Abstract
Memory impairment is a common feature of conditions that involve changes in inflammatory signaling in the brain, including traumatic brain injury, infection, neurodegenerative disorders, and normal aging. However, the causal importance of inflammatory mediators in cognitive impairments in these conditions remains unclear. Here we show that specific immune proteins, members of the major histocompatibility complex class I (MHC class I), are essential for normal hippocampus-dependent memory, and are specifically required for NMDAR-dependent forms of long-term depression (LTD) in the healthy adult hippocampus. In β2m−/−TAP−/−mice, which lack stable cell-surface expression of most MHC class I proteins, NMDAR-dependent LTD in area CA1 of adult hippocampus is abolished, while NMDAR-independent forms of potentiation, facilitation, and depression are unaffected. Altered NMDAR-dependent synaptic plasticity in the hippocampus of β2m−/−TAP−/−mice is accompanied by pervasive deficits in hippocampus-dependent memory, including contextual fear memory, object recognition memory, and social recognition memory. Thus normal MHC class I expression is essential for NMDAR-dependent hippocampal synaptic depression and hippocampus-dependent memory. These results suggest that changes in MHC class I expression could be an unexpected cause of disrupted synaptic plasticity and cognitive deficits in the aging, damaged, and diseased brain.
Changes in inflammatory signaling in the central nervous system (CNS) are frequently associated with cognitive impairments. Memory, in particular, is disrupted in states that trigger brain inflammation, including traumatic brain injury, infections, neurodegenerative disorders like Alzheimer’s disease (AD), and normal aging. Both chronic and acute inflammation have been associated with significant memory impairments in humans (e.g., Hilsabeck et al. 2002; Schmidt et al. 2006; Patanella et al. 2010). Neuronal damage and death are an immediate source of cognitive deficits in the wake of brain injury, infection, and neurodegeneration. However, increases or decreases in inflammatory cytokines alone are sufficient to impair learning and/or memory in animal models, even in the absence of other insults (e.g., Oitzl et al. 1993; Gibertini 1996; Banks et al. 2001; Derecki et al. 2010), suggesting that inflammatory signaling may contribute to a second wave of cognitive deficits in these and other conditions. Immune signaling may be involved in some cases of autism and schizophrenia (Adams et al. 1993; Torres et al. 2001; Brown 2006; Patterson 2009; Purcell et al. 2009; Shi et al. 2009; Stefansson et al. 2009), and patients with these disorders often show explicit memory dysfunction (Ben Shalom 2003; Barch and Ceaser 2012). The molecular mechanisms that might link inflammation to changes in learning and memory are a topic of ongoing research (for review, see, e.g., Yirmiya and Goshen 2011). Given accumulating evidence that inflammatory mediators play essential roles in development, homeostasis, and plasticity in the normal, healthy brain (for recent reviews, see, e.g., Merrill and Jonakait 1995; Ziv and Schwartz 2008; Boulanger 2009; McAllister and van de Water 2009; Shatz 2009; Kipnis et al. 2012; Stephan et al. 2012), it is possible that disruption of these newly discovered normal functions of specific immune effectors contributes to neuropathology in brain disorders.
The cellular substrates of learning and memory formation are thought to be activity-dependent changes in the strength of synaptic transmission, or synaptic plasticity (for reviews, see Bliss and Collingridge 1993; Bear 1996; Milner et al. 1998; Malenka and Nicoll 1999; Martin et al. 2000; Neves et al. 2008). In particular, long-term potentiation (LTP) and long-term depression (LTD) at synapses made onto CA1 pyramidal neurons in the adult hippocampus have been implicated in memory formation and retrieval (Coan et al. 1987; Bliss and Collingridge 1993). N-Methyl d-aspartate-type glutamate receptors (NMDARs) play an important role in hippocampal LTP and LTD, and are essential for learning and memory (Morris et al. 1986; Bliss and Collingridge 1993; Bear 1996; Milner et al. 1998; Martin et al. 2000; Neves et al. 2008). Because of the vital role of NMDARs in some forms of memory, regulation of NMDAR function is a potential strategy for preventing or reversing cognitive decline in the aging population (Collingridge et al. 2013).
Recent studies identified surprising endogenous regulators of NMDAR function: specific immune proteins, members of the major histocompatibility complex class I (MHC class I) (Fourgeaud et al. 2010). Although MHC class I is best-known for its role in adaptive immunity, MHC class I also has important roles in the normal, healthy developing and adult brain (e.g., Huh et al. 2000; Loconto et al. 2003; Leinders-Zufall et al. 2004; Goddard et al. 2007; Datwani et al. 2009; Fourgeaud et al. 2010; Glynn et al. 2011; Washburn et al. 2011; Bilousova et al. 2012; Chacon and Boulanger 2013; for reviews, see Boulanger et al. 2001; Boulanger and Shatz 2004; Boulanger 2009; McAllister and van de Water 2009; Shatz 2009; Fourgeaud and Boulanger 2010; Elmer and McAllister 2012). MHC class I mRNA is highly expressed in sites of ongoing adult plasticity, including adult hippocampus (Corriveau et al. 1998; Huh et al. 2000), where it is present in dendrites of CA1 pyramidal neurons (Zhong et al. 2006). MHC class I levels are regulated by synaptic activity (Corriveau et al. 1998; Huh et al. 2000) and by the plasticity-associated transcription factors CREB (Barco et al. 2005), MECP2 (Miralves et al. 2007), and Npas4 (Lin et al. 2008). MHC class I protein is enriched in synaptic fractions (Huh et al. 2000) and in hippocampal neurons in vitro, and MHC class I colocalizes with the postsynaptic density protein PSD-95 (Goddard et al. 2007). Thus MHC class I is expressed in the right times and places to regulate synaptic transmission and plasticity in the hippocampus.
Indeed, NMDAR-mediated synaptic transmission in the hippocampus is dramatically enhanced in MHC class I-deficient transgenic mice (Fourgeaud et al. 2010). Since the MHC class I is a large gene family, and multiple MHC class I genes are expressed in neurons (Huh et al. 2000; Loconto et al. 2003), these and other previous studies have taken advantage of transgenic mouse models in which cell-surface expression of most MHC class I proteins is reduced or abolished (e.g., Huh et al. 2000; Loconto et al. 2003; Goddard et al. 2007; McConnell et al. 2009; Glynn et al. 2011). These transgenics lack expression of β2 microglobulin (β2m), the light chain that is required for stable cell-surface expression of most MHC class I proteins (Zijlstra et al. 1989) and, in some cases, the transporter associated with antigen processing 1 (TAP1), which transports peptides across the endoplasmic reticulum membrane, and is required for peptide-presenting MHC class I proteins to be stably expressed at the cell surface (Van Kaer et al. 1992). Double-transgenic β2m−/−TAP−/− mice have impairments in the assembly and transport of MHC class I and a near-complete absence of MHC class I on the cell surface (Ljunggren et al. 1995; Dorfman et al. 1997). NMDAR-mediated synaptic transmission is dramatically enhanced in CA1 pyramidal neurons of adult hippocampus from MHC class I-deficient β2m−/−TAP−/− mice (Fourgeaud et al. 2010). Changes in synaptic plasticity are also observed at these synapses in MHC class I-deficient mice: Low-frequency stimulation-induced LTD (LFS-LTD) is abolished, while high-frequency stimulation-induced LTP (HFS-LTP) is enhanced (Huh et al. 2000). HFS-LTP and LFS-LTD both depend on NMDAR activation (Morris et al. 1986; Bliss and Collingridge 1993; Bear 1996), suggesting that the changes in HFS-LTP and LFS-LTD in MHC class I-deficient animals might result from their enhanced NMDAR-mediated currents (Fourgeaud et al. 2010). Alternatively, loss of cell surface MHC class I might disrupt plasticity more broadly. It remains unknown if cell surface MHC class I selectively regulates NMDAR-dependent, but not NMDAR-independent, forms of synaptic plasticity in the hippocampus, or if changes in MHC class I expression can affect NMDAR- and hippocampus-dependent learning or memory. In the present study, we tested these hypotheses using a combination of electrophysiological and behavioral assessments in wild type (WT) and MHC class I-deficient mice. Our results suggest a model in which MHC class I normally regulates the sign and magnitude of hippocampal synaptic plasticity through its effects on NMDAR function, and provide the first evidence that changes in MHC class I expression are sufficient to cause significant impairments in hippocampus-dependent memory.
Results
NMDA-LTD is converted to LTP in β2m−/−TAP−/− animals
NMDAR-mediated synaptic currents are enhanced in adult hippocampus if cell surface MHC class I expression is reduced, suggesting that MHC class I normally limits NMDAR function (Fourgeaud et al. 2010). In current models of synaptic plasticity, the magnitude of NMDAR-mediated responses is thought to encode the polarity of synaptic plasticity, such that responses above a given threshold induce long-term potentiation (LTP) and below it induce long-term depression (LTD) (Bear and Malenka 1994; but see Nabavi et al. 2013). In these models, increasing NMDAR-mediated responses should shift the input–output curve of NMDAR-dependent plasticity in favor of potentiation. Consistent with this model, NMDAR-dependent LFS-LTD is abolished at adult Schaffer collateral/CA3–CA1 synapses from β2m−/−TAP−/− animals, and HFS-LTP is enhanced (Huh et al. 2000). If changes in synaptic plasticity in MHC class I-deficient mice are due to an increase in NMDAR-mediated currents, a second prediction is that MHC class I should selectively affect NMDAR-dependent, but not NMDAR-independent, forms of synaptic plasticity. To test this prediction, we first examined LTD induced by brief bath application of NMDA (NMDA-LTD) in β2m−/−TAP−/− animals. NMDA-LTD, like LFS-LTD, depends on activation of NMDARs (Lee et al. 1998; Li et al. 2004). We reasoned that if MHC class I affects LFS-LTD through enhancement of NMDAR-mediated currents, then NMDA-LTD, like LFS-LTD, should be abolished or converted to potentiation in β2m−/−TAP−/− hippocampus.
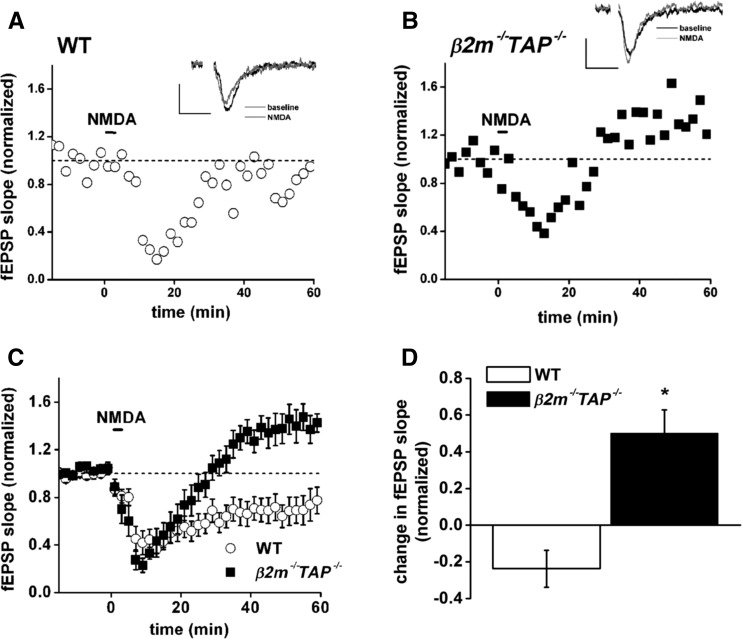
In WT hippocampal slices, bath application of NMDA causes transient desensitization, followed by long-lasting stable synaptic depression (LTD), as expected (Fig. 1A). In β2m−/−TAP−/−slices, however, NMDA-induced desensitization is, instead, followed by striking potentiation (Fig. 1B) that is stable for at least 1 h. Pooled data (Fig. 1C,D) show that while NMDA induces LTD in WT animals (to 76.2 ± 10% of baseline), it consistently induces significant potentiation in β2m−/−TAP−/− animals (to 150.2 ± 12.6% of baseline). This aberrant NMDA-induced potentiation in MHC class I-deficient mice is comparable in magnitude to tetanic stimulation-induced LTP in WT mice (Huh et al. 2000). Thus in hippocampal slices from animals with reduced cell surface MHC class I, both LFS-LTD and NMDA-LTD are abolished and converted to potentiation, suggesting a model in which MHC class I regulates the coupling between stimulation of NMDARs and appropriate potentiation or depression of synapses. Specifically, these results suggest that MHC class I is required for NMDAR-dependent LTD and limits NMDAR-dependent LTP.
Figure 1.
NMDA-LTD is converted to potentiation in β2m−/−TAP−/− hippocampal slices. (A,B) Responses to NMDA in WT (open circles [A]) or β2m−/−TAP−/− (filled squares [B]) slices. (Insets) Superimposed sample field excitatory postsynaptic potentials (fEPSPs; average of four) recorded 15 min before (black line) or 60 min after (gray line) application of NMDA. Note depression in WT, but potentiation in β2m−/−TAP−/−, in response to NMDA. Scale bar, 0.2 mV/20 msec. (C) Average response to NMDA in WT (open circles, n = 13 slices from 10 animals) or β2m−/−TAP−/− (filled squares, n = 7 slices from seven animals). (D) Plasticity in response to NMDA in WT and β2m−/−TAP−/− slices over the period 45–60 min after application of NMDA for the recordings shown in C. There is a significant difference between WT and β2m−/−TAP−/− synaptic plasticity in response to NMDA treatment (two-tailed Student’s t-test, [*]P < 0.005).
LFS induces complete depotentiation in β2m−/−TAP−/−animals
Hippocampal LFS-LTD is abolished in MHC class I-deficient mice (Huh et al. 2000). To determine whether MHC class I is required to translate LFS into appropriate plasticity, we examined depotentiation. LFS can induce either LTD or depotentiation, depending on the activity history of the synapse. In naive WT slices, LFS causes long-lasting depression of synaptic transmission below baseline (LFS-LTD). If synapses have recently undergone tetanic stimulation, however, LFS returns synaptic strength to a pretetanus baseline (termed depotentiation) through a mechanism that is distinct from LFS-LTD (Dudek and Bear 1992, 1993; Zhuo et al. 1999; Huang et al. 2001). Although depotentiation induced after some intervals post-tetanus is prevented by NMDAR blockers (Fujii et al. 1991; Massey et al. 2004; Zhang et al. 2009), depotentiation induced 30 min after tetanic stimulation is thought to be largely NMDAR-independent (Bashir and Collingridge 1994; Fitzjohn et al. 1998; Peineau et al. 2007; but see O’Dell and Kandel 1994). If MHC class I is required to translate LFS into appropriate changes in synaptic strength, or is broadly required for all forms of synaptic weakening at these synapses, both LFS-LTD and LFS-depotentiation should be impaired in β2m−/−TAP−/− hippocampus. Alternately, if MHC class I is specifically required for NMDAR-dependent synaptic depression, depotentiation induced 30 min post-tetanus should be intact.
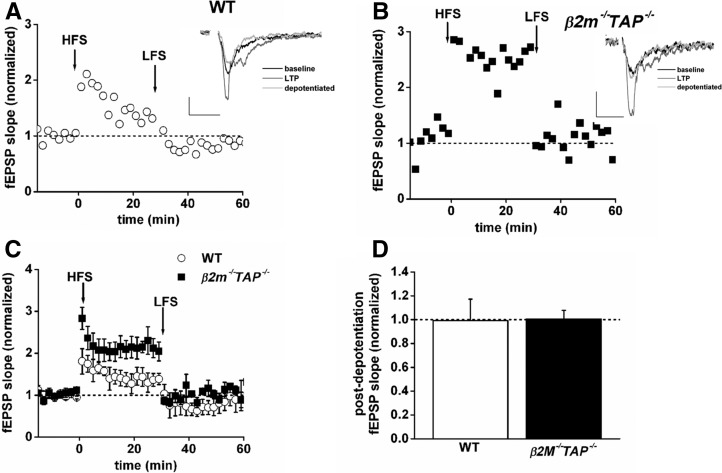
In WT animals, LFS applied 30 min after tetanic stimulation induced depotentiation that restored synaptic transmission to prepotentiated baseline values (Fig. 2A). In β2m−/−TAP−/− animals, tetanic stimulation induced LTP that was nearly twice the magnitude of LTP in WT animals, consistent with previous results (Huh et al. 2000). Strikingly, LFS fully restored synaptic transmission to baseline in β2m−/−TAP−/− slices (WT, n = 5, 99 ± 18% of baseline; β2m−/−TAP−/−, n = 5, 100 ± 7.3% of baseline) (Fig. 2A–D). Thus β2m−/−TAP−/− synapses can respond appropriately to LFS, depending on the activation history of the synapse, and are competent to undergo some forms of synaptic weakening. These results suggest that conversion of LFS-LTD to potentiation in β2m−/−TAP−/− animals is not due to general disruption of plasticity, but rather reflects a specific requirement for MHC class I in NMDAR-dependent synaptic depression.
Figure 2.
Depotentiation is complete in β2m−/−TAP−/− hippocampal slices. (A,B) fEPSP slopes, normalized to baseline, from an individual wild type (open circles [A]) or β2m−/−TAP−/− (filled squares [B]) slices exposed to HFS at time 0, followed by LFS 30 min later. Potentiation is roughly twice as large in the β2m−/−TAP−/− slice, as previously reported (Huh et al. 2000), and potentiation is fully reversed by LFS in both genotypes. (Insets) Superimposed sample fEPSPs (average of four) recorded 15 min before HFS (black line), 20 min after HFS (dark gray line), or 30 min after LFS (light gray line). Scale bar, 0.2 mV/20 msec. (C) Average depotentiation in wild type (open circles, n = 5 slices from five animals) or β2m−/−TAP−/− (filled squares, n = 5 slices from five animals). (D) Average depotentiation from recordings in C, measured 10–30 min after LFS. There is no significant difference between the extent of wild type and β2m−/−TAP−/− depotentiation.
mGluR-dependent LTD is normal in β2m−/−TAP−/− animals
The above experiments demonstrate that β2m−/−TAP−/− synapses are capable of depotentiation back to baseline, but it is possible that loss of MHC class I renders synapses incapable of synaptic depression below baseline, for example, due to structural or functional constraints on the malleability of synaptic connections. To test this directly, we attempted to induce a form of synaptic depression that differs in its expression mechanisms from both LFS-LTD and NMDA-LTD. Bath application of the group I metabotropic glutamate receptor (mGluR) agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG) produces stable, saturable, dose-dependent, protein-synthesis-dependent LTD at adult CA3–CA1 synapses (Oliet et al. 1997; Palmer et al. 1997; Nicoll et al. 1998; Fitzjohn et al. 1999; Huber et al. 2001). Importantly, DHPG-LTD is NMDAR-independent (Palmer et al. 1997; Fitzjohn et al. 2001; Huber et al. 2001; Watabe et al. 2002; Ireland and Abraham 2009).
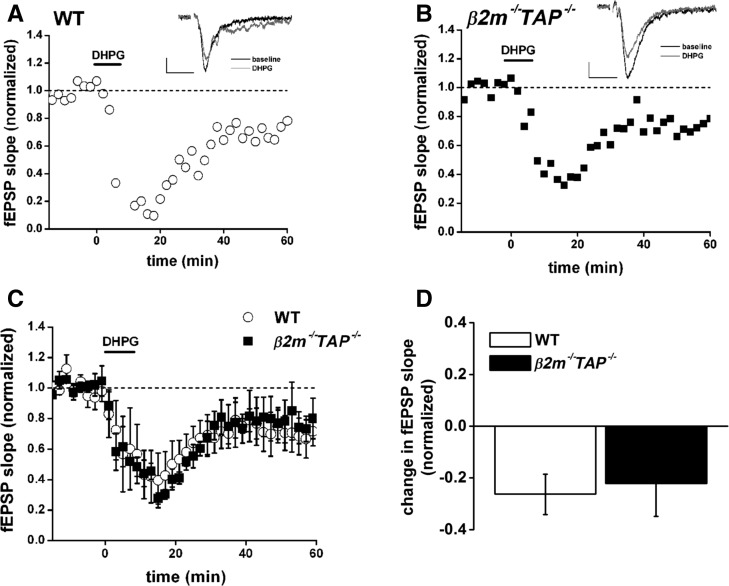
As previously reported, DHPG produces stable LTD in WT slices (Fig. 3A). β2m−/−TAP−/− slices also exhibit robust depression in response to DHPG (Fig. 3B). Pooled data demonstrate that the synaptic depression in response to DHPG in β2m−/−TAP−/− animals is indistinguishable from WT (WT, 73.7 ± 7.8% of baseline, versus β2m−/−TAP−/− 77.8 ± 12.6% of baseline, P > 0.05, Student’s t-test) (Fig. 3C,D). These results reveal that when cell surface MHC class I expression is inhibited, hippocampal synapses are still competent to undergo some forms of synaptic depression. This suggests that the failure to induce LTD in response to either LFS or NMDA in these animals cannot be explained by a structural inability to weaken synapses, but rather reflects selective disruption of NMDAR-dependent but not NMDAR-independent forms of synaptic weakening.
Figure 3.
Normal synaptic depression induced by the mGluR agonist DHPG in β2m−/−TAP−/− mice. (A,B) Field EPSP (fEPSP) slopes, normalized to baseline, from individual wild type (open circles [A]) or β2m−/−TAP−/− (filled squares [B]) slices exposed to DHPG in the bath (100 μM for 10 min) starting at time 0. (Insets) Superimposed sample fEPSPs (average of four) recorded 15 min before (black line) or 60 min after (gray line) application of DHPG. Scale bar, 0.2 mV/20 msec. (C) Averaged responses for WT (open circles, n = 8 slices from seven animals) or β2m−/−TAP−/− (filled squares, n = 5 slices from five animals) 15 min before to 60 min after application of DHPG. All points are averages of four consecutive fEPSPs (means ± SEM, normalized to baseline) recorded from CA1 fields. (D) Average change in fEPSPs in response to DHPG, measured 30–60 min after treatment, recorded from the WT and β2m−/−TAP−/− slices in C. DHPG-induced LTD is not significantly different in WT versus β2m−/−TAP−/− slices.
Presynaptic, short-term forms of plasticity are normal in β2m−/−TAP−/− animals
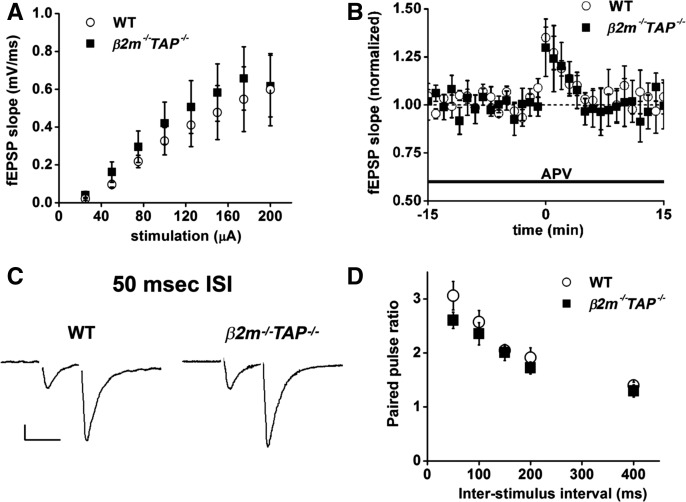
Basal hippocampal fEPSPs are of normal amplitude in β2m−/− TAP−/− animals (Huh et al. 2000; Fourgeaud et al. 2010), and loss of MHC class I does not affect the magnitude of whole-cell currents mediated by α-amino-3-hydroxy-5-methyl-4-isoxale propionic acid receptors (AMPARs) (Fourgeaud et al. 2010). However, synaptic vesicle density is slightly higher than WT in hippocampal slices from β2m−/−TAP−/− animals, and miniature excitatory postsynaptic current (mEPSC) frequency is higher in hippocampal cultures from these animals (Goddard et al. 2007), raising the possibility that presynaptic glutamate release may be altered. To test if loss of MHC class I might affect glutamate release, we stimulated Schaffer collaterals and recorded field excitatory postsynaptic potentials (fEPSPs) from groups of postsynaptic CA1 pyramidal neurons. The input/output relationship of the fEPSP is comparable between genotypes (Fig. 4A), indicating that at resting membrane potentials, when currents are largely AMPAR-mediated, responses of β2m−/−TAP−/− synapses are indistinguishable from WT across a range of stimulation intensities. This is consistent with evidence that postsynaptic responses are normal in MHC class I-deficient neurons if mediated by AMPARs (Huh et al. 2000; Goddard et al. 2007; Fourgeaud et al. 2010), but increased if mediated by NMDARs (Fourgeaud et al. 2010). Together, these results suggest that the magnitude of glutamate release from presynaptic terminals is normal in β2m−/−TAP−/− animals.
Figure 4.
Input–output ratio and short-term presynaptic plasticity are normal in β2m−/−TAP−/− hippocampal slices. (A) Basal input/output curve for WT (open circles, n = 8 slices from eight animals) or β2m−/−TAP−/− (filled squares, n = 12 slices from 10 animals). There was no significant difference in the fEPSP slope evoked at any level of stimulation tested. (B) PTP recorded from WT (open circles, n = 3 slices from three animals) or β2m−/−TAP−/− (filled squares, n = 6 slices from five animals) hippocampal slices. The amplitude and kinetics of PTP are indistinguishable from WT in β2m−/−TAP−/− slices. (C) Sample EPSCs (50-msec ISI) showing PPF recorded from CA1 pyramidal neurons voltage clamped at −70 mV. Scale 50 pA/50 ms. (D) Mean paired-pulse ratio (PPR) of EPSCs recorded from WT (open circles, n = 10 slices from nine animals) or β2m−/−TAP−/− (filled squares, n = 9 slices from nine animals) neurons. PPR does not differ significantly at any of the ISIs tested.
To specifically probe the presynaptic probability of release (Pr) of glutamate, we examined two forms of plasticity that are sensitive to changes in Pr: post-tetanic potentiation (PTP) and paired-pulse facilitation (PPF). Tetanic stimulation at hippocampal Schaffer collateral–CA1 synapses induces PTP, a form of NMDAR-independent, short-term potentiation that precedes LTP, and is thought to result from a transient increase in Pr due to acute accumulation of calcium in the presynaptic terminal (Zucker 1989). PTP was isolated from LTP by applying tetanic stimulation to the Schaffer collaterals in the presence of the N-methyl D-aspartate receptor (NMDAR) blocker (2R)-amino-5-phosphonovaleric acid (APV). In WT slices, HFS induced robust PTP which decayed back to baseline within ∼5 min. Both the amplitude and kinetics of PTP were indistinguishable from WT in β2m−/− TAP−/− slices (Fig. 4B), supporting the idea that MHC class I does not alter Pr.
The effects of MHC class I on Pr were also independently evaluated by measuring PPF. Stimulation of presynaptic axons with two pulses separated by a brief interval causes paired-pulse plasticity at many synapses, which is evident as a change in the amplitude of the second response relative to the first. Paired pulse ratios are inversely proportional to Pr, such that low-Pr synapses (including Shaffer collateral–CA3 synapses) normally show PPF. These synapses show enhanced facilitation when Pr is decreased, and become less facilitating, or even depressing, under conditions that increase Pr (Dobrunz and Stevens 1997; Zucker and Regehr 2002). PPF was induced in whole cell configuration by stimulating the Schaffer collaterals with paired stimuli spaced 10–200 msec apart. In WT slices, stimulation with two pulses spaced 50 msec apart caused significant PPF, and there was no difference in the magnitude of PPF induced in wild type or β2m−/−TAP−/− slices (Fig. 4C). PPF also showed the same dependence on inter-stimulus interval (ISI) in wild type and mutant mice (Fig. 4D). Thus three independent measures (fEPSP slope, PTP, and PPF) support the idea that changes in presynaptic glutamate release are unlikely to contribute to altered hippocampal synaptic plasticity in response to decreased MHC class I expression on the cell surface.
NMDAR-dependent LTD is normal in somatosensory cortex of β2m−/−TAP−/− animals
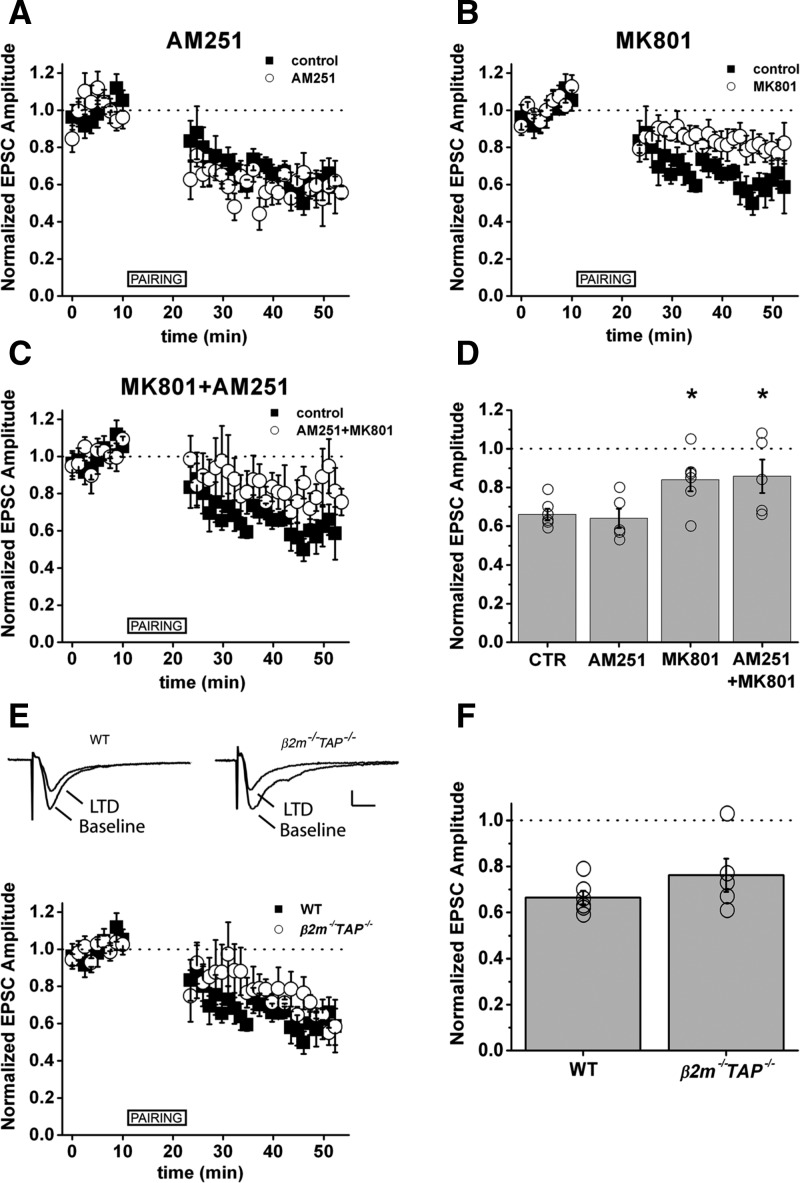
The above results indicate that MHC class I is an important regulator of NMDAR-dependent synaptic plasticity in the adult hippocampus. MHC class I genes are widely expressed in the adult mammalian brain (Corriveau et al. 1998; Lidman et al. 1999; Linda et al. 1999; Huh et al. 2000; Needleman et al. 2010), raising the possibility that loss of MHC class I may impair NMDAR-dependent plasticity in other circuits. To determine if MHC class I regulates NMDAR-dependent plasticity outside hippocampus, we examined NMDAR-dependent LTD at synapses between layer 4 and layer 2/3 (L4-2/3) in slices from adult mouse somatosensory cortex (S1). LTD at these synapses may be involved in development of somatosensory maps in rat barrel cortex (Allen et al. 2003). MHC class I mRNAs encoding the MHC class I proteins H2-D, T22, and Qa-1 are strongly expressed in area S1 of mouse (Huh et al. 2000). In rats, pairing presynaptic stimulation with postsynaptic depolarization at L4-2/3 synapses in S1 causes depression of synaptic transmission (pairing LTD; Feldman 2000). Similarly, in mice, pairing produced a persistent decrease in EPSC amplitude (66 ± 5% of baseline, n = 6) (Fig. 5A–D). In rats, some forms of LTD at these synapses are mediated by presynaptic NMDARs and CB1 cannabinoid receptors (CB1Rs), while others are mediated by postsynaptic NMDARs (Bender et al. 2006). To determine whether postsynaptic NMDARs are required for pairing-induced LTD at L4-L2/3 synapses in mice, we performed pairing in the presence of the CB1R antagonist AM251, or included the NMDAR antagonist MK801 in the recording pipette (iMK801), to selectively block postsynaptic (but not presynaptic) NMDARs. AM251 had no effect on pairing-induced LTD (64 ± 5% of baseline, n = 5, P = 0.67) (Fig. 5A,D), but iMK801 significantly attenuated pairing-induced LTD (84 ± 6% of baseline, n = 6, P = 0.04) (Fig. 5B,D). Thus the majority of pairing-induced LTD at L4-2/3 synapses in WT mouse S1 is mediated by postsynaptic NMDARs (Fig. 5A–D). Although it is strongly NMDAR-dependent, pairing-induced LTD in S1 was intact in β2m−/−TAP−/− mice (LTD/baseline = 0.76 ± 0.07, n = 5, P = 0.23) (Fig. 5E,F). Consistent with this, whisker barrel anatomy in S1 is grossly normal in β2m−/−TAP−/− mice when visualized by cytochrome oxidase staining (not shown). These results suggest that cell-surface expression of MHC class I is required for NMDAR-dependent LTD in hippocampus, but not in S1 of cortex, revealing that the requirement for MHC class I in NMDAR-dependent plasticity is spatially restricted.
Figure 5.
NMDAR-dependent LTD is intact in barrel cortex (S1) of β2m−/−TAP−/− mice. (A) The CB1R antagonist AM251 has no effect on pairing-induced LTD in mouse S1. (B) Pairing LTD is attenuated when postsynaptic NMDARs are blocked by inclusion of MK801 in the recording pipette. (C) AM251 and MK801 together do not attenuate LTD to a greater extent than MK801 alone. (D) Normalized EPSC amplitude averaged over 25 consecutive sweeps at least 15 min after LTD induction. (Circles) Individual cells, (bars) average of all cells. Error bars, SEM. (A–D) Control, n = 6 slices from six animals; AM251, n = 5 slices from five animals; MK801, n = 6 slices from six animals; AM251 + MK801, n = 5 slices from five animals. (E) Top traces, average of 25 consecutive EPSCs recorded in voltage clamp before (baseline) and after (LTD) pairing. Scale bar, 20 pA, 10 ms. (F) Normalized EPSC amplitude averaged over 25 consecutive sweeps at least 15 min after LTD induction. (Circles) Individual cells, (bars) average of all cells. Error bars, SEM. Same control data shown in panels A–C and E, collected from randomly interspersed trials. (E,F) WT n = 6 slices from six animals; β2m−/−TAP−/− n = 5 slices from five animals. (*) P < 0.05, Student's t-test.
Hippocampal learning deficits in β2m−/−TAP−/− animals
Prevailing models suggest that NMDAR-dependent LTP and LTD at CA3–CA1 synapses may underlie many forms of hippocampus-dependent learning and memory (Anagnostaras et al. 2001). Since our results indicate that MHC class I is required for normal NMDAR-dependent LTP and LTD in the adult hippocampus, we tested the hypothesis that hippocampus- (Anagnostaras et al. 2003) and NMDAR-dependent learning and memory is disrupted in β2m−/−TAP−/− animals. Pavlovian fear conditioning is an established model of hippocampus- and amygdala-dependent memory. Mice are given tone–shock pairings and fear of the tone (cued) or environmental context (contextual) is assessed by measuring freezing behavior at a later time (Anagnostaras et al. 2000, 2001). Contextual fear conditioning depends on NMDARs in the hippocampus, whereas acquisition of cued fear conditioning is hippocampus-independent; both contextual and cued fear depend on NMDARs in the basolateral/lateral complex of the amygdala (Maren 1999; Anagnostaras et al. 2001). Thus if MHC class I regulates NMDAR function in hippocampus, but not amygdyla, β2m−/−TAP−/− animals should show impairments in contextual, but not cued, fear conditioning.
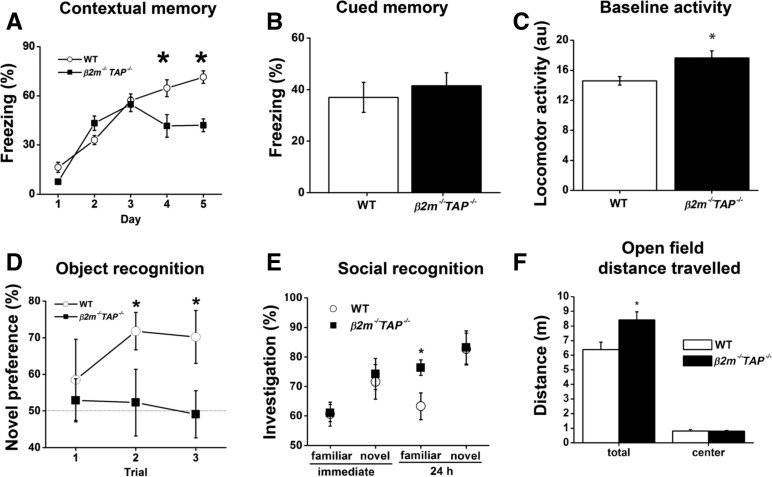
Figure 6A depicts the acquisition of contextual fear conditioning when mice were given one tone–shock pairing per day. Freezing is depicted for the baseline period prior to the tone each day. β2m−/−TAP−/− mice acquired some fear conditioning and did not differ from wild types on day 2 or 3 (F(1,18) values < 1, P values > 0.5), but failed to exhibit high levels of learning and exhibited significant deficits on days 4 and 5 (F(1,18) values > 11.6, P values < 0.01). Cued fear conditioning was also examined during the 30-sec period when the tone was on for each day. Because of considerable variability during the short tone presentations, baseline tone-elicited freezing during day 1 (prior to the first shock) was subtracted from tone-elicited freezing on days 2–5 (after training) and averaged across days 2–5 (Fig. 6B). There was no significant difference between groups in tone-elicited freezing (F(1,18) < 0.3, P > 0.5). Normal cued fear conditioning in β2m−/−TAP−/− animals suggests that their lack of context-dependent fear conditioning is not due to a problem in the amygdala or in the production of freezing. As a control, we examined locomotor activity during the 4-min baseline period prior to the tone–shock pairing on day 1 (Fig. 6C). Mutant mice exhibited a small but significant hyperactivity during the baseline period (F(1,18) = 8.2, P = 0.01). However, this was probably not the cause of their contextual fear deficit, because mutant mice froze normally to the tone, and there was no significant relationship between the level of baseline hyperactivity and the freezing deficit on any day (Fisher’s r-to-z, r values < 0.4, P values > 0.05; Maren et al. 1998). In order to more fully explore activity levels, mice were placed into an automated photocell based 25 cm × 25 cm open field chamber for 1 h in a room lit only by dim red light. Total distance traveled and distance traveled in the center of the field are depicted (Fig. 6F). As during the baseline period in the fear conditioning chambers, β2m−/−TAP−/− mice exhibited a small but significant hyperactivity (F(1,16) = 6.0, P < 0.05). This was not present in the center of the open field (F(1,16) < 0.1, P > 0.5) (Fig. 6F), suggesting these mice did not exhibit elevated anxiety. Combined with the evidence from Fig. 6C, mutant mice exhibit a significant but modest hyperactivity that is unlikely to interfere with other behavioral tasks (see, e.g., Maren et al. 1998). These results together suggest that normal levels of MHC class I on the cell surface are essential for context-dependent fear conditioning.
Figure 6.
Deficits in hippocampus-dependent but not hippocampus-independent learning in β2m−/−TAP–/- mice. (A) Context-dependent fear conditioning. Mean freezing is depicted for the baseline period prior to the tone–shock pairing each day. After a normal initial 3-d acquisition period, β2m−/−TAP−/− animals show a significant deficit in their ability to improve with further training. WT n = 8; β2m−/−TAP−/−, n = 6. (B) Cued fear conditioning measured over 5 d. Depicted are freezing during the 30-sec tone pretraining (day 1), subtracted from the average tone-elicited freezing post-training (days 2–5). (C) Locomotor activity measured during the 4-min period prior to the tone–shock pairing on day 1. (au) Arbitrary units. (A–C) WT n = 17; β2m−/−TAP−/−, n = 6. (D) Object recognition. Novel object percent preference (novel time/[novel + familiar time]) is depicted for each trial. By trial 2, WT mice exhibited significant preference, indicating memory, while β2m−/−TAP−/− mutants failed to acquire any preference, even with additional training. WT n = 9; β2m−/−TAP−/−, n = 9. (E) Social recognition. WT and β2m−/−TAP−/− mutants exhibited normal immediate (short-term) memory, evident as decreased investigation for the familiar mouse. While WT mice exhibited good memory, β2m−/−TAP−/− mutants exhibited deficient memory, evident as elevated exploration of the familiar female. WT n = 15; β2m−/−TAP−/−, n = 13. (F) Open field distance traveled in a dimly lit open field for 1 h. β2m−/−TAP−/− mice (filled bars) exhibited slight hyperactivity relative to WT mice, but when distance traveled in the center of the open field was scored, β2m−/−TAP−/− and WT mice exhibited similar locomotion. This can be taken as normal anxiety-like behavior. Error bars, SEM. WT n = 12; β2m−/−TAP−/−, n = 6.
Object recognition learning is a second form of hippocampus- and NMDAR-dependent learning that has been examined extensively in humans, monkeys, and rodents (Rampon et al. 2000; Broadbent et al. 2010). Mice are exposed to objects, and upon reexposure will tend to investigate these now-familiar objects less than novel objects. After initial training with two identical objects, mice were given three repeated test trials with the familiar and one of three novel objects. A recognition index from each trial, reflecting a preference for the novel object (and, therefore, a memory for the familiar object), is depicted in Fig. 6D. This task is relatively difficult for mice, and each trial serves as additional training. Neither wild type nor β2m−/−TAP−/− mice exhibited learning on trial 1 (one sample t-test, against chance value of 50%; t(8) values < 1, P values > 0.4). However, wild type mice exhibited significant learning on trials 2 and 3 (t(8) values > 2.8, P values < 0.03), whereas mutant mice did not (t(8) values < 0.3, P values > 0.5). Moreover, while wild type and mutant mice did not differ significantly on trial 1 (F(1,16) < 0.3, P > 0.5) or trial 2 (F(1,16) = 3.5, P = 0.08), they differed significantly by trial 3 (F(1,16) = 4.8, P < 0.05). The mean investigation time for each object type, for trials when the object was novel, did not vary between genotypes (see Methods). Thus, overall β2m−/−TAP−/− mice failed to acquire object recognition while wild type mice exhibited good learning after two trials.
Social recognition memory is a third form of hippocampus- and NMDAR-dependent memory (Kogan et al. 2000; Sanchez-Andrade et al. 2005). Male subjects are exposed to ovariectomized females, and the task takes advantage of the fact that males tend to explore novel females more than familiar females. This requires memory for the familiar female, which is hippocampus-dependent. After extensive prehabituation to the procedure, male mutant and wild type mice were exposed to a to-be-familiar ovariectomized female for four 1-min trials; this was followed by a fifth dishabituation trial with a novel female. This habituation–dishabituation procedure was repeated 24 h later with the same familiar female and different novel female. Data are presented in Figure 6E as investigation by the male (subject) of the female (target) as percent time for the fourth habituation trial (familiar) and the fifth dishabituation trial (novel) for the first (immediate) and second (24-h) day of training. For the immediate (day 1) testing, wild type and β2m−/−TAP−/− mice exhibited learning as evidenced by a difference between the novel and familiar female (F(1,26) values > 4.8, P values < 0.05) and did not differ from each other for either the novel or familiar female tests (F(1,26) values < 0.5, P values > 0.5). This evidence suggests that β2m−/−TAP−/− mice are able to acquire the task. However, when tested 24 h later, mutant mice failed to exhibit memory (F(1,12) = 1.6, P > 0.2), whereas wild type mice continued to exhibit good memory (F(1,14) = 15.9, P < 0.01). Moreover, mutant mice failed to explore the familiar female as much as the wild type mice (F(1,26) = 5.7, P < 0.05), but exhibited the same levels of exploration of the familiar and novel female (F(1,26) < 0.5, P > 0.5). These data suggest that β2m−/−TAP−/− mice have normal levels of exploration of novel females, suggesting relevant sensory and motor functions are intact, but they fail to acquire long-lasting social recognition memory. Taken together, these behavioral studies demonstrate that changes in cell-surface expression of MHC class I significantly impair hippocampus-dependent learning and memory.
Discussion
Here we demonstrate that cell surface MHC class I is essential for NMDAR-dependent hippocampal long-term depression and hippocampus-dependent memory. Reducing cell surface MHC class I levels abolishes NMDAR-dependent LTD in the hippocampus, but does not affect NMDAR-independent forms of synaptic plasticity at the same synapses. Together with previous studies showing that loss of MHC class I enhances HFS-LTP (Huh et al. 2000), which is also NMDAR-dependent (Harris et al. 1984; Morris et al. 1986; Bliss and Collingridge 1993), the current results suggest that MHC class I regulates both the sign and the magnitude of NMDAR-dependent synaptic plasticity expressed in the adult hippocampus. A second main conclusion of these studies is that loss of MHC class I on the cell surface severely disrupts an animal’s capacity for learning and memory. MHC class I-deficient β2m−/−TAP−/− mice show pervasive deficits in contextual memory and social and novel object recognition. Overall, these studies suggest that endogenous MHC class I levels are critical determinants of hippocampal synaptic plasticity and cognition.
A remarkable aspect of our findings is the specificity of the deficit in plasticity in β2m−/−TAP−/− animals. Forms of potentiation or depression that share an induction protocol (HFS-induced PTP and LTP, LFS-induced depotentiation and LTD) are dissociable based on their MHC class I-dependence, demonstrating that MHC class I is not simply required to translate HFS or LFS into appropriate synaptic plasticity. Notably, in both cases, in β2m−/−TAP−/− mice NMDAR-dependent forms of plasticity are altered, while NMDAR-independent forms are spared. Indeed, of the seven forms of synaptic plasticity examined in β2m−/−TAP−/− hippocampus to date (HFS-LTP, LFS-LTD (Huh et al. 2000), PPF, PTP, depotentiation, NMDA-LTD, and DHPG-LTD), only those that are NMDAR-dependent are altered in β2m−/−TAP−/− animals. It is also notable that in MHC class I-deficient hippocampus, all forms of NMDAR-dependent plasticity are shifted in favor of potentiation relative to WT, consistent with the possibility that there may be a single molecular event through which MHC class I regulates all of these forms of plasticity. A likely mechanism is the recently described restriction of hippocampal NMDAR function by endogenous MHC class I (Fourgeaud et al. 2010). The current results show that loss of MHC class I on the cell surface does not nonspecifically disrupt all synaptic plasticity, and are consistent with a model in which MHC class I influences plasticity as a direct consequence of its role in regulating NMDAR function.
Our paired-pulse facilitation and post-tetanic potentiation experiments (Fig. 4B–D) suggest that the probability and amount of neurotransmitter release is normal at Schaffer collateral/CA3–CA1 synapses in adult β2m−/−TAP−/− hippocampus. The frequency of miniature excitatory postsynaptic currents (mEPSCs) is elevated in mixed neonatal β2m−/−TAP−/− hippocampal neurons in vitro and in layer 4 neurons in acute slices of postnatal day 19–21 β2m−/−TAP−/− visual cortex (Goddard et al. 2007), suggesting that in these systems probability of release may be increased. However, mEPSC frequency is also influenced by synapse number, and synapse density is increased in β2m−/− cortex (Glynn et al. 2011), suggesting that increased mEPSC frequency observed in cortex could reflect an increase in number of synapses, rather than an increase in the probability of release. Synapse number was not significantly elevated in mixed hippocampal cultures from β2m−/−TAP−/− mice (Goddard et al. 2007), and further studies will be needed to determine if synapse number and/or mEPSC frequency are elevated in the CA1 region of β2m−/−TAP−/− hippocampal slices. Thus several independent lines of evidence (normal PPF and PTP [Fig. 4B–D] and normal AMPAR-mediated fEPSP [Huh et al. 2000; Fourgeaud et al. 2010], EPSC [Fourgeaud et al. 2010], and mEPSC [Goddard et al. 2007] amplitudes in β2m−/−TAP−/− animals) support the idea that MHC class I does not significantly affect the probability of presynaptic glutamate release. The fact that AMPAR-mediated responses to glutamate are unaffected by loss of MHC class I provides further support for the conclusion that, under basal conditions, MHC class I selectively affects postsynaptic responses to glutamate mediated by NMDARs (Fourgeaud et al. 2010).
In contrast to hippocampus, NMDAR-dependent LTD is intact in adult somatosensory cortex from β2m−/−TAP−/− animals, suggesting that MHC class I does not limit NMDAR function throughout the entire brain. This idea is further supported by the finding that memory tasks that rely on hippocampal NMDARs are impaired in β2m−/−TAP−/− mice, while a task that requires NMDARs in amygdyla but not hippocampus (cued fear conditioning) is spared. Given that individual MHC class I genes show distinct expression patterns in neurons (Huh et al. 2000; Loconto et al. 2003), one possible explanation for the spatial restriction of MHC class I effects on NMDAR-dependent plasticity is that specific MHC class I family members expressed in hippocampus, but not in S1 or amygdyla, are responsible. In the future, it will be important to determine if MHC class I affects NMDAR-dependent plasticity at any other sites in the developing or adult brain. The current studies show that MHC class I regulates NMDAR-mediated synaptic transmission and plasticity in the adult hippocampus at synapses which have been implicated in learning and memory.
Our results in β2m−/−TAP−/− mice also demonstrate that cell surface MHC class I is essential for some forms of hippocampus-dependent learning and memory. Mammals are thought to acquire and consolidate explicit memories through LTP- and LTD-like processes in the adult hippocampus. In particular, NMDAR-dependent LTP and LTD at CA3–CA1 synapses have been proposed to underlie many forms of learning and memory (Bear 1996; Milner et al. 1998; Martin et al. 2000; Neves et al. 2008). Consistent with these models, we find that selective loss of NMDAR-dependent LTD and enhancement of NMDAR-dependent LTP is associated with pervasive deficits in hippocampus-dependent memory in β2m−/−TAP−/− animals. These transgenics are unable to acquire contextual fear conditioning and object or social recognition memory, all prominent models of human hippocampus-dependent explicit memory in rodents (Anagnostaras et al. 2001). Unlike β2m−/−TAP−/− animals, β2m−/− mutants did not exhibit differences in contextual fear conditioning relative to wild type mice (see Methods). Similarly, a high-throughput screen found that mice genetically deficient for β2m alone did not have significant impairments in either immediate or remote memory of a context-dependent fear conditioning task (Matynia et al. 2008). This may be because a small population of MHC class I proteins may still reach the cell surface in β2m-deficient neurons (Glynn et al. 2011) and nonneuronal cell lines (Allen et al. 1986). Indeed, functional immune assays suggest that β2m−/−TAP−/−double mutants express even lower levels of MHC class I at the cell surface than β2m−/−single mutants (Dorfman et al. 1997). Repeating these experiments in mice that lack only the classical MHC class I H2-K and H2-D (Kb−/−Db−/− mice [Vugmeyster et al. 1998]) or overexpress the classical MHC class I H2-D specifically in neurons (NSE-Db mice [Rall et al. 1995]) may help clarify the role of specific members of the MHC class I family in regulating hippocampal synaptic plasticity and learning and memory.
NMDAR-dependent LTP and LTD are thought to be induced by intracellular signaling activated downstream of glutamate binding to NMDARs (Lisman 1989; Artola and Singer 1993; Malenka and Nicoll 1993; Nabavi et al. 2013). In many current models, the level and/or kinetics of NMDAR-mediated responses determine if LTP or LTD will occur, with a small, prolonged NMDAR response giving rise to LTD, and larger, faster NMDAR responses giving rise to LTP (Malenka and Bear 2004). In agreement with theoretical predictions (Bienenstock et al. 1982), the postsynaptic response point above which LTP is induced and below which LTD is induced (the “modification threshold”) is itself modifiable, an adjustment of plasticity rules termed metaplasticity (Abraham and Bear 1996; Yasuda et al. 2003; Nosyreva and Huber 2005; He et al. 2006; Jo et al. 2006). Modification of the relative ability to undergo LTP and LTD can preserve the dynamic range of neuronal responses, and may improve signal-to-noise ratios and preserve memory traces in the face of ongoing activity and plasticity (Abraham and Bear 1996). Metaplasticity can be state-dependent (where recent potentiation or depression of synapses affects the ability to induce subsequent plasticity), homeostatic (where the average activity level shifts plasticity), or neuromodulatory (where soluble factors shift plasticity) in origin. Metaplastic changes in the threshold for potentiation have been identified in diverse settings, including developmental critical periods (Crair and Malenka 1995; Kirkwood et al. 1996; Feldman et al. 1998), following learning (Zelcer et al. 2006), and after modification of the seizure threshold (Ullal et al. 1989).
Here we describe a novel form of metaplasticity that is driven by the levels of MHC class I expressed at the cell surface. In β2m−/−TAP−/− animals, LTP is enhanced (Huh et al. 2000) and NMDAR-dependent LTD is abolished. Furthermore, low-frequency (1-Hz) stimulation that does not cause appreciable plasticity in WTs is sufficient to induce LTP in MHC class I-deficient animals. Thus loss of MHC class I shifts the frequency–response curve for NMDAR-dependent plasticity in favor of potentiation, suggesting that endogenous MHC class I is essential for NMDAR-dependent LTD, and limits NMDAR-dependent LTP. It remains to be determined if MHC class I drives metaplasticity by modifying the parameters for induction of both LTP and LTD at individual synapses, or by biasing the proportion of synapses that undergo all-or-none potentiation vs depression (O’Connor et al. 2005a,b).
During early postnatal development, MHC class I expression is dynamic (Corriveau et al. 1998). The current results suggest that these naturally occurring changes in MHC class I levels could contribute to metaplasticity during developmental critical periods. In both the developing and adult brain, MHC class I levels increase in response to high levels of activity and drop following activity blockade (Corriveau et al. 1998; Huh et al. 2000), suggesting that under regimes of high activity, MHC class I levels will rise, promoting synaptic depression, while under conditions of low activity, MHC class I levels will drop, enhancing synaptic potentiation. In this way, MHC class I could also contribute to homeostatic metaplasticity, which preserves potentiation and depression in the face of chronic changes in activity levels (Bienenstock et al. 1982). MHC class I is required to translate chronic activity blockade into scaling-up of the size of PSD-95 puncta in hippocampal neurons in vitro (Goddard et al. 2007), and it will be of interest to determine if MHC class I’s effect on synaptic scaling is related to its ability to limit NMDAR function.
These results demonstrate, we believe for the first time, the plausibility of changes in the expression of MHC class I proteins as a source of cognitive deficits. A substantial body of literature suggests that inflammatory mediators are required for proper brain development and function and even some forms of learning and memory (for reviews, see, e.g., Merrill and Jonakait 1995; Ziv and Schwartz 2008; Boulanger 2009; McAllister and van de Water 2009; Shatz 2009; Yirmiya and Goshen 2011; Kipnis et al. 2012; Stephan et al. 2012). Microglia (Paolicelli et al. 2011), MHC class I (Huh et al. 2000; Datwani et al. 2009), and components of the complement system (Stevens et al. 2007; Schafer et al. 2012) are all required for normal synapse elimination in the developing rodent CNS. In adults, T cells are required for spatial learning and memory (e.g., Kipnis et al. 2004; Ziv et al. 2006; Radjavi et al. 2013; for review, see Kipnis et al. 2012). MHC class I-deficient β2m−/− mice have a normal distribution of γ δ, CD4+/CD8+, and CD4+/CD8– T cells, but both β2m−/− and TAP1−/− mice lack mature CD4–/CD8+ T cells, and are defective in CD4–/CD8+ T cell-mediated cytotoxicity (Zijlstra et al. 1990; Van Kaer et al. 1992). Cytotoxicity mediated by CD4–/CD8+ T cells involves interactions with MHC class I on the surface of antigen-presenting cells. Such interactions could potentially underlie the requirement for both MHC class I and T cells in learning and memory. Counter to this hypothesis, however, LTP is intact in RAG1−/−mice, which lack all T and B cells, but is significantly enhanced in mice lacking MHC class I (Huh et al. 2000), suggesting that enhanced LTP in MHC class I-deficient mice is not secondary to disrupted T cell function, but rather reflects a T cell-independent requirement for MHC class I in synaptic plasticity in the healthy adult brain.
Consistent with essential roles for immune effectors in the healthy brain, alterations in inflammatory signaling have been implicated in diverse causes of memory impairments, including brain injury, infections, neurodegenerative disorders like Alzheimer’s disease (AD), and normal aging. Changes in immune signaling may also play a part in memory impairments associated with neurodevelopmental disorders. Patients with autism or schizophrenia often show explicit memory dysfunction, which can be particularly severe for episodic memory (Ben Shalom 2003; Barch and Ceaser 2012). An inflammatory trigger has been proposed for these disorders (e.g., Patterson 2009), and genome-wide association studies (GWAS) have identified links between the relative risk of autism or schizophrenia and genetic variation in the MHC class I region (HLA in humans) (Torres et al. 2001; Purcell et al. 2009; Shi et al. 2009; Stefansson et al. 2009). Activation of the maternal immune response may slightly increase the risk of schizophrenia in the child (Adams et al. 1993; Brown 2006), and is sufficient to produce lasting changes in memory in the offspring in animal models (Meyer et al. 2005, 2008, 2010; Ozawa et al. 2006; Ibi et al. 2009; Bitanihirwe et al. 2010; Ito et al. 2010). The current results suggest that, alongside their critical role in the immune response to infection and cellular injury, MHC class I proteins are essential for normal cognition. Thus changes in MHC class I expression could unexpectedly contribute to pathological changes in synaptic plasticity and memory in the developing, diseased, and aging brain.
Materials and Methods
Animals
We made use of mice lacking two molecules required for the stable cell-surface expression of most MHC class I proteins: β2-microglobulin (β2m), a noncovalently associated class I light chain that is an obligatory subunit of most MHC class I molecules, and TAP1, a transporter required for loading peptides onto MHC class I molecules during their assembly in the endoplasmic reticulum. Double knockout (β2m−/−TAP−/−) mice lack stable cell-surface expression of most MHC class I proteins (Zijlstra et al. 1990; Van Kaer et al. 1992; Ljunggren et al. 1995; Dorfman et al. 1997), and are outwardly normal when housed in a clean facility. β2m−/−TAP−/− animals were backcrossed at least eight times to C57Bl/6. WT animals were purchased from Jackson Laboratories and periodically rederived from backcrosses to both transgenic lines. Sentinel mice were used to monitor colony health, and full pathology workups were performed semiannually to ensure pathogen-free status. Both male and female mice were used in approximately equal numbers. Mice were age-matched within each experiment and all experiments were conducted by an observer blind to the genotype.
Electrophysiology
For all electrophysiology experiments, only a single recording was performed for each brain slice. Most slices were from different animals, although in a small number of cases, two slices from the same animal were used. The n’s for both animals and recordings are reported in the text and/or figure legends for each experiment. For each set of experiments, mean plasticity was calculated for an interval when responses reached a new steady state. Since the kinetics of different forms of plasticity vary, the interval for this calculation was independently defined for each plasticity induction protocol (see descriptions below or figure legends for values).
Hippocampal recording
Slices of mouse brain 400 μM thick were prepared from 4 to 7-wk-old animals, deeply anesthetized to a surgical plane with halothane. After rapid dissection, slices were allowed to recover at 25°C in a holding chamber containing oxygenated artificial cerebrospinal fluid (ACSF [in mM]: 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 2.5 CaCl2, 26 NaHCO3, and 10 glucose) for a minimum of 1 h before recording. Individual slices were then transferred to a submerged recording chamber and perfused (1 ml/min) with oxygenated ACSF. Upon transfer to the recording chamber, connections to the CA3 region of the hippocampus were microsurgically cut, and 100 μM pictotoxin (Sigma), a noncompetitive antagonist of GABAA receptors, was added to the bath ACSF unless otherwise noted. Stainless-steel bipolar electrodes were used to stimulate Schaffer collateral/commissural fibers; glass microelectrodes filled with ACSF (2–6 mΩ) were inserted into the stratum radiatum to record field excitatory postsynaptic potentials (fEPSPs) from populations of CA1 pyramidal neurons. Test pulses (0.033 Hz) were applied to determine the stable baseline fEPSP (for stimulation intensities, see below). Each point represents an average of four consecutive fEPSPs, normalized to baseline. All values are reported as means ± SEM, where n is the number of slices (one slice per animal). Data collection was performed by an observer blind to genotype. Before the blind was dropped, recordings were omitted from analysis if the extracellular resistance changed significantly >15% or if the stimulating electrode had visibly drifted over the course of the recording. Stimulus artifacts were complete well before fEPSP onset and so were easily omitted from analysis. Statistical significance was assessed by a two-tailed one-way analysis of variance (ANOVA) or Student’s t-test.
High-frequency and low-frequency stimulation
Test pulses (0.033 Hz) were applied at a stimulation intensity required to produce an fEPSP that was 30% (for HFS) or 50% (for LFS) of the maximal response for each recording. After at least 30 min of baseline recording, high-frequency stimulation (HFS; four trains of 100 pulses at 100 Hz, intertrain interval 15 sec) or low-frequency stimulation (LFS; 900 pulses at1 Hz) were applied at time 0. LTP or LTD was calculated as the average responses between 30 and 60 min after stimulation, unless otherwise noted, normalized to a 15-min pretetanus control period.
Post-tetanic potentiation and paired-pulse facilitation
For post-tetanic potentiation (PTP), HFS was applied as above, but 50 μM D-amino-5-phosphorovaleric acid (D-APV) was present throughout the recording. For paired-pulse facilitation, presynaptic stimulation (30% of maximum) was delivered in paired pulses with an inter-stimulus interval (ISI) of 20–200 msec. Pairs of pulses were delivered at 0.033 Hz. PPF was calculated as the ratio of the slope of the second fEPSP to the slope of the first.
Depotentiation
Test pulses (0.033 Hz) were applied at a stimulation intensity required to produce an fEPSP that was 30% of the maximal response for each recording. After at least 30 min of baseline recording, high-frequency stimulation (HFS; four trains of 100 pulses at 100 Hz, intertrain interval 15 sec) was applied at time 0. Test pulses (0.33 Hz) were applied for another 30 min, and then low-frequency stimulation (LFS; 900 pulses at 1 Hz) was applied. Depotentiation was calculated as the averaged responses between 10 and 30 min after LFS, normalized to a 15-min pretetanus control period.
NMDA-LTD
Test pulses (0.033 Hz) were applied at a stimulation intensity required to produce an fEPSP that was 50% of the maximal response for each recording. After at least 30 min of stable baseline recording, NMDA (40 μM in ACSF) was bath-applied for 3 min and then washed out completely using high-flow-rate perfusion (>1 mL/min) and a low-volume chamber (<500 μL). NMDA-LTD was calculated as the averaged responses between 45 and 60 min after the start of NMDA perfusion, normalized to a 15-min pretetanus baseline.
DHPG-LTD
Test pulses (0.033 Hz) were applied at a stimulation intensity required to produce an fEPSP that was 30% of the maximal response for each recording. Following acquisition of a stable baseline recording for a minimum of 30 min, 100 μM DHPG (Sigma) was applied for 10 min in ACSF (as above, but containing 100 μM PTX, 1.9 mM Ca2+, and 1.9 mM Mg2+). DHPG-LTD was calculated as the averaged responses between 30 and 60 min following application of DHPG.
Barrel cortex recordings
Mice (P14-P19) were anesthetized by inhaled isofluorane and decapitated. Brains were removed and immediately plunged into cold sucrose slicing solution containing (in mM) 85 NaCl, 2.5 KCl, 1.25 NaH2PO4, 75 sucrose, 25 dextrose, 0.5 ascorbic acid, 0.5 CaCl2, 4 MgSO4, and 25 NaHCO3 bubbled with 95% O2/5% CO2. Four hundred micron coronal slices were made with a vibratome and transferred to ACSF containing (in mM) 119 NaCl, 2.5 KCl, 1 NaH2PO4, 11 dextrose, 2.5 CaCl2, 1.3 MgSO4, and 26 NaHCO3, at 32°C for 30 min, then returned to RT (∼23°C) and allowed to recover for 1 h prior to recording.
Slices were placed in a stage-mounted recording chamber and perfused with oxygenated ACSF at RT. Slices were visualized using infrared differential interference contrast (IR-DIC) video microscopy. A bipolar tungsten stimulating electrode was placed in a L4 barrel for presynaptic stimulation, and L2/3 pyramidal cells were targeted for whole-cell recording based on their characteristic pyramidal soma shape and thick proximal apical dendrite extending toward the cortical surface. Recording electrodes (4–6 μΩ) were filled with (in mM) 108 cesium gluconate, 20 HEPES, 0.4 EGTA, 2.8 NaCl, 5 TEACl, 4 MgATP, 0.3 NaGTP, 10 phosphocreatine, and 0.3% biocytin for voltage clamp, or 116 K gluconate, 20 HEPES, 6 KCl, 2 NaCl, 0.5 EGTA, 4 MgATP, 0.3 NaGTP, 10 phosphocreatine, and 0.3% biocytin for current clamp. Internal solutions were adjusted to pH 7.2 and ∼290 mOsm. To isolate excitatory synaptic responses in voltage clamp, GABA-A mediated inhibition was blocked locally via a glass pipette (10–15 µm diameter) filled with 1 mM bicuculine methiodide (BMI), placed 40–80 µm from the recorded cell. BMI diffuses locally from this electrode and effectively blocks inhibition in the recorded cell. Current or voltage traces were amplified, filtered at 2 kHz, digitized, and collected using custom software running in Igor. During recording, holding current (in voltage clamp) or membrane potential (in current clamp), series resistance, and input resistance were monitored as measures of recording quality and cell health. Cells were not used if any of these measures deviated >20% during the recording. ACSF was supplemented with AM251 or internal solution was supplemented with MK801 as indicated.
Barrel cortex pairing-induced LFS-LTD
Baseline responses were recorded while holding the postsynaptic L2/3 cell at −75 mV and stimulating presynaptically at 0.067 Hz. Pairing-induced LTD was triggered by depolarizing the postsynaptic L2/3 cell to –50 mV and stimulating presynaptically at 0.14 Hz for 100 stimuli, and then returning to baseline conditions. LFS-LTD was induced by low-frequency stimulation (900 stimuli at 1 Hz), after which baseline conditions were returned to. In both cases, LTD was quantified by averaging the peak response amplitude over 25 consecutive sweeps beginning 15 min after LTD induction and normalizing to the average peak response amplitude over 25 consecutive sweeps during the baseline.
Learning and memory tests
Fear conditioning
Mice were given a “slow acquisition” protocol to explore the learning deficits more finely (Anagnostaras et al. 2003). Mice were placed in a fear conditioning chamber and after a 4-min baseline were given one tone (2.8 kHz, 30 sec, 90 dBA)–shock (2 sec, 0.75 mA) pairing. After an additional 30 sec, they were returned to their home cage. Freezing and locomotor were assessed for the entire period using an automated algorithm (for complete procedures, see, e.g., Anagnostaras et al. [2000] and Wood and Anagnostaras [2009]). This was repeated for a total of 5 d. We also examined fear conditioning in β2m−/− and β2m–/+ single mutant mice. For acquisition of contextual fear across 5 d of training, cued fear conditioning, and locomotor activity for the baseline period prior to shock on the first day of training, β2m−/− and β2m–/+ mutants failed to exhibit differences relative to wild type mice (Fisher’s PLSD, P values > 0.05), and no further behavioral analysis of single mutants was pursued.
Object recognition
We began with a habituation procedure: Male and female mutant and wild type mice were individually placed in their home cages for two 10-min sessions (1-h interval) in a room with white noise, dimly lit with red light. Mice were returned to this room 24 h later and presented with two identical objects in their home cages for two 10-min sessions (1-h interval) to habituate to object presentation (these objects were not used again). Twenty-four hours later, object recognition training and testing was conducted across four 5-min test sessions (1-h intervals), as follows: training trial, two identical to-be-familiar objects; testing trials 1–3, one familiar object and one novel object. Four objects were used (a plastic toy block, a ceramic food cup, a plastic toy soldier, and a spiky rubber ball). The exact object used was completely counterbalanced for novelty, order, and genotype. Behavior was video-recorded and later scored for the duration of olfactory investigation of objects (defined as the subjects nose oriented toward and in close contact with the object). A novel preference percent score was generated to indicate learning: (novel time)/(novel + familiar time) for each testing trial. Basal object exploration was measured during the initial exposure to each object. Although differences existed for exploration time for the object types (F(3,46) = 6.4, P < 0.01), these were counterbalanced during training and testing, and there were no group differences (F(1,46) = 1.9, P > 0.1) or group × object type interaction (F(3,46) < 0.5, P > 0.5). This indicates that β2m−/−TAP−/− mutants exhibited the same amount of overall exploration as wild types and showed normal baseline preferences among the object types.
Social recognition
Male mutant and wild type mice were transferred from group to individual housing for 7 d prior to testing to allow home cage establishment (Ferguson et al. 2000). In a room with white noise and dimly lit with red light, novel adult female ovariectomized 129X1/SvJ mice (Jackson Laboratory, Bar Harbor, ME) were introduced into the home cage of each subject for 1-min intervals, on each of seven pre-test days. On the eighth day, a novel (to be familiar) ovariectomized female was repeatedly introduced for four 1-min habituation trials (10-min interval), followed by a 1-min dishabituation trial with a novel female. After a 24-h delay, male mice were retested on the habituation–dishabituation paradigm, using the same (familiar) ovariectomized female from the day prior, and a novel female on the dishabituation trial. Behavior was video-recorded, and later scored for the duration of olfactory investigation (defined as the subjects nose oriented toward and in contact with the female). Behaviors that could potentially confound social recognition, including mounting, male-on-female aggression, and female-on-male aggression, were scored. These behaviors were rare and not confounded with genotype, suggesting they were not the source of social recognition memory failure (mounting, WT 5.400 ± 9.920, β2m−/−TAP−/− 6.462 ± 10.705; male aggression, WT 0.933 ± 1.751, β2m−/−TAP−/− 0.615 ± 1.044; female aggression, WT 2.267 ± 2.915, β2m−/−TAP−/− 2.769 ± 2.006; all values reported as mean percent time ± standard error over the entire testing period). All behavior is reported as percent of the total trial time.
Statistical analysis
For behavioral experiments, data were entered into an analysis of variance (ANOVA). Because there were only two groups for most comparisons, a univariate ANOVA was used for group comparisons, unless otherwise noted. The level of significance was set at P < 0.05. For electrophysiology, all results are expressed ± SEM. For visual clarity, graphs showing the time course of plasticity over multiple neurons (e.g., Fig. 1C, 2C, 3C) show points representing an average of four consecutive fEPSPs. Bar graphs summarizing the extent of plasticity in pooled data (e.g., Fig. 1D, 2D, 3D) show averages of all individual events. T-tests were performed, and statistical significance was set at P < 0.05.
Acknowledgments
We thank P. Riquelme, N. Colaco, J. Kim, S. Carmack, and T. Czech for expert technical assistance, D. Feldman for lending equipment and expertise for the cortical LTD experiments, M. Scanziani for helpful discussions, and D. Raulet for providing TAP−/− mice. We are grateful for the support and guidance of C.J. Shatz, who was instrumental in initiating these experiments at Harvard Medical School, supported in part by MH071666 (C.J. Shatz). Additional support for these studies was provided by The Alfred P. Sloan Foundation, the Silvio Varon Chair in Neuroregeneration, and grants from the Whitehall Foundation, Cure Autism Now, and Autism Speaks (L.M.B), and a Hellman Fellowship (S.G.A.).
References
- Abraham WC, Bear MF 1996. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci 19: 126–130 [DOI] [PubMed] [Google Scholar]
- Adams W, Kendell RE, Hare EH, Munk-Jorgensen P 1993. Epidemiological evidence that maternal influenza contributes to the aetiology of schizophrenia. An analysis of Scottish, English, and Danish data. Br J Psychiatry 163: 522–534 [DOI] [PubMed] [Google Scholar]
- Allen H, Fraser J, Flyer D, Calvin S, Flavell R 1986. β 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc Natl Acad Sci 83: 7447–7451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CB, Celikel T, Feldman DE 2003. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci 6: 291–299 [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ 2000. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem 7: 58–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS 2001. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus 11: 8–17 [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ 2003. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6: 51–58 [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W 1993. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci 16: 480–487 [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, La Scola ME, Morley JE 2001. Intravenous human interleukin-1α impairs memory processing in mice: Dependence on blood–brain barrier transport into posterior division of the septum. J Pharmacol Exp Ther 299: 536–541 [PubMed] [Google Scholar]
- Barch DM, Ceaser A 2012. Cognition in schizophrenia: Core psychological and neural mechanisms. Trends Cogn Sci 16: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER 2005. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 48: 123–137 [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Collingridge GL 1994. An investigation of depotentiation of long-term potentiation in the CA1 region of the hippocampus. Exp Brain Res 100: 437–443 [DOI] [PubMed] [Google Scholar]
- Bear MF 1996. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci 93: 13453–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC 1994. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399 [DOI] [PubMed] [Google Scholar]
- Ben Shalom D 2003. Memory in autism: Review and synthesis. Cortex 39: 1129–1138 [DOI] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE 2006. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 26: 4166–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW 1982. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci 2: 32–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova T, Dang H, Xu W, Gustafson S, Jin Y, Wickramasinghe L, Won T, Bobarnac G, Middleton B, Tian J, et al. 2012. Major histocompatibility complex class I molecules modulate embryonic neuritogenesis and neuronal polarization. J Neuroimmunol 247: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U 2010. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol 13: 981–996 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL 1993. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Boulanger LM 2009. Immune proteins in brain development and synaptic plasticity. Neuron 64: 93–109 [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ 2004. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci 5: 521–531 [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Huh GS, Shatz CJ 2001. Neuronal plasticity and cellular immunity: Shared molecular mechanisms. Curr Opin Neurobiol 11: 568–578 [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE 2010. Object recognition memory and the rodent hippocampus. Learn Mem 17: 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS 2006. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull 32: 200–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon MA, Boulanger LM 2013. MHC class I protein is expressed by neurons and neural progenitors in mid-gestation mouse brain. Mol Cell Neurosci 52: 117–127 [DOI] [PubMed] [Google Scholar]
- Coan EJ, Saywood W, Collingridge GL 1987. MK-801 blocks NMDA receptor-mediated synaptic transmission and long term potentiation in rat hippocampal slices. Neurosci Lett 80: 111–114 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Volianskis A, Bannister N, France G, Hanna L, Mercier M, Tidball P, Fang G, Irvine MW, Costa BM, et al. 2013. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 64: 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ 1998. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21: 505–520 [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC 1995. A critical period for long-term potentiation at thalamocortical synapses. Nature 375: 325–328 [DOI] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ 2009. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron 64: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J 2010. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J Exp Med 207: 1067–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF 1997. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18: 995–1008 [DOI] [PubMed] [Google Scholar]
- Dorfman JR, Zerrahn J, Coles MC, Raulet DH 1997. The basis for self-tolerance of natural killer cells in β2-microglobulin- and TAP-1- mice. J Immunol 159: 5219–5225 [PubMed] [Google Scholar]
- Dudek SM, Bear MF 1992. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci 89: 4363–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF 1993. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci 13: 2910–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer BM, McAllister AK 2012. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci.35: 660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE 2000. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron 27: 45–56 [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT 1998. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron 21: 347–357 [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT 2000. Social amnesia in mice lacking the oxytocin gene. Nat Genet 253: 284–288 [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, Kingston AE, Lodge D, Collingridge GL 1998. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology 37: 1445–1458 [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL 1999. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology 38: 1577–1583 [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Palmer MJ, May JE, Neeson A, Morris SA, Collingridge GL 2001. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol 537: 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Boulanger LM 2010. Role of immune molecules in the establishment and plasticity of glutamatergic synapses. Eur J Neurosci 32: 207–217 [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer MB, Boulanger LM 2010. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc Natl Acad Sci 107: 22278–22283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H 1991. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res 555: 112–122 [DOI] [PubMed] [Google Scholar]
- Gibertini M 1996. IL1 β impairs relational but not procedural rodent learning in a water maze task. Adv Exp Med Biol 402: 207–217 [DOI] [PubMed] [Google Scholar]
- Glynn MW, Elmer BM, Garay PA, Liu XB, Needleman LA, El-Sabeawy F, McAllister AK 2011. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci 14: 442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ 2007. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci 104: 6828–6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, Ganong AH, Cotman CW 1984. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res 323: 132–137 [DOI] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM 2006. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci 26: 2951–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsabeck RC, Perry W, Hassanein TI 2002. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology 35: 440–446 [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS 2001. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J Biol Chem 276: 48108–48117 [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF 2001. Chemical induction of mGluR5- and protein synthesis—dependent long-term depression in hippocampal area CA1. J Neurophysiol 86: 321–325 [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ 2000. Functional requirement for class I MHC in CNS development and plasticity. Science 290: 2155–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, Takuma K, Kamei H, Noda Y, Nitta A, et al. 2009. Neonatal polyI: C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res 64: 297–305 [DOI] [PubMed] [Google Scholar]
- Ireland DR, Abraham WC 2009. Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral–CA1 synapses. J Neurophysiol 101: 1375–1385 [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH 2010. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun 24: 930–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Ball SM, Seok H, Oh SB, Massey PV, Molnar E, Bashir ZI, Cho K 2006. Experience-dependent modification of mechanisms of long-term depression. Nat Neurosci 9: 170–172 [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M 2004. T cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci 101: 8180–8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC 2012. Pro-cognitive properties of T cells. Nat Rev Immunol 12: 663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF 1996. Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381: 526–528 [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ 2000. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10: 47–56 [DOI] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF 1998. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21: 1151–1162 [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmayer P, Prashanth Chandramani S, Maul-Pavicic A, Jager M, Li XH, Breer H, Zufall F, Boehm T 2004. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306: 1033–1037 [DOI] [PubMed] [Google Scholar]
- Li R, Dozmorov M, Hellberg F, Tian Y, Jilderos B, Wigstrom H 2004. Characterization of NMDA induced depression in rat hippocampus: Involvement of AMPA and NMDA receptors. Neurosci Lett 357: 87–90 [DOI] [PubMed] [Google Scholar]
- Lidman O, Olsson T, Piehl F 1999. Expression of nonclassical MHC class I (RT1-U) in certain neuronal populations of the central nervous system. Eur J Neurosci 11: 4468–4472 [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME 2008. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455: 1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linda H, Hammarberg H, Piehl F, Khademi M, Olsson T 1999. Expression of MHC class I heavy chain and β2-microglobulin in rat brainstem motoneurons and nigral dopaminergic neurons. J Neuroimmunol 101: 76–86 [DOI] [PubMed] [Google Scholar]
- Lisman J 1989. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci 86: 9574–9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Van Kaer L, Sabatine MS, Auchincloss H Jr, Tonegawa S, Ploegh HL 1995. MHC class I expression and CD8+ T cell development in TAP1/β 2-microglobulin double mutant mice. Int Immunol 7: 975–984 [DOI] [PubMed] [Google Scholar]
- Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, Kumanovics A, Fischer Lindahl K, Dulac C 2003. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell 112: 607–618 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF 2004. LTP and LTD: An embarrassment of riches. Neuron 44: 5–21 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA 1993. NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci 16: 521–527 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA 1999. Long-term potentiation—a decade of progress? Science 285: 1870–1874 [DOI] [PubMed] [Google Scholar]
- Maren S 1999. Long-term potentiation in the amygdala: A mechanism for emotional learning and memory. Trends Neurosci 22: 561–567 [DOI] [PubMed] [Google Scholar]
- Maren S, Anagnostaras SG, Fanselow MS 1998. The startled seahorse: Is the hippocampus necessary for contextual fear conditioning? Trends Cogn Sci 2: 39–42 [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG 2000. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci 23: 649–711 [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI 2004. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 24: 7821–7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matynia A, Anagnostaras SG, Wiltgen BJ, Lacuesta M, Fanselow MS, Silva AJ 2008. A high through-put reverse genetic screen identifies two genes involved in remote memory in mice. PLoS ONE 3: e2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, van de Water J 2009. Breaking boundaries in neural-immune interactions. Neuron 64: 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MJ, Huang YH, Datwani A, Shatz CJ 2009. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci 106: 6784–6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Jonakait GM 1995. Interactions of the nervous and immune systems in development, normal brain homeostasis, and disease. FASEB J 9: 611–618 [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK 2005. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev 29: 913–947 [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J 2008. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun 22: 469–486 [DOI] [PubMed] [Google Scholar]
- Meyer U, Knuesel I, Nyffeler M, Feldon J 2010. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology (Berl) 208: 531–543 [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER 1998. Cognitive neuroscience and the study of memory. Neuron 20: 445–468 [DOI] [PubMed] [Google Scholar]
- Miralves J, Magdeleine E, Kaddoum L, Brun H, Peries S, Joly E 2007. High levels of MeCP2 depress MHC class I expression in neuronal cells. PLoS ONE 2: e1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M 1986. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319: 774–776 [DOI] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R 2013. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci 110: 4027–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman LA, Liu XB, El-Sabeawy F, Jones EG, McAllister AK 2010. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc Natl Acad Sci 107: 16999–17004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV 2008. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat Rev Neurosci 9: 65–75 [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Oliet SH, Malenka RC 1998. NMDA receptor-dependent and metabotropic glutamate receptor-dependent forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neurobiol Learn Mem 70: 62–72 [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM 2005. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci 25: 2992–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Wittenberg GM, Wang SS 2005a. Dissection of bidirectional synaptic plasticity into saturable unidirectional processes. J Neurophysiol 94: 1565–1573 [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Wittenberg GM, Wang SS 2005b. Graded bidirectional synaptic plasticity is composed of switch-like unitary events. Proc Natl Acad Sci 102: 9679–9684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER 1994. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem 1: 129–139 [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schobitz B, de Kloet ER 1993. Interleukin-1 β, but not interleukin-6, impairs spatial navigation learning. Brain Res 613: 160–163 [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA 1997. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron 18: 969–982 [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M 2006. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: A neurodevelopmental animal model of schizophrenia. Biol Psychiatry 59: 546–554 [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL 1997. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology 36: 1517–1532 [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. 2011. Synaptic pruning by microglia is necessary for normal brain development. Science 333: 1456–1458 [DOI] [PubMed] [Google Scholar]
- Patanella AK, Zinno M, Quaranta D, Nociti V, Frisullo G, Gainotti G, Tonali PA, Batocchi AP, Marra C 2010. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-α, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res 88: 1106–1112 [DOI] [PubMed] [Google Scholar]
- Patterson PH 2009. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res 204: 313–321 [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, et al. 2007. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron 53: 703–717 [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P 2009. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460: 748–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjavi A, Smirnov I, Derecki N, Kipnis J 2013. Dynamics of the meningeal CD4 T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol Psychiatry. 10.1038/mp.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB 1995. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med 182: 1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ 2000. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci 3: 238–244 [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, James BM, Kendrick KM 2005. Neural encoding of olfactory recognition memory. J Reprod Dev 51: 547–558 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74: 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Heimann B, Djukic M, Mazurek C, Fels C, Wallesch CW, Nau R 2006. Neuropsychological sequelae of bacterial and viral meningitis. Brain 129: 333–345 [DOI] [PubMed] [Google Scholar]
- Shatz CJ 2009. MHC class I: An unexpected role in neuronal plasticity. Neuron 64: 40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, et al. 2009. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460: 753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, et al. 2009. Common variants conferring risk of schizophrenia. Nature 460: 744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B 2012. The complement system: An unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35: 369–389 [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. 2007. The classical complement cascade mediates CNS synapse elimination. Cell 131: 1164–1178 [DOI] [PubMed] [Google Scholar]
- Torres AR, Maciulis A, Odell D 2001. The association of MHC genes with autism. Front Biosci 6: D936–943 [DOI] [PubMed] [Google Scholar]
- Ullal GR, Ninchoji T, Uemura K 1989. Low frequency stimulation induces an increase in after-discharge thresholds in hippocampal and amygdaloid kindling. Epilepsy Res 3: 232–235 [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S 1992. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4–8+ T cells. Cell 71: 1205–1214 [DOI] [PubMed] [Google Scholar]
- Vugmeyster Y, Glas R, Perarnau B, Lemonnier FA, Eisen H, Ploegh H 1998. Major histocompatibility complex (MHC) class I KbDb −/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci 95: 12492–12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn LR, Zekzer D, Eitan S, Lu Y, Dang H, Middleton B, Evans CJ, Tian J, Kaufman DL 2011. A potential role for shed soluble major histocompatibility class I molecules as modulators of neurite outgrowth. PLoS One 6: e18439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe AM, Carlisle HJ, O’Dell TJ 2002. Postsynaptic induction and presynaptic expression of group 1 mGluR-dependent LTD in the hippocampal CA1 region. J Neurophysiol 87: 1395–1403 [DOI] [PubMed] [Google Scholar]
- Wood SC, Anagnostaras SG 2009. Memory and psychostimulants: Modulation of Pavlovian fear conditioning by amphetamine in C57BL/6 mice. Psychopharmacology (Berl) 202: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC 2003. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci 6: 15–16 [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I 2011. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181–213 [DOI] [PubMed] [Google Scholar]
- Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E 2006. A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex 16: 460–468 [DOI] [PubMed] [Google Scholar]
- Zhang L, Meng K, Li YH, Han TZ 2009. NR2A-containing NMDA receptors are required for L-LTP induction and depotentiation in CA1 region of hippocampal slices. Eur J Neurosci 29: 2137–2144 [DOI] [PubMed] [Google Scholar]
- Zhong J, Zhang T, Bloch LM 2006. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Zhang W, Son H, Mansuy I, Sobel RA, Seidman J, Kandel ER 1999. A selective role of calcineurin aα in synaptic depotentiation in hippocampus. Proc Natl Acad Sci 96: 4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M, Li E, Sajjadi F, Subramani S, Jaenisch R 1989. Germ-line transmission of a disrupted β 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature 342: 435–438 [DOI] [PubMed] [Google Scholar]
- Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R 1990. β 2-microglobulin deficient mice lack CD4–8+ cytolytic T cells. Nature 344: 742–746 [DOI] [PubMed] [Google Scholar]
- Ziv Y, Schwartz M 2008. Immune-based regulation of adult neurogenesis: Implications for learning and memory. Brain Behav Immun 22: 167–176 [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M 2006. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9: 268–275 [DOI] [PubMed] [Google Scholar]
- Zucker RS 1989. Short-term synaptic plasticity. Annu Rev Neurosci 12: 13–31 [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG 2002. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405 [DOI] [PubMed] [Google Scholar]