
- Selassie AW, Varma A, Saunders LL, Welldaregay W. Determinants of in-hospital death after acute spinal cord injury: A population-based study. Spinal Cord. 2013;51(1):48–54 [DOI] [PubMed] [Google Scholar]
- Varma A, Hill EG, Nicholas J, Selassie A. Predictors of early mortality after traumatic spinal cord injury: A population-based study. Spine. 2010;35(7):778–783 [DOI] [PubMed] [Google Scholar]
- ICD-9-CM International Classification of Diseases, 9th rev. Clinical Modification. 3d edition, volumes 1, 2 and 3. Official authorized addendum effective October 1, 1990–HCFA. J Am Med Rec Assoc. 1990;61(8):suppl1–35 [PubMed] [Google Scholar]
- Thurman DJ, Sniezek JE, et al. Guidelines for Surveillance of Central Nervous System Injury. Atlanta, GA: Centers for Disease Control and Prevention; 1995 [Google Scholar]
- SAS Statistical Analytical Software Version 9.1.3 ed. Cary, NC: SAS Institute Inc; 2010 [Google Scholar]
- McCarthy ML, MacKenzie EJ, Durbin DR, et al. The Pediatric Quality of Life Inventory: An evaluation of its reliability and validity for children with traumatic brain injury. Arch Phys Med Rehabil. 2005;86(10):1901–1909 [DOI] [PubMed] [Google Scholar]
- Rivara FP, Koepsell TD, Wang J, et al. Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics. 2011;128(5):e1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27(4):412–422 [DOI] [PubMed] [Google Scholar]
- The Abbreviated Injury Scale 1990 Revision. Des Plaines, IL: Association for the Advancement of Automotive Medicine; 1990 [Google Scholar]
- Baker SP, O’Neill B, Haddon W, Jr., Long WB. The Injury Severity Score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196 [PubMed] [Google Scholar]
- Armitage P, Colton T. Encyclopedia of Biostatistics. Chichester, New York: J. Wiley; 1998 [Google Scholar]
- Hosmer DL. The multinomial logistic regression model. In: Logistic Regression. New York, NY: John Wiley & Sons; 2000 [Google Scholar]
- Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185(1):123–132 [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47 [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biologicalrisk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98(8):4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LL, Selassie AW, Hill EG, et al. Traumatic spinal cord injury mortality, 1981–1998. J Trauma. 2009;66(1):184–190 [DOI] [PubMed] [Google Scholar]
- Ferraris VF, SP; Sasha SP. The relationship between mortality and preexisting cardiac disease in 5,971 trauma patients. J Trauma. 2010;69:645–652 [DOI] [PubMed] [Google Scholar]
- Disability and health: overview. 2020 Topics & objectives. http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicId=9. [Accessed Jul 21;, 2011 ];2011
- Centers for Disease Control and Prevention. Fact sheet: Health disparities in obesity. http://www.cdc.gov/minorityhealth/reports/CHDIR11/FactSheets/Obesity.pdf. [Accessed Jul 13;, 2011 ];2011
- Centers for Disease Control and Prevention. Fact Sheet: Health disparities in diabetes. http://www.cdc.gov/minorityhealth/reports/CHDIR11/FactSheets/Diabetes.pdf. [Accessed Jul 13;, 2011 ];2011
- Centers for Disease Control and Prevention Fact sheet: Health disparities in education and income. 2011. http://www.cdc.gov/minorityhealth/reports/CHDIR11/FactSheets/EducationIncome.pdf Accessed July13, 2011
- Krause JS, Saunders LL, DeVivo MJ. Income and risk of mortality after spinal cord injury. Arch Phys Med Rehabil. 2011;92(3):339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett DM, Kolakowsky-Hayner SA, White JM, Cifu DX. Impact of minority status following traumatic spinal cord injury. NeuroRehabilitation. 2002;17(3):187–194 [PubMed] [Google Scholar]
- Selassie AW, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from pre-hypertension to hypertension in African Americans. Hypertension. 2011;58(4):579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab CW, Young G, Civil I; et al. DRG reimbursement for trauma: The demise of the trauma center (the use of ISS grouping as an early predictor of total hospital cost). J Trauma. 1988;28:939–946 [PubMed] [Google Scholar]
- Johnson RL, Gabella BA, Gerhart KA, McCray J, Menconi JC, Whiteneck GG. Evaluating sources of traumatic spinal cord injury surveillance data in Colorado. Am J Epidemiol. 1997;146(3):266–272 [DOI] [PubMed] [Google Scholar]
- Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control – continued disparities in adults: United States, 2005–2006. NCHS Data Brief 2008(3). http://www.cdc.gov/nchs/data/databriefs/db03.pdf Accessed July14, 2011 [PubMed]


