Abstract
Historically, metastatic renal cell carcinoma (mRCC) is more resistant to conventional cytotoxic chemotherapeutic agents than other solid tumors. Although significant progress has been made over the last decade with several novel therapeutics, these agents invariably go on to fail, largely due to either intrinsic or acquired resistance. To help overcome, or at least delay resistance, combinatorial therapies utilizing agents with disparate, and ideally complementary, mechanisms of actions are needed. In this report, we assess the novel combination of the mTOR inhibitor, temsirolimus, with the microtubule stabilizing drug ixabepilone in RCC. Our results demonstrate synergy in multiple cell lines of RCC and further evaluation of this combination is warranted in the clinical setting. Activation of the endoplasmic reticulum (ER) stress response pathway may in part explain the combinatorial synergy. We further propose that ER stress induced proteins may serve as early response biomarkers to combinatorial therapy in a clinical trial.
Keywords: Renal cell carcinoma, ixabepilone, microtubule stabilizer, mTOR inhibitor, temsirolimus, combination therapy
Introduction
There are approximately 65,000 new cases of kidney cancer along with 13,500 deaths each year in the US, with the majority of these cases being related to renal cell carcinoma (RCC) [1]. Over the last four decades, the incidence of RCC has steadily risen [2]. While patients presenting with early-stage disease respond well to surgical resection or other methods of local intervention, the five-year survival rate for metastatic RCC remains less than 10% [3].
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase involved in eukaryotic cell proliferation and survival and is an integral part of the phosphoinositide 3-kinase PI3K/Akt signaling pathway [4]. Increased PI3K/Akt/mTOR signaling is obser-ved in a large percentage of RCC patient tumor samples [5]. In addition, activated mTOR demonstrates pro-tumorigenic activity in RCC [5,6], and is correlated with poor prognostic features [6].
Inhibitors of mTOR are amongst the recently approved agents for mRCC [7,8]. Although these agents both have demonstrable activity in RCC, they typically produce disease stabilization, with relatively few objective responses. Furthermore, most patients will have disease progression within six months of initiation of therapy with this class of agent.
Temsirolimus (CCI-779; Torisel®) binds to the protein FKBP-12, forming a complex that primarily inhibits mTOR complex 1 (mTORc1) function [9,10]. Recent studies have shown the effectiveness of temsirolimus in patients with metastatic breast [11], pancreatic [12], and renal [13,14] carcinomas. It has also been safely combined with interferon [8] as well as other cytotoxic chemotherapeutics [15] in humans, suggesting that combinatorial therapy with other active agents in RCC may be feasible.
Ixabepilone, an epothilone B analog, is a microtubule (MT) stabilizer that functions by promoting microtubule polymerization and consequently arrests cells in the G2-M transition. [16-18]. Ixabepilone and other epothilone analogs have demonstrated anti-tumor activity in taxane-sensitive, as well as taxane-resistant tumors [19]. Two recent clinical trials evaluating ixabepilone in metastatic RCC have shown clinical benefit including objective responses and prolonged disease stabilization [16,20]. Furthermore, ixabepilone has demonstrated efficacy in many cancers that do not respond to standard of care treatments, such as triple negative breast cancer [21], metastatic melanoma [22] and non-small cell lung cancer [20].
Though the specific combination of temsirolimus and ixabepilone has not been tested, the combination of an mTOR inhibitor and a microtubule stabilizer has recently been shown to be effective in vitro in anaplastic thyroid carcinoma [23], hepatocellular carcinoma [24], and non-Hodgkin lymphoma [25]. The combination of these drugs enhanced mTOR downregulation and synergistically enhanced caspase-dependent induction of apoptosis [25], indicating the potent anti-tumor activity of this combination. To evaluate the efficacy of this combination in RCC, representative cell lines were selected for analysis and a series of molecular assays were conducted to assess potential synergistic effects of this combination of drugs. We found that the combination of temsirolimus and ixabepilone demonstrates synergistic, anti-proliferative effects through activation of the endoplasmic reticulum (ER) stress response pathway, and also enhances cell death via apoptosis.
Materials and methods
Chemicals
Ixabepilone was kindly provided by Bristol-Myers Squibb. Temsirolimus was purchased from LC labs (Woburn, MA). All drugs were diluted in DMSO (Sigma-Aldrich; St. Louis, MO) at various concentrations and stored at -20°C.
Cell culture and proliferation assays
The clear cell renal cell carcinoma cell lines included A498, Caki-1, Caki-2, and UMRC3. All were purchased from ATCC (Manassas, VA), except for UMRC3, which was a kind gift from Dr. Bart Grossman (UT MD Anderson Cancer Center). All cell lines were maintained in Dulbecco’s modified eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (Hyclone, Logan, UT) and 1% penicillin-streptomycin B (Cellgro, Herndon, VA) at 37°C under humidified atmosphere with 5% CO2. For proliferation analysis, cells were plated in 12-well plates (BD Biosciences, Bedford, MA) in triplicate at a concentration of 2 x 104 cells/well. After 24 hours, cells were treated with temsirolimus, ixabepilone, or a combination of various concentrations as indicated in figure legends for 72 hours. DMSO alone was used as control. After 72 hours, cells were washed with PBS (Cellgro), trypsinized and counted by Coulter Particle Counter (Beckman, Brea, CA). For single dose outs of temsirolimus and ixabepilone, IC50 was calculated via extrapolation of 50% growth on a log scale to determine corresponding drug concentration. For the synergy curves, various concentrations of ixabepilone were used alone and in combination with three fixed concentrations of temsirolimus (0.1 nM, 1 nM, and 10 nM) (RCC cell lines). Drug interactions were analyzed using CalcuSyn® [26] based upon the Chou-Talalay Method [27]. Determination of synergy, additivity or antagonism was quantified by the combination index (CI). CI=1 indicates an additive effect, <1 is synergy and >1 is antagonism [27].
Microtubule stabilization assay
Exponentially growing cells were treated with either DMSO control or 2 nM ixabepilone for 6 hours and then harvested, pelleted and washed in cold PBS (Cellgro). Pelleted cells were lysed in 50 μl hypotonic lysis buffer (1 mM MgCl2, 2 mM EGTA, 0.5% Nonidet P-40, 20 mM Tris-HCl (pH 6.8), and 2 mM PMSF) for 5 min at 37°C. Samples were centrifuged at ~ 15,000 x g for 10 min at 22°C in a temperature-controlled centrifuge to separate the pellet (polymerized cytoskeletal fraction) from the supernatant (soluble cytosolic fraction). The pellet was resuspended in a volume of lysis buffer equal to the supernatant, and both had an equal volume of 4X LDS sample buffer (Invitrogen) added. Equal volumes of samples were used for western analysis.
Western blot analysis
Cells were plated at 50% confluence prior to 24 hour drug treatment. Cell lysis was done using cold MPER buffer (Pierce, Rockford, IL) containing protease inhibitor cocktail (Roche, Mann-heim, Germany) and phosphatase inhibitor (Pierce) for 15 minutes followed by 15 minute centrifugation. Supernatants were collected and protein concentration was measured by BCA assay (Pierce). Twenty micrograms of protein was loaded on Bis-Tris/MES gels or a 18% tris-glycine gel for 4EBP1 (Invitrogen) and then transferred to 0.2 μm Immobilon-P (Millipore; Billerica, MA) membrane. The membranes were then hybridized overnight at 4°C with the following antibodies: PDGFRB, VEGFR, IGFR, PARP, mTOR, p-mTOR, p70s6k, p-p70s6k, p-p4EBP1, BIP (Cell Signaling, Beverly, MA); α-tubulin, β-actin (Sigma-Aldrich); p4EBP1 (R&D Biosys-tems, Minneapolis, MN); β-III-tubulin (Abcam, Cambridge, MA); EGFR (Labvision, Fremont, CA); XBP1 (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary species-specific horseradish peroxidase-labeled antibodies (Jackson Immunoresearch, West Grove, PA) were applied in 3% milk/TBS for 45 min at room temperature. Detection was performed using Super-signal chemiluminescence kit (Pierce). Protein expression from Western analysis was quantitated using Image Quant 5.0 (Molecular Dynamics, GE Healthcare, Piscataway, NJ) Blots were background corrected and normalized to loading controls.
RNA isolation and quantitative PCR
RNAqueous Midi Kit (Ambion, Austin, TX) was utilized to extract and purify RNA from cell lines. The O.D. 260/280 ratio of the mRNA was at least 1.8 and the 18 s / 28 s bands were verified on a 1% agarose gel. cDNA was prepared from purified RNA samples using High Capacity cDNA Reverse Transcriptase Kit (Applied Bio-systems, Foster City, CA) per manufacturer’s instruction. TaqManâFast Universal PCR Mas-ter Mix (Applied Biosystems) and TaqManâFAMTM dye-labeled probes including POLR2A (Hs00-172187_m1) (normalization control) and ATF6 (Hs00232586_m1) were combined with prepared cDNA samples to analyze relative mRNA expression via QPCR. Fold change values were calculated using the ΔΔCt method [28].
Flow cytometry
For cell death analysis, cells were plated at 7.5 x 104 cells/plate in 6 cm plates (Midwest Scientific) and treated with either 10 nM temsirolimus, 2 nM ixabepilone or both for 72 hrs in 0.5% serum media. Media was collected for floating cells and adhered cells were collected using 0.05% trypsin (Cellgro). Both floating and adhered cells were washed with cold PBS (Cellgro) and resuspended in cold binding buffer (BD Pharmingen, San Jose, CA) followed by staining with propidium iodide (PI) (BD Phar-mingen) for 10 minutes. FACS analysis was performed on Accuri C6 flow cytometer (Accuri, Ann Arbor, MI). Unstained cells were used as controls for setting the population parameters and overlay of histograms show no deviation or drift of channels.
Lentiviral infections
MISSION shRNA pLKO.1 constructs (Sigma-Aldrich) were used to make self-inactivating shRNA lentiviruses for human ATF6 (clones: NM_007348.1-332s1c1 (ATF6-332), NM_00-7348.1-690s1c1 (ATF-690)), and a non-target (NT) random scrambled sequence control (SHC002). Lipofectamine 2000 (Invitrogen) and ViraPower (Invitrogen) were used to generate lentiviruses in HEK293FT viral progenitor cells (Invitrogen). Lentivirus was applied to cells along with 5 μg/mL polybrene (American Bioanalytical, Natick, MA) for 24 hours prior to clonal selection with Puromycin (Cellgro).
Statistical analysis
Data are presented as the mean ± s.d. and comparisons were analyzed by 2–tailed paired Student’s t test. Data for comparison of multiple groups are presented as mean ± S.D. and were analyzed by ANOVA. p<0.05 was considered statistically significant.
Results
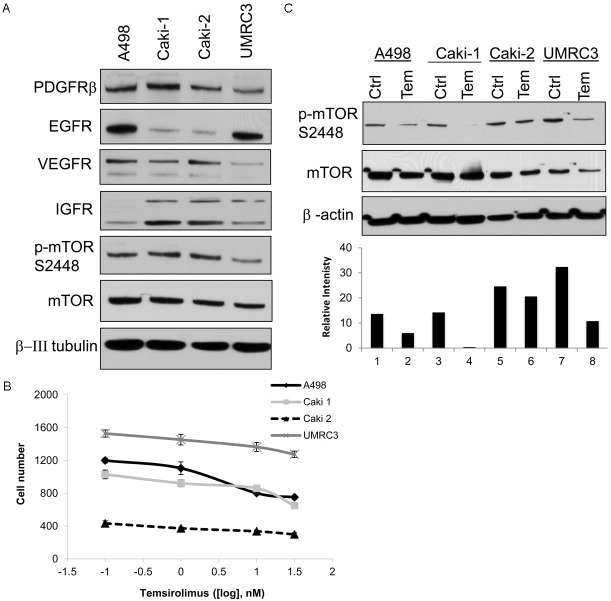
MTOR signaling is frequently activated through several different mechanisms by upstream tyrosine kinase membrane receptors (RTK) which promote tumor growth and survival signaling. Protein expression of several of these RTKs was examined by western blot (Figure 1A) in four RCC cell lines, two of which are VHL mutant (Caki-2 and UMRC3). Platelet derived growth factor beta (PDGFRβ) was expressed at high levels in all 4 cell lines examined, epidermal growth factor receptor (EGFR) was predominantly expressed in A498 and UMRC3, vascular endothelial growth factor receptor (VEGFR) demonstrated higher expression in A498 and Caki-2, and insulin growth factor receptor (IGFR) was found to be highly expressed in Caki-1, Caki-2, and UMRC3. Phosphorylation of mTOR at serine 2481 indicates activation of the mTORc1 complex. A498, Caki-1 and Caki-2 demonstrate high levels of phosphorylated mTOR, and UMRC3 to a lesser degree (Figure 1A). Dose response of temsirolimus (0.1–150 nM) demonstrated little growth inhibitory activity in all four RCC cell lines (Figure 1B). Temsirolimus treatment of cells for 24 hours at a 10 nM dose strongly down-regulated phosphorylated mTOR (p-mTOR) in all cell lines, except for Caki-2, which showed some resistance to temsirolimus (Figure 1C), indicating the drug is successfully abrogating mTORc1 activity. Thus, temsirolimus effectively blocked active p-mTOR, but had little growth effect upon four RCC cell lines when used alone.
Figure 1.
Temsirolimus down-regulates p-mTOR in RCC cell lines. A. Western blot panel of 4 RCC cell lines examining receptor and mTOR expression. B. Cell proliferation assay showing that temsirolimus after 72 hours does not have a dose effect. C. Western blot analysis of cells treated for 24 hours with 10 nM temsirolimus show p-MTOR expression decreases after treatment. β-actin is used as a loading control. Densitometry analysis of p-MTOR is normalized to β-actin.
In order to produce an anti-tumor growth response in RCC cells, the chemotoxic microtubule stabilizing drug Ixabepilone was examined for efficacy. Dose response curves demonstrated an IC50 of ~ 2 to 3 nM for ixabepilone for all four cell lines (Figure 2A). In order to verify that ixabepilone was effectively stabilizing the microtubules, a microtubule stabilization assay was performed as shown by western blot. β-III tubulin and its binding partner, α-tubulin, accumulated in the polymerized fractions (P) after 6 hour exposures to ixabepilone (Figure 2B).
Figure 2.
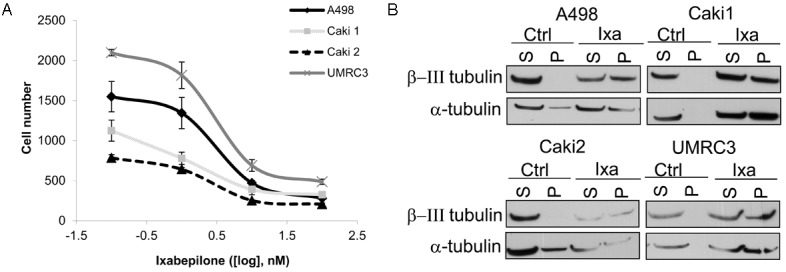
Ixabepilone dose responsively inhibits cell proliferation in RCC cell lines. A. Cell proliferation assay showing that ixabepilone has a dose effect after 72 hours and IC50 was determined to be ~ 2 nM overall. B. Western blot analysis of cells treated for 6 hours with 10 nM ixabepilone for microtubule stabilization assay shows that β-III tubulin and α-tubulin become polymerized in response to treatment. S=soluble, P=polymerized.
Since RCC cell lines were resistant to temsirolimus despite downregulation of p-MTOR, but sensitive to ixabepilone, the two chemotherapeutics were used in combination to examine synergistic effects (Figure 3). A dose range of ixabepilone (0.1-3 nM) was combined with fixed doses of Temsirolimus (0, 0.1, 1, or 10 nM). Overall, temsirolimus and ixabepilone are synergistic when combined at low concentrations (nM) as shown by Fa-CI plots (Figure 3). Dose ratios that did not exhibit synergy (>1) are marked by black circles in the plot legend. Interestingly, the renal cell line (Caki-1) that had complete loss of p-MTOR in response to temsirolimus (Figure 2C), exhibited the highest synergism as indicated by the Fa-CI plot (Figure 3B). The cell line (Caki-2) that was most resistant to p-mTOR downregulation (Figure 2C), only exhibited synergism when ixabepilone was used at concentrations higher than 1 nM (Figure 3C).
Figure 3.
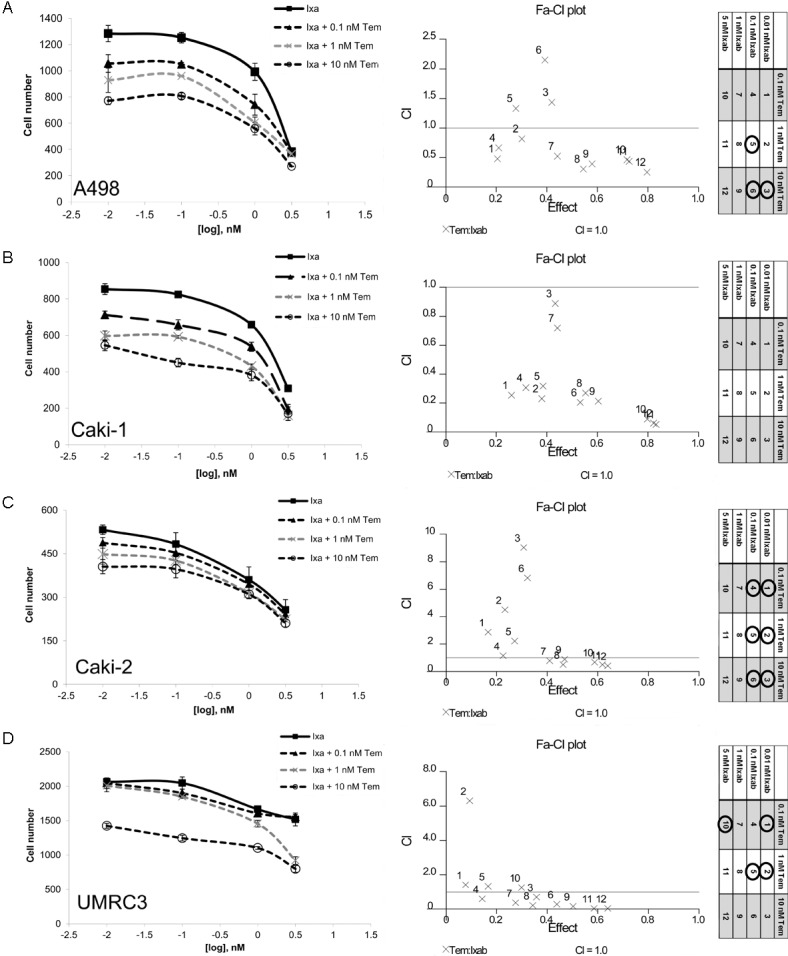
Combinatorial effects of temsirolimus and ixabepilone in RCC. Synergy curves using various concentrations of ixabepilone were used alone and in combination with three fixed concentrations of temsirolimus (0.1 nM, 1 nM, and 10 nM). Drug interactions were analyzed using CalcuSyn® [26] based upon the Chou-Talalay Method, whereas the combination index (CI) =1 indicates an additive effect, <1 is synergy and >1 is antagonism [27]. Dose curves of A. A498, B. Caki-1, C. Caki-2 and D. UMRC3 overall indicate that temsirolimus and ixabepilone are synergistic when combined as shown by Fa-CI plots. The legend is included and dose ratios that did not exhibit synergy (>1) are marked by black circles.
In order to identify whether the synergistic decrease in proliferation observed was due to decreased cell doubling or induction of cell death, cell death via propidium iodide staining as measured by flow cytometry was examined using 10 nM temosirolimus and 2 nM ixabepilone (Figure 4). Again, Caki-1 cells showed the highest combinatorial effects at 38.4% cell death (Figure 4B). Caki-2 cells, which previously showed the most resistance to Temsirolimus (Figures 2C, 3C), had 29.3% cell death in combination (Figure 4C). UMRC3 cells were more resistant to cell death with only a 1.6% difference in the combination when compared to ixabepilone alone, suggesting that synergy may be due to lower proliferative capacity and not induction of cell death (Figure 4D). Western analysis confirmed that cell death due to Ixabepilone and combination treatment in all four RCC cell lines was due to apoptosis as indicated by PARP cleavage (Figure 5). Combination treatment demonstrated elevated PARP cleavage when compared to all other treatment groups, with UMRC3 having the lowest levels of cleavage, reflective of the propidium idodide staining (Figure 5). Of note, Caki-1 and Caki-2 both demonstrated significant cell death via propidium iodide staining (Figure 4B, 4C) due to Temsirolimus monotherapy, however no corresponding PARP cleavage (Figure 5). This suggests that temsirolimus monotherapy may be inducing non-apoptotic mechanisms of cell death. Downstream targets of m-TOR were also examined and p-p70S6K was strongly down regulated with no change in p4EBP1 levels (Figure 5).
Figure 4.
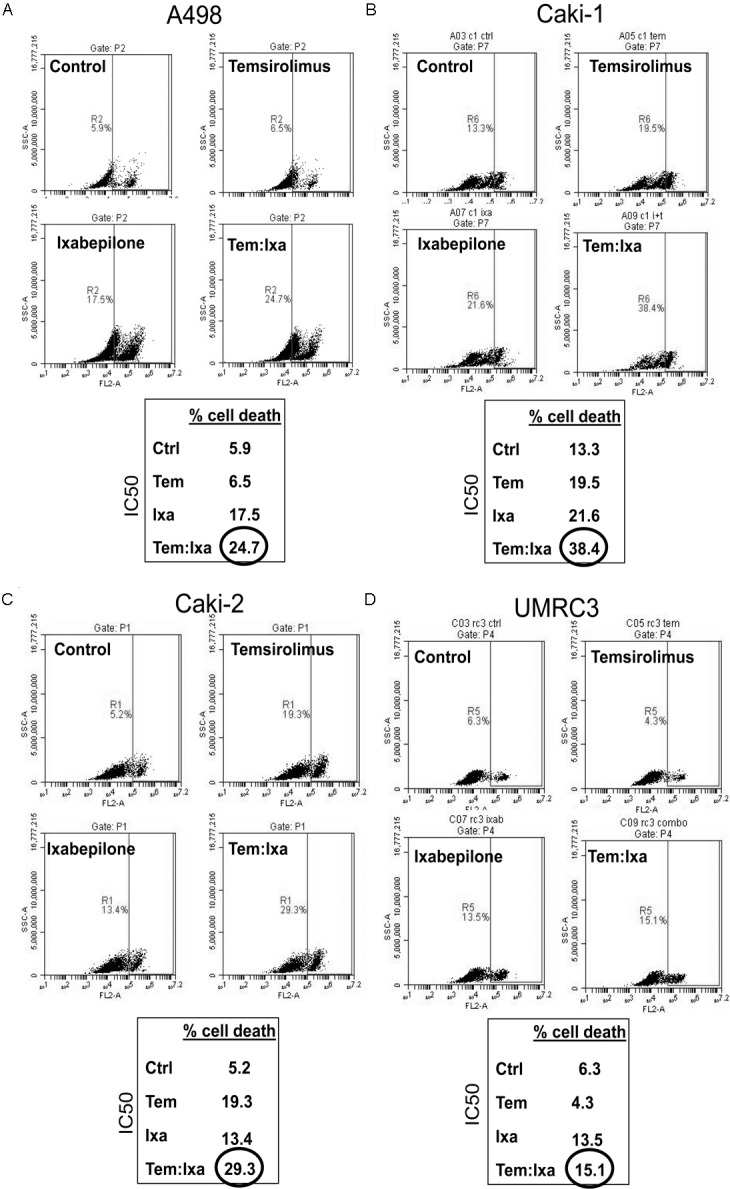
Cell death combinatorial effects of temsirolimus and ixabepilone in RCC. Cells were treated with 10 nM temsirolimus (Tem) and 2 nM ixabepilone (Ixa) for 48 hours followed by cell death analysis as described in Materials and Methods. A. A498, B. Caki-1, C. Caki-2 and D. UMRC3.
Figure 5.
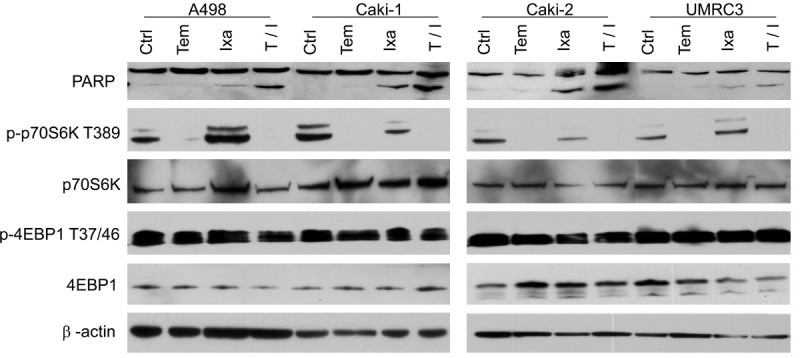
Effects of temsirolimus and ixabepilone on RCC protein expression. Western blot panel of 4 RCC cell lines treated with 10 nM temsirolimus (Tem) and 2 nM ixabepilone (Ixa) for 24 hours. PARP and proteins downstream of p-mTOR were examined. All cell lines show increased PARP cleavage, indicative of apoptosis, with the combinatorial treatment (T/I). p-p70s6K expression was decreased in response to Tem with no effects on p4EBP1. Proteins for 4EBP1 were run on a 18% tris-glycine gel for 1.5 hrs at 175 V.
In order to identify mechanisms of synergistic cell death in RCC cells, downstream components of the mTOR signaling pathway and Ixabepilone were examined. Microtubule stabilizers such as Ixabepilone function by binding to microtubule polymers and preventing them from dissociating into monomers, thereby disrupting the process of MT treadmilling [29-32]. MT dynamics are crucial for many cells processes, and a possible immediate consequence may be perturbation of endoplasmic reticulum (ER) homeostasis [30-32]. MTOR signaling also plays a role in ER functionality through mTORC1 by mediating protein translation, metabolism, autophagy, etc [33,34]. Thus, we examined ER stress as a convergence point of both Temsirolimus and Ixabepilone activity.
Combination therapy demonstrated the highest induction of endoplasmic reticulum (ER) stress signaling in both the Caki-1 (VHL wt) and Caki-2 (VHL mut) RCC cell lines treated with combinatorial therapy (10 nM temsirolimus and 2 nM ixabepilone) as shown by increased protein expression of downstream ER stress protein chaperone BIP and apoptosis inducer CHOP (Figure 6A) when compared to control or monotherapy. ATF6 is one of the primary ER stress sensor, and translocates to the nucleus to upregulate transcription of several ER stress response proteins when activated, including BiP and CHOP [35,36]. ATF6 expression was attenuated in Caki-1 and Caki-2 cells using two separate lentiviral shRNA constructs (ATF6-332 and ATF6-690) as shown by QPCR (Figure 6C). Both ATF6 shRNA constructs demonstrated significant recovery of cell proliferation in the presence of ixabepilone (2 nM) and temsirolimus (10 nM) combination therapy in both Caki-1 and Caki-2 cell lines compared to nontarget (NT) control (Figure 6D). We have thus demonstrated that ER stress likely mediates partof the antitumor synergy of combined mTOR inhibitor and a microtubule stabilizer.
Figure 6.
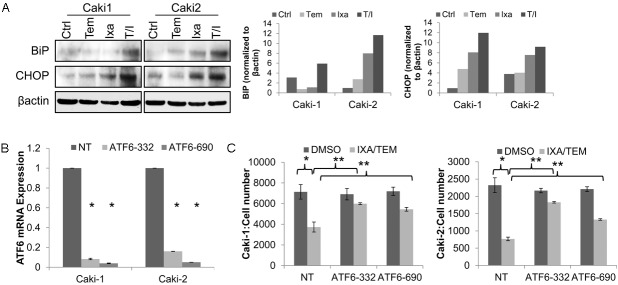
Combination of temsirolimus with ixabepilone induces ER stress. Endoplasmic Reticulum (ER) stress was observed in Caki-1 and Caki-2 RCC cells treated with temsirolimus (10 nM) and ixabepilone (2 nM) as shown by A. induced expression of the ER stress markers BIP and CHOP via western blot. B. Lentiviral suppression of ER stress regulator ATF6 using two separate shRNA constructs as demonstrated by QPCR revealed C. significant abrogation of growth inhibition induced by combination therapy when compared to non-target (NT) controls. **indicates p<0.05 when comparing ATF6 shRNA to NT control and *when comparing treatment to DMSO control.
Discussion
A new focus on development of combinatorial strategies to treat RCC has emerged in the past few years, however progress has been limited. Targeted therapies currently being investigated for combinatorial treatment of RCC include anti-angiogenic drugs such as bevacizumab, tyrosine kinase inhibitors such as sunitinib, immunotherapy, and mTOR inhibitors such as temsirolimus [37,38]. While combinatorial therapy may hold potential for more effective anti-cancer therapies, these combinations are not always better than single agent therapy and may produce intolerable adverse events in the clinic. Rational, mechanism-based combinations need to be evaluated at the bench, with promising combinations to be subsequently evaluated in the clinic.
In an effort to identify a novel, more effective regimen for patients with advanced RCC, we chose to evaluate the combination of the FDA approved mTOR inhibitor, temsirolimus, with the microtubule stabilizing drug, Ixabepilone. This combination was in part based on the clinical activity that both agents had demonstrated in the clinical setting, but more importantly on the potential for mechanism-based synergism with the two agents in this patient population. Temsirolimus blocks pro-survival signaling of the oncogenic PI3K/Akt/mTOR conduit, potentially crippling tumor cell translation, ribosome biogenesis, metabolism or other downstream effectors of this pathway [4,5]. Despite its efficacy in the clinic, we observed a lack of RCC tumor cell response to Temsirolimus in vitro, and therefore sought to combine it with a chemotoxic agent in an effort to enhance the anti-tumor effects of Temsirolimus. Microtubules are dynamic cytoskeletal constituents that not only regulate cellular transport and cell motility, but are critical for mitosis and cytokinesis during cell division [39,40]. Application of microtubule stabilizers such as ixabepilone effectively blocks dissociation of microtubule polymers and consequently arrests cell division [17,19]. Ixabepilone was of particular interest as it has demonstrated efficacy in tumor cells that have demonstrated resistance to other microtubule stabilizing drugs such as the taxane agents paclitaxel and docetaxel [19,41,42].
In this study we demonstrate synergistic anti-proliferative effects using temsirolimus in combination with ixabepilone in RCC. Combination treatment successfully attenuated p70S6K phosphorylation downstream of mTOR, resulted in an increase in polymerized microtubule fractions, and yielded an increase in cell death as shown by both PI staining via flow cytometry and PARP cleavage. In an effort to identify potential mechanisms of drug synergy, our group identified, for the first time, enhanced ER stress signaling in part responsible for drug response.
Some of the major functions of the ER include protein translation and refolding, storage and secretion of intracellular calcium, and it belongs to part of the secretory pathway. In the event of cellular stress induced by accumulation of misfolded protein, or protein aggregation, the ER is responsible for triggering the unfolded protein response (UPR) in an effort to restore cellular homeostasis or induce programmed cell death [43,44]. This signaling response is marked by the activation of three primary stress sensors: activating transcription factor 6 (ATF6), inositol-requiring protein 1 (IRE1), and/or protein kinase RNA-like endoplasmic reticulum kinase (PERK). These then regulate downstream activation of protein chaperones such as heat shock 70kDa protein 5 (BIP), ER-associated degradation (ERAD) proteins, or apoptosis mediators such as damage inducible transcript 3 (CHOP) [43,44].
Microtubules may influence ER homeostasis in several ways. They play an important role in the secretory pathway as they provide the avenues by which transport intermediates are shuttled between the ER and the Golgi [39,45]. Also, the dynamic instability of microtubules allows for rapid re-organization in response to various stimuli within the cell, including the structural integrity and expansion of the ER [30-32]. We demonstrate that interference of microtubule dynamics via treatment with ixabepilone induces expression of BIP and CHOP (Figure 6), suggesting that ER stress is a downstream consequence. Furthermore, while temsirolimus seemed to induce little ER stress on its own, the combination of temsirolimus with ixabepilone significantly enhanced this response (Figure 6). Based upon this data, we propose that ER stress induced proteins such as BIP and CHOP may serve as early biomarkers for positive response to combinatorial therapy. While recent undertakings in the elucidation of crosstalk between mTOR signaling and the ER stress response is currently taking place [46], further investigation is required in order to provide a suitable explanation for our observations.
In summary, we demonstrate that the novel combination of ixabepilone with temsirolimus synergistically attenuates tumor cell growth in highly aggressive, metastatic RCC. This drug combination also resulted in the induction of the ER stress response, which contributed in part to tumor cell death and provides a partial explanation for mechanism of synergy. A phase I clinical trial evaluating the safety of this combination in patients with advanced solid tumors is currently ongoing (Clinical Trials Identifier: NCT01375829).
Acknowledgements
This work was funded in part from NIH/NCI grants 2K12CA090628 (MEM) and R01CA10-4505 (JAC); a generous gift from the David & Lois Stulberg Endowed Fund for Kidney Cancer Research (JAC); Scheidel Foundation (JAC) and a grant for rare cancers from Dr. Ellis and Dona Brunton (JAC). This publication was made possible by the CTSA Grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosure of conflict of interest
The authors of this manuscript declare that they have no conflict of interest.
Abbreviations
- RCC
Renal cell carcinoma
- PI3K
phosphoino-sitide-3-kinase
- PDGFRβ
platelet derived growth factor beta
- EGFR
epidermal growth factor receptor
- VEGFR
vascular endothelial growth factor receptor
- IGFR
insulin growth factor receptor
- ER
endoplasmic reticulum
- UPR
unfolded protein response
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Russo P, Nanus DM, Berg WJ. Renal cell carcinoma. Curr Probl Cancer. 1997;21:185–232. doi: 10.1016/s0147-0272(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 5.Robb VA, Karbowniczek M, Klein-Szanto AJ, Henske EP. Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol. 2007;177:346–352. doi: 10.1016/j.juro.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 6.Pantuck AJ, Seligson DB, Klatte T, Yu H, Leppert JT, Moore L, O’Toole T, Gibbons J, Belldegrun AS, Figlin RA. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 9.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 10.Yazbeck VY, Georgakis GV, Li Y, Younes A. The mTOR inhibitor CCI-779 (temsirolimus) downregulates p21 and induces cell cycle arrest and autophagy in mantle cell lymphoma (MCL) Journal of Clinical Oncology (Meeting Abstracts) 2006;24:7573. [Google Scholar]
- 11.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illiger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 12.Garrido-Laguna I, Tan AC, Uson M, Angenendt M, Ma WW, Villaroel MC, Zhao M, Rajeshkumar NV, Jimeno A, Donehower R, Iacobuzio-Donahue C, Barrett M, Rudek MA, Rubio-Viqueira B, Laheru D, Hidalgo M. Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer. Br J Cancer. 2010;103:649–655. doi: 10.1038/sj.bjc.6605819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover S, Singh JC, Kekre NS. Temsirolimus, interferon alfa or both in advanced renal-cell carcinoma: One plus one does not always equal two. Indian J Urol. 2007;23:332–333. [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie MJ, Rini BI, Elson P, Schwandt A, Wood L, Trinkhaus M, Bjarnason G, Knox J. Temsirolimus in VEGF-refractory metastatic renal cell carcinoma. Ann Oncol. 2011;22:145–8. doi: 10.1093/annonc/mdq320. [DOI] [PubMed] [Google Scholar]
- 15.Oza AM, Kollmannsberger C, Hirte H, Welch S, Siu L, Mazurka J, Sederias J, Doyle LA, Eisenhauer E; NCIC Clinical Trials Group. Phase I study of temsirolimus (CCI-779), carboplatin, and paclitaxel in patients (pts) with advanced solid tumors: NCIC CTG IND 179. J. Clin. Oncol. 2009;27:3558. doi: 10.1093/annonc/mdr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Menefee M, Edgerly M, Zhuang S, Kotz H, Poruchynsky M, Huff LM, Bates S, Fojo T. A phase II clinical trial of ixabepilone (Ixempra; BMS-247550; NSC 710428), an epothilone B analog, in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2010;16:1634–1641. doi: 10.1158/1078-0432.CCR-09-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 18.Zhuang SH, Hung YE, Hung L, Robey RW, Sackett DL, Linehan WM, Bates SE, Fojo T, Poruchynsky MS. Evidence for microtubule target engagement in tumors of patients receiving ixabepilone. Clin Cancer Res. 2007;13:7480–7486. doi: 10.1158/1078-0432.CCR-06-2883. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)) J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 20.Posadas EM, Undevia S, Manchen E, Wade JL, Colevas AD, Karrison T, Vokes EE, Stadler WM. A phase II study of ixabepilone (BMS-247550) in metastatic renal-cell carcinoma. Cancer Biol Ther. 2007;6:490–493. doi: 10.4161/cbt.6.4.3831. [DOI] [PubMed] [Google Scholar]
- 21.Perez EA, Patel T, Moreno-Aspitia A. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res Treat. 2010;121:261–271. doi: 10.1007/s10549-010-0824-0. [DOI] [PubMed] [Google Scholar]
- 22.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SF, Huang YY, Lin JD, Chou TC, Hsueh C, Wong RJ. Utility of a PI3K/mTOR inhibitor (NVP-BEZ235) for thyroid cancer therapy. PLoS One. 2012;7:e46726. doi: 10.1371/journal.pone.0046726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Wong CH, Lau CP, Hui CW, Lui VW, Chan SL, Yeo W. Enhanced Antitumor Activity with Combining Effect of mTOR Inhibition and Microtubule Stabilization in Hepatocellular Carcinoma. Int J Hepatol. 2013;2013:103830. doi: 10.1155/2013/103830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang CT, Yeh PY, Gao M, Chen CW, Yeh LC, Feng WC, Kuo SH, Hsu CH, Lu YS, Cheng AL. Combinations of mTORC1 inhibitor RAD001 with gemcitabine and paclitaxel for treating non-Hodgkin lymphoma. Cancer Lett. 2010;298:195–203. doi: 10.1016/j.canlet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Chou TC, Hayball MP. CalcuSyn for Windows: multiple-drug dose effect analyzer and manual. Cambridge (UK): Biosoft 1997 [Google Scholar]
- 27.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 29.Honore S, Pasquier E, Braguer D. Understanding microtubule dynamics for improved cancer therapy. Cell Mol Life Sci. 2005;62:3039–3056. doi: 10.1007/s00018-005-5330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986;103:1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;8:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- 33.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012;22:274–282. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem. 2000;275:27013–27020. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- 36.Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol. 2009;112:e1–9. doi: 10.1159/000210573. [DOI] [PubMed] [Google Scholar]
- 37.Hutson TE. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist. 2011;16(Suppl 2):14–22. doi: 10.1634/theoncologist.2011-S2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escudier B, Szczylik C, Porta C, Gore M. Treatment selection in metastatic renal cell carcinoma: expert consensus. Nat Rev Clin Oncol. 2012;9:327–337. doi: 10.1038/nrclinonc.2012.59. [DOI] [PubMed] [Google Scholar]
- 39.Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- 40.Valiron O, Caudron N, Job D. Microtubule dynamics. Cell Mol Life Sci. 2001;58:2069–2084. doi: 10.1007/PL00000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edelman MJ, Shvartsbeyn M. Epothilones in development for non--small-cell lung cancer: novel anti-tubulin agents with the potential to overcome taxane resistance. Clin Lung Cancer. 2012;13:171–180. doi: 10.1016/j.cllc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Cobham MV, Donovan D. Ixabepilone: a new treatment option for the management of taxane-resistant metastatic breast cancer. Cancer Manag Res. 2009;1:69–77. [PMC free article] [PubMed] [Google Scholar]
- 43.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 44.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bloom GS, Goldstein LS. Cruising along microtubule highways: how membranes move through the secretory pathway. J Cell Biol. 1998;140:1277–1280. doi: 10.1083/jcb.140.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–176. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]