Abstract
Background:
Evidence suggests an elevated prevalence of cardiometabolic risks among persons with spinal cord injury (SCI); however, the unique clustering of risk factors in this population has not been fully explored.
Objective:
The purpose of this study was to describe unique clustering of cardiometabolic risk factors differentiated by level of injury.
Methods:
One hundred twenty-one subjects (mean 37 ± 12 years; range, 18–73) with chronic C5 to T12 motor complete SCI were studied. Assessments included medical histories, anthropometrics and blood pressure, and fasting serum lipids, glucose, insulin, and hemoglobin A1c (HbA1c).
Results:
The most common cardiometabolic risk factors were overweight/obesity, high levels of low-density lipoprotein (LDL-C), and low levels of high-density lipoprotein (HDL-C). Risk clustering was found in 76.9% of the population. Exploratory principal component factor analysis using varimax rotation revealed a 3–factor model in persons with paraplegia (65.4% variance) and a 4–factor solution in persons with tetraplegia (73.3% variance). The differences between groups were emphasized by the varied composition of the extracted factors: Lipid Profile A (total cholesterol [TC] and LDL-C), Body Mass-Hypertension Profile (body mass index [BMI], systolic blood pressure [SBP], and fasting insulin [FI]); Glycemic Profile (fasting glucose and HbA1c), and Lipid Profile B (TG and HDL-C). BMI and SBP formed a separate factor only in persons with tetraplegia. Conclusions: Although the majority of the population with SCI has risk clustering, the composition of the risk clusters may be dependent on level of injury, based on a factor analysis group comparison. This is clinically plausible and relevant as tetraplegics tend to be hypo- to normotensive and more sedentary, resulting in lower HDL-C and a greater propensity toward impaired carbohydrate metabolism.
Key words: body composition, cardiometabolic syndrome, cardiovascular disease, diabetes, dyslipidemia, factor analysis, spinal cord injury
Risk factor clustering is thought to better characterize cardiovascular disease (CVD) and endocrine risks that impart a health hazard. Cardiometabolic syndrome is defined through a clustering of cardiovascular risk factors, primarily identified as overweight/obesity, atherogenic dyslipidemia, hypertension, and insulin resistance.1,2 To a lesser extent, the defined risks include a pro-thrombotic state and elevated levels of pro-inflammatory cytokines. Cardiometabolic risk (CMR) is currently considered so threatening to health and well-being that it has fueled a named initiative, “Heart of Diabetes,” by the American Diabetes Association (ADA) and American Heart Association (AHA), targeting greater focus on evidence-based prevention, recognition, and treatment of all risk factors for diabetes and CVD.3
While the evidence base in spinal cord injury (SCI) is fairly robust with studies of risk factors, there is very little evidence focusing on the unique clustering of risk factors in people with SCI. Using the National Heart, Lung and Blood Institute’s National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (NCEP ATP III) guidelines,4,5 Nash and Mendez6 found that the combination of abdominal obesity, elevated fasting triglycerides (TG), low levels of fasting high-density lipoprotein cholesterol (HDL-C), hypertension, and fasting hyperglycemia was observed in more than 1 in 3 young, healthy persons with paraplegia. Wahman and Nash followed with an examination of risk and risk clustering in Swedish patients with paraplegia and found pervasive clustering of dyslipidemia, hypertension, and overweight.7
The prevailing clinical opinion is that CVD is a relatively rare but major health concern for persons with SCI.8–10 However, in the 2008 Evidence Report on Carbohydrate and Lipid Disorders After Spinal Cord Injury, which was conducted by the Minnesota Evidence-based Practice Center and published by the Agency for Healthcare Research and Quality (AHRQ),11 it was concluded that evidence for carbohydrate and lipid disorders after SCI is weak and limited due to the number of published studies, study designs, and variation in study outcomes. In response to this statement of need, the purpose of this article is to advance the strength of the evidence on cardiometabolic risk factor clustering in people with SCI utilizing more advanced analytic methods of factor analysis, a multivariate analytic technique.
Methods
Design
This was a multicenter study of CMR in subjects with chronic SCI from the National Rehabilitation Hospital in Washington, DC (NRH) and the University of Miami Miller School of Medicine/Miami Project to Cure Paralysis in Miami, FL (UM). The study was based on a cross-sectional research design and was approved by the Institutional Review Boards at both NRH and UM. Informed consent was obtained prior to subject enrollment.
Subjects
One hundred twenty-one individuals with chronic traumatic SCI residing in the community were enrolled over a 5-year period. Participants were identified through database and medical record queries and via newsletter advertisements, flyers, and Web site announcements. Individuals were screened for study inclusion according to the following criteria: motor complete (ASIA Impairment Scale [AIS] A or B) injury between C5 and T12, 18 years of age or older, at least 1 year post injury, and no prior history of traumatic brain injury (TBI), CVD, or diabetes.
Procedures
Health history and anthropometric and blood pressure assessments
A concise medical history was obtained using a structured questionnaire and included demographic information, previous surgeries, current medications, current and past smoking status, and history of CVD, diabetes, and TBI. Abbreviated neurologic exams augmented medical record reviews to confirm level and completeness of SCI.12 Resting blood pressure was determined in the seated position using the standard auscultation method previously described by the Joint National Committee (JNC),13 and subjects using hypertensive medications were identified. Height was determined by measurement in the supine position. Participants were weighed on a calibrated portable wheelchair scale. Body mass index (BMI) was calculated as the quotient of body mass and the squared height (kg/m2).
Serum analysis
Subjects were instructed to fast for 12 hours prior to their blood draw and to avoid smoking, strenuous exercise, alcohol, and caffeine for 24 hours before their appointment. Fasting blood samples were collected under antiseptic conditions using antecubital venous blood between 8:00 a.m. and 10:00 a.m. Samples collected in a glycolytic inhibitor were assayed for glucose, and those collected in a gel and lysis activator were assayed for the lipid profile and fasting insulin. Samples used for lipid, insulin, and glucose assays were centrifuged at 3,000 x g for 30 minutes to isolate platelet poor plasma and were frozen at -70°C (-94°F). At the end of each month, samples from NRH were packed in dry ice and sent to the core laboratory at UM.
Total cholesterol (TC), TG, and HDL-C were assayed on an automated analyzer (Roche Cobas-Mira) utilizing commercially available kits according to manufacturer’s instructions and run procedures.14 Low-density lipoprotein cholesterol (LDL-C) was calculated using the method of Friedewald et al15:
Glucose was assayed using the glucose oxidase method. Insulin resistance was determined using the fasting insulin concentrations and by the homeostasis model of Matthews (HOMA-IR)16 derived from the equation:
Cardiometabolic risk factors were identified utilizing threshold values as shown in Table 1. Because routine BMI tables have little applicability to the SCI population,17 Laughton’s adjustment for the SCI population was used (BMI > 22 was considered overweight and > 25 obese)18 in addition to standard BMI tables. In accordance with the National Cholesterol Education Program’s Adult Treatment Panel III,4 the authors chose to use 2 or more risk factors to define “metabolically unhealthy.” The NCEP ATP III identifies 2 or more risk factors as the classification of “multiple risk factors,” and this benchmark also determines differences in cholesterol goals and therapy options. Although the ATP III also uses 3 or more risk factors to define metabolic syndrome, absolute number of risk factors and cholesterol goals are considered primary targets, and metabolic syndrome classification is used secondarily.
Table 1. Risk criteria for inclusion into cardiometabolic risk cluster.
| Risk factor | Risk criteria |
|---|---|
| BMI, kg/m2 | Standard ≥ 25 |
| SCI-specific ≥ 22 | |
| SBP, mm Hg | < 120 |
| TC, mg/dL | ≥ 200 |
| LDL-C, mg/dL | ≥ 100 |
| HDL-C, mg/dL | Men ≤ 40 Women ≤ 50 |
| TG, mg/dL | ≥ 150 |
| HbA1c, % | < 4.4 or >6.4a |
| FBG, mg/dL | ≥ 100 |
| FI, µIU/mL | ≥20a |
| Tobacco use | Any tobacco use in the previous month |
Note: BMI = body mass index; FBG = fasting blood glucose; FI = fasting insulin; HbA1c = glycated hemoglobin; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; SBP = systolic blood pressure; TC = total cholesterol. TG, triglycerides.
Variable depending on laboratory.
Data analysis
The first phase of analysis included descriptive univariate and bivariate statistics using frequency distributions, means, and standard deviations for the main outcome variables calculated separately for 2 main study subgroups: persons with paraplegia and persons with tetraplegia. During the second phase, a group analysis based on cardiometabolic risk factors was conducted using a 2-tailed Mann-Whitney test to compare continuous data. In phase III, an exploratory principal component factor analysis of all identified CMR variables was conducted for each of the subgroups separately employing a varimax method of rotation using PASW Statistics 17 software (Predictive Analytic Software, formerly SPSS - Statistical Package for the Social Sciences). Risk factors were considered “abnormal” if values fell outside the “optimal” range listed in Table 1. Statistical significance was defined a priori to be at the .05 level.
Results
A total of 121 subjects participated in the study. Mean age of the participants was 37 ± 12 years (range, 18–73 years) and mean duration of injury was 11 ± 8 years (range, 1–36 years). Total and subgroup demographic characteristics of the participants are shown in Table 2. The gender distribution was approximately 80% male and 20% female, and race was evenly distributed among Caucasians, African Americans, and Hispanics. Approximately 60% of the population had a thoracic or lower level of injury, and approximately 40% had a cervical level of injury.
Table 2. Subject demographics.
| N (% of group) |
|||
|---|---|---|---|
| Demographic | Para (n = 73; 60.3%) | Tetra (n = 48; 39.7%) | Total N (% of total) |
| Gender | |||
| Male | 56 (76.7%) | 41 (85.4%) | 97 (80.2%) |
| Female | 17 (23.3%) | 7 (14.6%) | 24 (19.8%) |
| Race | |||
| Caucasian | 20 (27.4%) | 20 (41.7%) | 40 (33.1%) |
| African American | 29 (39.7%) | 17 (35.4%) | 46 (38.0%) |
| Hispanic | 23 (31.5%) | 10 (20.8%) | 33 (27.3%) |
| Asian | 0 | 1 (2.1%) | 1 (0.8%) |
| Other | 1 (1.4%) | 0 | 1 (0.8%) |
| Para (average ± SD) | Tetra (average ± SD) | Total (average ± SD) | |
| Age, years | 37.47 ± 13.20 | 36.94 ± 11.44 | 37 ± 12 |
| Duration of injury, years | 10.43 ± 8.27 | 10.83 ± 8.90 | 11 ± 8 |
Note: Para = persons with paraglegia; Tetra = persons with tetraplegia.
Phase I analysis
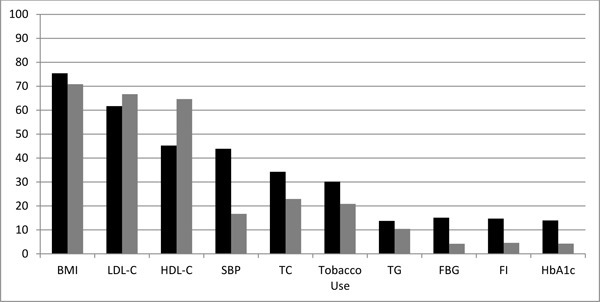
Figure 1 demonstrates that the most prevalent cardiometabolic risk factors for persons with tetraplegia and paraplegia were overweight/obesity (74%), high LDL-C (64%), and low HDL-C (42% of males; 11% of females). High systolic blood pressure was prevalent only among persons with paraplegia.
Figure 1. The cardiometabolic risk factors found in persons with paraplegia (black bar) and tetraplegia (gray bar) are displayed, with the bars representing the percent of the subject population with each risk factor. BMI = body mass index; HbA1c = glycated hemoglobin; HDL-C = high-density lipoprotein cholesterol; FBG = fasting blood glucose; FI = fasting insulin; LDL-C = low-density lipoprotein cholesterol; SBP = systolic blood pressure; TC = total cholesterol; TG = triglycerides.
Cardiometabolic risk clustering
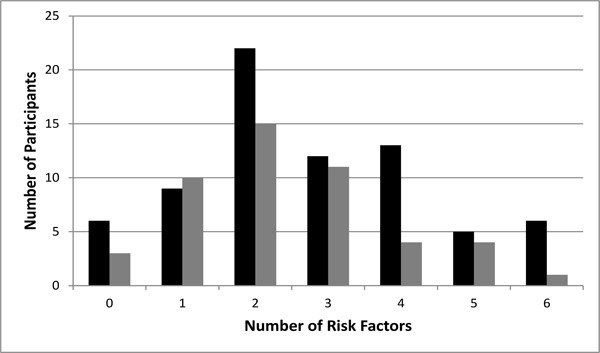
Figure 2 shows that 7.4% and 15.7% of the population had the presence of either 0 or 1 cardiometabolic risk factors, respectively, and 76.9% of the overall study population had 2 or more cardiometabolic risk factors present. Such a disposition of the CMR factors in our population suggested further detailed inquiry applying more rigorous factor analysis technique to better delineate the CMR clustering in the SCI population.
Figure 2. Cardiometabolic risk clustering in the subject population was examined. The subject population was stratified by the number of risk factors found in each subject. The absolute number of subjects with the corresponding number of cardiometabolic risk factors is shown, with black bars representing persons with paraplegia and gray bars representing persons with tetraplegia.
Phase II analysis
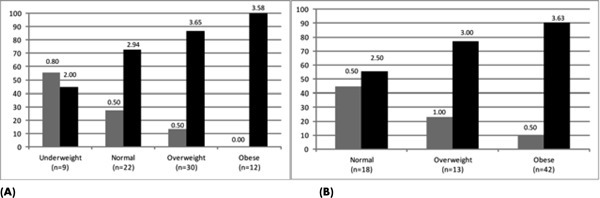
For Phase II, the study population was stratified by BMI subgroup, because of the high prevalence of overweight/obesity in the SCI population and because of anticipated physiologic differences in body composition related to severity and completeness of injury. Figures 3A and 3B show that all BMI classification levels except underweight paraplegics have at least 50% of subjects classified as metabolically unhealthy. Using standard BMI criteria, the proportion of subjects classified as underweight and normal weight who were found to be metabolically unhealthy was considerable (44% and 73% for persons with paraplegia and 60% and 69% for persons with tetraplegia, respectively). The proportion of metabolically unhealthy subjects classified as normal weight using SCI-adjusted BMI classification remained high (55% for persons with paraplegia and 71% for persons with tetraplegia).
Figure 3. Cardiometabolic risk clustering was stratified by body mass index (BMI) classification for persons with paraplegia. (A) The proportion of paraplegic subjects metabolically healthy (0 or 1 risk factor; gray bar) or unhealthy (2 or more risk factors; black bar) is shown according to standard BMI criteria. (B) The proportion of paraplegic subjects metabolically healthy (0 or 1 risk factor; gray bar) or unhealthy (2 or more risk factors; black bar) is shown according to the SCI-adjusted BMI criteria. The data label above each bar is the mean number of risk factors for each group.
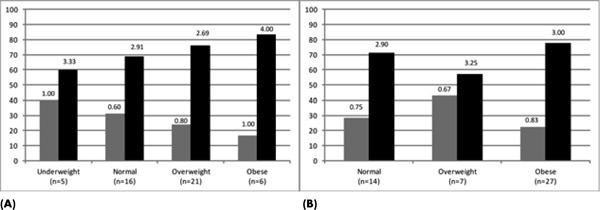
Using the stratified BMI data, a nonparametric comparison method was used to explore whether there was a difference between the groups of persons with paraplegia and tetraplegia in the prevalence of cardiometabolic risk factors (Figures 4A and 4B). A comparison analysis based on the nonparametric Kruskal-Wallis test demonstrated significant differences in the number of cardiometabolic risk factors present between all BMI subgroups in persons with tetraplegia (P = .001) and a borderline significance (P = .053) in persons with paraplegia.
Table 3A. A three-factor solution of CMR in paraplegia.
| Component | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| SBP | 0.521 | 0.139 | 0.260 |
| TC | 0.119 | 0.974 | 0.083 |
| HDL-C | -0.678 | 0.242 | 0.041 |
| TG | 0.654 | 0.096 | 0.080 |
| LDL-C | 0.146 | 0.932 | 0.048 |
| FBG | 0.088 | 0.031 | 0.891 |
| HbA1c | 0.173 | 0.080 | 0.853 |
| FI | 0.611 | 0.151 | 0.115 |
| BMI | 0.722 | 0.298 | 0.103 |
Note: Variables with high loading are indicated in bold, BMI = body mass index; FBG = fasting blood glucose; FI = fasting insulin; HbA1c = glycated hemoglobin; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; SBP = systolic blood pressure; TC = total cholesterol; TG = triglycerides.
Figure 4. Cardiometabolic risk clustering was stratified by body mass index (BMI) classification for persons with tetraplegia. (A) The proportion of tetraplegic subjects metabolically healthy (0 or 1 risk factor; gray bar) or unhealthy (2 or more risk factors; black bar) is shown according to standard BMI criteria. (B) The proportion of tetraplegic subjects metabolically healthy (0 or 1 risk factor; gray bar) or unhealthy (2 or more risk factors; black bar) is shown according to the SCI-adjusted BMI criteria. The data label above each bar is the mean number of risk factors for each group.
Phase III: Factor analysis by subgroups
The goal of the factor analysis was to identify whether the variables in question form clusters and how these clusters relate to each other in the main subgroups of paraplegia and tetraplegia. Nine cardiometabolic variables (both Framingham and non-Framingham) were subjected to exploratory factor analysis (EFA) using squared multiple correlations as prior communality estimates. Factors were extracted by the principal factor method, and a varimax (orthogonal) rotation was applied to the results. An exploratory principal component factor analysis using the varimax method of rotation revealed that each subgroup has an interpretable factor solution of both Framingham and non-Framingham risk factors. Cardiometabolic variables included in the analysis were TC, HDL-C, systolic blood pressure (SBP), BMI, TG, LDL-C, fasting blood glucose (FBG), HbA1c, and fasting insulin. Age, gender, and smoking constituted a hypothetical secondary level in our factor model and were excluded in the first step so that interrelations among the cardiometabolic risk factors could be further explored.
Factor solution for persons with paraplegia
A resulting 3-factor model, similar in its structure to the model explored while using orthogonal algorithm, represented 65.4% of the variance. The first factor accounted for 23.6% of the total variance, with the second and third factors accounting for 22.5% and 18% total variance, respectively (see Table 3A).
The identified clusters and related factor description are as follows: SBP, BMI, HDL, TG, ISI_0, with a composition that combines Factors 3 (Lipid Profile B) and 4 (Body Mass-Hypertension Profile) can be labeled as General CVD Risk Factor 1; Factor 2 composed of TC and LDL-C indicators can be defined as Lipid Profile A; and Factor 3 composed of FBG and HbA1c indicators can be labeled as Glycemic Profile.
Table 3B. A four-factor solution of cardiometabolic risk in tetraplegia.
| Component | ||||||||
| 1 | 2 | 3 | 4 | |||||
| SBP | -0.143 | -0.227 | -0.181 | 0.737 | ||||
| TC | 0.971 | 0.098 | 0.117 | 0.091 | ||||
| HDL-C | 0.093 | 0.115 | -0.760 | 0.287 | ||||
| TG | 0.372 | 0.348 | 0.661 | 0.116 | ||||
| LDL-C | 0.914 | -0.090 | 0.066 | -0.138 | ||||
| FBG | -0.086 | 0.866 | -0.083 | -0.171 | ||||
| HbA1c | 0.086 | 0.844 | 0.158 | -0.028 | ||||
| FI | 0.143 | 0.058 | 0.656 | 0.295 | ||||
| BMI | 0.070 | -0.003 | 0.207 | 0.770 | ||||
Note: Variables with high loading are indicated in bold. BMI = body mass index;
FBG = fasting blood glucose; FI = fasting insulin; HbA1c = glycated hemoglobin; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol;
SBP = systolic blood pressure; TC = total cholesterol; TG = triglycerides.
Factor solution for persons with tetraplegia
Factor analysis of variables in the group of persons with tetraplegia revealed a 4–factor model, similar in its structure to the model explored while using the orthogonal algorithm, representing 73.3% of the variance. The first factor accounted for 22% of the total variance, with the second and third factors accounting for 18% and 17% total variance, respectively. The fourth factor accounted for 15% of the total variance (see Table 3B).
The identified clusters and related factor description were as follows: Factor 1 (Lipid Profile A) consists of TC and LDL-C; Factor 2 (Glycemic Profile) includes FBG and HbA1c; Factor 3 (Lipid Profile B) is formed by the TG, HDL-C (with a reverse trend for HDL-C), and fasting insulin (ISI_0); and Factor 4 (Body Mass Hypertension) includes SBP and BMI.
Discussion
This study describes the presence and clustering of cardiometabolic risk factors in a sample of community-dwelling people with chronic SCI. Overweight/obesity was the prevailing risk factor, followed by LDL-C, HDL-C, SBP, and TC. Despite lower total caloric intake in people with SCI,19 the disturbingly high percentage (approximately three-quarters) of persons with SCI found to be overweight or obese in this study is consistent with that reported in the literature.
BMI is known to be an inaccurate predictor of fat mass in people with SCI.19 As a result, Laughton18 has suggested using the “recommended” range of 22 kg/m2 for people with SCI (where 22–25 kg/ m2 is considered overweight and 25 kg/m2 and greater is considered obese). Three-quarters of the population were overweight or obese utilizing these cut points. Had standard BMI tables been used, this proportion would have been 57%. This compares with a range of 40% to 66% being overweight or obese reported in the literature.20–25 Our results are very similar to those reported in a large study of veterans (N = 7,959) with SCI utilizing the lowered BMI cut points, in which 68% were found to be overweight (37%) or obese (31%).24
The health implications of this obesity trend are clearly more serious for persons with SCI than without disability, as body fat gains are associated with increased dyslipidemia, defects in carbohydrate metabolism, and early CVD.26 Liang et al27 examined risk pattern differences between men with SCI and nondisabled men and found that although metabolic syndrome prevalence rates were similar in each group, the men with SCI were at higher risk for abdominal obesity and low HDL-C. Additionally, Inskip et al28 found a greater accumulation of visceral fat in persons with upper thoracic SCI compared to persons with lower thoracic SCI and control groups. Visceral fat in both the able-bodied29 and SCI populations30 is associated with an increased risk of CVD and a greater incidence of metabolic syndrome. This places individuals with high thoracic SCI at a significantly greater risk than able-bodied and lower thoracic SCI individuals.
Dyslipidemia has been widely reported in SCI, and depressed plasma concentration of HDL-C14,31–36 has been a consistent finding. Confirming previous findings, we found abnormalities in LDL-C (64%) with greater frequency than HDL-C (42% in males). This is consistent with recent findings reported by Wahman et al7 in Swedish paraplegics, in which 57% had abnormal LDL-C and 43% had abnormal HDL-C.
Also similar to findings by Wahman,7 the prevalence of impaired FBG was approximately 11%. We found, though, a much higher prevalence of impaired glucose tolerance on 2-hour testing (28%). In Bauman’s 1994 study, 50% of paraplegics and 62% of tetraplegics were found to have either impaired glucose tolerance or diabetes mellitus when using a 75 g oral glucose load for testing.37 The discrepancy in impaired glucose tolerance results between our study and Bauman’s can be explained by differences in demographics of the 2 populations. Bauman’s sample was comprised of 100 male veterans, whereas our population was only 80% male. His population was older (51 years for persons with paraplegia and 47 years for persons with tetraplegia vs 37 years in our sample), had a longer duration of injury (17 years for persons with paraplegia and 19 years for persons with tetraplegia vs 10 and 11 years), and was comprised of more persons with tetraplegia (50% vs 40% in our sample).
The importance of these findings is related to subsequent cardiometabolic risk clustering. Clustering of risk factors refers to the unique combinations of risk factors in populations that may impart a health hazard. Throughout the last decade, clusters of cardiovascular risk factors have been described as a distinct metabolic syndrome (currently referred to as cardiometabolic syndrome), which was primarily defined as the presence of 3 or more of the following: overweight/ obesity, atherogenic dyslipidemia, hypertension, and insulin resistance.1,2 To a lesser extent, the defined risks include a pro-thrombotic state and elevated levels of pro-inflammatory cytokines, both of which have been reported after SCI.38–40 Risk clustering has been linked with increases in CVD-related morbidity and mortality and worsens non-linearly when additional risk factors are identified.
Our results demonstrate a disturbingly high proportion of risk clustering (76.9%) in this population with SCI. Nash and Mendez6 conducted one of the initial studies of risk factor clustering in people with SCI. Utilizing ATP III guidelines, the authors found that the combination of abdominal obesity, elevated fasting TG, low levels of HDL-C, hypertension, and fasting hyperglycemia was observed in more than 1 of 3 young, healthy persons with paraplegia. Defining 2 or more cardiometabolic risk factors as “metabolically unhealthy” and stratifying by BMI, the majority of the population was metabolically unhealthy, and the proportion of the population with risk clustering increased with increasing BMI. Because roughly 65% of the population with “normal” BMI had between 2 and 3 risk factors indicates that BMI may not be an adequate predictor of risk. Examination of the presence of cardiometabolic risk clustering by BMI subgroup suggests that Laughton’s18 adjustment for SCI only marginally improves risk prediction based on CMR clustering, hence more refinement is needed. For example, there was only minimal reduction in the proportion of individuals classified as “normal weight” who were metabolically unhealthy utilizing the cut point of 22 kg/m2. In fact, there was still a greater proportion of metabolically unhealthy (62%; 2.7 risk factors) compared with metabolically healthy in this group. This indicates that if BMI is to be used for risk prediction in SCI, an even more restrictive classification is needed, especially in the “lower” BMI categories. Alternatively, the possibility remains that the use of BMI in SCI be jettisoned in favor of more accurate body fat assessment, such as by dual x-ray absorptiometry, or altogether due to the significant body composition changes that occur after SCI.
The factor analysis conducted in this study provides more in-depth details on risk clustering and interactions between risk factors in persons with SCI. Our main finding revealed variations in factor structure between persons with paraplegia and tetraplegia, indicating the influence of level of injury on risk factor development and interactions. Three main factors identified in persons with paraplegia demonstrated a more intense interaction between SBP, BMI, HDL, TG, and fasting insulin. At the same time, factor analysis identified a similarity between tetraplegia and paraplegia groups on the clustering of TC and LDL-C and FBG and HbA1c that formed 2 distinct factors in both populations, suggesting that association between TC and LDL-C, as well as FBG and HbA1c, are not impacted by the level of injury.
It is important to emphasize that the main difference between the groups was found in the clustering of risk factors that include BMI and SBP, which is consistent with the clinical observation of the uncertain predictive status of the BMI and SBP in the population with SCI. The amount of variance explained by each factor in each subgroup is substantial, which supports the hypothesis that CMR clustering does occur in SCI. These results are compared with those of a study by Jones, Legge, and Goulding,17 which had a similar study population (age range of 16–52 years and mean duration of injury of 10.3 years) and analyzed cardiometabolic risk factor clustering utilizing factor analysis in a sample of 20 men with SCI. A 3-factor model in the subgroup of persons with paraplegia and a 4-factor model in the subgroup analysis of persons with tetraplegia were characterized by clustering of adiposity (fat percent, trunk fat) and impaired glucose tolerance (postload insulin and glucose), dyslipidemia and insulin, and a postabsorptive factor (fasting plasma insulin and fasting glucose). Our model utilized slightly different risk factors in subgroups of persons with paraplegia and tetraplegia and found the dyslipidemia factor to be strongest, followed by adiposity interacting with blood pressure and insulin, glycemic indicators, and then dyslipidemia associated with low HDL-C and elevated TG. This supports the hypothesis of multiple etiologies in the development of cardiometabolic risk in people with SCI.41 It also interesting to note that the BMI and SBP were the only variables that resulted in different factor composition in 2 study subgroups. This is consistent with well-established clinical differences in SBP between persons with paraplegia and tetraplegia and a likely greater disruption in body composition based on level of injury. The most loaded (first) factor in a 3-factor solution for the paraplegic group included BMI and SBP variables merged with the HDL, TG, and fasting insulin; whereas in the group of persons with tetraplegia, the BMI and SBP formed a separate factor. The latter can be attributed to the fact that persons with tetraplegia are characterized by a low blood pressure and more disrupted body composition compared to the persons with paraplegia. We can further suggest that considering those indicators as consistent predictors of cardiometabolic risk in persons with SCI should be individualized depending on the level of injury in the target population.
Our results are consistent with the findings from a previous study42 and suggest that cardiometabolic risk factor clustering may be dependent on level of injury. This is clinically plausible and relevant as persons with tetraplegia tend to be hypo- to normotensive and less physically active, resulting in lower HDL-C than in persons with paraplegia. Further, persons with tetraplegia tend to have a greater propensity toward impairment of carbohydrate metabolism.
Study limitations
There are several limitations of this study. The cross-sectional design did not allow us to explore the interplay between identified factors over time. Also, relatively small sample size in each of the studies subgroups limited our use of various factor models to the traditional varimax rotation, not allowing greater exploration of the nature of relationships between the factors. Finally, we did not utilize age and gender as a covariate for our outcome model. An exploration of the SCI demographics could be beneficial to clarify the clustering of risk factors depending on age and gender differences.
Conclusion
Excessive body fat, elevated LDL-C, SBP and TC, and low HDL-C were the predominant risk factors. Factor analysis indicates multiple interactions, most likely high body fat interacting with glucose intolerance and insulin resistance and dyslipidemia, all of which develop insidiously over many years. The majority of the population had cardiometabolic risk clustering, which was not predicted by BMI category.
Many, if not all, of these risk factors are modifiable by therapeutic lifestyle changes (diet and physical activity) and pharmacotherapy. Therefore, based on these findings in the context of the evidence base, we recommend early and regular monitoring for these cardiometabolic risk factors and characteristic risk clustering in people with SCI so that opportunities for prevention can be taken before disease develops. For persons found to be at higher risk of disease, we recommend that clinicians critically apply existing guidelines (NCEP ATP III, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, and the American Diabetes Association) to the care of people with SCI.4,13,43
Acknowledgments
Financial support/disclosures: This project was funded by NIDRR grants H133B0331114 and H133B090002, the NRH Rehabilitation Research and Training Center in the Rehabilitation of Individuals with Spinal Cord Injury. Statistical support for this project was provided through the Medstar Health Research Institute, a component of the Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) and supported by grant U54 RR026076-01 from the NCRR, a component of the National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. The authors have no conflicts of interest.
References
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359 [DOI] [PubMed] [Google Scholar]
- Haffner SM. Obesity and the metabolic syndrome: The San Antonio Heart Study. Br J Nutr. 2000;83(suppl 1):S67–S70 [DOI] [PubMed] [Google Scholar]
- AHA/ADA. Causes, initiatives and programs. Heart of diabetes. http://americanheaert.mediaroom.com/index.php?s=23&cat+9. [Accessed Aug 3;, 2009 ];2008
- Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Castelli WP. Epidemiology of coronary heart disease: The Framingham study. Am J Med. 1984;76:4–12 [DOI] [PubMed] [Google Scholar]
- Nash MS, Mendez AJ. A guideline-driven assessment of need for cardiovascular disease risk intervention in persons with chronic paraplegia. Arch Phys Med Rehabil. 2007;88:751–757 [DOI] [PubMed] [Google Scholar]
- Wahman K, Nash MS, Westgren N, et al. Cardiovascular disease risk factors in persons with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med. 2010;42(3):272–278 [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: Assessment of risk factors. Spinal Cord. 2008;46:466–476 [DOI] [PubMed] [Google Scholar]
- Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: An overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–152 [DOI] [PubMed] [Google Scholar]
- Nash MS, Gintel SL, Mendez AJ, et al. Elevated CRP and vascular disease after SCI: Inflammatory epiphenomenon or pathologic agent? [abstract]. J Spinal Cord Med. 2006;29(3):252 [Google Scholar]
- Wilt TJ, Carlson FK, Goldish GD, et al. Carbohydrate and lipid disorders and relevant considerations in persons with spinal cord injury. Evidence Report/ Technology Assessment No. 163 (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-02-0009). AHRQ Publication No. 08-E005. Rockville, MD: Agency for Healthcare Research and Quality; January2008 [PMC free article] [PubMed] [Google Scholar]
- ASIA Neurological Standards Committee International Standards for Neurological Classification of Spinal Cord Injury. 6th ed. Chicago IL: American Spinal Injury Association; 2002 [Google Scholar]
- National High JNC-Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7). Hypertension. 2003;42(6):1206–1252 [DOI] [PubMed] [Google Scholar]
- Nash MS, Jacobs PL, Mendez AJ, et al. Circuit resistance training improves the atherogenic lipid profiles of persons with chronic paraplegia. J Spinal Cord Med. 2001;24:2–9 [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugation. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- Jones LM, Legge M, Goulding A. Factor analysis of the metabolic syndrome in spinal cord-injured men. Metabolism. 2004;53(10):1372–1377 [DOI] [PubMed] [Google Scholar]
- Laughton GE, Buchholz AC, Martin Ginis KA, et al. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–762 [DOI] [PubMed] [Google Scholar]
- Groah SL, Nash MS, Ljungberg I, et al. Nutrient intake and body habitus after spinal cord injury: An analysis by sex and level of injury. J Spinal Cord Med. 2009;32(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury: A retrospective study. Spinal Cord. 2006;44:92–94 [DOI] [PubMed] [Google Scholar]
- Tomey K, Chen D, Wang X, et al. Dietary intake and nutritional status of urban community-dwelling males with paraplegia. Arch Phys Med Rehabil. 2005;86:664–671 [DOI] [PubMed] [Google Scholar]
- Johnston MV, Diab ME, Chu BC, et al. Preventive services and health behaviors among people with spinal cord injury. J Spinal Cord Med. 2005;28:43–54 [DOI] [PubMed] [Google Scholar]
- Anson CA, Shepherd C. Incidence of secondary complications in spinal cord injury. Int J Rehabil Res. 1996;19:55–66 [DOI] [PubMed] [Google Scholar]
- Weaver FM, Collins EG, Kirichi K, et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: A retrospective review. Am J Phys Med Rehabil. 2007;86:22–29 [DOI] [PubMed] [Google Scholar]
- Rajan S, McNeely MJ, Warms C, et al. Clinical assessment and management of obesity in individuals with spinal cord injury: A review. J Spinal Cord Med. 2008;31:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki KC, Briones ER, Langbein WE, et al. Associations between serum lipids and indicators of adiposity in men with spinal cord injury. Paraplegia. 1995;33(2):102–109 [DOI] [PubMed] [Google Scholar]
- Liang H, Chen D, Wang Y, et al. Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil. 2007;88:1198–1204 [DOI] [PubMed] [Google Scholar]
- Inskip J, Plunet W, Ramer L, et al. Cardiometabolic risk factors in experimental spinal cord injury. J Neurotrauma. 2010;27:275–285 [DOI] [PubMed] [Google Scholar]
- Kuk JL, Katzmarzyk PT, Nichaman MZ, et al. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14:336–341 [DOI] [PubMed] [Google Scholar]
- Gater DR., Jr.Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18(2):333–351 [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Zhong YG, et al. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia. 1992;30:697–703 [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Raza M, et al. Coronary artery disease: Metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med. 1992;59(2):163–168 [PubMed] [Google Scholar]
- Bauman WA, Adkins RH, Spungen AM, et al. Is immobilization associated with an abnormal lipoprotein profile? Observations from a diverse cohort. Spinal Cord. 1999;37(7):485–493 [DOI] [PubMed] [Google Scholar]
- Bostom AG, Toner MM, McArdle WD, et al. Lipid and lipoprotein profiles relate to peak aerobic power in spinal cord injured men. Med Sci Sports Exerc. 1991;23:409–414 [PubMed] [Google Scholar]
- Zlotolow SP, Levy E, Bauman WA. The serum lipoprotein profile in veterans with paraplegia: The relationship to nutritional factors and body mass index. J Am Paraplegia Soc. 1992;15:158–162 [DOI] [PubMed] [Google Scholar]
- Washburn RA, Figoni SF. High density lipoprotein cholesterol in individuals with spinal cord injury: The potential role of physical activity. Spinal Cord. 1999;37:685–695 [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: A model of premature aging. Metabolism. 1994;43:749–756 [DOI] [PubMed] [Google Scholar]
- Frost F, Roach MJ, Kushner I, et al. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86:312–317 [DOI] [PubMed] [Google Scholar]
- Liang H, Mojtahedi MC, Chen D, et al. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil. 2008;89:36–41 [DOI] [PubMed] [Google Scholar]
- Wang TD, Wang YH, Huang TS, et al. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc. 2007;106:919–928 [DOI] [PubMed] [Google Scholar]
- Hanson RL, Imperatore G, Bennett PH, et al. Components of the metabolic syndrome and incidence of type 2 diabetes. Diabetes. 2002;51:2642–3127 [DOI] [PubMed] [Google Scholar]
- Groah SL, Nash MS, Ward EA, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31(2):73–80 [DOI] [PubMed] [Google Scholar]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]


