Abstract
Our group recently demonstrated in a rat model that pretreatment with morphine facilitates doxorubicin delivery to the brain in the absence of signs of increased acute systemic toxicity. Morphine and other drugs such as dexamethasone or ondansetron seem to inhibit MDR proteins localized on blood-brain barrier, neurons and glial cells increasing the access of doxorubicin to the brain by efflux transporters competition. We explored the feasibility of active modification of the blood-brain barrier protection, by using morphine dexamethasone or ondansetron pretreatment, to allow doxorubicin accumulation into the brain in a rodent model. Rats were pretreated with morphine (10 mg/kg, i.p.), dexamethasone (2 mg/kg, i.p.) or ondansetron (2 mg/kg, i.p.) before injection of doxorubicin (12 mg/kg, i.p.). Quantitative analysis of doxorubicin was performed by mass spectrometry. Acute hearth and kidney damage was analyzed by measuring doxorubicin accumulation, LDH activity and malondialdehyde plasma levels. The concentration of doxorubicin was significantly higher in all brain areas of rats pretreated with morphine (P < 0.001) or ondansetron (P < 0.05) than in control tissues. The concentration of doxorubicin was significantly higher in cerebral hemispheres and brainstem (P < 0.05) but not in cerebellum of rats pretreated with dexamethasone than in control tissues. Pretreatment with any of these drugs did not increase LDH activity or lipid peroxidation compared to controls. Our data suggest that morphine, dexamethasone or ondansetron pretreatment is able to allow doxorubicin penetration inside the brain by modulating the BBB. This effect is not associated with acute cardiac or renal toxicity. This finding might provide the rationale for clinical applications in the treatment of refractory brain tumors and pave the way to novel applications of active but currently inapplicable chemotherapeutic drugs.
Keywords: Doxorubicin, morphine, dexamethasone, ondansetron, blood-brain barrier, rodent model, MDR transporters, mass spectrometry
Introduction
Cure rate of CNS tumors is hampered by limited diffusion of chemotherapeutic agents within the nervous parenchyma. A prerequisite for the efficacy of an anti-neoplastic agent is that it reaches the tumor at an efficacious concentration. In CNS the achievement of therapeu-tic concentration of antineoplastic agents is complicated by the presence of P-glycopro-tein (P-gp) efflux transporter localized on the blood-brain barrier (BBB), a physiological barrier that protects the brain from toxic insults [1,2].
Anthracyclines have been shown to inhibit tumor cell growth in several tumor lines and are now used to treat different types of neoplasms. Doxorubicin is a potent inhibitor of glioma in vitro and in vivo and is toxic to glioblastoma cell lines [3,4].
Unfortunately, doxorubicin has a poor penetration into the brain when systemically administered due to the lack of drug penetration through the BBB.
Our group recently verified in an animal model that morphine pretreatment increases the level of doxorubicin into the brain [5,6].
It has lately emerged that the mechanism of BBB-mediated drug resistance is complicated by the cooperation of P-glycoprotein (P-gp, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). They very efficiently remove several molecules and drugs from the CNS, thus limiting their entry into the brain [7]. These two “gatekeeper” efflux pumps work in synergy on the BBB and are usually present on the plasma membrane of many brain tumors. Inhibition of P-gp or BCRP can be compensated by the respective other transporter [8]. Interestingly, P-gp and BCRP have broad substrate specificity and interact with a range of chemically assorted molecules, including chemotherapeutic agents, such as doxorubicin, methotrexate, etoposide, paclitaxel and vincristine [7,9]. Moreover, many other drugs as morphine, dexamethasone and ondansetron are substrate of these efflux transporters. Thus, it is conceivable that morphine and other chemical agents could inhibit P-gp and BCRP localized on BBB, neurons and glial cells and thus increase the access of doxorubicin to the brain.
Materials and methods
Drugs and reagents
Doxorubicin (12 mg/kg, i.p. solution for injection, Sandoz Spa, Varese, Italy); Morphine (10 mg/kg, i.p. solution for injection, Molteni SpA, Firenze, Italy); Ondansetron (Zofran® 2 mg/kg, i.p. solution for injection, Glaxosmithkline SpA, Verona, Italy). Dexamethasone phosphate (2 mg/kg, i.p. solution for injection, Hospira, Napoli, Italy).
The internal standard Doxorubicin-13C4 (98% D purity) was purchased from Medical Isotopes Inc. (Pelham, USA). All other chemicals and solvents were of the highest purity grade commercially available.
Animals
Male adult Wistar rats (260-280 g, Harlan Italia, Milano, Italy) were used. All animal manipulations were carried out according to the European Community guidelines for animal care (DL 116/92, application of the European Communities Council Directive 86/609/EEC). All efforts were made to minimize animal sufferings and to use only the number of animals necessary to produce reliable scientific data.
Rats were randomly allocated to one of the following experimental groups (N=4-10):
Group 1 (Morph+DOXO): on day 1 rats were treated twice with morphine (10 mg/kg, i.p., at 10.00 a.m. and again at 5.00 p.m.); on day 2 at 10.00 a.m., the rats received a third dose of morphine; one hour later they received doxorubicin (12 mg/kg, i.p.).
Group 2 (Dexa+DOXO): on day 1 rats were treated twice with dexamethasone (2 mg/kg, i.p., at 10.00 a.m. and again at 5.00 p.m.); on day 2 at 10.00 a.m., the rats received a third dose of dexamethasone; one or 2 h later they received doxorubicin (12 mg/kg, i.p.).
Group 3 (Onda+DOXO): on day 1 rats were treated twice with ondansetron (2 mg/kg, i.p., at 10.00 a.m. and again at 5.00 p.m.); on day 2 at 10.00 a.m., the rats received a third dose of ondansetron; one or 2 h later they received doxorubicin (12 mg/kg, i.p.).
Group 4 rats (DOXO): rats were treated with doxorubicin alone (12 mg/kg, i.p.).
Group 5 rats (Control): naïve rats received no drugs.
Collection of brain tissue samples
One hour after administration of doxorubicin, the rats were anesthetized with chloral hydrate (400 mg/kg, i.p.). The heart was exposed and animals were perfused transcardially with ice-cold saline (500 mL, 50 mL/min). At the end of perfusion, cerebral hemispheres, cerebellum, brainstem were collected, immediately frozen on dry ice and kept at -80°C until assay.
Doxorubicin determination by mass spectrometry
Doxorubicin determination on plasma
To quantify plasma concentration of doxorubicin, samples (25 μL) were spotted on filter paper (903®, Whatman GmbH, Dassel Germany) and air-dried. Dried plasma spots were punched and two 3.2 mm circles (containing about 1.75 μL of plasma each) were extracted together with 200 μL of 20/80 water/methanol solution containing 10 μmol/L of 13C4- doxorubicin. Samples were put in an orbital shaker and kept at 37°C for 20 min.
For the setting-up of this study, a pooled mixture of plasma samples from untreated rats was spiked with increasing concentrations of doxorubicin and 25 μL were spotted on 903® filter paper.
Tissue sample preparation
Thawed brain sections (cerebral hemispheres, brainstem, and whole brain) were weighed and spiked with 2 μL of 13C4- doxorubicin (10 μmol/L) directly injected into the tissue. After 10 min, the sections were diluted with 2.5 mL H2O. Sample homogenization was performed using a mechanical homogenizer (UltraTurrex, IKA-Werke, Staufen, Germany) followed by 10 min of intermittent ultrasonication in the ice bath. Samples were defatted using 2 mL of chloroform with vigorous shaking for 5 min, followed by centrifugation at 3,000 rpm for 10 min. The aqueous upper phase was transferred to a new tube, and the chloroform extraction step was repeated 6 times. After the last extraction acetonitrile (400 μL) was added and precipitates removed by centrifugation at 13,200 rpm for 3 min. The samples were dried out and rehydrated with acidified methanol (0.1% formic acid, v/v) 300 μL. To determine the extraction yield of doxorubicin, brain tissue samples from an untreated rat were spiked with doxorubicin (5 μmol/L) before and after extraction process.
Mass spectrometry
An Applied Biosystems-Sciex (Toronto, Canada) API 4000 bench-top TurboIonSpray-Triple-Quad Mass Spectrometer (MS/MS) was used for this study. The ion source operated under positive ion mode (5,500 V).
Declustering Potential (DP), Collision Exit Potential (CXP) and Collision Energy (CE) were automatically optimized for doxorubicin and 13C4- doxorubicin. The resulting DP was 35 V. Optimal CE and CXP were found at 16 V and 10 V, respectively. From these experiments, the resulting most selective ion-pair transitions for the quantitative experiment (SRM) are 544.3>397.2 for doxorubicin and 548.3>401.2 for 13C4- doxorubicin. We have chosen one additional transition as qualifier: 544.3>379.2 for doxorubicin and 548.3>383.2 for 13C4- doxo-rubicin.
Quantitative analysis was undertaken using an HPLC Series 1100 Agilent Technologies (Waldbronn, Germany). Liquid chromatography was performed using a Phenomenex Synergi 4 μm Polar-RP 80A 4 μm, 2 x 150 mm HPLC column (Phenomenex, Anzola Emilia, Italy) injecting 5 μL of the extracted sample. Column flow was 0.2 mL/min using an isocratic aqueous solution of 80% methanol containing 0.1% formic acid. The eluent from the column was directed to the TurboIonSpray probe without split ratio.
LDH activity and tissue lipid peroxidation
Lactate dehydrogenase (LDH) activity was measured in rat plasma using the Cytotoxicity Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany) and absorbance read at 490 nm.
Tissue lipid peroxidation in terms of malondialdehyde (MDA) production (thiobarbituric acid-reactive species) was determined as previously described [10].
Statistical analysis
Statistical significance was evaluated using one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test as a post-hoc analysis or by Student’s t-test, asappropriate. A P value of 0.05 or less was taken as the criterion for statistically significant difference.
Results
In control samples from the brain of naïve, untreated rats no ion-pair transition from 544.4 to 397.2, co-eluting with doxorubicin was observed indicating that in brain tissues no endogenous compound which may interfere with the quantitative analysis of doxorubicin was present. Indeed, quantitative analysis of doxorubicin in control brain samples gave the amount of 0.001±0.001 ng/mg fresh tissue (n=3). The estimated limit of detection of doxorubicin (signal to noise ratio>3) in the brain was 0.1 ng/g fresh tissue, and the limit of quantitation (signal to noise ratio >5) was 0.2 ng/g fresh tissue.
Mean plasma levels of doxorubicin in rats receiving doxorubicin alone (13.26±2.12 μmol/L plasma, n=5) did not differ from those found in animals pretreated with morphine (12.89±0.71 μmol/L plasma, n=5), with ondansetron (9.34±0.88 μmol/L plasma, n=7) or with dexamethasone (12.66±1.78 μmol/L plasma, n=8) (n.s., one-way ANOVA followed by NewmanKeuls).
The concentration of doxorubicin was significantly higher in the cerebral cortex (+220%, P<0.0001, Student’s t test), in the brainstem (+156%, P<0.0001, Student’s t test) or in the cerebellum (+208%, P<0.0001, Student’s t test) of rats pre-treated with morphine (10 mg/kg, i.p., than in matched control brain areas of rats treated with doxorubicin alone at the same dose.
Cardiac and renal toxicity after treatment with an anthracycline is a matter of great concern. We therefore investigated whether pretreatment with morphine might increase the doxorubicin concentrations in the heart and kidney, thus predicting increased toxicity in these two target organs. Pretreatment with morphine did not increase the levels of doxorubicin in either tissue at 1 h after administration (P>0.05, Student’s t test).
We addressed the issue of cardiac and renal toxicity by analysis of plasma LDH activity and MDA levels. Doxorubicin induced a non-significant 60% increase of plasma LDH activity 1 h after treatment in treated versus control rats (P>0.05, Student’s t test ). No difference in LDH activity was found between rats treated with doxorubicin alone and those receiving morphine plus doxorubicin (P>0.05, Student’s t test).
We performed a time-course of the effect of pretreatment with dexamethasone (2 mg/kg, i.p., three times in 24 h) on doxorubicin penetration into the brain of the rat, administering doxorubicin 1 or 2 h after the last injection of dexamethasone. The results are presented in Figure 1. Doxorubicin concentration was higher in all the different brain areas of animals pretreated with dexamethasone both at 1 and 2 h.
Figure 1.
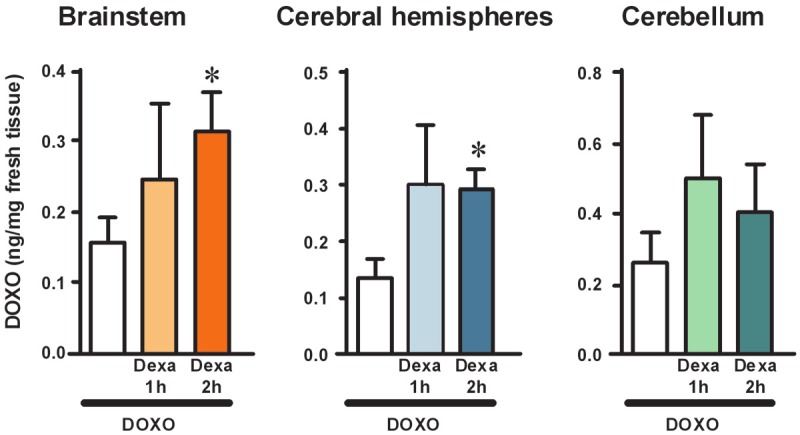
Time-course of the effect of pretreatment with dexamethasone (2 mg/kg, i.p., three times in 24 h) on doxorubicin penetration into the brain of the rat, administering doxorubicin 1 or 2 h after the last injection of dexamethasone. Levels of doxorubicin in brainstem, cerebral hemispheres, and cerebellum of the rat were quantified collecting brain structures 1 h after a single administration of doxorubicin (12 mg/kg, i.p.). Doxorubicin was administered to different groups of rats either alone (white bars, n=5) or at different times after pretreatment with 3 doses of dexamethasone (10 mg/kg each, three times in 24 h). Doxorubicin was administered either 1 h (Dexa 1 h) or 2 h (Dexa 2 h) after the last administration of dexamethasone. Tissue levels of doxorubicin are expressed as ng/mg fresh tissue (mean±SEM). Statistical analysis was performed separately within each brain area investigated. Significance: *P<0.05 versus doxorubicin alone (one-way ANOVA and Dunnett’s Multiple Comparison Test).
The mean concentration of doxorubicin in the brainstem of rats treated with doxorubicin alone was 0.16±0.04 ng/mg fresh tissue (n=7), versus 0.25±0.11 ng/mg fresh tissue in those treated with doxorubicin plus dexamethasone at 1 h (+56%, n=3), and 0.32±0.05 ng/mg fresh tissue in those treated with doxorubicin plus dexamethasone at 2 h (+100%, n=6). This latter effect was statistically significant (one-way ANOVA F[2,13]=4.364, P<0.05; *P<0.05 vs doxorubicin alone, Dunnett’s Multiple Comparison Test).
The mean concentration of doxorubicin in the cerebral hemispheres of rats treated with doxorubicin alone was 0.14±0.03 ng/mg fresh tissue (n=10), versus 0.30±0.11 ng/mg fresh tissue in those treated with doxorubicin plus dexamethasone at 1 h (+114%, n=3), and 0.30±0.03 ng/mg fresh tissue in those treated with doxorubicin plus dexamethasone at 2 h (+114%, n=7). This latter effect was statistically significant (one-way ANOVA F[2,17]=5.157, P<0.05; *P<0.05 vs doxorubicin alone, Dunnett’s Multiple Comparison Test).
The mean concentration of doxorubicin in the cerebellum of rats treated with doxorubicin alone was 0.26±0.09 ng/mg fresh tissue (n=4), versus 0.50±0.18 ng/mg fresh tissue in those treated with doxorubicin plus dexamethasone at 1 h (+92%, n=3), and 0.40±0.14 ng/mg fresh tissue in those treated with doxorubicin plus dexamethasone at 2 h (+54%, n=6). Neither effect was statistically significant (one-way ANOVA F[2,10]=0.635, P>0.05).
We performed a time-course of the effect of pretreatment with ondansetron (2 mg/kg, i.p., three times in 24 h) on doxorubicin penetration into the brain of the rat, administering doxorubicin 1 or 2 h after the last injection of ondansetron. The results are presented in Figure 2. Doxorubicin concentration was higher in all the different brain areas of animals pretreated with ondansetron (Figure 3).
Figure 2.
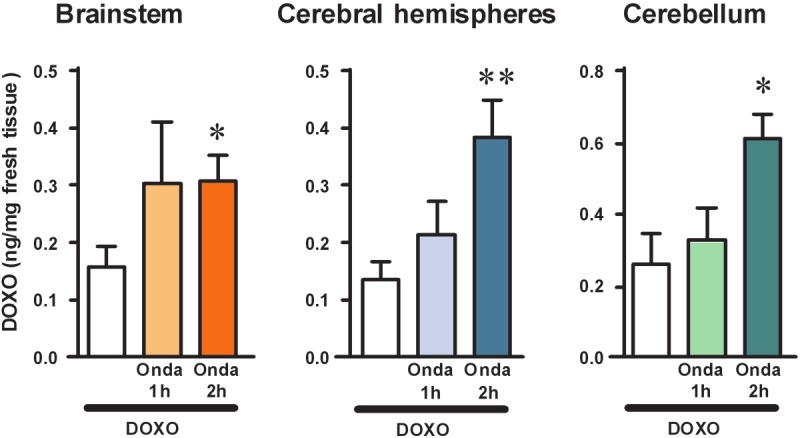
Time-course of the effect of pretreatment with ondansetron (2 mg/kg, i.p., three times in 24 h) on doxorubicin penetration into the brain of the rat, administering doxorubicin 1 or 2 h after the last injection of ondansetron. Levels of doxorubicin in brainstem, cerebral hemispheres, and cerebellum of the rat were quantified collecting brain structures 1 h after a single administration of doxorubicin (12 mg/kg, i.p.). Doxorubicin was administered to different groups of rats either alone (white bars, n=5) or at different times after pretreatment with 3 doses of ondansetron (10 mg/kg each, three times in 24 h). Doxorubicin was administered either 1 h (Desa 1 h) or 2 h (Desa 2 h) after the last administration of ondansetron. Tissue levels of doxorubicin are expressed as ng/mg fresh tissue (mean±SEM). Statistical analysis was performed separately within each brain area investigated. Significance: *P<0.05 and **P<0.01 versus doxorubicin alone (one-way ANOVA and Dunnett’s Multiple Comparison Test).
Figure 3.
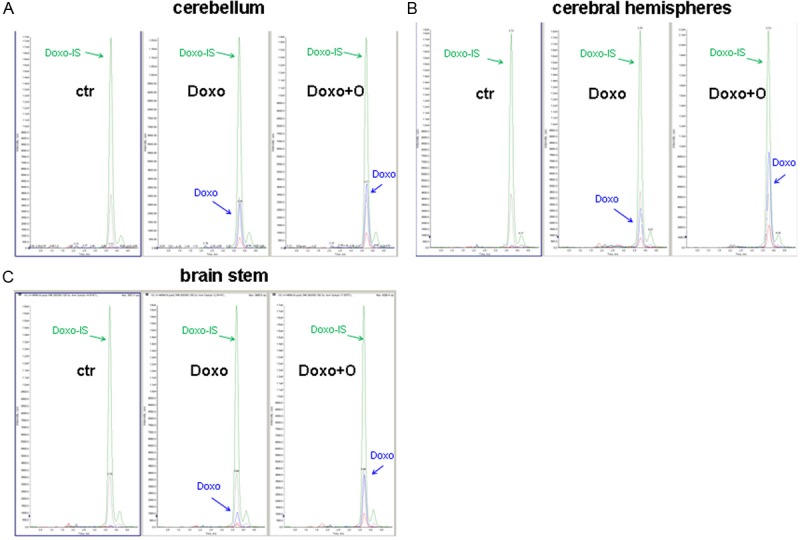
Extracted ion chromatograms of doxorubicin after pretreatment with ondansetron in rat brain (blu signal: quantifier ion; red signal, qualifier ion) and labeled internal standard (IS) (green signal: quantifier ion; gray signal, qualifier ion) in cerebral hemisphere (A), cerebellum (B) and brainstem (C). Doxorubicin signal is undetectable in all control samples.
The mean concentration of doxorubicin in the brainstem of rats treated with doxorubicin alone was 0.16±0.04 ng/mg fresh tissue (n=7), versus 0.30±0.10 ng/mg fresh tissue in those treated with doxorubicin plus ondansetron at 1 h (+87%, n=4), and 0.31±0.04 ng/mg fresh tissue in those treated with doxorubicin plus ondansetron at 2 h (+94%, n=9). This latter effect was statistically significant (one-way ANOVA F[2,17]=3.140, P<0.05; *P<0.05 vs doxorubicin alone, Dunnett’s Multiple Comparison Test).
The mean concentration of doxorubicin in the cerebral hemispheres of rats treated with doxorubicin alone was 0.14±0.03 ng/mg fresh tissue (n=10), versus 0.21±0.06 ng/mg fresh tissue in those treated with doxorubicin plus ondansetron at 1 h (+50%, n=7), and 0.39±0.06 ng/mg fresh tissue in those treated with doxorubicin plus ondansetron at 2 h (+179%, n=12). This latter effect was statistically significant (one-way ANOVA F[2,26]=5.930, P<0.01; **P< 0.01 vs doxorubicin alone, Dunnett’s Multiple Comparison Test).
The mean concentration of doxorubicin in the cerebellum of rats treated with doxorubicin alone was 0.26±0.08 ng/mg fresh tissue (n=4), versus 0.33±0.09 ng/mg fresh tissue in those treated with doxorubicin plus ondansetron at 1 h (+27%, n=4), and 0.61±0.07 ng/mg fresh tissue in those treated with doxorubicin plus ondansetron at 2 h (+135%, n=10). This latter effect was statistically significant (one-way ANOVA F[2,15]=5.426, P<0.02; *P<0.05 vs doxorubicin alone, Dunnett’s Multiple Comparison Test).
Discussion
The core result of our study is that pretreatment with therapeutic dose of morphine, dexamethasone or ondansetron allows delivery of doxorubicin within the brain tissue, which is otherwise disallowed by the BBB. Our previous study had already demonstrated that doxorubicin penetration into the brain of rat is significantly enhanced when it is administered in the presence of therapeutic plasma levels of morphine [5].
Doxorubicin, an anthracycline antibiotic which works by intercalating DNA and inhibiting topoisomerase II, is commonly used in the treatment of a wide range of tumors, including hematological malignancies, many types of carcinoma and soft tissue sarcomas [11]. The anthracyclines have got a very limited efficacy in the treatment of brain tumors because of their poor penetration through BBB mediated by MDR efflux transporters [12].
Interestingly, in vitro and in vivo data on models of malignant glioma suggest that doxorubicin is an effective anti-tumor agent [13-15]. Moreover, Eramo et al. have demonstrated that doxorubicin displays high antitumor activity in glioblastoma stem cells [16].
Therefore, several approaches have been tested for improving anthracyclines delivery to CNS through circumvention of the BBB. Recently, intracerebral drug delivery of doxorubicin via a polymer matrix in brain tumors of rat has been successfully performed [3]. A clinical study has shown promising results with intralesional doxorubicin infusion via an Ommaya reservoir without significant systemic side effects for therapy of malignant gliomas [17].
In vitro studies have recognized MDR efflux pumps such as P-gp, BCRP and other MDR-associated proteins in primary cultures of cerebral endothelial cells. These molecules are located at both the luminal and apical surfaces of the BBB to protect the CNS against xenobiotic agents [18-21] and they are therefore capable of transporting a wide assortment of chemotherapeutic agents such as doxorubicin, methotrexate, etoposide, paclitaxel and vincristine [7,9].
Morphine, dexamethasone and ondansetron, are reported in literature as a substrate of the P-gp [7,22]. Thus, it is conceivable that these chemical agents inhibiting MDR molecules localized on BBB could increase the access of doxorubicin to the brain competing with the same efflux transporters as P-gp and BCRP, that very efficiently removes these drugs from the CNS [7].
The ability of morphine, dexamethasone and ondansetron to modulate physiological MDR efflux pumps is of clinical interest. Indeed, these drugs are usually included in chemotherapy regimens for the treatment of pain, edema and nausea associated to chemotherapy.
Morphine is the most used narcotic analgesic. In a recent report Sharma and coworkers using a rodent model documented that morphine induced a transient alteration of BBB permeability to large molecules [23].
Dexamethasone is routinely used in the management of patients with intracranial tumors to reduce brain edema [24]. This agent is known to differentially alter the expression of efflux proteins depending on the type of tissue or origin of the cells. Interestingly, Narang et al. showed that corticosteroids such as dexamethasone may diminish the effectiveness of drugs that are required to penetrate the BBB increasing the expression of MDR transporters in a rat model [25].
Ondansetron is a selective 5-hydroxytryptamine( 3) (5-HT(3)) receptor antagonist, mainly used in clinical practice as an antiemetic for prophylaxis and treatment of nausea and vomiting related to chemotherapy and anesthesia. It affects both the peripheral and the central nervous system. However, CSF concentrations are less than 15% of plasma concentrations, indicating that the rate of penetration of the blood brain barrier by ondansetron is very low [26].
In the previous experiments we demonstrated the concentration of doxorubicin was significantly higher in all brain areas of rats pretreated with morphine than in control tissues (P<0.001). This was evident only at therapeutic morphine dose (10 mg/kg, three times over 24 hours), while lower doses (2.5 and 5 mg/kg) were not associated with doxorubicin accumulation. Pretreatment with morphine did not induce a raise of LDH activity or of lipid peroxidation compared to controls.
In this study we confirmed the previous results on morphine pre-treatment facilitates doxorubicin delivery beyond the BBB to the brain in the absence of signs of increased acute systemic toxicity in a rat model. We confirmed a significantly higher doxorubicin level in all brain areas after pretreatment with morphine (P<0.001). Dexamethasone pretreatment facilitates doxorubicin penetration in brainstem and cerebral hemispheres (P<0.05), whereas does not determine change of doxorubicin level in cerebellum (>0.05). Lastly, we demonstrated a significantly higher doxorubicin level in all brain areas after pretreatment with ondansetron (brainstem and cerebellum P<0.05; cerebral hemispheres P<0.01).
The present study validated that the concentration of doxorubicin after morphine pretreatment was significantly higher in cerebral hemispheres than in cerebellum and brainstem (P<0.05). This could depend on a different composition of MDR efflux transporters in these brain areas, making the brainstem less accessible to many molecules.
Anthracyclines may induce acute cardiotoxicity, which is characterized by hypotension, tachycardia, arrhythmia, and transient depression of left ventricular function. In addition, high cumulative doses are associated with late-onset cardiomyopathy that is refractory to standard treatment [27]. Free radical generation and p53-dependent apoptosis by modulation of mTOR activity are supposed to contribute to doxorubicin-induced cardiotoxicity [28,29].
To evaluate whether doxorubicin toxicity might be enhanced by co-administration of morphine, dexamethasone or ondansetron, thus leading to acute cardiotoxicity and/or nephrotoxicity, we quantified doxorubicin levels in heart and kidney of pretreated rats. Co-administration of morphine, dexamethasone or ondansetron did not increase the levels of doxorubicin in either tissue 1 hour (morphine) and 1-2 hours (dexamethasone, ondansetron) after administration. We also evaluated plasma LDH activity and MDA levels, as markers of acute cardiotoxicity, and found no difference between rats treated with doxorubicin alone and those also receiving morphine, dexamethasone or ondansetron.
In conclusion our data suggest that blocking the MDR efflux transporters by pretreatment with morphine, dexamethasone or ondansetron is able to enhance significantly doxorubicin penetration into the brain of rat. This is not associated with acute cardiac or renal toxicity. These preliminary results on a rodent model will enable us to novel therapeutic approaches to refractory or recurrent brain tumours in which molecules usually stopped by the BBB may have a therapeutic impact.
Acknowledgements
Associazione Italiana per la Ricerca sul Cancro (AIRC), grant RG-6232; ‘‘Amicodivalerio’’ Onlus; “Noi per Voi” Onlus, Fondazione Tommasino Bacciotti.
Disclosure of conflict of interest
The authors indicated no potential conflicts of interest.
References
- 1.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnár Z, O'Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25:3825–3831. [PMC free article] [PubMed] [Google Scholar]
- 4.Stan AC, Casares S, Radu D, Walter GF, Brumeanu TD. Doxorubicin-induced cell death in highly invasive human gliomas. Anticancer Res. 1999;19:941–950. [PubMed] [Google Scholar]
- 5.Sardi I, la Marca G, Giovannini MG, Malvagia S, Guerrini R, Genitori L, Massimino M, Aricò M. Detection of doxorubicin hydrochloride accumulation in the rat brain after morphine treatment by mass spectrometry. Cancer Chemother Pharmacol. 2011;67:1333–1340. doi: 10.1007/s00280-010-1429-3. [DOI] [PubMed] [Google Scholar]
- 6.Sardi I. Morphine facilitates doxorubicin penetration in the central nervous system: a new prospect for therapy of brain tumors. J Neurooncol. 2011;104:619–620. doi: 10.1007/s11060-010-0518-9. [DOI] [PubMed] [Google Scholar]
- 7.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S, Hartz AM, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17:2793–2802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama M, Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin RS, Riggs CE Jr, Bachur NR. Pharmacokinetics and metabolism of adriamycin in man. Clin Pharmacol Ther. 1973;14:592–600. doi: 10.1002/cpt1973144part1592. [DOI] [PubMed] [Google Scholar]
- 12.Ohnishi T, Tamai I, Sakanaka K, Sakata A, Yamashima T, Yamashita J, Tsuji A. In vivo and in vitro evidence for ATP-dependency of P-glycoprotein-mediated efflux of doxorubicin at the blood-brain barrier. Biochem Pharmacol. 1995;49:1541–1544. doi: 10.1016/0006-2952(95)00082-b. [DOI] [PubMed] [Google Scholar]
- 13.Abe T, Hasegawa S, Taniguchi K, Yokomizo A, Kuwano T, Ono M, Mori T, Hori S, Kohno K, Kuwano M. Possible involvement of multidrug-resistance-associated protein (MRP) gene expression in spontaneous drug resistance to vincristine, etoposide and adriamycin in human glioma cells. Int J Cancer. 1994;58:860–864. doi: 10.1002/ijc.2910580619. [DOI] [PubMed] [Google Scholar]
- 14.Wolff JE, Trilling T, Mölenkamp G, Egeler RM, Jürgens H. Chemosensitivity of glioma cells in vitro: a meta analysis. J Cancer Res Clin Oncol. 1999;125:481–486. doi: 10.1007/s004320050305. [DOI] [PubMed] [Google Scholar]
- 15.Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, Severin SE, Uhl R, Kock M, Geiger KD, Gelperina SE. Chemotherapy of glioblastoma in rats using doxorubicin- loaded nanoparticles. Int J Cancer. 2004;109:759–767. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 16.Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 17.Voulgaris S, Partheni M, Karamouzis M, Dimopoulos P, Papadakis N, Kalofonos HP. Intratumoral doxorubicin in patients with malignant brain gliomas. Am J Clin Oncol. 2002;25:60–4. doi: 10.1097/00000421-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- 19.Kusch-Poddar M, Drewe J, Fux I, Gutmann H. Evaluation of the immortalized human brain capillary endothelial cell line BB19 as a human cell culture model for the blood-brain barrier. Brain Res. 2005;1064:21–31. doi: 10.1016/j.brainres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Kubota H, Ishihara H, Langmann T, Schmitz G, Stieger B, Wieser HG, Yonekawa Y, Frei K. Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006;68:213–228. doi: 10.1016/j.eplepsyres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma HS, Ali SF. Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci. 2006;1074:198–224. doi: 10.1196/annals.1369.020. [DOI] [PubMed] [Google Scholar]
- 24.Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr Opin Oncol. 2004;16:593–600. doi: 10.1097/01.cco.0000142076.52721.b3. [DOI] [PubMed] [Google Scholar]
- 25.Narang VS, Fraga C, Kumar N, Shen J, Throm S, Stewart CF, Waters CM. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol. 2008;295:C440–450. doi: 10.1152/ajpcell.00491.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson KH, Hicks FM. Clinical pharmacokinetics of ondansetron. A review. J Pharm Pharmacol. 1996;48:774–781. doi: 10.1111/j.2042-7158.1996.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 27.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–777. [PubMed] [Google Scholar]
- 29.Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, Caldwell RL, Shou W, Field LJ. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation. 2009;119:99–106. doi: 10.1161/CIRCULATIONAHA.108.799700. [DOI] [PMC free article] [PubMed] [Google Scholar]


