Summary
Notch signaling is an evolutionarily conserved pathway that plays a central role in numerous developmental and disease processes. The versatility of the Notch pathway relies on the activity of context-dependent regulators. These include rab11, sec15, arp3 and Drosophila EHBP1 (dEHBP1), which control Notch signaling and cell fate acquisition in asymmetrically dividing mechanosensory lineages by regulating the trafficking of the ligand Delta. Here, we show that dEHBP1 also controls the specification of R8 photoreceptors, as its loss results in the emergence of supernumerary R8 photoreceptors. Given the requirements for Notch signaling during lateral inhibition, we propose that dEHBP1 regulates distinct aspects of Notch signaling in different developmental contexts. We show that dEHBP1 regulates the exocytosis of Scabrous, a positive regulator of Notch signaling. In conclusion, dEHBP1 provides developmental versatility of intercellular signaling by regulating the trafficking of distinct Notch signaling components.
Key words: Drosophila, EHBP1, Lateral inhibition, Photoreceptor development, Scabrous, Notch
Introduction
Notch signaling is an evolutionarily conserved pathway that is involved in numerous processes during development and adult tissue homeostasis (Artavanis-Tsakonas and Muskavitch, 2010; Kopan and Ilagan, 2009). The pathway is often used reiteratively in different contexts, suggesting a very tight regulation of its signaling output. Hence, dysfunction of Notch signaling leads to multiple diseases (Louvi and Artavanis-Tsakonas, 2012).
Although the core of the Notch transduction pathway is similar, if not identical, in most Notch-dependent processes, the players that often fine-tune the pathway are distinct in different developmental contexts (Bray, 1998; Bray, 2006). The identification of context dependent regulators provides valuable insights into how Notch signaling is implemented at the molecular and cell biological level. Furthermore, the identification and characterization of context dependent regulators of Notch signaling should enable the design of more specific and efficient therapeutic strategies (Yamamoto et al., 2010).
We have recently reported that dEHBP1 regulates Notch signaling in asymmetrically dividing external sensory organ lineages (ESO) (Giagtzoglou et al., 2012). In this context, dEHBP1 controls the abundance of Sanpodo (Spdo), a regulator of Notch signaling (Couturier et al., 2012; Dye et al., 1998; O'Connor-Giles and Skeath, 2003; Skeath and Doe, 1998), as well as the intracellular trafficking of Delta (Dl), which is the ligand of the Notch receptor (Kimble and Simpson, 1997). Interestingly, dEHBP1 physically interacts with both the recycling endosome Rab11 GTPase and its effector, Sec15 (Giagtzoglou et al., 2012). Moreover, Rab11 and Sec15 physically interact (Wu et al., 2005) and regulate Dl trafficking (Emery et al., 2005; Jafar-Nejad et al., 2005) towards the apical portion of the actin-rich structure (ARS), which is the actin-rich plasma membrane microdomain at the interface of the pIIa/pIIb progeny in the ESO lineage, promoted by the activity of the WASP–Arp2/3 actin regulatory complex (Rajan et al., 2009). Hence, the identification of dEHBP1 as a regulator of Notch signaling raises additional questions: Is dEHBP1 a component of Notch signaling in other developmental contexts? If dEHBP1 is required for Notch signaling in other tissues, does dEHBP1 function by regulating intracellular trafficking of Spdo and Dl?
To address these questions, we assessed the role of dEHBP1 in Notch signaling in the developing Drosophila eye. The adult Drosophila eye is comprised of a highly specialized lattice of ∼800 ommatidia (reviewed by Kumar, 2012; Tsachaki and Sprecher, 2012). Each ommatidium contains eight photoreceptors, R1–R8, that emerge sequentially from undifferentiated imaginal tissue in a tightly regulated manner. The R8 photoreceptors are the first to be specified. Each R8 photoreceptor is singled out via Notch-signaling-mediated lateral inhibition from a group of developmentally equivalent cells, which all initially express the proneural basic helix-loop-helix (bHLH) transcription factor Atonal (Ato) (Jarman et al., 1994) followed by the Zn-finger transcription factor Senseless (Sens) (Frankfort et al., 2001; Nolo et al., 2000).
The efficacy of lateral inhibition in the eye relies on the activity of Scabrous (Sca), a secreted glycoprotein that is expressed in a subset of Ato-expressing cells and is later restricted to the R8 photoreceptors (Baker et al., 1990; Lee et al., 1996; Mlodzik et al., 1990). Sca is secreted and functions non-autonomously in the surrounding cells (Baker et al., 1990). Sca binds to and stabilizes the Notch receptor at the cell surface (Powell et al., 2001). Sca is then endocytosed by the cells in the vicinity of R8, where it may activate the Notch receptor or protect it from degradation and/or inactivation (Li et al., 2003).
Here, we report that dEHBP1 is a context dependent regulator of Notch signaling. While dEHBP1 regulates Notch signaling in the asymmetrically dividing mechanoreceptors via Dl and Spdo (Giagtzoglou et al., 2012), dEHBP1 controls Notch signaling during lateral inhibition in the developing eye via intracellular trafficking and exocytosis of Scabrous, a protein required for lateral inhibition. Our findings emphasize that regulators of intracellular trafficking like dEHBP1 modulate the output of distinct developmental modes of Notch signaling by controlling the intracellular trafficking of different components of the pathway.
Results
dEHBP1 affects eye development
dEHBP1 controls Notch signaling during the development of ESO lineages where it controls the abundance and subcellular localization of Sanpodo (Spdo) and Delta (Dl) (Giagtzoglou et al., 2012). To explore whether dEHBP1 regulates Notch signaling in developmental processes other than the asymmetric divisions of the mechanosensory bristle lineages, we generated homozygous mutant clones of loss of function alleles of dEHBP1 in several tissues that depend on Notch signaling for their proper development, using the FLP/FRT technique (Xu and Rubin, 1993).
We first assessed whether the loss of dEHBP1 affects the formation of the wing margin. The wing margin is specified via Notch-mediated inductive signaling at the dorsal-ventral (DV) boundary of larval wing imaginal discs, where Cut is expressed (de Celis and Bray, 1997; de Celis et al., 1996; Micchelli et al., 1997; Neumann and Cohen, 1996). We did not observe defects in dEHBP1 mutant clones, judged by the expression of Cut (supplementary material Fig. S1). Next, as Notch has been shown to regulate the expression of Hindsight (Hnt) and Cut in follicle cells during oogenesis (Sun and Deng, 2005; Sun and Deng, 2007), we assessed the expression of Hnt and Cut in somatic follicle cells upon the loss of dEHBP1 in either follicle or germ cells, using MARCM analysis (Lee and Luo, 1999). As shown in supplementary material Fig. S1, loss of dEHBP1 does not affect the expression of Hnt and Cut. Hence, the data suggest that dEHBP1 is a context dependent regulator of Notch signaling.
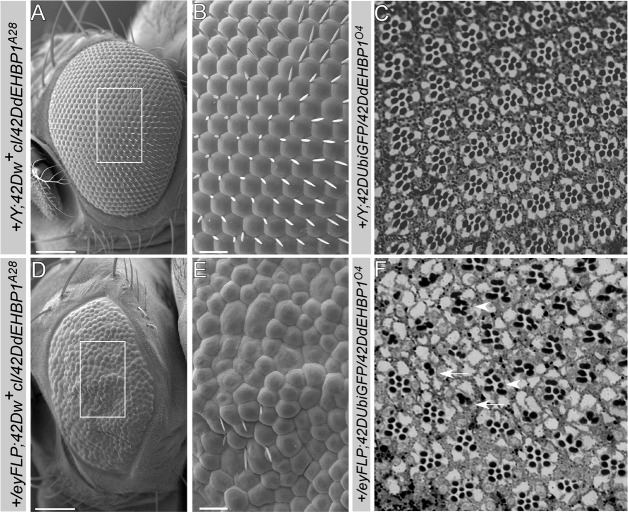
In contrast to the lack of obvious defects in the wing and the ovary, loss of dEHBP1 in eye clones using the eyFLP causes rough adult eyes. Scanning Electron Microscopy of an eye that is almost entirely mutant for dEHBP1 (see Materials and Methods) revealed a severe loss of mechanosensory bristles, as expected, and irregularly shaped and fused ommatidia (Fig. 1A,B,D,E). Furthermore, sections of adult retinas show that dEHBP1 mutant ommatidia contain aberrant numbers of photoreceptors (Fig. 1F, arrows) as well as fused rhabdomeres (Fig. 1F, arrowheads) and exhibit an overall abnormal patterning (Fig. 1C,F). In summary, in addition to defects in mechanosensory bristle development, loss of dEHBP1 affects multiple aspects of eye development.
Fig. 1.
dEHBP1 regulates the development of photoreceptors. Analysis of adult Drosophila retinas from (A–C) control flies (A,B are +/Y;42DUbiGFP/42DdEHBP1A28, C is +/Y;42DUbiGFP/42DdEHBP1O4 ), and (D–F) flies bearing dEHBP1 mutant clones (D,E are eyFLP;42Dcl/42D dEHBPA28, F is eyFLP;42DUbiGFP/42D dEHBPO4). (A,B,D,E) SEM analysis of adult retinas shows that in the absence of dEHBP1, the eyes develop to a smaller size and that the development of photoreceptors is disturbed, as evident by the lack of mechanosensory bristles, the fused ommatidia, and the loss of the characteristic hexagonal lattice. (C,F) Thick sections of adult retinas of control flies or bearing clones homozygous for dEHBP1O4, stained with Toluidine Blue, indicate that in the absence of dEHBP1, the ommatidia contain an aberrant number of photoreceptors. Arrows and arrowheads indicate ommatidia with less or more photoreceptors than normal, respectively. Scale bars: 100 µm (A,D), 20 µm (B,E).
Lateral inhibition is impaired in the absence of dEHBP1
During the development of the eye imaginal disc, Notch signaling is reiteratively utilized to regulate multiple processes, including the initiation and progression of the morphogenetic furrow, proneural enhancement, lateral inhibition, cell fate determination, cell proliferation, and apoptosis (Baker, 2007; Carthew, 2007; Roignant and Treisman, 2009; Sundaram, 2005). Lateral inhibition by Notch signaling mediates the singling out of R8 photoreceptors from developmentally equipotent groups of Ato positive cells (Baker, 2007; Carthew, 2007; Frankfort and Mardon, 2002). Early R8 photoreceptor cells express Ato and Sens (Frankfort et al., 2001; Jarman et al., 1994; Nolo et al., 2000). In the absence of dEHBP1, we observe supernumerary R8 photoreceptors in mutant clones (Fig. 2A–F). Note that the stripe of proneural expression of Ato at the morphogenetic furrow remains unaffected in the absence of dEHBP1 (Fig. 2A–C), indicating that dEHBP1 is dispensable for the process of proneural enhancement at the morphogenetic furrow, which has also been shown to be mediated by Notch signaling (Li and Baker, 2001).
Fig. 2.
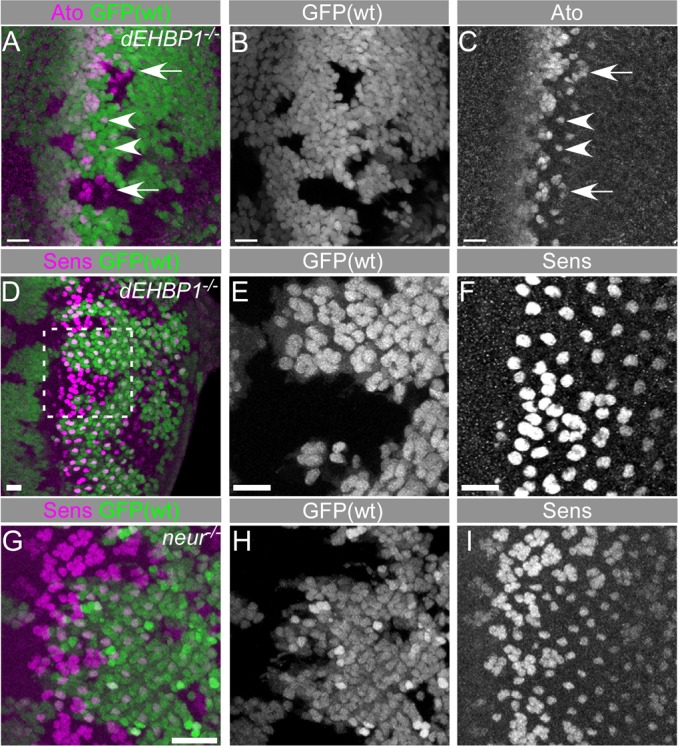
dEHBP1 controls the development of R8 photoreceptor cells. Loss of dEHBP1 within clones of cells negatively marked by nuclear GFP leads to the emergence of supernumerary Ato positive R8 phtotoreceptor cells (A–C) and of supernumerary Sens-positive R8 photoreceptor cells (D–F). The absence of neur within clones of cells negatively marked by nuclear GFP leads to the emergence of supernumerary Sens-positive cells (G–I). Note the greater severity of the Sens phenotype in neur clones (I) than in dEHBP1 clones (F). Scale bars: 10 nm.
The emergence of additional R8 photoreceptors in dEHBP1 homozygous mutant cells is weaker than what is observed upon loss of neuralized (Fig. 2G–I), an E3 ubiquitin ligase that is necessary for the internalization of the ligand Dl and its ability to signal to the Notch receptor (Lai et al., 2001; Lai and Rubin, 2001a; Lai and Rubin, 2001b; Le Borgne and Schweisguth, 2003; Li and Baker, 2001; Pavlopoulos et al., 2001). On the basis of this observation, we determined whether signaling pathways other than Notch may contribute to the development of supernumerary R8 photoreceptors based on known mutants, including loss of E-cadherin (Mirkovic and Mlodzik, 2006), loss of Rough (Pepple et al., 2008) or aberrant EGFR signaling (Rawlins et al., 2003; Spencer and Cagan, 2003). However, expression of E-cadherin, Rough, as well as dpERK and pointed-LacZ are not altered in dEHBP1 mutant clones (supplementary material Fig. S2). Hence, the supernumerary R8 cells caused by loss of dEHBP1 function are most likely due to a defect in some aspect of Notch signaling.
Scabrous fails to be secreted in the absence of dEHBP1
To test which component of the Notch signaling pathway is affected in dEHBP1 mutant cells, we first assessed the abundance and distribution of the Notch receptor, the Dl ligand and the E(spl) trancription factors, targets of Notch signaling during lateral inhibition (Bailey and Posakony, 1995; Delidakis and Artavanis-Tsakonas, 1992; Jennings et al., 1994; Lecourtois and Schweisguth, 1995). As shown in Fig. 3A–L, the expression levels and subcellular localization of Notch, E(spl) and Dl remain unaffected in dEHBP1 mutant clones (Fig. 3A–L).
Fig. 3.
Loss of dEHBP1 does not affect the expression of key components of Notch pathway, such as Notch, Dl and E(spl). Loss of dEHBP1 within clones of cells that are negatively marked by nuclear GFP does not affect the expression pattern of Notch (A–D), E(spl) (E–H) and Dl (I–L), or the endocytosis of Dl (M–O). Scale bars: 10 nm.
Previously, we found a striking defect in the dynamic intracellular trafficking of Dl in dEHBP1 mutant cells while standard immunofluorescence techniques failed to reveal obvious defects in asymmetrically dividing thoracic mechanosensory lineages (Giagtzoglou et al., 2012). To assess if trafficking of Dl is impaired, we performed endocytosis assays of Delta (Le Borgne et al., 2005) in the developing eye. Again, endocytosis of Dl appears to be normal in the absence of dEHBP1 (Fig. 3M–O), suggesting that dEHBP1 affects different components of Notch signaling in different cells (Giagtzoglou et al., 2012).
The lateral inhibition defects associated with the loss of dEHBP1 in the developing eyes is also reminiscent of the phenotype associated with the loss of scabrous (sca), a regulator of Notch signaling (Baker et al., 1990; Mlodzik et al., 1990). Immunostaining with an anti-Sca antibody in dEHBP1−/− mutant clones revealed that the Sca protein levels are upregulated in long cellular processes that emanate from the morphogenetic furrow (Fig. 4A–C). These processes correspond to actin rich filopodia, that regulate the trafficking and exocytosis of vesicles containing Sca several cell diameters away from the morphogenetic furrow (Chou and Chien, 2002). Similarly, we also observe an accumulation of Sca in the sensory organ precursors of the thoracic mechanosensory lineages (Fig. 4D–G). Hence, the lateral inhibition phenotype that we observe in the absence of dEHBP1 is likely to be caused by a defect in Sca trafficking.
Fig. 4.
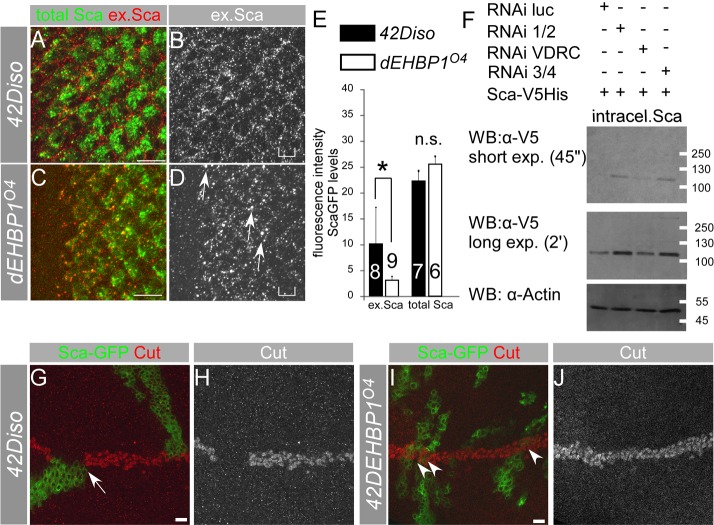
Loss of dEHBP1 results in accumulation of Sca, which together function to control Notch-mediated lateral inhibition. (A–G) Loss of dEHBP1 within clones of cells negatively marked by nuclear GFP leads to accumulation of Sca in the developing eye imaginal discs (A–C), where Sca decorates long actin-rich processes, and in pupal nota 17 hours after puparium formation (APF) (D–G). (H–O) dEHBP1 and sca function in the same pathway, because the phenotype of supernumerary R8 photoreceptors is indistinguishable in single and double mutants. (P) Quantification of the R8 photoreceptors that emerge per unit of area in single and double mutant regions in comparison to corresponding heterozygous control regions. Numbers shown at the base of the bars correspond to number of animals. Scale bars: 10 nm.
To test if dEHBP1 and sca function in the same pathway, we quantified the number of Sens positive R8 photoreceptors that emerge in the absence of only sca, only dEHBP1, or in the absence of both dEHBP1 and sca. We compared the number of R8 precursors in mutant clones with the corresponding heterozygous control tissue. The phenotype of single and double mutants are indistinguishable from each other, suggesting that the loss of dEHBP1 is equivalent to loss of function of sca (Fig. 4H–P). It is therefore likely that the loss of dEHBP1 causes a loss of sca function, even though the levels of Sca protein are upregulated.
The accumulation of Sca upon loss of dEHBP1 may represent either a defect in exocytosis of Sca from the R8 cells or a defect in endocytosis of Sca in neighboring cells. To examine whether exocytosis or endocytosis of Sca are defective in dEHBP1 mutant cells, we performed a series of experiments. In the first set of experiments, we overexpressed Sca–GFP in developing wild-type or dEHBP1 mutant photoreceptors (Newsome et al., 2000). We assessed the abundance of the extracellular portion of Sca–GFP and observed that more extracellular Sca–GFP is present in wild-type than in dEHBP1 mutant eye imaginal discs (Fig. 5A–E). Thus, loss of dEHBP1 impairs the abundance of extracellular Sca.
Fig. 5.
Loss of dEHBP1 disrupts exocytosis of Sca modifying its effect on Notch-mediated signaling. (A–D) The abundance of extracellular Sca (ex.Sca, shown in B and D) is greater in wild-type eye imaginal dics (A,B) compared with that in a mutant dEHBP1 background (C,D), whereas the total levels of overexpressed Sca–GFP (A,C) are comparable between the two different genetic backgrounds. This suggests that dEHBP1 regulates the exocytosis of Sca–GFP. (E) Quantification of the abundance of extracellular Sca–GFP pixels (red) that colocalize with pixels of total Sca–GFP (green) (see Materials and Methods), as reflected by the pixel intensity (mean grey value) of colocalized pixels, in wild-type and dEHPB1 mutant eye imaginal discs overexpressing Sca–GFP (shown with the square brackets in C and D, respectively). Sca–GFP is driven by neur-Gal4. In addition, total levels of Sca–GFP, as evident in the green channel, are indistinguishable between the two genotypes. Numbers shown at the base of the bars correspond to number of animals. (F) RNAi-mediated knockdown of EHBP1 (RNAi 1/2, VDRC, 3/4), but not of luciferase (RNAi luc-control) results in an increase in levels of intracellular Sca–V5His. Actin is a loading control. WB, western blot. (G–J) MARCM clonal analysis of wild-type (G,H) or dEHBP1 mutant clones (I–J) overexpressing Sca–GFP reveals that, in the absence of dEHBP1, Sca–GFP does not interrupt Notch signaling, as judged by expression of Cut (shown as a single channel representation in H,J). Scale bars: 10 nm.
To further corroborate our results, we performed RNAi-mediated knockdown of dEHBP1 in S2 cells which were subsequently transfected transiently with Sca–V5His. Under normal conditions, Sca is secreted in the culture medium (Lee et al., 1996). We reasoned that if dEHBP1 controls the secretion of Sca, then reduction of dEHBP1 will result in intracellular retention of Sca and consequently, higher levels of intracellular Sca will be detected by western blot analysis. Indeed, we observed that reduction of dEHBP1 results increased Sca intracellular levels (Fig. 5F), suggesting that upon reduction of dEHBP1, Sca secretion is impaired.
Finally, we took advantage of the fact that Sca acts as an extracellular antagonist of Notch signaling at the wing margin (Lee et al., 2000). As described previously for untagged Sca, overexpression of Sca–GFP in wild-type, positively marked, MARCM clones that abut the dorsoventral (D/V) boundary of wing imaginal discs disrupts Notch signaling and interrupts the stripe of expression of the transcription factor Cut (Fig. 5G–H). However, overexpression of Sca–GFP in dEHBP1 mutant clones has no effect on the expression of Cut at the D/V boundary (Fig. 5I–J). Therefore, dEHBP1 is required for the negative function of Sca–GFP upon Notch signaling at the D/V boundary of the wing imaginal discs.
In summary, we propose that dEHBP1 regulates secretion of Sca during the development of R8 photoreceptors. In the absence of dEHBP1, Sca positive vesicles are allowed to travel towards the exocytic sites, where they fail to be secreted and accumulate intracellularly.
dEHBP1 acts at a late step of exocytosis of Sca
The role of actin in the development of single R8 photoreceptor (Legent et al., 2012) raised the possibility that the multiple R8 phenotype upon loss of dEHBP1 may be due to the disruption of the actin cytoskeleton. However, F-actin appears normal in dEHBP1−/− tissue, suggesting that in the absence of dEHBP1, the actin cytoskeleton is not disrupted (Fig. 6A–D).
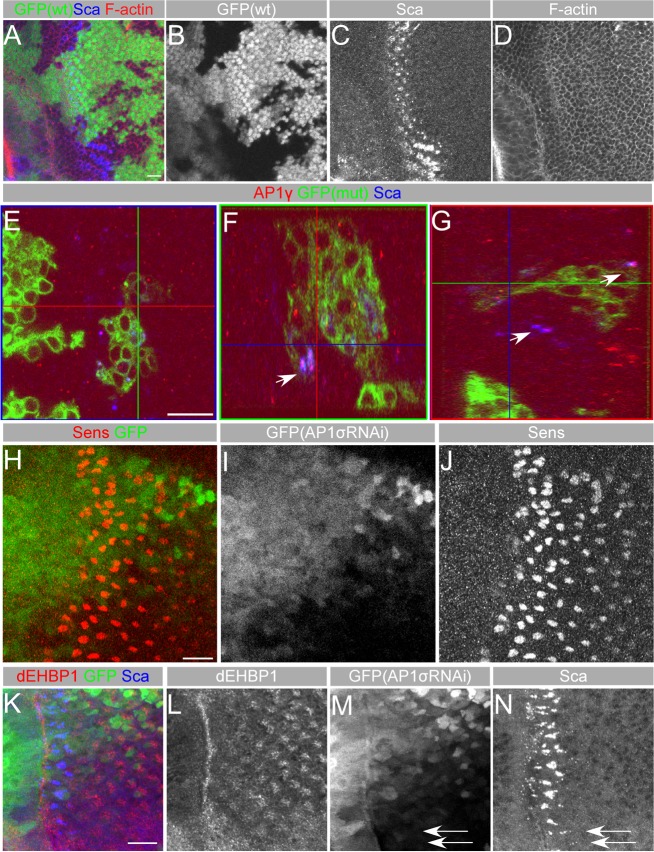
Fig. 6.
dEHBP1 does not affect the integrity of cytoskeleton but results in the accumulation of AP-1γ along with Sca. (A–D) Loss of dEHBP1 within clones of cells negatively marked by GFP (B) does not affect distribution of fluorescently marked phalloidin, a marker of F-actin (D). (E–G) Loss of dEHBP1 within clones of cells positively marked by nuclear GFP results in accumulation of AP-1γ along with Sca, as evident by the arrows in the YZ (F) and XZ (G) confocal slices of the XY view (E). Please note that AP-1γ appears to colocalize with Sca in wild-type tissue as well, suggesting that Sca vesicles are of AP-1γ identity. (H–J) eyg-Gal4-driven RNAi-mediated knockdown of the AP-1σ subunit of the AP-1 complex in a domain marked by the expression of GFP (I) results in development of supernumerary R8 photoreceptors, as seen by expression of Sens (J) in agreement with previously published data (Kametaka et al., 2012). (K–N) eyg-Gal4-driven RNAi-mediated knockdown of the AP-1σ subunit of the AP-1 complex in a domain marked by the expression of GFP (M) results in intracellular accumulation of Sca (N), in agreement with previously published data (Kametaka et al., 2012), without affecting the subcellular localization of dEHBP1. Scale bars: 10 nm.
The severe upregulation of Sca in filopodia in developing eye imaginal discs prompted us to determine the nature of the vesicular compartment. To estimate the integrity and abundance of various subcellular compartments, we analyzed the distribution and levels of various markers, such as VAP33 (endoplasmatic reticulum), Rab1 (Golgi), GM130 (Golgi), Syx16 (Golgi), Aftiphilin (Golgi), Rab5 (early endosomes), Hrs (multivesicular bodies), Rab11 (recycling endosomes), Spi (late endosomes), Vps16 (late endosomes) and Rab7 (late endosomes) (supplementary material Fig. S3). With the exception of the Golgi markers Syx16 and GM130, which accumulate in cells that are located posterior to the morphogenetic furrow and thereby, have arisen at earlier stages of development, we failed to observe an aberrant distribution of any other markers in dEHBP1 mutant clones (supplementary material Fig. S3). These data suggest that loss of dEHBP1 does not result in any overt perturbations of subcellular organization at the morphogenic furrow where lateral inhibition is happening.
Despite the partial localization of Sca with Rab7 in both wild-type and mutant tissue (arrowheads in supplementary material Fig. S3H–I), as proposed previously (Li et al., 2003), we observe that the majority of Sca, does not colocalize with any of the aforementioned markers. It was recently shown that depletion of the AP-1 clathrin adaptor complex at the morphogenetic furrow leads to an intracellular accumulation of Sca, accompanied by a loss of function of Notch signaling phenotype (Kametaka et al., 2012), suggesting that the AP-1 complex is responsible for trafficking of Sca. The accumulation of Sca upon either knockdown of AP-1 activity (Kametaka et al., 2012) (Fig. 6J) or in the absence of dEHBP1 (Fig. 4A,C; supplementary material Fig. S3) raised the possibility that AP-1 complex and dEHBP1 may function together in Sca exocytosis. To address this hypothesis, we asked whether dEHBP1 interacts and colocalizes with AP-1γ, the gamma subunit of the AP-1 complex. We could not detect any co-localization between dEHBP1 and AP-1γ in developing eye discs by immunofluorescent experiments (data not shown). Neither were we able to detect any physical interaction between Sca or AP-47 (the μ subunit of the AP-1 complex) with dEHBP1 in co-immunoprecipitation experiments from whole cell protein extracts of transiently transfected S2 cells (data not shown). We then asked whether the subcellular localization of AP-1γ and dEHBP1 are interdependent. We find that AP-1γ co-localizes with Sca in both mutant dEHBP1 and wild-type neighboring tissue to the same extent (Fig. 6E–G). Importantly, the absence of dEHBP1 is accompanied by an accumulation of AP-1γ along with the Sca accumulation, thus placing dEHBP1 at a later step in the trafficking of AP-1γ–Sca vesicles. Indeed, a reduction of AP-1 activity via RNAi-mediated knockdown of AP-1σ results in accumulation of Scabrous but it does not affect the localization of dEHBP1 (Fig. 6K–N). These observations further support the notion that dEHBP1 is critical for exocytosis of Sca at a later step than AP-1γ.
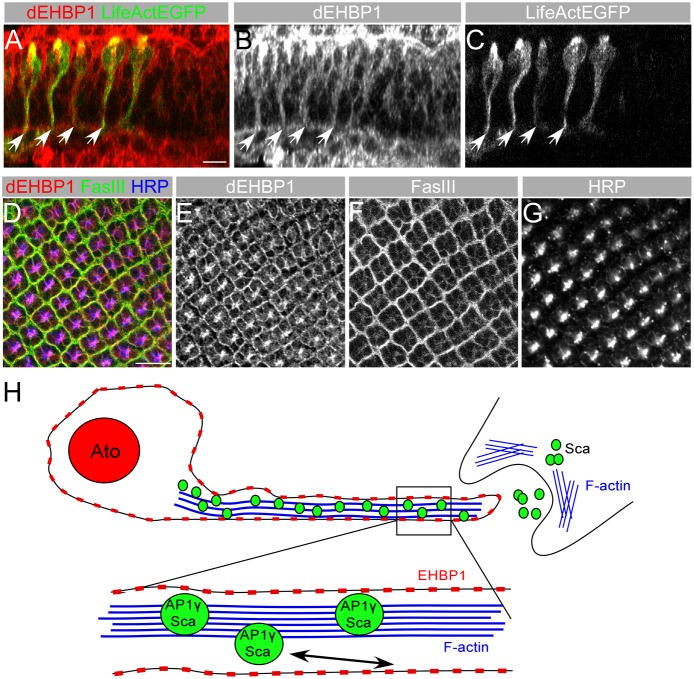
dEHBP1 contains a calponin homology (CH) domain, which is classified as an actin binding domain (Korenbaum and Rivero, 2002). In addition, dEHBP1 is enriched at the actin rich cellular interphase in asymmetrically dividing sensory organ precursor cells (Giagtzoglou et al., 2012). We therefore tested whether endogenous dEHBP1 co-localizes with F-actin in photoreceptors cells using LifeAct–EGFP, expressed under the control of neur-GAL4 to specifically label F-Actin in these cells. Upon closer examination of the endogenous pattern of dEHBP1 in the developing eye, we observe that dEHBP1 colocalizes with actin, as visualized by LifeAct–EGFP (Fig. 7A–C). Furthermore, dEHBP1 mostly localizes near the plasma membrane, marked by the neuronal membrane antigen HRP or the lateral membrane marker Fas-III, in the developing photoreceptor neurons (Fig. 7D–G). These results suggest that dEHBP1 functions at the plasma membrane at a late step of exocytosis of Sca/AP-1γ vesicles, trafficked along the actin cytoskeleton (Fig. 7H).
Fig. 7.
dEHBP1 is localized at the plasma membrane of photoreceptors. (A–C) In an XZ single confocal slice of third-instar eye imaginal disc expressing the actin marker LifeAct EGFP (C), endogenous dEHBP1 (B) colocalizes with the actin cytoskeleton. (B) (D–G) dEHBP1 (E) colocalizes with membrane molecules FasIII (F) and HRP (G) in developing neurons in larval eye imaginal discs. Scale bars: 10 nm. (H) Schematic model of Sca secretion in R8 photorecetors where AP-1γ vesicles bearing Sca travel down actin-rich filopodia. dEHBP1 localizes at the plasma membrane where it regulates a late step in the exocytosis of Sca. When the integrity of the actin cytoskeleton is challenged, as for example upon application of cytochalasin D, transfer of Sca vesicles down the actin-rich tracks is known to be perturbed (Chou and Chien, 2002). However, we propose that actin might also affect other aspects of Notch signaling, such as the endocytosis of Sca, which might also result in defective lateral inhibition during development of R8 photoreceptors (Li et al., 2003). In the absence of dEHBP1, Sca vesicles travel down their path but fail to exocytose, resulting in intracellular accumulation of Sca.
Discussion
dEHBP1 is a context-dependent regulator of Notch signaling
By examining several distinct developmental events that are known to depend on Notch signaling, we show that dEHBP1 is a context dependent regulator of Notch signaling. dEHBP1 does not affect wing margin formation, ovary development or proneural enhancement of the Ato stripe of expression at the morphogenetic furrow. However, dEHBP1 regulates the singling of External Sensory Organ precursors in developing thoracic epithelia (data not shown) and of R8 photoreceptors in the eye imaginal discs of Drosophila (Figs 2, 4). Hence, dEHBP1 regulates Notch signaling in at least two different developmental contexts, i.e. asymmetric divisions of the mechanosensory lineages (Giagtzoglou et al., 2012) and during lateral inhibition of the R8 photoreceptors (this study). Importantly, our data reveal that dEHBP1 regulates Notch signaling in these two instances by regulating different substrates, i.e. exocytosis of Dl and trafficking of Sca, respectively. Although we cannot exclude a role of dEHBP1 in the endocytosis of Scabrous in signal receiving cells, our data suggest that dEHBP1 is required for the exocytosis of Sca from signal sending cells.
Intracellular trafficking pathways establish multiple layers of regulation within Notch signaling pathway
The identification of numerous intracellular trafficking regulators within the Notch signaling signifies the importance of intracellular trafficking in controlling the efficacy and robustness of signaling output. Furthermore, the plethora of different context dependent intracellular trafficking regulators provides evidence that different modes of intercellular signaling rely on distinct intracellular trafficking pathways. For example, although endocytosis of Notch ligands always appears to rely on the activity of dynamin, it is not always dependent clathrin and auxillin (Banks et al., 2011; Windler and Bilder, 2010). Similarly, endosomal recycling of Notch ligands, controlled by rab11 and its effector, sec15, is required for the development of asymmetrically dividing mechanosensory lineages (Benhra et al., 2010; Emery et al., 2005; Jafar-Nejad et al., 2005), but not during photoreceptor development or ovary development (Banks et al., 2011; Mehta et al., 2005; Windler and Bilder, 2010).
Intriguingly, the role of certain intracellular trafficking regulators uncover an additional level of complexity within Notch signaling pathway. For instance, arp3 organizes the emergence of actin rich microvillae at the apical side of the pIIa/pIIb sibling cells of the asymmetrically dividing mechanosensory lineages. In the absence of arp3, Dl recycling towards this specialized microenvironment of the plasma membrane is impaired (Rajan et al., 2009). However, in other tissues, where Dl recycling does not play a role, as for example during development of ovaries (Windler and Bilder, 2010) or photoreceptors (Banks et al., 2011), arp3 may regulate different aspects of Notch signaling. Similarly, our studies support the conclusion that dEHBP1 regulates Notch signaling in different developmental contexts by controlling the intracellular trafficking of different substrates, as for example Dl and Spdo during asymmetric divisions and Sca during lateral inhibition. The AP-1 complex represents another interesting case of how Notch signaling can be differentially regulated at the level of intracellular trafficking. In the thoracic mechanosensory lineages, loss of AP-1 complex activity results in a gain of function of Notch signaling, because it results in retention of Spdo at the plasma membrane and higher Notch signaling activity (Benhra et al., 2011). However, loss of function of the AP-1 complex activity results in loss of function of Notch signaling in developing photoreceptors, as it regulates trafficking of Sca, which has been reported to accumulate intracellularly (Kametaka et al., 2012). Based on the subcellular localization of AP-1γ and Sca in dEHBP1 mutant cells as well as our inability to detect any colocalization among dEHBP1 and Sca or AP-1γ (data not shown) we propose that dEHBP1 functions at a late exocytic step of Sca vesicles.
The role of intracellular trafficking and Notch signaling in adult neuronal circuits
Notch signaling is utilized reiteratively during metazoan development in a plethora of processes, including proliferation, cell fate specification and apoptosis. An expanding body of evidence indicates that Notch signaling is also involved in regulation of neuronal function at both structural and functional levels. In mammals, Notch and its ligands are localized at synapses, where they regulate synaptic plasticity (Alberi et al., 2011; Dahlhaus et al., 2008; Wang et al., 2004). In Drosophila, Notch regulates synaptic plasticity at the larval neuromuscular junction (de Bivort et al., 2009), while activation of adult olfactory neurons leads to presenilin-mediated cleavage of Notch (Lieber et al., 2011). Notch has also been implicated in the control of sleep (Seugnet et al., 2011; Singh et al., 2011). Finally, Notch activity is required for memory formation in Drosophila and mice (Costa et al., 2003; Ge et al., 2004; Matsuno et al., 2009; Presente et al., 2004). Intriguingly, loss of function of sca disrupts the formation of memories for ethanol reward without disrupting apparent features of the corresponding neuronal circuitry (Kaun et al., 2011).
Conceivably, and similarly to what is observed during development, distinct intracellular trafficking pathways may underlie the activation of Notch signaling pathway in different neuronal circuitries or different aspects of neuronal function. Therefore, context specific regulators, including arp3, AP-1γ and dEHBP1, provide unique molecular handles to dissect the roles of intracellular trafficking pathways and Notch signaling in the adult brain.
Materials and Methods
Fly stocks and genetics
The following stocks were used in this study: FRT42D dEHBP1A28 and FRT42D dEHBP1O4 (Giagtzoglou et al., 2012), cn scabp2 bw (BDSC), FRT42D scaBP2dEHBP1O4, eyFLP; FRT42D cl w+/CyO, eyFLP; FRT42D UbiGFP, UbxFLP; FRT42D UbiGFP, eyFLP; T(2;3)apXa/FRT42D cl w+;neur-Gal4, UAS-CD8::GFP hsFLP; FRT42D tub-GAL80; tub-GAL4/TM6B, Tb, eyg-Gal UAS-GFP, UAS-sca-GFP (Chou and Chien, 2002), UAS-LifeAct-EGFP, Vienna (VDRC) RNAi lines against AP-1σ (CG5864- VDRC 107322/KK, VDRC 101356/KK).
FRT42D dEHBP1A28 and FRT42D dEHBP1O4 are considered null alleles based on immunofluorescent stainings with anti-dEHBP1 as well as on similar lethal stages of homozygous and hemizygous (point mutation over corresponding chromosomal deficiency) mutants (Giagtzoglou et al., 2012). scaBP2 is also a null allele (Mlodzik et al., 1990; Renaud and Simpson, 2001).
Flies bearing adult eyes mostly comprised by large clones of mutant dEHBP1 tissue (Fig. 1) were generated using the w eyFLP; FRT42D cl w+ technique (Newsome et al., 2000).
Constructs
The full length Sca cDNA was PCR amplified without a stop codon from MT-Sca, a gift from Dr Nick Baker, and cloned as a Not/Xba fragment in frame with V5-his composite tag into pAc-V5His B (Invitrogen). pUASTattB-LifeAct::EGFP was constructed by PCR-mediated fusion of a Kozak consensus sequence (GCCACC) and the LifeAct–EGFP sequence, which endodes the 17 a.a. peptide ‘MGVADLIKKFESISKEE’ (Riedl et al., 2008), fused in frame with EGFP. PCR products were cloned into the BglII/XbaI sites of pUASTattB (Bischof et al., 2007). After sequencing verification, this construct (pUASTattB-LifeAct-EGFP) was injected into docking sites VK37 (2nd chromosome) or VK33 (3rd chromosome) by phiC31-mediated integration to obtain transgenic flies. All constructs were verified by sequencing before use.
Antibodies, immunofluorescent stainings, confocal microscopy and imaging
The following monoclonal antibodies were provided by the Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA and used at following concentration: mouse anti-Notch 1∶100 [(C17.9C6) (Fehon et al., 1990)], rat anti-DE-cadherin 1∶100 [DCAD2 (Oda et al., 1994)], mouse anti-E(spl) 1∶100 [mAb323 (Jennings et al., 1994)], mouse anti-Delta 1∶100 [C594.9B (Klueg et al., 1998)], mouse anti-Scabrous 1∶100 [sca1 (Lee et al., 1996)], mouse anti-Cut 1∶100 [2B10 (Blochlinger et al., 1990; Blochlinger et al., 1993)], mouse anti Hindsight 1∶100 [1G9 (Yip et al., 1997)], and mouse anti-FasIII 1∶100 [7G10 (Patel et al., 1987)], mouse anti-Rough 1∶100 [ro-62C2A8 (Kimmel et al., 1990)], mouse anti-V5 (Invitrogen) 1∶1000, Mouse anti-Actin (clone C4, MP Biomedicals LLC) 1∶1000. Mouse monoclonal anti-Rab11 was purchased from by BD Biosciences and used at a dilution of 1∶100 (Khodosh et al., 2006).
The following polyclonal antibodies were used: guinea pig anti-Atonal 1∶1000 (Chang et al., 2002), rabbit dpERK 1∶50 (Sigma), rabbit anti-β-galactosidase 1∶1000 (Abcam), rabbit anti-GM130 1∶500 (Abcam), chicken anti-GFP (Abcam) 1∶500, rabbit anti-GFP (Invitrogen) 1∶500, rabbit anti-Syx16 1∶500 (Abcam), guinea pig anti-Senseless 1∶1500 (Nolo et al., 2000), guinea pig anti-dEHBP1 1∶1500 (Giagtzoglou et al., 2012), purified rabbit anti-AP-1γ 1∶100 (Benhra et al., 2011), rabbit anti-HRP 1∶500 (Jackson Immunoresearch), rabbit anti-Vap33 1∶1000 (Tsuda et al., 2008), rabbit anti-Rab5 1∶500 (Abcam), rabbit anti-Rab7 (Chinchore et al., 2009), guinea pig anti-Hrs 1∶1000 (Lloyd et al., 2002), guinea pig anti-Spinster 1∶500 (Sweeney and Davis, 2002), rabbit anti Vps16a 1∶500 (Pulipparacharuvil et al., 2005), mouse anti-Aftiphilin 1∶500 (Kametaka et al., 2012), mouse anti-Rab1 1∶500 (Satoh et al., 1997).
For immunostaining of larval eye discs, the dissected eye antennal imaginal discs were fixed in 3.7% PFA in 1×PBS for 20 minutes followed by incubation with the primary antibodies in 1×PBS containing 0.2% Triton X-100 (1×PBT) and 5% normal goat serum for 18 hours at 4°C. After washing with 1×PBT, samples were incubated with appropriate secondary antibodies (Jackson InmunoResearch Laboratories, Inc.) at a final dilution of 1∶500 for 2 hours at room temperature.
In Delta endocytosis assays, dissections of larval eye–brain tissue and their subsequent incubations with anti-Delta were performed in Schneider's medium (Invitrogen) at room temperature (RT) for 20 minutes (Le Borgne and Schweisguth, 2003). The samples were then washed three times in Schneider's medium and fixed in 3.7% paraformaldehyde/Schneider's medium for 20 min at RT. After washing with Schneider's medium, eye imaginal discs were permeabilized in 1×PBS, 0.2% Triton X-100 (1×PBT) and incubated with secondary antibodies in 1×PBT, 5% normal goat serum (NGS) for 2 hours at room temperature.
We find that the available monoclonal antibody against Sca does not produce reliable results in our assays for the assessment of steady state levels of extracellular Sca, as initially suggested (Lee et al., 1996), despite its usage for similar purposes in later studies (Chou and Chien, 2002; Kametaka et al., 2012). Therefore, to estimate the steady state levels of exocytosis of Sca in vivo, we performed the following experiment.
We drove the overexpression of the Sca–GFP fusion protein with neur-Gal4 in larval photoreceptors and their precursors, in an either wild-type or a dEHBP1 mutant genetic background using the UAS/GAL4 system (Brand and Perrimon, 1993). Larval eye brain complexes were dissected under non-permeabilizing conditions in Schneider's medium. After exchanging the dissection media with fresh, cold Schneider's medium, larval eye-brain complexes were immediately placed on ice to minimize endocytosis of Sca, and incubated with an excess of chicken anti-GFP antibody at a final dilution of 1∶100 for 1–2 hours. After rinsing with cold Schneider's medium, we incubated the tissue samples with Cy3-conjugated secondary anti chicken antibody.
Fluorescent images were captured with a Zeiss 510 LSM confocal microscope. Image processing and quantifications were performed using ImageJ software (National Institutes of Health). To avoid any background noise and achieve precise measurements of the abundance of extracellular Sca we first split the channels in each stack of images. We then applied the Colocalization Threshold plugin, which is part of the GDSC suite of plugins, available for download along with its manual from the following website: http://www.sussex.ac.uk/gdsc/intranet/microscopy/imagej/colocalisation. We retained the default values in our analysis. The fluorescence intensity levels of extracellular Sca–GFP pixels (red channel) that colocalize with total ScaGFP pixels (green channel) were measured throughout the stack of confocal images automatically at the end of the process.
The colocalized pixels, which are represented in the blue channel of the output images of the aforementioned procedure were automatically thresholded using inbuilt functions. We then used the inbuilt Measure Stacks plugin to quantify throughout the whole 3D stack of images the average mean pixel intensity of colocalized pixels in a randomly demarcated 256×256 pixel region of interest (ROI) behind the morphogenetic furrow. The results of our analysis reflect the abundance of extracellular Sca–GFP, shown in Fig. 5E as ‘ex.Sca’. We calculated the levels of total Sca–GFP in the green channel through the stack of confocal images using ‘Measure Stacks’ inbuilt plugin, and the results are now shown in Fig. 5E as ‘total Sca’. The axis in Fig. 5E is labeled as ‘fluorescence intensity of Sca–GFP levels’.
Final image sets were assembled in Adobe Photoshop 7.0 (Adobe systems) and Canvas X software (ACDSee Products). Scale bars correspond to 10 nm, unless otherwise indicated.
Statistical tests were performed using GraphPad Prism 3.0 software (GraphPad Software). Hypothesis testing was based on Student's t-test between two groups (two tailed, unequal variance, statistically significant for P<0.05), as for example in the case of the abundance of extracellular Sca in Fig. 5 or the number of Sens R8 cells between homozygous and heterozygous tissue in Fig. 4, or on a Kruskal–Wallis test (non parametric ANOVA) followed by Dunn's multiple comparison test among all groups with one control group, namely the dEHBP14O4 heterozygous tissue in Fig. 4 (statistically significant for P<0.05).
RNAi treatment of S2 cells
RNAi treatment of S2 cells was performed by modification of standard methods (Ramadan et al., 2007).
For generation of dsRNA, we designed primers targeting three different regions of dEHBP1, all bearing T7 promoter sequences at their 5′ end. For comparison, one primer pair targets the region that dsRNA from Vienna Drosophila RNAi Center has been designed against. As a control, RNAi against luciferase was designed. Sequences are available upon request. dsRNA were generated and purified using Megascript RNAi kit (Ambion) following the manufacturer's instructions.
Briefly, S2 cells were seeded at a concentration of 106 cells/ml in 12-well plates. Each well containing 1 ml of cell culture in Schneider's medium (Invitrogen), supplemented with 10% heat inactivated and filter sterilized Fetal Bovine Serum (Invitrogen), as well as a cocktail of Penicillin and Streptomycin antibiotics (Invitrogen). The following day, we performed RNAi treatment of S2 cells in triplicates following the bathing protocol, without however removing the serum from the media. We added ∼9 µg of purified dsRNA to 106 S2 cells and incubated them overnight. On the third day, we transfected all cells with pAc-Sca-V5His using Effectene transfection reagent (Qiagen) and according to manufacturer's instructions. S2 cells were harvested 48 hours after transfection. After centrifugation at room temperature at 3000 rpm for 2′, cells were washed from remnants of supernatant medium by resuspension in 1×PBS. S2 cells were then centrifuged as above, resuspendend in 100 µl/106cells of lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5% glycerol, 0.5% NP-40, 1 mM DTT) and incubated on ice for 30 minutes. After a final centrifugation at 15,000 rpm at 4°C for 5 min, the supernatant was kept for western blot analysis of intracellular Sca–V5His. Actin served as a loading control of the extracts. The whole experiment was repeated three times.
Supplementary Material
Acknowledgments
Confocal microscopy at Baylor College of Medicine was supported by the Intellectual and Developmental Disabilities Research Center (IDDRC). We thank the Drosophila Bloomington Stock Center at the University of Indiana, Bloomington, Vienna Drosophila Stock Center and the Developmental Studies Hybridoma Bank at the University of Iowa. We thank Dr Chien for providing us with UAS-Sca-GFP flies. We also thank Dr Roland Le Borgne for his gift of AP-1γ antibody and helpful discussions, Dr Satoshi Kametaka and Dr Satoshi Waguri for their gift of anti-Aftiphilin, and Dr Nicholas Baker for providing us with sca cDNA constructs and suggestions. We also thank Dr Wan Hee Yoon, Nele Haelterman and Dongxue Mao for comments on the manuscript. No competing interests declared.
Footnotes
Author contributions
N.G. designed and performed experiments and wrote the manuscript, T.L. and S.Y. performed experiments, H.J.B. designed experiments and wrote the manuscript.
Funding
N.G. was supported by a long-term European Molecular Biology Organization postdoctoral fellowship and the Howard Hughes Medical Institute. S.Y. was supported by the Nakajima Foundation. H.J.B. is a Howard Hughes Medical Institute Investigator. This work has been supported by the Howard Hughes Medical Institute, by grant number 5P30HD024064 to the BCM IDDRC from NICHD, and grant number C06RR029965 from NCRR. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.126292/-/DC1
References
- Alberi L., Liu S., Wang Y., Badie R., Smith-Hicks C., Wu J., Pierfelice T. J., Abazyan B., Mattson M. P., Kuhl D. et al. (2011). Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 69, 437–444 10.1016/j.neuron.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Muskavitch M. A. (2010). Notch: the past, the present, and the future. Curr. Top. Dev. Biol. 92, 1–29 10.1016/S0070-2153(10)92001-2 [DOI] [PubMed] [Google Scholar]
- Bailey A. M., Posakony J. W. (1995). Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 9, 2609–2622 10.1101/gad.9.21.2609 [DOI] [PubMed] [Google Scholar]
- Baker N. E. (2007). Patterning signals and proliferation in Drosophila imaginal discs. Curr. Opin. Genet. Dev. 17, 287–293 10.1016/j.gde.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Baker N. E., Mlodzik M., Rubin G. M. (1990). Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science 250, 1370–1377 10.1126/science.2175046 [DOI] [PubMed] [Google Scholar]
- Banks S. M., Cho B., Eun S. H., Lee J. H., Windler S. L., Xie X., Bilder D., Fischer J. A. (2011). The functions of auxilin and Rab11 in Drosophila suggest that the fundamental role of ligand endocytosis in notch signaling cells is not recycling. PLoS ONE 6, e18259 10.1371/journal.pone.0018259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhra N., Vignaux F., Dussert A., Schweisguth F., Le Borgne R. (2010). Neuralized promotes basal to apical transcytosis of delta in epithelial cells. Mol. Biol. Cell 21, 2078–2086 10.1091/mbc.E09-11-0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhra N., Lallet S., Cotton M., Le Bras S., Dussert A., Le Borgne R. (2011). AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors. Curr. Biol. 21, 87–95 10.1016/j.cub.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312–3317 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Bodmer R., Jan L. Y., Jan Y. N. (1990). Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 4, 1322–1331 10.1101/gad.4.8.1322 [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Jan L. Y., Jan Y. N. (1993). Postembryonic patterns of expression of cut, a locus regulating sensory organ identity in Drosophila. Development 117, 441–450 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- Bray S. (1998). Notch signalling in Drosophila: three ways to use a pathway. Semin. Cell Dev. Biol. 9, 591–597 10.1006/scdb.1998.0262 [DOI] [PubMed] [Google Scholar]
- Bray S. J. (2006). Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- Carthew R. W. (2007). Pattern formation in the Drosophila eye. Curr. Opin. Genet. Dev. 17, 309–313 10.1016/j.gde.2007.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Newmyer S. L., Hull M. J., Ebersold M., Schmid S. L., Mellman I. (2002). Hsc70 is required for endocytosis and clathrin function in Drosophila. J. Cell Biol. 159, 477–487 10.1083/jcb.200205086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchore Y., Mitra A., Dolph P. J. (2009). Accumulation of rhodopsin in late endosomes triggers photoreceptor cell degeneration. PLoS Genet. 5, e1000377 10.1371/journal.pgen.1000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. H., Chien C. T. (2002). Scabrous controls ommatidial rotation in the Drosophila compound eye. Dev. Cell 3, 839–850 10.1016/S1534-5807(02)00362-3 [DOI] [PubMed] [Google Scholar]
- Costa R. M., Honjo T., Silva A. J. (2003). Learning and memory deficits in Notch mutant mice. Curr. Biol. 13, 1348–1354 10.1016/S0960-9822(03)00492-5 [DOI] [PubMed] [Google Scholar]
- Couturier L., Vodovar N., Schweisguth F. (2012). Endocytosis by Numb breaks Notch symmetry at cytokinesis. Nat. Cell Biol. 14, 131–139 10.1038/ncb2419 [DOI] [PubMed] [Google Scholar]
- Dahlhaus M., Hermans J. M., Van Woerden L. H., Saiepour M. H., Nakazawa K., Mansvelder H. D., Heimel J. A., Levelt C. N. (2008). Notch1 signaling in pyramidal neurons regulates synaptic connectivity and experience-dependent modifications of acuity in the visual cortex. J. Neurosci. 28, 10794–10802 10.1523/JNEUROSCI.1348-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bivort B. L., Guo H. F., Zhong Y. (2009). Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction. J. Neurogenet. 23, 395–404 10.3109/01677060902878481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis J. F., Bray S. (1997). Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124, 3241–3251 [DOI] [PubMed] [Google Scholar]
- de Celis J. F., Garcia-Bellido A., Bray S. J. (1996). Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122, 359–369 [DOI] [PubMed] [Google Scholar]
- Delidakis C., Artavanis-Tsakonas S. (1992). The Enhancer of split [E(spl)] locus of Drosophila encodes seven independent helix-loop-helix proteins. Proc. Natl. Acad. Sci. USA 89, 8731–8735 10.1073/pnas.89.18.8731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C. A., Lee J. K., Atkinson R. C., Brewster R., Han P. L., Bellen H. J. (1998). The Drosophila sanpodo gene controls sibling cell fate and encodes a tropomodulin homolog, an actin/tropomyosin-associated protein. Development 125, 1845–1856 [DOI] [PubMed] [Google Scholar]
- Emery G., Hutterer A., Berdnik D., Mayer B., Wirtz-Peitz F., Gaitan M. G., Knoblich J. A. (2005). Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122, 763–773 10.1016/j.cell.2005.08.017 [DOI] [PubMed] [Google Scholar]
- Fehon R. G., Kooh P. J., Rebay I., Regan C. L., Xu T., Muskavitch M. A., Artavanis-Tsakonas S. (1990). Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61, 523–534 10.1016/0092-8674(90)90534-L [DOI] [PubMed] [Google Scholar]
- Frankfort B. J., Mardon G. (2002). R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development 129, 1295–1306 [DOI] [PubMed] [Google Scholar]
- Frankfort B. J., Nolo R., Zhang Z., Bellen H., Mardon G. (2001). senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron 32, 403–414 10.1016/S0896-6273(01)00480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Hannan F., Xie Z., Feng C., Tully T., Zhou H., Xie Z., Zhong Y. (2004). Notch signaling in Drosophila long-term memory formation. Proc. Natl. Acad. Sci. USA 101, 10172–10176 10.1073/pnas.0403497101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagtzoglou N., Yamamoto S., Zitserman D., Graves H. K., Schulze K. L., Wang H., Klein H., Roegiers F., Bellen H. J. (2012). dEHBP1 controls exocytosis and recycling of Delta during asymmetric divisions. J. Cell Biol. 196, 65–83 10.1083/jcb.201106088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H., Andrews H. K., Acar M., Bayat V., Wirtz-Peitz F., Mehta S. Q., Knoblich J. A., Bellen H. J. (2005). Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell 9, 351–363 10.1016/j.devcel.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Grell E. H., Ackerman L., Jan L. Y., Jan Y. N. (1994). Atonal is the proneural gene for Drosophila photoreceptors. Nature 369, 398–400 10.1038/369398a0 [DOI] [PubMed] [Google Scholar]
- Jennings B., Preiss A., Delidakis C., Bray S. (1994). The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development 120, 3537–3548 [DOI] [PubMed] [Google Scholar]
- Kametaka S., Kametaka A., Yonekura S., Haruta M., Takenoshita S., Goto S., Waguri S. (2012). AP-1 clathrin adaptor and CG8538/Aftiphilin are involved in Notch signaling during eye development in Drosophila melanogaster. J. Cell Sci. 125, 634–648 10.1242/jcs.090167 [DOI] [PubMed] [Google Scholar]
- Kaun K. R., Azanchi R., Maung Z., Hirsh J., Heberlein U. (2011). A Drosophila model for alcohol reward. Nat. Neurosci. 14, 612–619 10.1038/nn.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodosh R., Augsburger A., Schwarz T. L., Garrity P. A. (2006). Bchs, a BEACH domain protein, antagonizes Rab11 in synapse morphogenesis and other developmental events. Development 133, 4655–4665 10.1242/dev.02650 [DOI] [PubMed] [Google Scholar]
- Kimble J., Simpson P. (1997). The LIN-12/Notch signaling pathway and its regulation. Annu. Rev. Cell Dev. Biol. 13, 333–361 10.1146/annurev.cellbio.13.1.333 [DOI] [PubMed] [Google Scholar]
- Kimmel B. E., Heberlein U., Rubin G. M. (1990). The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 4, 712–727 10.1101/gad.4.5.712 [DOI] [PubMed] [Google Scholar]
- Klueg K. M., Parody T. R., Muskavitch M. A. (1998). Complex proteolytic processing acts on Delta, a transmembrane ligand for Notch, during Drosophila development. Mol. Biol. Cell 9, 1709–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Ilagan M. X. (2009). The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbaum E., Rivero F. (2002). Calponin homology domains at a glance. J. Cell Sci. 115, 3543–3545 10.1242/jcs.00003 [DOI] [PubMed] [Google Scholar]
- Kumar J. P. (2012). Building an ommatidium one cell at a time. Dev. Dyn. 241, 136–149 10.1002/dvdy.23707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Rubin G. M. (2001a). neuralized functions cell-autonomously to regulate a subset of notch-dependent processes during adult Drosophila development. Dev. Biol. 231, 217–233 10.1006/dbio.2000.0124 [DOI] [PubMed] [Google Scholar]
- Lai E. C., Rubin G. M. (2001b). Neuralized is essential for a subset of Notch pathway-dependent cell fate decisions during Drosophila eye development. Proc. Natl. Acad. Sci. USA 98, 5637–5642 10.1073/pnas.101135498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Deblandre G. A., Kintner C., Rubin G. M. (2001). Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev. Cell 1, 783–794 10.1016/S1534-5807(01)00092-2 [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Schweisguth F. (2003). Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell 5, 139–148 10.1016/S1534-5807(03)00187-4 [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Remaud S., Hamel S., Schweisguth F. (2005). Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 3, e96 10.1371/journal.pbio.0030096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtois M., Schweisguth F. (1995). The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev. 9, 2598–2608 10.1101/gad.9.21.2598 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Lee E. C., Hu X., Yu S. Y., Baker N. E. (1996). The scabrous gene encodes a secreted glycoprotein dimer and regulates proneural development in Drosophila eyes. Mol. Cell. Biol. 16, 1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. C., Yu S. Y., Baker N. E. (2000). The scabrous protein can act as an extracellular antagonist of notch signaling in the Drosophila wing. Curr. Biol. 10, 931–934 10.1016/S0960-9822(00)00622-9 [DOI] [PubMed] [Google Scholar]
- Legent K., Steinhauer J., Richard M., Treisman J. E. (2012). A screen for X-linked mutations affecting Drosophila photoreceptor differentiation identifies Casein kinase 1α as an essential negative regulator of wingless signaling. Genetics 190, 601–616 10.1534/genetics.111.133827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Baker N. E. (2001). Proneural enhancement by Notch overcomes Suppressor-of-Hairless repressor function in the developing Drosophila eye. Curr. Biol. 11, 330–338 10.1016/S0960-9822(01)00093-8 [DOI] [PubMed] [Google Scholar]
- Li Y., Fetchko M., Lai Z. C., Baker N. E. (2003). Scabrous and Gp150 are endosomal proteins that regulate Notch activity. Development 130, 2819–2827 10.1242/dev.00495 [DOI] [PubMed] [Google Scholar]
- Lieber T., Kidd S., Struhl G. (2011). DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron 69, 468–481 10.1016/j.neuron.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. (2002). Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261–269 10.1016/S0092-8674(02)00611-6 [DOI] [PubMed] [Google Scholar]
- Louvi A., Artavanis-Tsakonas S. (2012). Notch and disease: a growing field. Semin. Cell Dev. Biol. 23, 473–480 10.1016/j.semcdb.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno M., Horiuchi J., Tully T., Saitoe M. (2009). The Drosophila cell adhesion molecule klingon is required for long-term memory formation and is regulated by Notch. Proc. Natl. Acad. Sci. USA 106, 310–315 10.1073/pnas.0807665106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. Q., Hiesinger P. R., Beronja S., Zhai R. G., Schulze K. L., Verstreken P., Cao Y., Zhou Y., Tepass U., Crair M. C. et al. (2005). Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 46, 219–232 10.1016/j.neuron.2005.02.029 [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., Rulifson E. J., Blair S. S. (1997). The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124, 1485–1495 [DOI] [PubMed] [Google Scholar]
- Mirkovic I., Mlodzik M. (2006). Cooperative activities of drosophila DE-cadherin and DN-cadherin regulate the cell motility process of ommatidial rotation. Development 133, 3283–3293 10.1242/dev.02468 [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Baker N. E., Rubin G. M. (1990). Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 4, 1848–1861 10.1101/gad.4.11.1848 [DOI] [PubMed] [Google Scholar]
- Neumann C. J., Cohen S. M. (1996). A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development 122, 3477–3485 [DOI] [PubMed] [Google Scholar]
- Newsome T. P., Asling B., Dickson B. J. (2000). Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860 [DOI] [PubMed] [Google Scholar]
- Nolo R., Abbott L. A., Bellen H. J. (2000). Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349–362 10.1016/S0092-8674(00)00040-4 [DOI] [PubMed] [Google Scholar]
- O'Connor-Giles K. M., Skeath J. B. (2003). Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev. Cell 5, 231–243 10.1016/S1534-5807(03)00226-0 [DOI] [PubMed] [Google Scholar]
- Oda H., Uemura T., Harada Y., Iwai Y., Takeichi M. (1994). A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev. Biol. 165, 716–726 10.1006/dbio.1994.1287 [DOI] [PubMed] [Google Scholar]
- Patel N. H., Snow P. M., Goodman C. S. (1987). Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell 48, 975–988 10.1016/0092-8674(87)90706-9 [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E., Pitsouli C., Klueg K. M., Muskavitch M. A., Moschonas N. K., Delidakis C. (2001). neuralized Encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev. Cell 1, 807–816 10.1016/S1534-5807(01)00093-4 [DOI] [PubMed] [Google Scholar]
- Pepple K. L., Atkins M., Venken K., Wellnitz K., Harding M., Frankfort B., Mardon G. (2008). Two-step selection of a single R8 photoreceptor: a bistable loop between senseless and rough locks in R8 fate. Development 135, 4071–4079 10.1242/dev.028951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell P. A., Wesley C., Spencer S., Cagan R. L. (2001). Scabrous complexes with Notch to mediate boundary formation. Nature 409, 626–630 10.1038/35054566 [DOI] [PubMed] [Google Scholar]
- Presente A., Boyles R. S., Serway C. N., de Belle J. S., Andres A. J. (2004). Notch is required for long-term memory in Drosophila. Proc. Natl. Acad. Sci. USA 101, 1764–1768 10.1073/pnas.0308259100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S., Akbar M. A., Ray S., Sevrioukov E. A., Haberman A. S., Rohrer J., Krämer H. (2005). Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118, 3663–3673 10.1242/jcs.02502 [DOI] [PubMed] [Google Scholar]
- Rajan A., Tien A. C., Haueter C. M., Schulze K. L., Bellen H. J. (2009). The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat. Cell Biol. 11, 815–824 10.1038/ncb1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan N., Flockhart I., Booker M., Perrimon N., Mathey-Prevot B. (2007). Design and implementation of high-throughput RNAi screens in cultured Drosophila cells. Nat. Protoc. 2, 2245–2264 10.1038/nprot.2007.250 [DOI] [PubMed] [Google Scholar]
- Rawlins E. L., White N. M., Jarman A. P. (2003). Echinoid limits R8 photoreceptor specification by inhibiting inappropriate EGF receptor signalling within R8 equivalence groups. Development 130, 3715–3724 10.1242/dev.00602 [DOI] [PubMed] [Google Scholar]
- Renaud O., Simpson P. (2001). scabrous modifies epithelial cell adhesion and extends the range of lateral signalling during development of the spaced bristle pattern in Drosophila. Dev. Biol. 240, 361–376 10.1006/dbio.2001.0482 [DOI] [PubMed] [Google Scholar]
- Riedl J., Crevenna A. H., Kessenbrock K., Yu J. H., Neukirchen D., Bista M., Bradke F., Jenne D., Holak T. A., Werb Z. et al. (2008). Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 10.1038/nmeth.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roignant J. Y., Treisman J. E. (2009). Pattern formation in the Drosophila eye disc. Int. J. Dev. Biol. 53, 795–804 10.1387/ijdb.072483jr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A., Tokunaga F., Kawamura S., Ozaki K. (1997). In situ inhibition of vesicle transport and protein processing in the dominant negative Rab1 mutant of Drosophila. J. Cell Sci. 110, 2943–2953 [DOI] [PubMed] [Google Scholar]
- Seugnet L., Suzuki Y., Merlin G., Gottschalk L., Duntley S. P., Shaw P. J. (2011). Notch signaling modulates sleep homeostasis and learning after sleep deprivation in Drosophila. Curr. Biol. 21, 835–840 10.1016/j.cub.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Chao M. Y., Somers G. A., Komatsu H., Corkins M. E., Larkins-Ford J., Tucey T., Dionne H. M., Walsh M. B., Beaumont E. K. et al. (2011). C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr. Biol. 21, 825–834 10.1016/j.cub.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath J. B., Doe C. Q. (1998). Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development 125, 1857–1865 [DOI] [PubMed] [Google Scholar]
- Spencer S. A., Cagan R. L. (2003). Echinoid is essential for regulation of Egfr signaling and R8 formation during Drosophila eye development. Development 130, 3725–3733 10.1242/dev.00605 [DOI] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2005). Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development 132, 4299–4308 10.1242/dev.02015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2007). Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell 12, 431–442 10.1016/j.devcel.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M. V. (2005). The love-hate relationship between Ras and Notch. Genes Dev. 19, 1825–1839 10.1101/gad.1330605 [DOI] [PubMed] [Google Scholar]
- Sweeney S. T., Davis G. W. (2002). Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 36, 403–416 10.1016/S0896-6273(02)01014-0 [DOI] [PubMed] [Google Scholar]
- Tsachaki M., Sprecher S. G. (2012). Genetic and developmental mechanisms underlying the formation of the Drosophila compound eye. Dev. Dyn. 241, 40–56 10.1002/dvdy.22738 [DOI] [PubMed] [Google Scholar]
- Tsuda H., Han S. M., Yang Y., Tong C., Lin Y. Q., Mohan K., Haueter C., Zoghbi A., Harati Y., Kwan J. et al. (2008). The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell 133, 963–977 10.1016/j.cell.2008.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chan S. L., Miele L., Yao P. J., Mackes J., Ingram D. K., Mattson M. P., Furukawa K. (2004). Involvement of Notch signaling in hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. USA 101, 9458–9462 10.1073/pnas.0308126101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler S. L., Bilder D. (2010). Endocytic internalization routes required for delta/notch signaling. Curr. Biol. 20, 538–543 10.1016/j.cub.2010.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Mehta S. Q., Pichaud F., Bellen H. J., Quiocho F. A. (2005). Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol. 12, 879–885 10.1038/nsmb987 [DOI] [PubMed] [Google Scholar]
- Xu T., Rubin G. M. (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Charng W. L., Bellen H. J. (2010). Endocytosis and intracellular trafficking of Notch and its ligands. Curr. Top. Dev. Biol. 92, 165–200 10.1016/S0070-2153(10)92005-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip M. L., Lamka M. L., Lipshitz H. D. (1997). Control of germ-band retraction in Drosophila by the zinc-finger protein HINDSIGHT. Development 124, 2129–2141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.