Summary
Neural stem cells (NSCs) typically show efficient self-renewal and selective differentiation. Their invasion potential, however, is not well studied. In this study, Sox2-positive NSCs from the E14.5 rat cortex were found to be non-invasive and showed only limited migration in vitro. By contrast, FGF2-expanded NSCs showed a strong migratory and invasive phenotype in response to the combination of FGF2 and BMP4. Invasive NSCs expressed Podoplanin (PDPN) and p75NGFR (Ngfr) at the plasma membrane after exposure to FGF2 and BMP4. FGF2 and BMP4 together upregulated the expression of Msx1, Snail1, Snail2, Ngfr, which are all found in neural crest (NC) cells during or after epithelial–mesenchymal transition (EMT), but not in forebrain stem cells. Invasive cells downregulated the expression of Olig2, Sox10, Egfr, Pdgfra, Gsh1/Gsx1 and Gsh2/Gsx2. Migrating and invasive NSCs had elevated expression of mRNA encoding Pax6, Tenascin C (TNC), PDPN, Hey1, SPARC, p75NGFR and Gli3. On the basis of the strongest upregulation in invasion-induced NSCs, we defined a group of five key invasion-related genes: Ngfr, Sparc, Snail1, Pdpn and Tnc. These genes were co-expressed and upregulated in seven samples of glioblastoma multiforme (GBM) compared with normal human brain controls. Induction of invasion and migration led to low expression of differentiation markers and repressed proliferation in NSCs. Our results indicate that normal forebrain stem cells have the inherent ability to adopt a glioma-like invasiveness. The results provide a novel in vitro system to study stem cell invasion and a novel glioma invasion model: tumoral abuse of the developmental dorsoventral identity regulation.
Key words: BMP4, FGF2, Invasion, Migration, Neural stem cell
Introduction
Neural stem cells (NSCs) from the developing forebrain can recapitulate in vitro the proliferation and differentiation of all major cell types in the central nervous system (CNS) (Davis and Temple, 1994; Johe et al., 1996; Raff et al., 1983; Ray et al., 1997; Reid et al., 1995). To correctly form the cortex layers NSCs are involved in complex migration patterns (Borello and Pierani, 2010; Guillemot, 2005; Liu, 2011; Rakic et al., 2009; Sur and Rubenstein, 2005). In contrast, only sparse data exist on invasion within the forebrain, although invasion may be critical for correct migration and generation of distinct forebrain structures, such as the interhemispheric mesenchyme (IHM) (Sailer et al., 2005). Indeed, invasion is a complex multistep process that requires proteolytic activities for degradation of extracellular matrix, followed by migration that provides substrate adhesion and traction for the cells to move (Sánchez-Tilló et al., 2012). Invasion has been well described for tumor cells, including gliomas (Nakada et al., 2007), but NSCs are not considered to be invasive. In the present study, we investigated whether NSCs may become migratory and invasive in defined culture conditions. Multiple signaling pathways are active during midterm rat forebrain development, including fibroblast growth factor (FGF), bone morphogenetic protein (BMP), Wingless-type proteins (Wnt) and Notch signaling (Ford-Perriss et al., 2001; Gaiano, 2008; Grove and Fukuchi-Shimogori, 2003; Hébert and Fishell, 2008). We focused on FGF2 and BMP4 signaling because FGF2 is one of the standard mitogens for NSCs, and BMPs are involved in dorsalization and NC induction in the caudal neural tube (Lee and Jessell, 1999; Panchision and McKay, 2002; Sauka-Spengler and Bronner-Fraser, 2008). Our findings reveal a novel mechanism to induce both migration and invasion in normal NSCs, with a molecular expression pattern resembling the highly invasive nature of malignant gliomas. To our knowledge, this study is the first describing similarities between induction of stem cell invasion and glioma invasion pathways.
Results and Discussion
NSCs become invasive by a distinct sequence of external growth factors
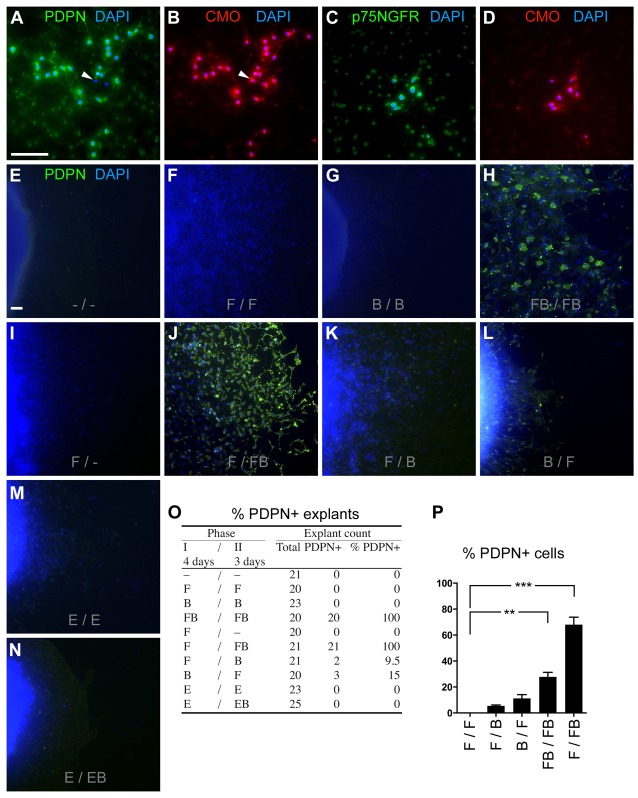
Multipotent NSCs isolated from the E14.5 rat cortical ventricular zone (CVZ) were expanded in FGF2 during 4 days and passaged. NSCs were then tested whether they were invasive by studying their ability to cross an extracellular (EC) matrix containing basal membrane (BM) extract during a 3 day period with different growth factors (Fig. 1A). FGF2-expanded NSCs were further cultured without any factor or with BMP4 alone (F/- or F/B, respectively). They did not show any invasive character (Fig. 1B,D,F). FGF2-expanded NSCs followed by continued culture in FGF2 (F/F) showed a low frequency of crossing the matrix (Fig. 1C,F). We found that the sequence of FGF2 followed by FGF2 and BMP4 (F/FB) effectively caused NSCs to cross a BM/EC matrix (Fig. 1A–F), with both factors being necessary to induce invasion. Interestingly, the freshly isolated cells did not respond to the combination of FGF2/BMP4, whereas FGF2-expanded cells did (supplementary material Fig. S1). This indicates that naïve cells have to be exposed to FGF2 factors for a period of time to allow the reprogramming of gene expression. Therefore, F/FB describes a specific sequence/code to induce invasion in otherwise non-invasive NSCs.
Fig. 1.
Non-invasive NSCs become invasive and migratory during combined BMP4 and FGF2 exposure. (A) Boyden invasion assay. NSCs from the E14.5 rat cortex were cultured first for 4 days in FGF2, then plated in the top chamber of the Boyden chamber and further exposed for 3 days to growth factors as indicated. Prior to fixation cells were stained with CMO, a lipophilic membrane dye. (B–E) Representative images of cells crossing the matrix. No Factor, FGF2 or BMP4 alone led to no significant crossing of the EC/BM matrix (B–D). Only the combination FGF2/BMP4 induced efficient invasion through the EC/BM matrix (E), as quantified (F). *P<0.01, FB versus others; n = 3. (G–P) Representative phase images of explant migration assay. Explants (containing NSCs) from the identical region as above were exposed for a total of 7 days (first phase/second phase with 4 and 3 days, respectively) to growth factors as indicated. (Q) Migration quantification. *P<0.0001, F/F versus F/FB and FB/FB; n = 3. Only the conditions combining F and B show an efficient migration and elongated migrating cells (Q, and supplementary material Fig. S2). F, FGF2; B, BMP4; E, EGF; -, no factor. Scale bars: 50 µm (B–E); 200 µm (G–P).
The F/FB sequence/code induces a strong migration in NSCs
Next, we tested whether the invasion of NSCs was associated with migration. NSCs migration was investigated in an outgrowth assay: E14.5 CVZ explants containing NSCs were cultured under various conditions as indicated (Fig. 1G–Q). Explants were identically cultured as above for the invasion experiments. Control explants without factor did not show any significant cell outgrowth (Fig. 1G), indicating that there is very little spontaneous migration. Also the combinations BMP4/BMP4 (B/B), F/-, F/B, BMP4/FGF2 (B/F) and the combinations with epidermal growth factor (EGF) EGF/EGF (E/E) and EGF/EGF+BMP4 (E/EB) did not show any strong migration (Fig. 1I–N,Q). Relative to control (168±19 µm), F/F caused a migration of 864±47 µm, F/FB of 1687±123 µm and FB/FB of 2541±97 µm (Fig. 1H,O,P, respectively, and 1Q). Some of the outgrowth of the F/F group was probably due to strong proliferation, as the cells were round, dividing and tiny (supplementary material Fig. S2B). The F/FB and FB/FB cells at the explant edge, in contrast, demonstrated a flat elongated morphology with a leading edge as expected of migratory cells (supplementary material Fig. S2I–L). Single cells in the F/FB and FB/FB groups moved without any obvious particular direction. The overall migration was centrifugal from the explant center. Taken together, these results demonstrate that the F/FB sequence/code induced both the strongest invasion and a very strong (visible without a microscope) migration.
Invasive NSCs express PDPN, which is also found during migration
Next, we characterized the invasive cells on the protein level. The F/FB cells crossing the BM/EC matrix (on the bottom of the chamber) were stained for various genes expressed in the dorsoventral axis. We observed that 95.9% of the cells expressed Podoplanin (PDPN) and 92.2% expressed p75NGFR (Fig. 2A–D), but not Sox10 and Olig2 (data not shown). We could not test Snail1/2 because we did not find an antibody that worked reliably in rat tissue.
Fig. 2.
Invading/migrating NSCs express PDPN. Double staining of invasive NSCs (bottom of Boyden chamber) showing PDPN (A) and p75NGFR (C) expression of CMO/DAPI-positive cells (B and D). Only few cells (arrowheads) did not express PDPN. (E–N) PDPN was used as an invasion marker under growth factor combinations as indicated, and was found in migrating cells at the edge furthest away from the explant center. PDPN and DAPI staining of migrating NSCs showed PDPN expression in the conditions F/FB, FB/FB, F/B and B/F only. (O,P) Number of PDPN+ explants and the percentage of PDPN+ cells among the PDPN+ explants are indicated. F/F, B/B, F/-, E/E, E/EB did not contain PDPN+ cells (P). Scale bars: 100 µm (A–D), 50 µm (E–N). **P = 0.0015; ***P<0.0001.
PDPN has been shown to be expressed in various tumors during migration and metastasis, modulating the actin cytoskeleton (Astarita et al., 2012). As PDPN was expressed in invasive cells (Fig. 2A,B), we screened for it as marker for invasive cells under various conditions in CVZ explants (Fig. 2E–N). We found the highest PDPN expression in the F/FB and FB/FB groups (Fig. 2O,P, respectively). Only explants with the combination of BMP4 and FGF2 contained PDPN-positive cells (Fig. 2O). Notably, EGF, an important mitogen in NSCs, could not substitute for FGF2. This corroborates the data above that FGF2 is both mitogenic, as expected, but also confers the competence for invasion in response to BMP4.
Invasion in NSCs is associated with expression of neural crest and glioblastoma genes and repression of genes expressed in the ventral telencephalon
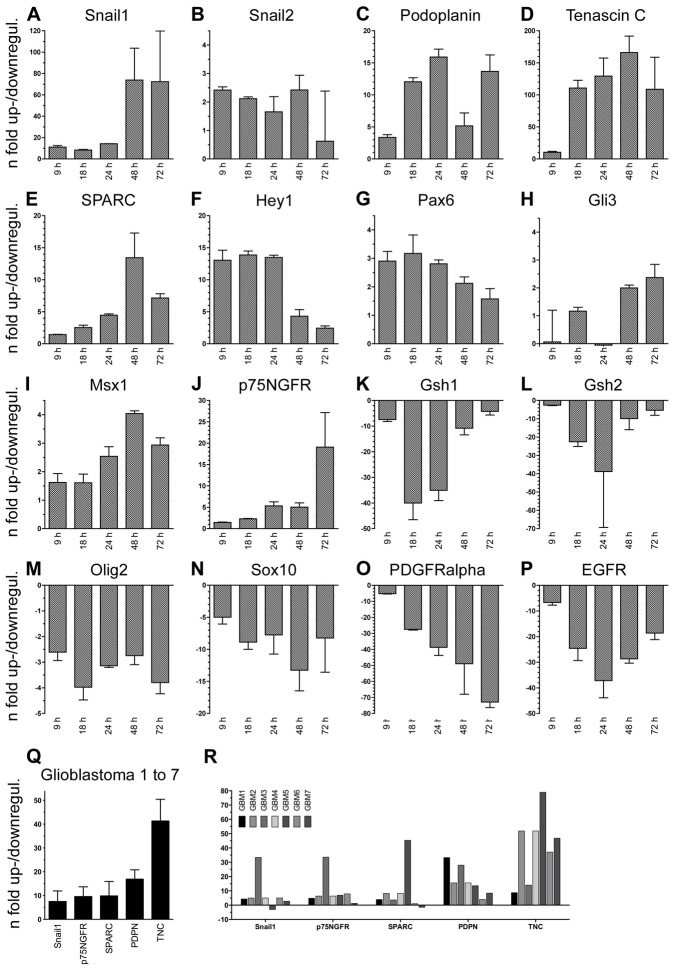
In order to characterize the invasive cells on the molecular level, we performed a time course analysis of gene expression using quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). To reproduce the invasive condition used above (F/FB), cortical NSCs were first expanded in FGF2 and then exposed to FGF2/BMP4. RNA was harvested for qRT-PCR after 9, 18, 24, 48 or 72 h in order to compare the gene expression with and without BMP4. Since BMP4 is known to be dorsalizing, we screened for genes in the dorsoventral axis including developmental invasion genes (Astarita et al., 2012; Nieto, 2002; Raica et al., 2008). Developmental invasion genes were upregulated during the F/FB time course, starting at 9 h (Fig. 3A–C). Among these, Snail1 showed the strongest upregulation of over 70-fold after 48 h (Fig. 3A). Snail3 was not expressed (data not shown). BMP4 also induced the expression of Tnc, Sparc and Hey1 (Fig. 3D–F), genes known to be highly expressed in human gliomas (Hirata et al., 2009; Hulleman et al., 2009; Rempel et al., 1998). Interestingly, Tnc, which is known to strongly promote glioma invasion (Hirata et al., 2009), was upregulated over 100-fold after 18 to 72 h of BMP4 exposure in NSCs. Further, Pax6, Gli3 and Msx1, transcription factors expressed in the dorsal forebrain (Furuta et al., 1997; Quinn et al., 2009), were significantly upregulated after 24 to 72 h of BMP4 exposure (Fig. 3G–I). P75NGFR (Ngfr), which is found in the NC and in the mesenchyme of the forebrain (Dupin and Coelho-Aguiar, 2013; Morrison et al., 1999; Sailer et al., 2005; Wong et al., 2006), was upregulated over 15-fold at 72 h (Fig. 3J, compare with Fig. 4M). Sox2 was expressed at high absolute levels, but did not significantly change during the time course (supplementary material Fig. S3). The induction of these dorsal genes was paralleled by the downregulation of genes from the ventral pallidal region Gsh1/Gsx1, Gsh2/Gsx2, Sox10, Olig2 and Pdgfra (PDGFRα), and of Egfr (Fig. 3K–P).
Fig. 3.
The combination of FGF2 and BMP4 upregulates genes involved in neural crest formation and glioblastoma invasion. (A–P) Time-course analysis of relative expression in FGF2-expanded NSCs submitted to FGF2 and BMP4 compared with FGF2 alone determined by qRT-PCR showing fold changes in genes involved in invasion, dorsal differentiation and glioma invasion (A–J) and in ventral pallidal identity (K–P). (Q,R) Relative expressions of key invasion genes in human GBM samples compared with normal brain tissue. Mean fold changes of the seven glioma samples (Q) and fold changes (R) in each individual glioma sample (GBM1 to GBM7).
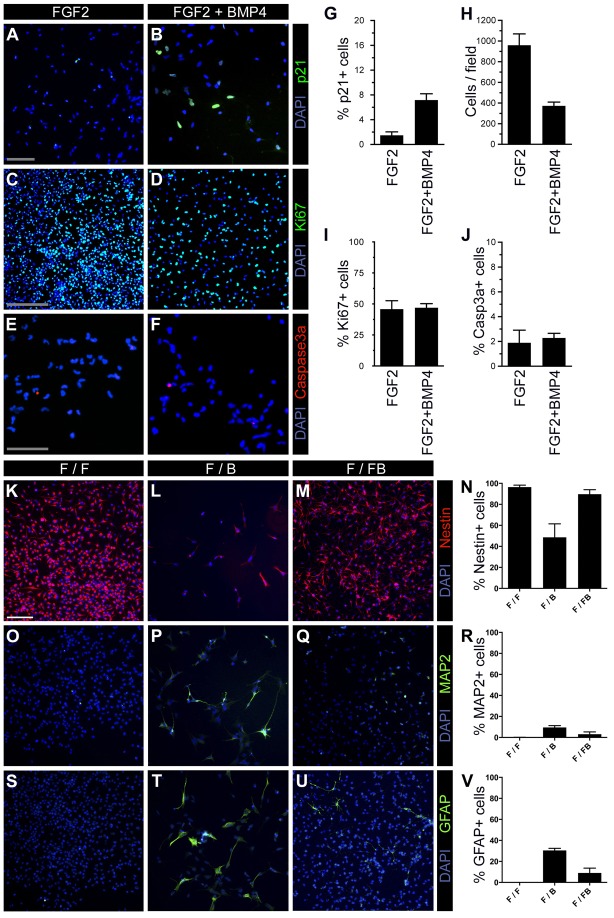
Fig. 4.
FGF2/BMP4 induces cell cycle inhibition, and slows down proliferation and differentiation. NSCs were exposed to FGF2 for 4 days then passaged and treated with or without BMP4 in combination with FGF2 for 5 days (A–J) and for 3 days (K–V), and were stained for p21/WAF1 (A,B), Ki67 (C,D), Caspase3a (E,F), Nestin (K–N), MAP2 (O–R), GFAP (S–V), together with DAPI. The F/FB group showed a higher p21-expressing cell proportion and lower total cell number (G,H, n = 3, P<0.0001 and P = 0.001, respectively). The proportion expressing Ki67 and Caspase3a did not show significant changes (I,J, n = 3). Nestin was strongly expressed in the F/F and F/FB group, whereas the F/B cells showed a marked reduction (K–N, n = 3, P<0.05 in F/F vs F/B, F/FB vs F/B, but no significant difference between F/FB and F/F). MAP2 was not found in the F/F group (O). A higher proportion of MAP2-positive cells was found in the F/B compared with the F/FB group (P–R, n = 3, P<0.05 F/F vs F/B, no significant difference in F/FB vs F/B and in F/F vs F/FB). No GFAP signal was observed in the F/F cells (S–V, n = 3, P<0.05 in F/F vs F/B and F/B vs F/FB, no significant difference between F/FB and F/F). Scale bars: 100 µm (A–D, K–M, O–Q, S–U); 50 µm (E,F).
Based on upregulation of over 10-fold, we defined Snail1, Pdpn, Tnc, Sparc and Ngfr as the key invasion genes in normal stem cells. To compare these genes from normal stem cells with malignant gliomas and to validate NSC invasion as a tumor model, we tested the five key invasion genes on primary GBM samples. Compared to normal brain controls, the mean expressions of these genes were all upregulated in the most malignant grade IV gliomas (Fig. 3Q). Interestingly, the same gene combination was almost conformly expressed in all tested human glioma samples (Fig. 3R).
Invasion is negatively correlated with proliferation speed
Since migration and proliferation are antagonistic cellular behaviors (Schiffer et al., 1997), we tested whether invasion affected proliferation. We compared proliferation and cell cycle arrest in NSCs exposed to the combinations F/F (no migration) and F/FB (inducing migration). We found that the cell cycle inhibitor p21 was more highly expressed in F/FB as compared to F/F (Fig. 4A,B,G). Interestingly, the cell proliferation marker Ki67 was similarly expressed in both groups, indicating that the cells in F/FB group had a slower cell cycle time (Fig. 4C,D,I). As a consequence, the density of cells was more than 2-fold higher in F/F compared to F/FB (Fig. 4H). This observation is consistent with the notion that Snail-dependent tumor invasion is correlated with reduced proliferation (Nieto, 2002; Nieto, 2009). This dichotomy is illustrated in supplementary material Fig. S4, showing the time course of gene expression inducing migration/invasion and reduction of cell cycle speed.
The reduced cell density in F/FB was not due to increased apoptosis, since similar low levels of about 2% of NSCs expressed activated caspase 3 in the F/F and F/FB groups (Fig. 4E,F,J).
Invasive NSCs maintain nestin expression and show impaired differentiation
It has been shown that BMP4 signaling promotes differentiation of brain tumor stem-like cells (Piccirillo et al., 2006). To investigate if invasive/migratory NSCs undergo differentiation or retain their stem cell fate, we performed immunostaining of NSCs treated with the combinations F/F, F/B or F/FB for nestin, MAP2 and GFAP (Fig. 4K–V). FGF2 is known to preserve the stem cell fate. Indeed, 96±1.8% NSCs were nestin-positive in F/F (Fig. 4K). A similar proportion was found for cells treated with F/FB (90±4.2%, Fig. 4M). In contrast, only 49±13.0% were nestin-positive with F/B (Fig. 4L). A small proportion of NSCs expressed the neuronal marker MAP2 in F/F and F/FB compared to F/B (0.1±0.1% and 3.1±2.2%, compared to 9.6±1.7%, respectively, Fig. 4O–R). Whereas no GFAP-positive cells were observed in F/F (Fig. 4S), few cells (9.0±4.6%, Fig. 4U) expressed this astrocytic marker in F/FB and 30.0±2.0% in F/B (Fig. 4T). Interestingly, F/FB therefore promoted invasion, but not differentiation, whereas F/B promoted differentation, but not invasion. This observation in NSCs is consistent with the findings in brain tumor stem-like cells (Piccirillo et al., 2006).
Why do forebrain stem cells become invasive/mesenchymal?
The results demonstrate that otherwise non-invasive stem cells become invasive although the forebrain does not have any obvious need for invasion. In the posterior neural tube dorsal precursors have to become invasive to form the NC (Dupin and Sommer, 2012). NC derivatives, such as melanocytes, show controlled invasion to form additional cell types within an already existing tissue. For the anterior neural tube, however, it is unclear why it contains potentially invasive cells. The last NC cells emigrate from the mouse neural tube at E9.5 (Serbedzija et al., 1992) and only from regions caudal to the mid-diencephalon (Couly and Le Douarin, 1987; Le Douarin and Kalcheim, 1999). Hence, our experiments demonstrate that NSCs from the forebrain retain the potential to become invasive resembling the EMT of NC cells, even after NC formation of the posterior neural tube. Our results support the hypothesis that the choroid plexus mesenchyme is derived from forebrain NSCs. Consistent with this view, Snail transcription factors have been found in the choroid plexus mesenchyme (Marin and Nieto, 2004). It remains to be shown in lineage-tracing experiments if, when and how this happens during forebrain development in vivo.
We reported previously that pericytes of the choroid plexus express p75NGFR (Sailer et al., 2005), a receptor also found expressed by invasive cells. Thus, the ability to become invasive may be necessary to contribute to mesenchymal perivascular cells within the brain. Likewise, NSCs may also differentiate into peripheral Schwann cells within the adult CNS (Nazarenko et al., 2012).
Neural stem cell invasion as a model for glioma invasion
Gliomas represent the majority (∼80%) of all primary malignant brain tumors and all of them, in particular glioblastoma multiforme (GBM), are strongly invasive (Schwartzbaum et al., 2006). Increasing evidence supports the hypothesis that gliomas derive from a cancer stem cell (Das et al., 2008). Genes encoding the following proteins are expressed in gliomas: Tenascin C (Hirata et al., 2009; Mariani et al., 2001), Hey1 (Hulleman et al., 2009), SPARC (Rempel et al., 1998), Snail1 and Snail2 (Han et al., 2011; Yang et al., 2010), FGFR1 (Morrison et al., 1994), BMPR1a (Yamada et al., 1996), EGFR (Hegi et al., 2012), PDGFRα (Andrae et al., 2008; Nazarenko et al., 2012), Sox2 (Annovazzi et al., 2011), Podoplanin (Mishima et al., 2006), Gli3 (Shahi et al., 2008) and p75NGFR (Johnston et al., 2007). All these glioma-related genes were also expressed in normal NSCs during their transformation into invasive stem cells in the present study, including the constitutively expressed Fgfr1 and Bmpr1a genes (Panchision et al., 2001; Vescovi et al., 1993). Further, invasion was negatively correlated to proliferation, an observation that has also been observed for GBM stem cells (Piccirillo et al., 2006; Vescovi et al., 2006). This study, therefore, unveils an unexpected similarity between invasive NSCs and invasive glioma cells at the transcriptional level. On the basis of these observations we propose that NSC invasion may be used as a model to understand glioma invasion.
If this NSC model is applied to glioma invasion, it suggests that the dorsoventral developmental identity is deregulated in adult glioma cells. This developmental transition between non-invasive (ventral) neuroepithelium to invasive (dorsal) forebrain mesenchyme may be falsely reactivated in adult gliomas. Our results suggest that blocking FGF and BMP signaling may interfere with invasion and migration. These observations are both relevant to understand normal forebrain as well as cancer development.
Materials and Methods
NSC and cortical explant culture
All animal procedures followed the ‘Principles of laboratory animal care’ (NIH publication No. 86-23, revised 1985) and were approved by the Animal Welfare Committee of Basel City (Swiss Guidelines for the Care and Use of Animals). NSCs were isolated from E14.5 timed-pregnant Sprague-Dawley rats (Harlan, Horst, The Netherlands) and cultured as described (Johe et al., 1996; Sailer et al., 2005). Noon of day of plug detection equaled E0.5. Adult pregnant rats (8 to 12 weeks old) were deeply anesthetized in isoflurane aerosol before decapitation, cesarean section and dissection of the CVZ. E14.5 rat forebrain was dissected free of meninges and a cortex section of 800 µm by 1600 µm along the length of the medial ganglionic eminence (MGE) was isolated and either used for stem cell or explant preparations. The CVZ started at the anterior MGE pole and followed the MGE anteroposteriorly at a distance of 800 µm from the lateral edge of the MGE. Explants were cut by microsurgical needle to a diameter size of 500 µm. Further trituration yielded single stem cells as previously described (Johe et al., 1996; Kim et al., 2003; Sailer et al., 2005).
NSCs and CVZ explants were cultured in DMEM/F12 (with N2, omitting progesterone) and on poly-ornithine/fibronectin-coated dishes, as described (Sailer et al., 2005). Medium containing growth factors was replaced every other day. The explants were washed twice with basal medium between the first and second phases of treatment. The following concentrations of growth factors were used: 10 ng/ml recombinant human (rh) FGF2 (146 aa), 10 ng/ml rhBMP4, 10 ng/ml rhEGF (all from R&D Systems; with BSA as carrier protein) and bovine pancreatic insulin at 25 µg/ml (Sigma).
Invasion assay and migration measurements
Cell invasion was tested using the CytoSelect cell invasion assay (Cell Biolabs) with 8 µm pore size. The inserts are coated with EC/BM matrix. The matrix is formed by 200 µl 0.4 mg/ml-matrigel that is lyophilized on a 60 mm2 bottom area (manufacturer specifications). The EC/BM matrix consists of laminin (56%), collagen type IV (31%), entactin (8%) and heparan sulfate proteoglycan (<5%) (Kleinman et al., 1982). Cells were added to the upper chamber and incubated for 72 h in medium with or without growth factors as indicated in the legends of the figures. Growth factors were identical in top and bottom chambers. The non-invaded cells from the upper side of the filter were scraped using moist cotton swabs. The invaded cells in the reverse side of the filter were stained with CellMask Orange plasma membrane stain (CMO, 1∶1000, Invitrogen), antibodies or with Crystal violet, lysed and quantified at OD 560 nm.
Migration distance was measured from the explant edge to the outermost 200×200 µm2 area containing at least 50 cells. We used Fiji/ImageJ v1.45i software (Schindelin et al., 2012) to define the outermost cell using a tangent to the explant surface with a perpendicular radial line, using four sample distances per explant.
Immunocytochemistry
Explants or NSCs were fixed and stained with fresh cold 4% paraformaldehyde (PFA)/PBS as described (Sailer et al., 2005). Briefly, primary antibodies were diluted in 5% NGS in PBS, applied after 1 h blocking in 0.2% Triton X-100/10% NGS in PBS and incubated overnight at 4°C. After washing in PBS, appropriate secondary antibodies coupled with fluorescent dyes (Cy3, 1∶300, from Jackson Immunoresearch, or Alexa Fluor 488, 1∶500 from Molecular Probes) were applied for 1 h at RT. Primary antibodies were as follows: anti-Caspase3a (rabbit, 1∶100, Chemicon), anti-MAP-2 (mouse, 1∶500, Millipore), anti-nestin (rabbit, 1∶500, Novus), anti-GFAP (rabbit, 1∶1000, DAKO), anti-p21/WAF1 (rabbit, 1∶800, Abcam), anti-p75NGFR (rabbit, 1∶200, Millipore), anti-PDPN (mouse, 1∶100, Acris). Nuclei were labeled with 0.25 µg/ml DAPI (Sigma) for 15 min and slides were mounted in Vectashield (Vector Labs). Images were photographed using a DMRE (Leica Microsystems) microscope combined with an F-view camera (Soft Imaging) or with an Axiovert 25 microscope (Carl Zeiss) combined with a C-5060 camera (Olympus).
Brain material
The human brain material was obtained from the Neurosurgery clinic, University Hospital of Basel, Switzerland, in accordance with Swiss law and the Ethical Committee Beider Basel. Patient consent was obtained for every sample. Seven human glioma tissue samples (GBM, grade IV) and two epileptogenic tissue samples were collected after resection surgery. The samples were immediately frozen in liquid nitrogen before further RNA extraction.
Quantitative reverse transcription-PCR analysis
For gene expression analysis, rat NSCs or human glioma tissue samples (GBM grade IV), and control human brain tissue (epileptogenic brain tissue) were harvested in Trizol reagent (Invitrogen) for isolation of RNA using RNeasy Mini Kit (Qiagen) and treated with DNase I (Turbo DNA-free Kit, Ambion). First strand cDNA was created using the QuantiTect Reverse Transcription Kit (Qiagen). All rat and human specific primers are commercially available (QuantiTect Primer Assays, Qiagen). Quantitative RT-PCR was performed on 300 ng to 1 µg of total cDNA per well using the QuantiFast SYBR Green PCR kit (Qiagen). Each sample was run in triplicate on the Bio-Rad detection system (C1000 and CFX96, Bio-Rad). The comparative cycle threshold [C(t)] method was used to calculate relative quantification of gene expression. Each measurement was made in duplicate and expressed relative to the internal control GAPDH [ΔC(t)]. The following formula was used to calculate the relative amount of the transcripts in the FGF2-treated conditions samples and the FGF2+BMP4 conditions: ΔΔC(t) = ΔC(t)FGF2+BMP4−ΔC(t)FGF2. The formula 2E-ΔΔC(t) expressed the fold-change for the FGF2+BMP4-treated relative to FGF2-treated samples. For human glioma samples, the relative amounts of transcripts were calculated relative to the mean amounts of the two epileptogenic tissue samples.
Statistical analysis
Samples were analyzed using the Student's t-test for unpaired data doublets and one way ANOVA for unpaired groups. Values describe means ± s.e.m. Statistical analysis was performed using Prism 6.0 (Graphpad).
Supplementary Material
Acknowledgments
We would like to thank Elisabeth Taylor for technical assistance. We thank Jean-Louis Boulay, Martin E. Schwab and Daniel Bodmer for discussions on the manuscript. The work is dedicated to Prof. Hanna Keidel.
Footnotes
Author contributions
M.S.: conception and design, collection of data, data analysis and interpretation, financial support, manuscript writing. A.G.: Collection of data, data analysis and interpretation. C.T.: Collection of data. G.H.: Conception and design. D.C.: Conception and design, critical revision of the manuscript. L.M.: financial support, critical revision of the manuscript. M.F.R.: study conception and design, acquisition of data, analysis and interpretation, manuscript writing.
Funding
The research was supported by the Research Fund of the University of Basel; the Swiss National Science Foundation (SNSF) [grant number 3135-54876.98]; and a National Institutes of Health visiting fellow award [grant number VFGU008683 to M.S.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.125757/-/DC1
References
- Andrae J., Gallini R., Betsholtz C. (2008). Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22, 1276–1312 10.1101/gad.1653708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annovazzi L., Mellai M., Caldera V., Valente G., Schiffer D. (2011). SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics Proteomics 8, 139–147 [PubMed] [Google Scholar]
- Astarita J. L., Acton S. E., Turley S. J. (2012). Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 3, 283 10.3389/fimmu.2012.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U., Pierani A. (2010). Patterning the cerebral cortex: traveling with morphogens. Curr. Opin. Genet. Dev. 20, 408–415 10.1016/j.gde.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Couly G. F., Le Douarin N. M. (1987). Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev. Biol. 120, 198–214 10.1016/0012-1606(87)90118-7 [DOI] [PubMed] [Google Scholar]
- Das S., Srikanth M., Kessler J. A. (2008). Cancer stem cells and glioma. Nat. Clin. Pract. Neurol. 4, 427–435 10.1038/ncpneuro0862 [DOI] [PubMed] [Google Scholar]
- Davis A. A., Temple S. (1994). A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature 372, 263–266 10.1038/372263a0 [DOI] [PubMed] [Google Scholar]
- Dupin E., Coelho-Aguiar J. M. (2013). Isolation and differentiation properties of neural crest stem cells. Cytometry A 83, 38–47 [DOI] [PubMed] [Google Scholar]
- Dupin E., Sommer L. (2012). Neural crest progenitors and stem cells: from early development to adulthood. Dev. Biol. 366, 83–95 10.1016/j.ydbio.2012.02.035 [DOI] [PubMed] [Google Scholar]
- Ford-Perriss M., Abud H., Murphy M. (2001). Fibroblast growth factors in the developing central nervous system. Clin. Exp. Pharmacol. Physiol. 28, 493–503 10.1046/j.1440-1681.2001.03477.x [DOI] [PubMed] [Google Scholar]
- Furuta Y., Piston D. W., Hogan B. L. (1997). Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124, 2203–2212 [DOI] [PubMed] [Google Scholar]
- Gaiano N. (2008). Strange bedfellows: Reelin and Notch signaling interact to regulate cell migration in the developing neocortex. Neuron 60, 189–191 10.1016/j.neuron.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Grove E. A., Fukuchi-Shimogori T. (2003). Generating the cerebral cortical area map. Annu. Rev. Neurosci. 26, 355–380 10.1146/annurev.neuro.26.041002.131137 [DOI] [PubMed] [Google Scholar]
- Guillemot F. (2005). Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr. Opin. Cell Biol. 17, 639–647 10.1016/j.ceb.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Han S. P., Kim J. H., Han M. E., Sim H. E., Kim K. S., Yoon S., Baek S. Y., Kim B. S., Oh S. O. (2011). SNAI1 is involved in the proliferation and migration of glioblastoma cells. Cell. Mol. Neurobiol. 31, 489–496 10.1007/s10571-010-9643-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert J. M., Fishell G. (2008). The genetics of early telencephalon patterning: some assembly required. Nat. Rev. Neurosci. 9, 678–685 10.1038/nrn2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi M. E., Rajakannu P., Weller M. (2012). Epidermal growth factor receptor: a re-emerging target in glioblastoma. Curr. Opin. Neurol. 25, 774–779 10.1097/WCO.0b013e328359b0bc [DOI] [PubMed] [Google Scholar]
- Hirata E., Arakawa Y., Shirahata M., Yamaguchi M., Kishi Y., Okada T., Takahashi J. A., Matsuda M., Hashimoto N. (2009). Endogenous tenascin-C enhances glioblastoma invasion with reactive change of surrounding brain tissue. Cancer Sci. 100, 1451–1459 10.1111/j.1349-7006.2009.01189.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulleman E., Quarto M., Vernell R., Masserdotti G., Colli E., Kros J. M., Levi D., Gaetani P., Tunici P., Finocchiaro G. et al. (2009). A role for the transcription factor HEY1 in glioblastoma. J. Cell. Mol. Med. 13, 136–146 10.1111/j.1582-4934.2008.00307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johe K. K., Hazel T. G., Muller T., Dugich-Djordjevic M. M., McKay R. D. (1996). Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 10, 3129–3140 10.1101/gad.10.24.3129 [DOI] [PubMed] [Google Scholar]
- Johnston A. L., Lun X., Rahn J. J., Liacini A., Wang L., Hamilton M. G., Parney I. F., Hempstead B. L., Robbins S. M., Forsyth P. A. et al. (2007). The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 5, e212 10.1371/journal.pbio.0050212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H., Panchision D. M., Kittappa R., McKay R. D. (2003). Generating CNS neurons from embryonic, fetal and adult stem cells. Differentiation of Embryonic Stem Cells New York, NY: Academic Press; [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. (1982). Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21, 6188–6193 10.1021/bi00267a025 [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Kalcheim C. (1999). The Neural Crest Cambridge University Press [Google Scholar]
- Lee K. J., Jessell T. M. (1999). The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 22, 261–294 10.1146/annurev.neuro.22.1.261 [DOI] [PubMed] [Google Scholar]
- Liu J. S. (2011). Molecular genetics of neuronal migration disorders. Curr. Neurol. Neurosci. Rep. 11, 171–178 10.1007/s11910-010-0176-5 [DOI] [PubMed] [Google Scholar]
- Mariani L., Beaudry C., McDonough W. S., Hoelzinger D. B., Demuth T., Ross K. R., Berens T., Coons S. W., Watts G., Trent J. M. et al. (2001). Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J. Neurooncol. 53, 161–176 10.1023/A:1012253317934 [DOI] [PubMed] [Google Scholar]
- Marin F., Nieto M. A. (2004). Expression of chicken slug and snail in mesenchymal components of the developing central nervous system. Dev. Dyn. 230, 144–148 10.1002/dvdy.20027 [DOI] [PubMed] [Google Scholar]
- Mishima K., Kato Y., Kaneko M. K., Nishikawa R., Hirose T., Matsutani M. (2006). Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 111, 483–488 10.1007/s00401-006-0063-y [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Yamaguchi F., Saya H., Bruner J. M., Yahanda A. M., Donehower L. A., Berger M. (1994). Basic fibroblast growth factor and fibroblast growth factor receptor I are implicated in the growth of human astrocytomas. J. Neurooncol. 18, 207–216 10.1007/BF01328955 [DOI] [PubMed] [Google Scholar]
- Morrison S. J., White P. M., Zock C., Anderson D. J. (1999). Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96, 737–749 10.1016/S0092-8674(00)80583-8 [DOI] [PubMed] [Google Scholar]
- Nakada M., Nakada S., Demuth T., Tran N. L., Hoelzinger D. B., Berens M. E. (2007). Molecular targets of glioma invasion. Cell. Mol. Life Sci. 64, 458–478 10.1007/s00018-007-6342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko I., Hede S. M., He X., Hedrén A., Thompson J., Lindström M. S., Nistér M. (2012). PDGF and PDGF receptors in glioma. Ups. J. Med. Sci. 117, 99–112 10.3109/03009734.2012.665097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A. (2002). The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3, 155–166 10.1038/nrm757 [DOI] [PubMed] [Google Scholar]
- Nieto M. A. (2009). Epithelial-mesenchymal transitions in development and disease: old views and new perspectives. Int. J. Dev. Biol. 53, 1541–1547 10.1387/ijdb.072410mn [DOI] [PubMed] [Google Scholar]
- Panchision D. M., McKay R. D. (2002). The control of neural stem cells by morphogenic signals. Curr. Opin. Genet. Dev. 12, 478–487 10.1016/S0959-437X(02)00329-5 [DOI] [PubMed] [Google Scholar]
- Panchision D. M., Pickel J. M., Studer L., Lee S. H., Turner P. A., Hazel T. G., McKay R. D. (2001). Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 15, 2094–2110 10.1101/gad.894701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo S. G., Reynolds B. A., Zanetti N., Lamorte G., Binda E., Broggi G., Brem H., Olivi A., Dimeco F., Vescovi A. L. (2006). Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444, 761–765 10.1038/nature05349 [DOI] [PubMed] [Google Scholar]
- Quinn J. C., Molinek M., Mason J. O., Price D. J. (2009). Gli3 is required autonomously for dorsal telencephalic cells to adopt appropriate fates during embryonic forebrain development. Dev. Biol. 327, 204–215 10.1016/j.ydbio.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. (1983). A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303, 390–396 10.1038/303390a0 [DOI] [PubMed] [Google Scholar]
- Raica M., Cimpean A. M., Ribatti D. (2008). The role of podoplanin in tumor progression and metastasis. Anticancer Res. 28 5B, 2997–3006 [PubMed] [Google Scholar]
- Rakic P., Ayoub A. E., Breunig J. J., Dominguez M. H. (2009). Decision by division: making cortical maps. Trends Neurosci. 32, 291–301 10.1016/j.tins.2009.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J., Baird A., Gage F. H. (1997). A 10-amino acid sequence of fibroblast growth factor 2 is sufficient for its mitogenic activity on neural progenitor cells. Proc. Natl. Acad. Sci. USA 94, 7047–7052 10.1073/pnas.94.13.7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. B., Liang I., Walsh C. (1995). Systematic widespread clonal organization in cerebral cortex. Neuron 15, 299–310 10.1016/0896-6273(95)90035-7 [DOI] [PubMed] [Google Scholar]
- Rempel S. A., Golembieski W. A., Ge S., Lemke N., Elisevich K., Mikkelsen T., Gutiérrez J. A. (1998). SPARC: a signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J. Neuropathol. Exp. Neurol. 57, 1112–1121 10.1097/00005072-199812000-00002 [DOI] [PubMed] [Google Scholar]
- Sailer M. H., Hazel T. G., Panchision D. M., Hoeppner D. J., Schwab M. E., McKay R. D. (2005). BMP2 and FGF2 cooperate to induce neural-crest-like fates from fetal and adult CNS stem cells. J. Cell Sci. 118, 5849–5860 10.1242/jcs.02708 [DOI] [PubMed] [Google Scholar]
- Sánchez-Tilló E., Liu Y., de Barrios O., Siles L., Fanlo L., Cuatrecasas M., Darling D. S., Dean D. C., Castells A., Postigo A. (2012). EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell. Mol. Life Sci. 69, 3429–3456 10.1007/s00018-012-1122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T., Bronner-Fraser M. (2008). A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 9, 557–568 10.1038/nrm2428 [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum J. A., Fisher J. L., Aldape K. D., Wrensch M. (2006). Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2, 494–503quiz 1 p following 516 [DOI] [PubMed] [Google Scholar]
- Serbedzija G. N., Bronner-Fraser M., Fraser S. E. (1992). Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development 116, 297–307 [DOI] [PubMed] [Google Scholar]
- Shahi M. H., Lorente A., Castresana J. S. (2008). Hedgehog signalling in medulloblastoma, glioblastoma and neuroblastoma. Oncol. Rep. 19, 681–688 [PubMed] [Google Scholar]
- Sur M., Rubenstein J. L. (2005). Patterning and plasticity of the cerebral cortex. Science 310, 805–810 10.1126/science.1112070 [DOI] [PubMed] [Google Scholar]
- Vescovi A. L., Reynolds B. A., Fraser D. D., Weiss S. (1993). bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron 11, 951–966 10.1016/0896-6273(93)90124-A [DOI] [PubMed] [Google Scholar]
- Vescovi A. L., Galli R., Reynolds B. A. (2006). Brain tumour stem cells. Nat. Rev. Cancer 6, 425–436 10.1038/nrc1889 [DOI] [PubMed] [Google Scholar]
- Wong C. E., Paratore C., Dours-Zimmermann M. T., Rochat A., Pietri T., Suter U., Zimmermann D. R., Dufour S., Thiery J. P., Meijer D. et al. (2006). Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J. Cell Biol. 175, 1005–1015 10.1083/jcb.200606062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Kato M., ten Dijke P., Yamashita H., Sampath T. K., Heldin C. H., Miyazono K., Funa K. (1996). Bone morphogenetic protein type IB receptor is progressively expressed in malignant glioma tumours. Br. J. Cancer 73, 624–629 10.1038/bjc.1996.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. W., Menon L. G., Black P. M., Carroll R. S., Johnson M. D. (2010). SNAI2/Slug promotes growth and invasion in human gliomas. BMC Cancer 10, 301 10.1186/1471-2407-10-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.