Abstract
Background:
Obesity and its consequences affect patients with spinal cord injury (SCI). There is a paucity of data with regard to the dietary intake patterns of patients with SCI in the acute inpatient rehabilitation setting. Our hypothesis is that acute rehabilitation inpatients with SCI consume significantly more calories and protein than other inpatient rehabilitation diagnoses.
Objective:
To compare calorie and protein intake in patients with new SCI versus other diagnoses (new traumatic brain injury [TBI], new stroke, and Parkinson’s disease [PD]) in the acute inpatient rehabilitation setting.
Methods:
The intake of 78 acute rehabilitation inpatients was recorded by registered dieticians utilizing once-weekly calorie and protein intake calculations.
Results:
Mean ± SD calorie intake (kcal) for the SCI, TBI, stroke, and PD groups was 1,967.9 ± 611.6, 1,546.8 ± 352.3, 1,459.7 ± 443.2, and 1,459.4 ± 434.6, respectively. ANOVA revealed a significant overall group difference, F(3, 74) = 4.74, P = .004. Mean ± SD protein intake (g) for the SCI, TBI, stroke, and PD groups was 71.5 ± 25.0, 61.1 ± 12.8, 57.6 ± 16.6, and 55.1 ± 19.1, respectively. ANOVA did not reveal an overall group difference, F(3, 74) = 2.50, P = .066.
Conclusions:
Given the diet-related comorbidities and energy balance abnormalities associated with SCI, combined with the intake levels demonstrated in this study, education with regard to appropriate calorie intake in patients with SCI should be given in the acute inpatient rehabilitation setting.
Key words: nutritional requirements, obesity, spinal cord injuries
It is estimated that more than two-thirds of individuals with spinal cord injury (SCI) are obese.1 Persons with SCI have been shown to be at increased risk of prematurely developing coronary heart disease and possess a higher prevalence of altered glucose metabolism compared to age-matched controls.2–9 Additionally, obesity in this population is associated with increased likelihood of deep tissue injury10 and fatal pulmonary embolism,11 higher rehospitalization rates and pain scores,12 decreased lung volumes,13 and worse functional outcomes after rehabilitation.14 Given reports that cardiovascular disease is the leading cause of mortality in long-term SCI when excluding deaths during the first year after injury,15 obesity in this population demands attention.
Total daily energy expenditure is the sum of basal metabolic rate, thermic effect of food digestion, and thermic effect of physical activity. Due to muscle atrophy and the subsequent reduction in fat-free lean body mass, SCI leads to drastic reductions in basal metabolic rate and thermic effect of physical activity.1,2 This profoundly impacts energy balance and necessitates equivalent reductions in energy intake to prevent the accumulation of adipose tissue.1 Given that total daily energy expenditure after SCI is reduced by 12% to 54% depending on level of injury, fat-free lean mass, and activity level,1 coupled with the difficulties the SCI population faces with regard to physical activity participation,2 an appropriate diet is of paramount importance in these individuals.
The acute phase after SCI is unlike most trauma in that it is associated with a reduction in metabolic activity and an uncorrectable negative nitrogen balance.16Additionally, it has been reported that predicted energy expenditure is greater than actual energy expenditure in the first weeks after SCI and, as a result of this discrepancy, acute SCI patients have historically been overfed.17 As a result, there has been a call to develop specific nutritional treatment protocols for persons with acute SCI.16 Given that the initial decrease in metabolic rate continues into the chronic spine injury phase, there has also been a call to develop nutritional guidelines specific to subacute and chronic SCI.18
There is a paucity of data with regard to dietary intake among individuals with SCI in the rehabilitation setting. This study attempts to characterize calorie and protein intake in patients with SCI in the acute inpatient rehabilitation setting and compare the intake of patients with SCI to other diagnoses commonly found in this setting. Understanding the dietary behavior of persons with SCI in the context of their immediate and chronic energy balance abnormalities and their long-term risk of obesity may lead to alterations in nutritional education provided to patients with new SCI undergoing rehabilitation. Additionally, comparing SCI intake to other diagnoses may serve to identify excessive dietary intake as a modifiable, SCI-specific medical problem.
Methods
Study group
This study was approved by the Northwestern University Institutional Review Board in Evanston, Illinois, and was made possible by a grant from the Spastic Paralysis Foundation of the Illinois-Eastern Kiwanis Club (DeKalb, Illinois). Consecutive patients age 18 and older newly admitted to an acute inpatient rehabilitation facility from September 1, 2009, to August 31, 2010 (with a hold on enrollment from July 12, 2010, to August 3, 2010, for consent form adjustment) carrying the primary diagnosis of new traumatic SCI, new traumatic brain injury (TBI), new stroke, or Parkinson’s disease (PD) were studied prospectively.
Patients were not included for study if they carried the diagnosis of end-stage liver disease, end-stage renal disease, or burn; were admitted on enteral (tube) feeding; presented with cognitive deficits (defined by a score <20 on the Mini-Mental State Examination); presented with communication deficits (including aphasia and non–English-speaking status); were admitted on medications that may have served as appetite stimulants (megestrol, dronabinol, mirtazapine, or steroids); or were admitted on dietary supplements. Subjects were excluded if they were started on any of the aforementioned medications or started on dietary supplements after enrollment. Subjects were not included or excluded for enteral feeding, appetite stimulants, and dietary supplements to eliminate potentially confounding variables that would have altered the natural intake levels of the patients in this study. Finally, subjects with dysphagia were included given that, for example, Foley et al19 reported no difference in the intakes of stroke patients receiving dysphagia versus regular diets.
Measures
Demographic data (age, gender, race) and weight (kilograms [kg]) were collected on each subject on admission, and length of stay was collected on each subject on discharge. Additionally, a full day of calorie (kilocalorie [kcal]) and protein (gram [g]) intake calculations were obtained on all subjects once per week by registered dietitians employed by the Rehabilitation Institute of Chicago. Breslow et al concluded that 1-day calorie intake calculations are a valid alternative to consecutive 3-day calorie intake calculations and, thus, were utilized here.20 The registered dieticians directly examined food trays after meals to calculate calorie and protein intake based on amount of food consumed. If direct examination was not possible, reports from speech language pathology, nursing, patient care technicians, and the patient and/or family were used to calculate intake. Food items brought in from outside the hospital were included in the intake calculations. The total number of days that calorie and protein intake calculations were obtained on each patient during their hospitalization was documented as well. So, if a patient was hospitalized for 3 weeks, he or she would have 3 calorie and 3 protein intake calculations that would then be used to generate 1 mean calorie intake result and 1 mean protein intake result for the entire hospitalization. Patients with a longer length of stay would have more intake calculations compared to those with a shorter length of stay. As the number of intake calculations had the potential to affect mean calorie and protein intake results, statistical investigation was required (see later discussion).
Data analysis
Analysis was performed using IBM SPSS 20. A series of one-way analysis of variance (ANOVA) was performed with post hoc Tukey HSD pairwise comparisons to compare mean age (years), length of stay, calorie intake (kcal), protein intake (g), calorie intake per body weight (kcal/kg), protein intake per body weight (g/kg), number of calorie intake calculations during hospitalization, and number of protein intake calculations during hospitalization between the groups. A t test was performed to compare intake variables between patients diagnosed with tetraplegia and paraplegia within the SCI group. Chi-square analysis was performed to compare distribution of gender and race between the groups. Multiple linear regression analysis was performed to assess the impact of age, gender, race, admission weight, and length of stay along with group (SCI vs combined group) on calorie and protein intake.
Results
Patient demographics
Eighty-eight subjects were studied prospectively. Three subjects withdrew (2 stroke, 1 SCI; all requested dietary supplementation), and 7 subjects were excluded after inclusion (4 stroke, 2 SCI, 1 TBI; 3 for supplementation initiation, 3 for steroid initiation, 1 for appetite stimulant initiation). As a result, data for 78 subjects were analyzed (SCI, n = 16 [tetraplegia, n = 8; paraplegia, n = 8]; TBI, n = 9; stroke, n = 43; PD, n = 10).For the entire sample, mean age was 56.3 ± 19.7 years; 64.1% were male (n = 50); and 51.3% were Caucasian (n = 40), 39.7% were African American (n = 31), and 14.6% were of other racial background (n = 7). There was no difference in gender nor racial distribution between the groups, χ2(3, N=78) = 5.03, P = .17, and χ2(6, N=78) = 9.60, P = .14, respectively. Demographic information and length of stay data for the groups are summarized in Table 1.
Table 1. Patient demographics.
| Group | SCI (n=16) | TBI (n=9) | Stroke (n=43) | PD (n=10) |
|---|---|---|---|---|
| Mean age, years (±SD) | 41.1±21.2a | 53.3±23.4 | 58.5±16.0a | 73.9±9.3a |
| Gender, n (%) | ||||
| Male | 13 (81.3) | 7 (77.8) | 23 (53.5) | 7 (70.0) |
| Female | 3 (18.7) | 2 (22.2) | 20 (46.5) | 3 (30.0) |
| Race, n (%) | ||||
| Caucasian | 8 (50.0) | 8 (88.9) | 17 (39.5) | 7 (70.0) |
| African American | 6 (37.5) | 1 (11.1) | 21 (48.8) | 3 (30.0) |
| Other | 2 (12.5) | 0 (00.0) | 5 (11.6) | 0 (00.0) |
| Length of stay, days (±SD) | 54.3±19.3b | 17.7±8.3b | 21.0±8.9b | 20.0±10.1b |
Note: PD = Parkinson’s disease; SCI = spinal cord injury; TBI = traumatic brain injury.
Mean age was significantly different between the SCI and Stroke (P = .006) and SCI and PD (P = .0005) groups.
LOS was significantly different between the SCI and TBI (P < .05), SCI and Stroke (P < .05), and SCI and PD (P < .05) groups.
Calorie intake
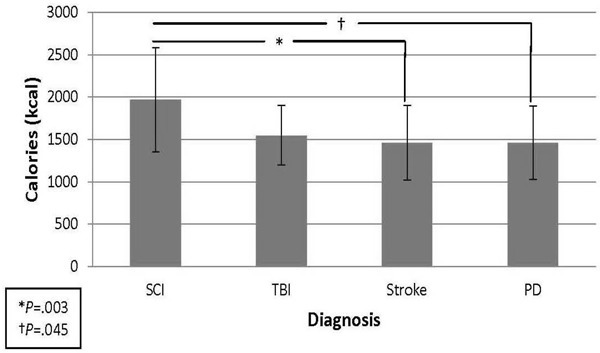
Mean calorie intake (kcal) for the SCI, TBI, stroke, and PD groups was 1,967.9 ± 611.6, 1,546.8 ± 352.3, 1,459.7 ± 443.2, and 1,459.4 ± 434.6, respectively. ANOVA revealed a significant overall group difference, F(3, 74) = 4.74, P = .004. Pairwise comparisons showed significant differences between SCI and stroke (P = .003) and SCI and PD (P = .045) (Figure 1). Mean calorie intake per body weight (kcal/kg) for the SCI, TBI, stroke, and PD groups was 24.4 ± 9.8, 20.4 ± 5.3, 17.4 ± 8.4, and 19.2 ± 6.6, respectively. ANOVA again revealed a significant overall group difference, F(3, 74) = 2.84, P = .044, however, pairwise comparison only found a significant difference between SCI and stroke (P = .025). Mean number of calorie intake calculations during hospitalization for the SCI, TBI, stroke, and PD groups was 5.4 ± 2.9, 2.1 ± 1.1, 2.3 ± 1.2, and 2.1 ± 1.2, respectively. ANOVA revealed a significant overall group difference, F(3, 74) = 15.86, P = .0001. Pairwise comparisons showed significant differences between the SCI group and the other 3 groups (P < .05). There was no significant difference in mean calorie intake (2,095.8 ± 734.1 vs 1,840.0 ± 474.8; P = .422) or mean calorie intake per body weight (24.0 ± 9.4 vs 24.8 ± 10.9; P = .873) between patients with tetraplegia versus paraplegia within the SCI group.
Figure 1. Mean calorie intake by diagnosis. PD = Parkinson’s disease; SCI = spinal cord injury; TBI = traumatic brain injury.
Multiple linear regression analysis revealed that age (P = .0001), gender (P = .023), and admission weight (P = .025) were significant predictors of calorie intake, whereas group (P = .384), length of stay (P = .806), and race (P = .813) were not. Younger age, male gender, and lower weight were associated with increased calorie intake.
Protein intake
Mean protein intake (g) for the SCI, TBI, stroke, and PD groups was 71.5 ± 25.0, 61.1 ± 12.8, 57.6 ± 16.6, and 55.1 ± 19.1, respectively. ANOVA indicated a nonsignificant trend, F(3, 74) = 2.50, P = .066. Mean protein intake per body weight (g/kg) for the SCI, TBI, stroke, and PD groups was 0.89 ± 0.39, 0.81 ± 0.18, 0.68 ± 0.29, and 0.73 ± 0.28, respectively. ANOVA did not reveal a significant difference between the groups, F(3, 74) = 1.97, P = .126. Mean number of protein intake calculations during hospitalization for the SCI, TBI, stroke, and PD groups was 5.4 ± 2.8, 2.1 ± 1.1, 2.3 ± 1.2, and 2.1 ± 1.2, respectively. ANOVA revealed a significant overall group difference, F(3, 74) = 15.97, P = .0001. Pairwise comparisons showed significant differences between the SCI group and the other 3 groups (P < .05). There was no significant difference in mean protein intake (74.9 ± 29.4 vs 68.0 ± 21.0; P = .599) or mean protein intake per body weight (0.86 ± 0.37 vs 0.92 ± 0.43; P = .751) between patients with tetraplegia and paraplegia within the SCI group.
Multiple linear regression analysis revealed that age (P = .002) and gender (P = .037) were significant predictors of protein intake, whereas group (P = .837), length of stay (P = .667), race (P = .923), and weight (P = .121) were not. Younger age and male gender were associated with increased protein intake.
Discussion
With regard to intake patterns among SCI patients in the rehabilitation setting, Laven et al reported average calorie and protein intake of 1,729 kcal/day and 65 g/day, respectively, in SCI patients at 4 weeks after injury; the authors suggested that intakes of 1,500 kcal/day may be sufficient in this population to prevent most untoward nutrition-related secondary complications.21 Using indirect calorimetry, Cox et al demonstrated mean energy expenditure of 22.7 kcal/kg/day for tetraplegic and 27.9 kcal/kg/day for paraplegic rehabilitation inpatients. Combining the tetraplegic and paraplegic groups, they reported that the SCI rehabilitation inpatients in their study required 23.4 kcal/kg/day (1,556 kcal/ day), however, they consumed 27.4 kcal/kg/day (1,774 kcal/day) on an uncontrolled diet, which lead to a weight gain of 1.7 kg/week.22 The study described here demonstrated higher absolute mean calorie and protein intake (1,967.9 kcal/ day and 71.5 g/day, respectively) compared to the findings described previously and demonstrated higher mean calorie intake per body weight (24.4 kcal/kg/day) than suggested previously. Multiple linear regression analysis in our study revealed that age was a significant predictor of calorie and protein intake (suggesting that younger age was associated with increased intake). Given that the mean age for the SCI patients in Laven’s study (29.5 years) and Cox’s study (29.8 years) were both less than that described here (41.1 years), we cannot suggest that the age difference between the patients in the 3 studies explains the higher intake values recorded in our study. Regardless, all 3 studies demonstrate intake levels consistent with a positive energy balance that would lead to obesity and other related comorbid conditions.
Initial data analysis suggested significantly higher mean calorie intake in rehabilitation inpatients with SCI compared to stroke and PD and higher mean protein intake in patients with SCI compared to stroke; however, multiple linear regression analysis revealed that diagnosis was not a predictor of calorie or protein intake. Instead, younger age and male gender were identified as significant predictors of increased calorie and protein intake in this study. Given the documented decreased food intake in the elderly23 coupled with the fact that the SCI group was significantly younger than the stroke and PD groups, these results are not surprising. With regard to gender, Groah et al reported a significant difference in daily calorie intake between men and women in a community-dwelling SCI population (2,049.0 kcal/day vs 1,662.5 kcal/day, respectively).24
Despite the findings here suggesting that diagnosis does not predict intake, diagnosis does remains as an important component in the determination of appropriate intake parameters. For example, in addition to SCI, diagnoses such as stroke, amyotrophic lateral sclerosis, obesity, and cerebral palsy have all been identified as disease states where hypocaloric nutrition may be appropriate and beneficial.25 This suggests that SCI patients are not unique in their potential to easily overconsume based on the effects that diagnosis has on energy balance. Nutrition counseling in these hypometabolic diagnoses should occur as soon as possible after initial diagnosis to prevent the untoward effects of a positive energy balance. Given the results of this study, younger, male patients with a hypometabolic diagnosis are at greatest risk of overeating and should receive appropriate, early education.
In a prospective study, de Groot et al showed an increase from 56% to 75% in the percentage of SCI patients classified as overweight or obese when comparing initial acute inpatient rehabilitation measurements to 5 years after discharge. Additionally, they noted a significant increase in body mass index (BMI) in the first year after rehabilitation discharge and recommended that attention be paid to weight management protocols focused on diet and an active lifestyle.26 Numerous studies have documented the poor nutritional status and dietary choices24,27–30 and decreased levels of physical activity18,31,32 in community-dwelling patients with SCI. There has been documented success in programs for SCI outpatients focused on weight loss, nutrition education, and physical fitness/activity participation,33–35 but ideally, primary prevention of poor diet choices and inactivity via education in the acute inpatient rehabilitation setting should be the focus. As a high level of interest in proper nutrition in community-dwelling chronic SCI patients has been reported,24 perhaps it can be assumed that acute inpatient rehabilitation SCI patients would also be receptive to a comprehensive nutrition and fitness education program (with implementation) during acute inpatient rehabilitation to prevent obesity.
Limitations
This study had a small number of participants, was performed in a single facility, and did not specifically characterize disease onset. Additionally, we did not measure weight at any time point other than admission. Assuming (reasonably) that the patients’ weights changed during the study period, the sole admission weight measurement may have altered the accuracy of our intake per body weight data. Further, we did not analyze patients with tetraplegia and paraplegia separately in the group comparisons. We did not evaluate the SCI patients based on completeness of injury. Also, due to differences in length of stay, there was a higher number of calorie and protein intake calculations comparing the SCI group to the other groups. Given the use of 1-day calorie counts by Breslow et al20 and given that length of stay did not predict intake in the regression model, the difference in intake calculations was likely inconsequential. Finally, fat and carbohydrate intake calculations were not completed, which would have been relevant here.
Conclusions
Given the diet-related comorbidities and energy balance abnormalities associated with SCI combined with the intake levels demonstrated in this study, education with regard to appropriate calorie intake in patients with SCI should be given in the acute inpatient rehabilitation setting as primary prevention against obesity in this vulnerable population.
Acknowledgments
Financial support/disclosures: This study was made possible by a grant from the Spastic Paralysis Foundation of the Illinois-Eastern Kiwanis Club (DeKalb, Illinois). The authors received no financial benefits.
Previous publication: Data were presented in the form of a poster with an associated abstract at the Annual Assembly of the American Academy of Physical Medicine and Rehabilitation in Atlanta, Georgia, November 2012.
Additional contributions: We acknowledge Rachel Scanlan, RD, and Victoria Jones, MS, RD (protein and calorie data collection); Jungwha Lee, PhD, MPH (statistical analysis); Barbara Lillwitz, BSN, RN, CRRN (technical support; acquisition of functional outcome scores); Thomas Snyder, MHA (technical support; acquisition of laboratory data); Kim Do, MD, Kate Temme, MD, Matthew Oswald, MD, Anjum Sayyad, MD, and Douglas D’Agati, MD (patient recruitment and data collection) for their efforts on this project.
References
- 1.Gater DR., Jr.Obesity after spinal cord injury. Phys Med Rehabil Clinics North Am. 2007; 18(2):333–351, vii [DOI] [PubMed] [Google Scholar]
- 2.Lavis TD, Scelza WM, Bockenek WL. Cardiovascular health and fitness in persons with spinal cord injury. Phys Med Rehabil Clinics North Am. 2007;18(2):317–331, vii [DOI] [PubMed] [Google Scholar]
- 3.Brenes G, Dearwater S, Shapera R, LaPorte RE, Collins E. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil. 1986;67(7):445–450 [PubMed] [Google Scholar]
- 4.Bauman WA, Adkins RH, Spungen AM, Kemp BJ, Waters RL. The effect of residual neurological deficit on serum lipoproteins in individuals with chronic spinal cord injury. Spinal Cord. 1998;36(1):13–17 [DOI] [PubMed] [Google Scholar]
- 5.Yekutiel M, Brooks ME, Ohry A, Yarom J, Carel R. The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia. 1989;27(1):58–62 [DOI] [PubMed] [Google Scholar]
- 6.Bauman WA, Kahn NN, Grimm DR, Spungen AM. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord. 1999;37(9):601–616 [DOI] [PubMed] [Google Scholar]
- 7.Castro MJ, Apple DF, Jr, Staron RS, Campos GER, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373–378 [DOI] [PubMed] [Google Scholar]
- 8.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: A model of premature aging. Metabol Clin Exper. 1994;43(6):749–756 [DOI] [PubMed] [Google Scholar]
- 9.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266–277 [DOI] [PubMed] [Google Scholar]
- 10.Elsner JJ, Gefen A. Is obesity a risk factor for deep tissue injury in patients with spinal cord injury? J Biomechanics. 2008;41(16):3322–3331 [DOI] [PubMed] [Google Scholar]
- 11.Green D, Twardowski P, Wei R, Rademaker AW. Fatal pulmonary embolism in spinal cord injury. Chest. 1994;105(3):853–855 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Cao Y, Allen V, Richards JS. Weight matters: Physical and psychosocial well being of persons with spinal cord injury in relation to body mass index. Arch Phys Med Rehabil. 2011;92(3): 391–398 [DOI] [PubMed] [Google Scholar]
- 13.Stepp EL, Brown R, Tun CG, Gagnon DR, Jain NB, Garshick E. Determinants of lung volumes in chronic spinal cord injury. Arch Phys Med Rehabil. 2008;89(8):1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenson KW, Deutsch A, Heinemann AW, Chen D. Obesity and inpatient rehabilitation outcomes for patients with a traumatic spinal cord injury. Arch Phys Med Rehabil. 2011;92(3):384–390 [DOI] [PubMed] [Google Scholar]
- 15.DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil.1999;80(11):1411–1419 [DOI] [PubMed] [Google Scholar]
- 16.Thibault-Halman G, Casha S, Singer S, Christie S. Acute management of nutritional demands after spinal cord injury. J Neurotrauma. 2011;28(8):1497–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez DJ, Benzel EC, Clevenger FW. The metabolic response to spinal cord injury. Spinal Cord. 1997;35(9):599–604 [DOI] [PubMed] [Google Scholar]
- 18.Buchholz AC, Pencharz PB. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004;7(6):635–639 [DOI] [PubMed] [Google Scholar]
- 19.Foley N, Finestone H, Woodbury MG, Teasell R, Green Finestone L. Energy and protein intakes of acute stroke patients. J Nutr Health Aging. 2006;10(3):171–175 [PubMed] [Google Scholar]
- 20.Breslow RA, Sorkin JD. Comparison of one-day and three-day calorie counts in hospitalized patients: A pilot study. J Am Geriatr Soc. 1993;41(9):923–927 [DOI] [PubMed] [Google Scholar]
- 21.Laven GT, Huang CT, DeVivo MJ, Stover SL, Kuhlemeier KV, Fine PR. Nutritional status during the acute stage of spinal cord injury. Arch Phys Med Rehabil. 1989;70(4):277–282 [PubMed] [Google Scholar]
- 22.Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G. Energy expenditure after spinal cord injury: An evaluation of stable rehabilitating patients. J Trauma. 1985;25(5):419–423 [PubMed] [Google Scholar]
- 23.Donini LM, Savina C, Cannella C. Eating habits and appetite control in the elderly: The anorexia of aging. Int Psychogeriatrics. 2003;15(1): 73–87 [DOI] [PubMed] [Google Scholar]
- 24.Groah SL, Nash MS, Ljungberg IH, et al. Nutrient intake and body habitus after spinal cord injury: An analysis by sex and level of injury. J Spinal Cord Med. 2009;32(1): 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnuson B, Peppard A, Auer Flomenhoft D. Hypocaloric considerations in patients with potentially hypometabolic disease states. Nutr Clin Pract. 2011;26(3):253–260 [DOI] [PubMed] [Google Scholar]
- 26.de Groot S, et al. Prospective analysis of body mass index during and up to 5 years after discharge from inpatient spinal cord injury rehabilitation. J Rehabil Med. 2010;42(10): 922–928 [DOI] [PubMed] [Google Scholar]
- 27.Perret C, Stoffel-Kurt N. Comparison of nutritional intake between individuals with acute and chronic spinal cord injury. J Spinal Cord Med. 2011;34(6):569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabour H, et al. Calorie and macronutrients intake in people with spinal cord injuries: An analysis by sex and injury-related variables. Nutrition. 2012;28(2):143–147 [DOI] [PubMed] [Google Scholar]
- 29.Tomey KM, Chen DM, Wang X, Braunschweig CL. Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Arch Phys Med Rehabil. 2005;86(4):664–671 [DOI] [PubMed] [Google Scholar]
- 30.Levine AM, Nash MS, Green BA, Shea JD, Aronica MJ. An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Paraplegia. 1992;30(12):880–889 [DOI] [PubMed] [Google Scholar]
- 31.Buchholz AC, McGillivray CF, Pencharz PB. Physical activity levels are low in free-living adults with chronic paraplegia. Obesity Res. 2003;11(4):563–570 [DOI] [PubMed] [Google Scholar]
- 32.Yamasaki M, Irizawa M, Komura T, et al. Daily energy expenditure in active and inactive persons with spinal cord injury. J Human Ergol. 1992;21(2):125–133 [PubMed] [Google Scholar]
- 33.Chen Y, Henson S, Jackson AB, Richards JS. Obesity intervention in persons with spinal cord injury. Spinal Cord. 2006; 44(2):82–91 [DOI] [PubMed] [Google Scholar]
- 34.Liusuwan RA, Widman LM, Abresch RT, Johnson AJ, McDonald CM. Behavioral intervention, exercise, and nutrition education to improve health and fitness (BENEfit) in adolescents with mobility impairment due to spinal cord dysfunction. J Spinal Cord Med. 2007;30(suppl 1):S119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Block P, et al. Shake-It-Up: Health promotion and capacity building for people with spinal cord injuries and related neurological disabilities. Disabil Rehabil. 2005;27(4):185–190 [DOI] [PubMed] [Google Scholar]


