Abstract
Background:
Pressure ulcers (PUs) are a common secondary condition associated with spinal cord injury (SCI). PUs can potentially interfere with activities of daily living, occupational duties, and rehabilitation programs, and in severe cases they may threaten life. Functional electrical stimulation (FES) cycling has been proposed as an activity that may decrease the risk of PUs through the promotion of increased blood flow and thickening of the gluteus maximus. The purpose of this pilot study was to measure the effects of home-based FES cycling on the average and maximal seat pressure of wheelchair-reliant individuals with SCI.
Method:
Eight male veterans with C5-T6 SCI participated in FES cycling 3 times per week. Cycling parameters were individualized depending on the comfort of the participants and the amount of current needed to perform the cycling activity. Pressure mapping was completed immediately before and after the 8 weeks of FES cycling with the measurement performed by a force sensitive application (FSA) 4 pressure mapping system.
Results:
The mean average seat pressure decreased by 3.69 ± 4.46 mm Hg (35.57 ± 11.99 to 31.88 ± 13.02), while the mean maximum seat pressure decreased by 14.56 ±18.45 mm Hg (112 ± 34.73 to 98.36 ± 25.89). Although neither measurement was statistically significant, there was a strong trend toward a reduction in average and maximal seat pressure (P = .052 and P = .061, respectively).
Conclusion:
The positive trend of decreased seat pressure in our study creates incentive for further investigation of the effects of electrical stimulation activities on seat pressure and the prevention of PUs.
Key words: functional electrical stimulation cycling, pressure ulcer, seat pressure mapping
Pressure ulcers (PUs) are among the most common secondary conditions associated with spinal cord injury (SCI). According to the Model SCI System Statistical Center, approximately 15% of individuals with SCI will develop a PU within the first year of injury and approximately 27% over the first 25-year period post injury.1 Additionally, 40% to 80% of those who develop a PU will have a recurrence.2 PUs are also among the most troublesome as they potentially interfere with activities of daily living, occupational duties, and rehabilitation programs. In severe cases, PUs may be life threatening. Monetarily, they account for approximately 25% of the cost associated with SCI in the United States, totaling 1.6 billion dollars annually and 70,000 dollars per full-thickness PU.3,4
PUs are also commonly known as pressure sores, pressure wounds, or decubitus ulcers. Typically, PUs result from constant pressure on the skin in areas of the body where there is a boney prominence near the surface, such as ischial tuberosities, sacrum, greater trochanters, and calcaneus and olecranon processes.3
Although there are many factors that play a role in the development of PUs, such as immobility, malnutrition, decreased blood circulation, and poor hygiene, the primary cause is the restriction of blood supply to soft tissue as a result of tissue compression between the external barrier to the skin (ie, bed, wheelchair seat, etc) and the internal bony prominence.5 When compression is sustained, the decrease in blood supply and oxygen to the local tissue results in tissue damage.6,7 Sprigle and Sonenblum assert that even though the underlying causes of PU are quite complex, with multiple factors influencing tissue breakdown, PUs do not occur without forces or pressure being placed on soft tissue. Because the loading is the defining characteristic of PUs, it is reasonable to believe that the magnitude and duration of loading are key factors.8
PUs are divided into 4 stages with increasing degree of severity. In stage 1, the skin is not broken but is red or discolored. The redness or change in color does not fade within 30 minutes after pressure is removed. In stage 2, the epidermis or topmost layer of the skin is broken, creating a shallow open sore. Drainage may or may not be present. For stage 3, the break in the skin extends through the dermis (second skin layer) into the subcutaneous and fat tissue. The wound is deeper than in stage 2. Finally, in stage 4, the breakdown extends into the muscle and can extend as far down as the bone. Usually lots of dead tissue and drainage are present.9
Persons with SCI are particularly at risk due to their reliance on wheelchairs and their loss of sensation, which hinders their ability to feel pressure. The atrophied state of the gluteal muscles also decreases the amount of soft tissue and vascularity, which predisposes individuals with SCI to PUs.7
Because of prolonged sitting due to paralysis, high pressure areas such as the ishium, greater trochanters, and the sacrum are especially at risk. Pressure relief techniques such as wheelchair pushups and directional trunk leaning in combination with mechanical pressure relief via tilt-in-space wheelchairs are designed to help decrease the risk of PUs. Likewise, a variety of seat cushions containing foam, gel, air, or a combination of these have been devised to maintain a healthy seat-buttocks interface. However, PUs remain a frequent problem for many of the 1.4 million wheelchair users in the United States.10 In fact, the incidence of PUs is reported to be as high as 38% in acute care, 23% in long-term care, and 17% in home environments.11
Garber and Rintala conducted a 3-year retrospective study on VA Medical Center out-patient veterans with SCI and found that 39% had been treated for PUs. Their study illustrates the magnitude of the problems of PUs associated with community-dwelling veterans with SCI.12
Electrical stimulation has been used over the past 4 decades in an attempt to assist in the prevention of PUs. Strategies include altering the contour of the buttocks to enhance its surface interface with the seat and increasing the blood flow and soft tissue area of the gluteus maximus.13–16
In 1989, Levine and associates applied bilateral electrical stimulation to the guteal muscles and found simultaneous reduction of pressure under the ischial tuberosities with redistribution of pressure to lower risk areas of the seat.13 This demonstrated that relatively low levels of electrical stimulation could positively alter the seating interface. Later, Levine and colleagues studied the effectiveness of reducing seat pressure through the reconfiguration of the shape of the buttocks during low-level electrical stimulation of the gluteal muscles.17 Again the result was reduced seat pressure. The authors theorized that reduced seat pressure may assist in the prevention of PUs. Ferguson et al applied electrical stimulation to the quadriceps muscles and found that the greater the knee extension movement, the more significant the decreases in pressure at the ischial tuberosity.18 A decade later, Bogie and Triolo used implanted neuromuscular stimulation of the gluteal muscles to assist individuals with standing and transfer activities.14 In addition, they measured seat interface pressures and found that after 8 weeks of electrical stimulation, the ischial regions of the buttocks showed decreased pressure in a seated position. This alteration was matched with increases in tissue oxygen levels in the ischial area. These changes provided evidence that neuromuscular electrical stimulation can benefit tissue health.14
Curtis and colleagues studied the use of intermittent electrical stimulation on the triceps surae of rats for 5 to 10 seconds every 10 minutes and found that it was effective in reducing deep muscle tissue damage caused by 28% and 38% body-weight pressures.16 Van Londen and associates completed a similar study on humans and found that the seat–buttocks interface pressure decreased whether the gluteal muscles were electrically stimulated simultaneously or alternately.19
Functional electrical stimulation (FES) cycling has been proposed as an activity that may decrease the risk of PUs through the promotion of increased blood flow and thickening of the gluteus maximus.20 Petrofsky et al compared the incidence of PUs in persons who performed FES cycling over a 2-year period with a non–FES cycling control group and found that the FES cycling group developed 90% fewer PUs.21 Nevertheless, research is lacking concerning the effects of FES cycling on seat pressure and its association with PUs. The purpose of this pilot study was to measure the effects of home-based FES cycling on the average and maximal seat pressure of wheelchair-reliant individuals with SCI.
Methods
Subjects
Eight male veterans with posttraumatic C5-T6 American Spinal Injury Association Impairment Scale (AIS) A-C SCI participated in the current study. The veterans were all wheelchair-reliant and at least 6 months post injury. The study inclusion criteria included US veterans (18–70 years of age) with C5-L4 AIS A-C SCI. Participants needed to be wheelchair reliant and have the ability to respond with electrically stimulated muscle contractions in the paralyzed limbs. The exclusion criteria included uncontrolled hypertension, uncontrolled coronary artery disease, uncontrolled autonomic dysreflexia, uncontrolled pain, fragility bone fracture, PUs greater than stage 2, deep venous thrombosis within the past 3 months, pregnancy, and any physical limitation that would preclude the ability to perform the FES cycling activity.
All participants reviewed and signed a written VA Human Subjects Research Consent form. This study was approved by a VA Medical Center institutional review board. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed.
FES cycling
All participants were screened and cleared for participation by their physician prior to starting the home-based FES cycling program. The participants also safely completed a successful trial of FES cycling at our SCI exercise laboratory prior to beginning the home-based FES cycling program.
During this study, an RT300SL FES cycle (Restorative Therapies Inc., Baltimore, MD) was placed in each participant’s home. The RT300 FES cycling system electrically stimulated the gluteal, quadriceps, and hamstring muscles through wires connected to surface electrodes. The Internet connectivity of the RT300SL allowed clinicians to remotely make alterations to optimize cycle parameters and exercise levels.
All participants were asked to cycle 3 times per week with at least 1 day of noncycling between sessions. Cycle parameters were individualized depending on the comfort of the participants and the amount of current needed to produce strong visible muscle contractions resulting in active cycling activity without motor support. The cycling parameters ranged from 70 to 140 milliamps (mA) for electrical current amplitude and 250 to 400 microseconds (µs) for pulse width, and 33 hertz (Hz) was maintained for frequency. Speed was advanced between 30 and 50 revolutions per minute (rpm) with an initial resistance of 0.5 newton meters (Nm). The RT300 possessed an option allowing automatic adjustment of pedal resistance to fit a preset speed. Cycling duration was increased over the 8-week period until a goal of between 40 and 60 minutes of continuous active FES cycling was achieved. Participants and participant helpers were provided training concerning the placement of electrodes and the FES cycling system.
Electrodes were placed on the skin as follows: gluteus maximus – 2 electrodes were placed parallel and on the bulk of the muscle belly of each buttock with 3 to 4 centimeters (cm) between electrodes; quadriceps – 1 electrode was placed on the skin 2 to 3 cm above the superior aspect of the patella over the vastus medialis muscle and the other lateral to and 30 cm above the patella over the vastus lateralis muscle; hamstrings – 1 electrode was placed 2 to 3 cm above the popliteal fossa and the other electrode 30 cm above the popliteal fossa.
Pressure mapping
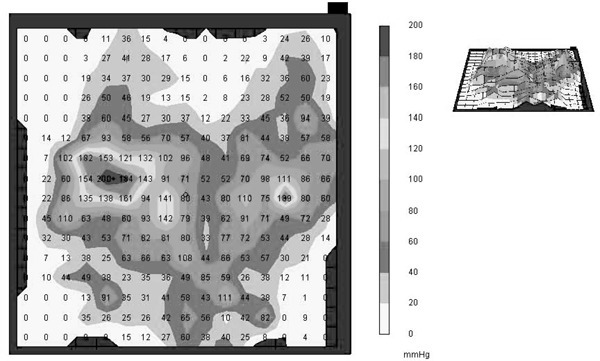
Interface pressure mapping is a valuable tool for identifying areas of high pressure at the buttocks–seat interface. It is commonly used to determine which type of seat cushion is most advantageous for maintaining safe seat pressures for persons who are wheelchair reliant.8 However, for this study, it was used to detect changes in seat pressure before and after an 8-week FES cycling program.8 Pressure mapping was completed immediately before and after the 8 weeks of FES cycling with the measurement performed by a force sensitive application (FSA) 4 pressure mapping system (Vista Medical, Henlow Bay, Fort Garry, Manitoba, Canada). This process involves using a thin sensor cover that is placed on the seating surface to quantify the pressure between the seat surface and the participant’s buttocks. Pressure was measured statically as the participant sat in an upright position on his or her seat cushion and wheelchair. Both maximal and average seat pressure readings were taken after 1 minute of nonmovement by the participant to allow for stabilizing and settling of the buttocks-seat interface (Figure 1). The FSA4 pressure mapping device was calibrated prior to testing procedures to increase accuracy.
Figure 1. Representation of a FSA4 pressure map showing average and maximal pressures measured.
Analyses
Paired samples t tests were used to compare both average seat pressure and maximal seat pressure to determine possible differences between the variables. Two-tailed paired samples t tests were used rather than 1-tailed because of limited prior evidence and the absence of a hypothesis-driven direction. The results were reported as the mean differences.
Results
The mean age of participants was 39.1 ±14.70 years, with an age range from 22 to 64 years. The average time since injury (TSI) was 6.38 ± 7.02 years, with a range from 0.5 to 19 years (Table 1). All participants completed the 8 weeks of FES cycling with no reports of PUs (Table 1).
Table 1. Physical characteristics of subjects.
| Subject | Age, years | Weight, kg | Height, cm | LOI | AISa | TSI, years | Sex |
|---|---|---|---|---|---|---|---|
| 1 | 64 | 74.62 | 170.18 | C5 | B | 1.0 | M |
| 2 | 31 | 68.04 | 175.26 | T4 | A | 12 | M |
| 3 | 31 | 88.09 | 185.42 | C7 | B | 0.5 | M |
| 4 | 22 | 58.2 | 182.88 | C6 | C | 0.5 | M |
| 5 | 31 | 73.94 | 172.72 | C7 | A | 4.0 | M |
| 6 | 37 | 68.17 | 182.37 | C6 | B | 12 | M |
| 7 | 38 | 73.94 | 185.42 | C6 | C | 19 | M |
| 8 | 59 | 77.31 | 54.94 | T6 | A | 2.0 | F |
Note: AIS = American Spinal Injury Association Impairment Scale; F = female; LOI = level of injury; M = male; TSI = time since injury.
A = no motor or sensation below injury; B = no motor but some sensation below the level of injury; C = some motor and some sensation below the level of injury.
Five of the 8 participants showed decreases in maximal seat pressure, while all but 1 participant showed decreases in average seat pressure. The mean average seat pressure decreased by 3.69 ± 4.46 mm Hg (35.57 ± 11.99 to 31.88 ± 13.02), while the mean maximum seat pressure decreased by 14.56 ± 18.45 mm Hg (112 ± 34.73 to 98.36 ± 25.89). Although neither measurement was statistically significant, there was a strong trend toward a reduction in average and maximal seat pressure (P = .052 and P= .061, respectively).
Discussion
The development of therapeutic modalities to decrease seat pressure and prevent PUs remains a requisite to improving the quality of life of persons who are wheelchair reliant. In our pilot study of 8 participants, we were able to demonstrate a trend toward reduced average and maximal pressure at the seat–buttocks interface. This provides encouragement for further investigation concerning the use of FES cycling for the reduction of seat pressure, albeit with a larger sample size.
It has been proposed that reducing the seat–buttocks interface pressure may assist in the prevention of PUs.20,21 The results of our pilot study support the idea set forth by Bogie and Triolo that electrical stimulation to the gluteus maximus may reduce seat pressure due to the increase in soft tissue through the hypertrophy of the muscle cells.14 In their study, they observed a decrease in pressure under the ischial tuberosities after 8 weeks of electrical stimulation during standing and transfer activities. Our pilot work showed a trend toward generalized average and maximal seat pressure at the seat–buttocks interface after 8 weeks of electrical stimulation during FES cycling. In both studies, the gluteus maximus received electrical stimulation. Furthermore, these results may help explain the findings of Petrofsky et al, who reported a 90% decrease in prevalence of PUs in participants after 2 years of FES cycling.21
Kim and associates tested sub–motor-threshold electrical stimulation on 6 adult males with complete SCI. There was an initial increase in transcutaneous oxygen tension, but it was not sustained. In addition, there were no changes in gluteal muscle area. These results caused the authors to conclude that sub–motor-threshold electrical stimulation is unlikely to prevent ulcers and that muscle contractive responses are critically important to this process.22
Due to the positive trend of decreased seat pressure in our study, the statistically significant decrease in surface pressure under the ischial tuberosities found by Bogie and Triolo and the marked decrease in prevalence of PUs among persons performing FES cycling found by Petrofsky, it is important to continue to investigate the effects of electrical stimulation activities on seat pressure and the prevention of PUs.
Limitations
Limitations of the current study include the small number of participants (8) and the relatively short period of FES cycling intervention (8 weeks). We did not record pressure variations in specific locations of high risk of PUs, such as the ischial tuberosities. Additionally, we did not use a standard seat surface, but rather measured seat pressure while the participants sat on their own seat cushions. We recorded and verified that the same cushions were used for measurements over the 8 weeks, however we could not account for possible shape or property changes of the respective cushions. Finally, there was a wide range of ages, levels of injury, and time since injury among the participants that could also affect tissue resilience and the results of the study.
Conclusion
Because the results of our study showed a trend and not statistically significant results, we cannot make declarative statements concerning the decrease of seat pressure or the benefits concerning PUs. However, our results do show a trend that supports the evidence set forth by others. We believe that our study helps to provide impetus toward the further study of seat pressure reduction via electrical stimulation activities including FES cycling.
Acknowledgments
All authors listed on this manuscript participated in its completion, there are no conflicts of interest, and ethical adherence was maintained throughout the process.
Financial disclosures/support: This research project was conducted from January 2011 to July 2012 and was sponsored by the Spinal Cord Injury and Disorders Service, McGuire VA Medical Center.
References
- 1.National Spinal Cord Injury Statistical Center 2005 Annual Report for the Model Spinal Cord Injury Care Systems. Birmingham, AL: The University of Alabama at Birmingham; July2005:120 [Google Scholar]
- 2.Kierney PC, Engrav LH, Isik FF, Esselman PC, Cardenas DD, Rand RP. Results of 268 pressure sores in 158 patients managed jointly by plastic surgery and rehabilitation medicine. Plastic Reconstr Surg. 1998;102(3):765–772 [DOI] [PubMed] [Google Scholar]
- 3.Gordon MD, Gottschlich MM, Helvig EI, et al. Review of evidence-based practice for the prevention of pressure sores in burn patients. J Burn Care Rehabil. 2004;25:388–410 [DOI] [PubMed] [Google Scholar]
- 4.Krause JS, Vines CL, Farley TL, Sniezek J, Coker J. An exploratory study of pressure ulcers after spinal cord injury: Relationship to protective behaviors and risk factors. Arch Phys Med Rehabil. 2001;82(1):107–113 [DOI] [PubMed] [Google Scholar]
- 5.Bluestein D, Ashkan J. Pressure ulcers: Prevention, evaluation, and management. Am Family Physician. 2008;78(10):1186–1194 [PubMed] [Google Scholar]
- 6.Zacharkow D. Wheelchair Posture and Pressure Sores. Springfield, IL: Charles C. Thomas Publishing Co; 1984 [Google Scholar]
- 7.Liu LQ, Nicholson GP, Knight SL, et al. Pressure changes under the ischial tuberosities of seated individuals during sacral nerve root stimulation. J Rehabil Res Dev. 2006;43(2):209–218 [DOI] [PubMed] [Google Scholar]
- 8.Sprigle S, Sonenblum S. Assessing evidence supporting redistribution of pressure ulcer prevention: A review. J Rehabil Res Dev.2011;48(3):203–214 [DOI] [PubMed] [Google Scholar]
- 9.http://www.spinal-injury.net/pressure-sore-stages-sci.htm Retrieved October27, 2012
- 10.Lim D, Chen KJ. Can functional electrical stimulation for pressure ulcer prevention reduce efficiently the incidence of deep-tissue injury? Int J Biol Biomed Eng. 2009;1(3):6–9 [Google Scholar]
- 11.Lyder CH. Pressure ulcer prevention and management. JAMA. 2003;28(9):223–226 [DOI] [PubMed] [Google Scholar]
- 12.Garber SL, Rintala DH. Pressure ulcers in veterans with spinal cord injury: A retrospective study. J Rehabil Res Dev. 2003;40(5):433–442 [DOI] [PubMed] [Google Scholar]
- 13.Levine SP, Kett RL, Cederna PS, Bowers LD, Brooks SV. Electrical muscle stimulation for pressure variation at the seating interface. J Rehabil Res Dev. 1989;26(4):1–8 [PubMed] [Google Scholar]
- 14.Bogie KM, Triolo RJ. Effects of regular use of neuromuscular electrical stimulation on tissue health. J Rehabil Res Dev. 2003;40(6):469–476 [DOI] [PubMed] [Google Scholar]
- 15.Bogie KM, Wang X, Triolo RJ. Long-term prevention of pressure ulcers in high-risk patients: A single case study of the use of gluteal neuromuscular electrical stimulation. Arch Phys Med Rehabil. 2006;87(4):585–591 [DOI] [PubMed] [Google Scholar]
- 16.Curtis CA, Chong SL, Kornelsen I, Uwiera RR, Seres P, Mushahwar VK. The effects of intermittent electrical stimulation on the prevention of deep tissue injury: Varying loads and stimulation paradigms. Artificial Organs. 2011;35(3):226–236 [DOI] [PubMed] [Google Scholar]
- 17.Levine SP, Kett RL, Cederna PS, Brooks SV. Electrical muscle stimulation for pressure sore prevention: tissue shape variation. Arch Phys Med Rehabil. 1990:71(3):210–215 [PubMed] [Google Scholar]
- 18.Ferguson AC, Keating JF, Delargy MA, Andrews BJ. Reduction of seating pressure using FES in patients with spinal cord injury. A preliminary report. Paraplegia. 1992;30(7):474–478 [DOI] [PubMed] [Google Scholar]
- 19.van Londen A, Herwegh M, van der Zee CH, et al. The effect of surface electrical stimulation of the gluteal muscles on the interface pressure in seated people with spinal cord injury. Arch Phys Med Rehabil. 2008;89:1724–1732 [DOI] [PubMed] [Google Scholar]
- 20.Berkelmans R. FES cycling. J Automatic Control. 2008;18(2):73–76 [Google Scholar]
- 21.Petrofsky JS. Functional electrical stimulation, a two year study. J Rehabil. 1992;58(3):29–34 [Google Scholar]
- 22.Kim J, Ho CH, Wang X, Bogie K. The use of sensory electrical stimulation for pressure ulcer prevention. Physiother Theory Pract. 2010;26(8):528–536 [DOI] [PubMed] [Google Scholar]


