Abstract
Uterine arteriovenous malformation (AVM) is a rare condition, with fewer than 100 cases reported in the literature. Despite it being rare, it is a potentially life-threatening condition. This case report describes a 33-year-old woman who presented with secondary post-partum hemorrhage. Transabdominal ultrasound (US) of the pelvis showed increased vascularity with multidirectional flow of the uterus and a prominent vessel, located on the left lateral wall. She also had retained product of conception, which complicated the diagnosis. A uterine artery angiogram confirmed an AVM in the fundal region with an early draining vein. Embolisation of the AVM was performed successfully.
Keywords: arteriovenous malformation, embolisation, post-partum hemorrhage, ultrasound, uterus, uterine, uterine artery
Introduction
Uterine arteriovenous malformation (AVM) is a rare condition, with fewer than 100 cases reported in the literature (1). It is a potentially life-threatening condition, as patients may present with profuse bleeding. Colour Doppler ultrasound (US) provides a non invasive method for initially diagnosing this rare condition and confirmation can be made using diagnostic angiography. Conservative management or embolisation is a preferable method of treatment in order to avoid a hysterectomy in patients of child-bearing age.
This case report highlights our experience with a patient having this rare gynaecological condition in our medical center.
Case Report
A 33-year-old woman was seen at our facility who presented with two episodes of heavy vaginal bleed that was separated with periods of moderate bleeding. Three weeks earlier, she had delivered her third baby at full term through spontaneous vaginal delivery. The patient had a retained placenta that required manual removal.
Her obstetrics history was uneventful. She had delivered three healthy babies of average weight (3–3.7 kg) at full term through spontaneous vaginal delivery and did not have any miscarriages. The patient denied having any curettage procedures performed.
Upon examination, she was afebrile and hemodynamically stable with a hemoglobin (Hb) level of 12.4 g/dL. Vaginal examination showed a small amount of blood at the external orifice of the uterus, but no active bleed was observed. In addition, her human chorionic gonadotropin (hCG) level was less than 2 mIU/mL.
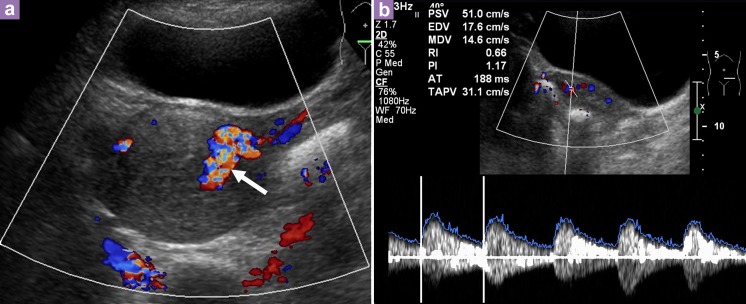
Transabdominal US of the pelvis showed a bulky uterus measuring 8.4 cm × 4.9 cm × 6.8 cm with an endometrial thickness of 1.5 cm. There was increased vascularity of the uterus with a prominent vessel seen on the left lateral wall of the uterus, which likely originated from the left uterine artery. Spectral Doppler US showed a peak systolic velocity (PSV) of 51 cm/s and resistive index (RI) of 0.66 (Figure 1). A diagnosis of an AVM was considered and required an angiogram for confirmation. The uterine artery angiogram confirmed the presence of an arteriovenous malformation in the fundal region. There were multiple feeding arteries, mainly from the left uterine arteries, with smaller feeding arteries from the right. An early draining vein was also observed at the fundus (Figure 2).
Figure 1:
Transabdominal ultrasound of the pelvis shows bulky uterus with increased vascularity and multidirectional flow. Prominent vessel is seen on the left lateral wall of the uterus (arrow), likely to arise from the left uterine artery. b) Spectral Doppler showed a peak systolic velocity (PSV) of 51 cm/s and resistive index (RI) of 0.66.
Figure 2:
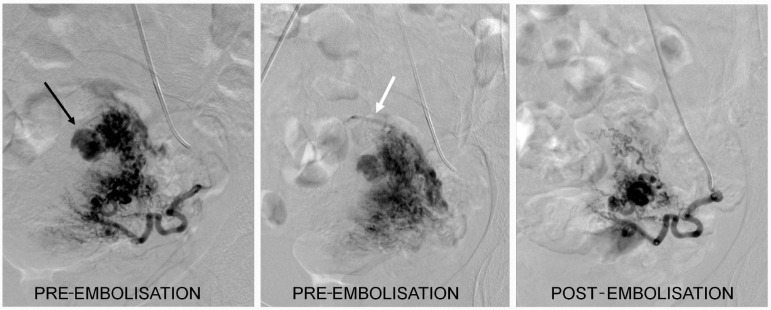
Angiogram of the left uterine artery showed an arteriovenous malformation (AVM) (black arrow) with an early draining vein seen at the fundus (white arrow). Post-embolisation run showed complete embolisation of the AVM.
We then proceeded with embolisation of the AVM. Embolisations of both uterine arteries were performed with 300–500 μm and 700–1000 μm polyvinyl alcohol (PVA). The post-embolisation arteriogram showed complete embolisation of the AVM with slow flow of contrast in both uterine arteries (Figure 2). No complications were encountered. The patient’s vaginal bleeding decreased post-embolisation and she was eventually discharged.
However, during follow up five days later, she still complained of vaginal bleeding. Transabdominal US showed a regular uterus with an endometrial thickness of 2.1 cm, and we could not entirely rule out retained product of conception (POC). She was admitted again for a hysteroscopy under anaesthesia, which showed irregular tissue in the uterus that was gently curetted. Histopathological examination of the tissue showed necrotic chorionic villi consistent with the recent pregnancy. Her vaginal bleeding decreased after the procedure and she was discharged.
During the follow up two weeks later, the bleeding had stopped, with her last episode of bleeding occurring approximately one week after the hysteroscopy.
One year and five months post-embolisation, the patient has not become pregnant and currently does not use contraception. She reports having a regular menstrual cycle and has remained asymptomatic. The patient has also indicated here desire to conceive again in the future.
Discussion
Uterine vascular abnormalities are rare entities in gynaecology. However, it is a potentially life-threatening disorder in which patients present with vaginal bleeding that may be profuse and cause hemodynamic instability. Thus, it is an important differential to be considered in women of reproductive age with unexplained vaginal bleeding and in post-menopausal women when anechoic structures are identified by US (2).
Dubreuil and Loubat reported the first case of uterine AVM in 1926 (3). To date, there are fewer than 100 cases reported in the literature (1). AVM consists of proliferation of arterial and venous channels with fistula formation and a mixture of capillary-like vessels. Distinction between arteries and veins is difficult because secondary intimal thickening occurs in the veins due to increased intraluminal pressure (3).
Uterine AVM may be congenital or acquired (2–4). Congenital AVM is believed to arise from arrested vascular embryologic development resulting in anomalous differentiation in the capillaries and abnormal communication, between arteries and veins (2). Moreover, congenital AVMs can have multiple vascular connections and may invade surrounding structures. They have been found as isolated cases, but have also been reported with AVMs occurring at other sites (4). Acquired AVM are more common and usually follows a history of previous uterine trauma, such as curettage procedures, caesarean section, or pelvic surgery. The potential to develop abnormal communication between arteries and veins occurs during the healing process, typically when a single artery joins a single vein. Acquired AVM is also associated with infection, retained POC, gestational trophoblastic disease, gynaecologic malignancies, and exposure to diethylstilboestrol.
In this case, it is possible that a congenital form of uterine AVM was present in the patient given the absence of uterine trauma, and presence of small feeding arteries. However, if it was truly congenital rather than acquired, it is unclear why the patient did not present earlier in life. In order for the endometrium to bleed, endometrial blood vessels and surface epithelium must both breakdown (1). We can only postulate that the AVM was not large or superficial enough for the vessels to be exposed at the endometrium and subsequently bleed.
AVM has been traditionally diagnosed by laparotomy or during examination of the uterus after a hysterectomy (4). However, with the availability of colour Doppler US, a non invasive method can now be used to detect this rare condition. Nevertheless, digital subtraction angiography remains the gold standard of diagnosis (4).
Gray scale ultrasound (US) can detect the presence of multiple tubular or “spongy” anechoic or hypoechoic areas within the myometrium of a normal endometrium (2,4,5). However, other conditions may present a similar appearance, such as retained products of conception, hemangioma, gestational trophoblastic disease, multilocular ovarian cysts, or hydrosalpinx. Thus, using colour and spectral Doppler US is important for obtaining more accurate information. A normal myometrial signal will show a PSV of 9–44 cm/s and RI of 0.6–0.8. In addition, uterine AVM will exhibit intensely vascular and multidirectional flow (regions of juxtaposed reds and blues caused by multiple tortuous vessels of varying orientations). Spectral Doppler US will show high velocity (mean PSV: 136 cm/s), low resistance (mean RI: 0.3) flow, low pulsatility of the arterial waveform, and pulsatile high-velocity venous waveform (4–5). Differentiation between the venous and arterial waveform is often difficult, and the pelvic veins distal to the AVM may show pulsatile flow instead of the normal monophasic flow (4).
As mentioned above, retained products of conception may also give a hypervascular appearance with turbulent flow. Timmerman et al,. reported two cases in which residual placental tissue was removed from patients diagnosed with uterine vascular malformation, thereby complicating the diagnosis further (6). Gestational trophoblastic disease (GTD), particularly in patients with associated uterine arteriovenous fistula, may also have similar US findings of increased uterine vascularity with a low RI (7). This patient did not have elevated hCG levels, which would be expected in GTD. However, a rare form of GTD, called placental site trophoblastic disease (PSTT), does not produce high levels of hCG, and instead produces high levels of human placental lactogen (hPL) (8). However, the hPL levels were not measured in this patient because GTD and PSTT were not considered in the differential diagnosis.
Digital subtraction angiography (DSA) remains the gold standard for the diagnosis of AVM. Findings with DSA include bilateral hypertrophy of uterine arteries that feed a tortuous, hypertrophic arterial mass with large accessory feeding vessels, and early drainage into enlarged hypertrophic veins (4). However, DSA is rarely performed for diagnosis alone due to its invasive nature and is usually reserved when a patient requires surgical intervention or embolisation.
We noted that our patient had also previously undergone a procedure for POC removal, but even with this information together with the US findings, a definite definitive diagnosis of uterine AVM would be difficult to obtain. The findings during DSA in this case confirmed the presence of an AVM, which underscores DSA as the gold standard for diagnosis. However, we do not advocate performing DSA on all patients with a hypervascular uterine mass, as it is indeed an invasive procedure.
Management of uterine AVM depends on the hemodynamic status, degree of bleeding, patient age, and desire for future fertility. Acute treatment involves stabilising the patient’s hemodynamic status, and stopping blood loss. Traditionally, a hysterectomy was the treatment of choice. However, the patient’s desire for future pregnancy is an important consideration, as there are now options available to avoid a hysterectomy. In stable patients who have the ability for close follow-up, expectant, and long-term medical management may be appropriate. Timmerman et al. presented 10 cases that demonstrated uterine AVM features by color Doppler US; of these, six cases spontaneously resolved (6). Moreover, oral contraception as well as intramuscular and subsequent oral methylergonovine maleate have been shown to be associated with regression of lesions based on US (4).
Since the first description of a successful embolisation treatment for uterine AVM in 1986, it has been commonly used in the emergency setting as well as less urgent circumstances. Various embolic materials have been used, including polyvinyl alcohol, histoacryl (glue), stainless steel coils, detachable balloons, and haemostatic gelatine. Some cases may require repeat embolisation (4). In addition, because uterine AVM is commonly diagnosed in women of childbearing age, angiographic embolisation has made hysterectomy no longer necessary. However, hysterectomy remains the treatment of choice in post-menopausal patients or as an emergency treatment in life-threatening situations (9).
This case report highlights the use of US and DSA for diagnosing uterine AVM in a patient of childbearing age who presented with secondary post-partum haemorrhage. It also highlights our experience in performing embolisation in this patient, which provided an alternative option for her to retain her fertility.
Acknowledgments
Angio Suite, Department of Biomedical Imaging and Department of Obstetrics and Gynaecology, Universiti Malaya Medical Center.
Footnotes
Conflict of interest
Nil.
Funds
Nil.
Authors’ Contributions
Conception and design, critical revision of the article for the important intellectual content and final approval of the article: HH, ON
Drafting of the article: HH
References
- 1.Hickey M, Fraser I. Clinical Implications of Disturbances of Uterine Vascular Morphology and Function. Baillieres Clin ObstetGynaecol. 2000;14(6):937–951. doi: 10.1053/beog.2000.0136. [DOI] [PubMed] [Google Scholar]
- 2.Polat P, Suma S, Kantarcy M, Alper F, Levent A. Colour Doppler Ultrasound in the Evaluation of Uterine Vascular Abnormalities. Radiographics. 2002;22:47–53. doi: 10.1148/radiographics.22.1.g02ja0947. [DOI] [PubMed] [Google Scholar]
- 3.Fleming H, Ostor A, Pickel H, Fortune D. Arteriovenous Malformations of the Uterus. Obstet Gynaecol. 1989;73(2):209–213. [PubMed] [Google Scholar]
- 4.Grivell R, Reid K, Mellor A. Uterine Arteriovenous Malformations: A review of the Current Literature. Obstet Gynaecol Survey. 2005;60(11):761–767. doi: 10.1097/01.ogx.0000183684.67656.ba. [DOI] [PubMed] [Google Scholar]
- 5.Huang M, Muradali D, Thurnston W, Burns P, Wilson S. Uterine Arteriovenous Malformations: Gray-Scale and Doppler Ultrasound features with MR Imaging Correlation. Radiology. 1998;206(1):115–123. doi: 10.1148/radiology.206.1.9423660. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman D, Bosch TVd, Peeraer K. Vascular Malformations in the Uterus: Ultrasonographic Diagnosis and Conservative Management. Euro J Obstet Gynaecol Reprod Biol. 2000;92:171–178. doi: 10.1016/s0301-2115(00)00443-7. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa Y, Nakauchi T, Sato T, Oki A, Tsunoda H, Yoshikawa H. Ultrasound diagnosis of uterine arteriovenous fistula associated with placental site trophoblastic tumor. Ultrasound Obstet Gynecol. 2003;21(6):606–608. doi: 10.1002/uog.145. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie A, Hancock B. In: Gestational Trophoblastic Disease. 3rd ed. Hancock B, Seckl M, Berkowitz R, Cole L, editors. International Society for the Study of Trophoblastic Diseases: 2009. Placental Site Trophoblastic Tumour; pp. 420–429. [Google Scholar]
- 9.Delotte J, Chevallier P, Benoit B, Castillon J, Bongain A. Pregnancy after Embolisation Therapy for Uterine Arteriovenous Malformation. Fertility and Sterility. 2006;85(1):228e1–6. doi: 10.1016/j.fertnstert.2005.06.058. [DOI] [PubMed] [Google Scholar]