Abstract
Objectives.
In 2 experiments, we examined the oft-replicated finding of age-related differences in accuracy at retrieving items stored in working memory, but outside the focus of attention. Specifically, we investigated whether such differences could be explained by (a) age-related differences in coping with the dual-task nature of swapping items into and out of the focus of attention and/or (b) age-related differences in resistance to interference.
Method.
We used a modified version of the N-Back task with stimuli of different levels of difficulty, and experimental manipulations aimed at isolating the dual-task and interference effects.
Results.
We found both explanations lacking: We obtained a dual-task cost (Experiment 1) and an interference cost (Experiment 2), as well as a large age effect (Cohen’s d = 1.6 in Experiment 1 and 0.7 in Experiment 2) but neither the dual task nor the interference effect was sensitive to age.
Discussion.
These findings, combined with previous failures to find an explanation for the age effects, suggest that item availability after a focus switch might be an important new and fundamental variable—a cognitive primitive—potentially necessary for a full understanding of age effects in higher order cognition.
Key Words: Cognition, Executive function, Working memory
Working memory is a temporary memory buffer—lasting for a few seconds at most—serving to both passively store and actively manipulate information (Baddeley & Hitch, 1974; Kane, Bleckley, Conway, & Engle, 2001; Miyake & Shah, 1999). A crucial feature of this system is its limited capacity, known to decrease considerably with advancing age: Older adults’ capacity on working memory tasks such as reading span or operation span is about 74% that of younger adults (Bopp & Verhaeghen, 2005). One particular determinant of working memory capacity that appears to be consistently age sensitive is the availability of information once it leaves the core structure of working memory, that is, the focus of attention (Chen & Li, 2007; Oberauer, 2002; Vaughan, Basak, Hartman, & Verhaeghen, 2008; Verhaeghen, 2012; Verhaeghen & Basak, 2005; Zhang, Verhaeghen, & Cerella, 2012).
What is this focus of attention? Many models of working memory, most notably embedded-process model of Cowan (1995, 2001), posit a hierarchy of availability and accessibility within working memory. Such models maintain that only a small subset of the information available in the world (one to four items, depending on the task; Verhaeghen, Cerella, & Basak, 2004) is immediately accessible to the individual; these items are said to reside in the focus of attention (Cowan, 1995). If more information needs to be stored than the focus can handle, the excess is held inside a larger, “activated” portion of long-term memory, subject to interference and possibly decay (we have labeled this “the outer store”; Verhaeghen et al., 2004). Most tasks that tap into working memory, such as operation span, N-Back, or running span, create overflow of the focus of attention and thus require material to be stored into and retrieved from the outer store. In the case of N-Back, for instance (a modified version of which we will use here), subjects pay attention to only a single item at a time, store the intervening items into the outer store, and access them one at a time as needed for comparison with the item on screen. This operation has been labeled focus switching (Voigt & Hagendorf, 2002); its two constituent processes (the swapping in and the swapping out, respectively) are (a) transfer of items from the focus into the outer store and (b) access to representations stored inside the outer store, which are then loaded into the focus of attention.
In our own work with the N-Back task, we have noted a particular age sensitivity in the process of focus switching: Older and younger adults are equally (and highly) accurate in a 1-Back version of the task, that is, when comparisons occur within the focus of attention, but age differences in accuracy emerge when N > 1, that is, when items need to be transferred into and accessed from the outer store. (The presence of the focus switching process in this paradigm is verified by a step in response time [RT] between N = 1 and N = 2.) Importantly, these age differences do not interact with N, or the number of items stored inside the outer store, suggesting that it is the focus-switching process per se and not the working memory load that drives the age effect (Bopp & Verhaeghen, 2009).
This finding leads to an obvious question: Is this loss in accuracy of items after a focus switch a fundamental aspect of the cognitive system, one that is particularly vulnerable to aging, or can this result be reduced to a deeper deficit in a known process?
Some of our recent work has tackled this question. So far, we have found the former proposition more attractive than the latter. Specifically, we examined three alternative mechanisms and found them all lacking. A first possible alternative explanation is age-related slowing—older adults may lose information at a higher rate than younger adults if they are disproportionally slower at executing the focus switch, by which time the to-be-accessed item has possibly disappeared from the outer store. In all of our studies, we have consistently found the dynamics of the focus-switching operation to be intact: The focus-switch cost in response time is identical for younger and older adults once general slowing is taken into account. A second possible explanation concerns the dynamics of search processes. If older adults are slower at accessing the “location” of a representation in the outer store, or use different search processes altogether, accuracy might suffer. In two experiments, we (Lange & Verhaeghen, 2009) examined serial position curves for search times in (among others) forward, backward, and random search through working memory. The serial position curves of older adults echoed those of younger adults closely; a simple linear rescaling of the younger adult data reproduced the older-adult data. This, then, strongly suggests that younger and older adults use the same retrieval processes, regardless of the type of search; older adults are just overall slower in executing those processes. A third possible explanation concerns the keeping-track requirement inherent in these tasks—if older adults are less capable of associating the item with its position in the sequence, item information might get lost more often. This, however, turns out not to be the case: Older adults seem to be just as able as younger adults to keep track of an item’s position in a sequence (Bopp & Verhaeghen, 2009; Lange & Verhaeghen, 2009; see also Vaughan et al., 2008, Experiment 2).
Recent work of ours has uncovered one new potential source of the accuracy decline after a focus switch. When transfer to the outer store and access to that store are combined on a single trial, accuracy suffers (Zhang et al., 2012; this study examined younger adults only). This dual-task situation is endemic in N-Back tasks, the task most often used to measure age deficits in accuracy after a focus switch.
The task we used to uncover this accuracy decline is a variant on the N-Back task. In this task, we require subjects to make an identity judgment on each trial (i.e., is the item currently on the screen identical to the one seen N positions back?), and we record both response time and accuracy. Items are presented in N columns; when a column is “filled,” stimulus presentation proceeds on the next “row.” Columnizing the presentation reduces the participant’s need to keep track. The task now effectively becomes: Is the item currently onscreen the same as the one presented earlier in the same column? To examine dual-task (i.e., transfer-and-access) costs, we restricted the number of rows to three (i.e., the number of stimuli are 3N). By doing so, we were able to isolate the component processes of the focus-switch operation: The first row requires only transfer to the outer store, the third row requires only access to the items from the second row, and the second row requires both access to the items from the first row and transfer of the items from the second row. The stimuli we used were abstract figures of varying complexity (obtained by varying the number of blocks in a 3 ×3 grid; Figure 1). Our experiment revealed that the necessity to access and transfer items within the same trial altered the nature of processing: Access times consistently increased with complexity, but transfer was only sensitive to complexity when performed in isolation; when performed in conjunction with access, transfer became indifferent to stimulus complexity. Crucial in the present context is that the need to simultaneously access and transfer items also incurred an accuracy cost: Accuracy for the third row (i.e., accuracy for items studied when both access and transfer were engaged) was markedly lower than that of the second row (i.e., accuracy for items studied when only transfer was engaged). This suggests that the dual-task situation (i.e., access-and-transfer) must be responsible for at least part of the decline of accuracy after a focus switch.
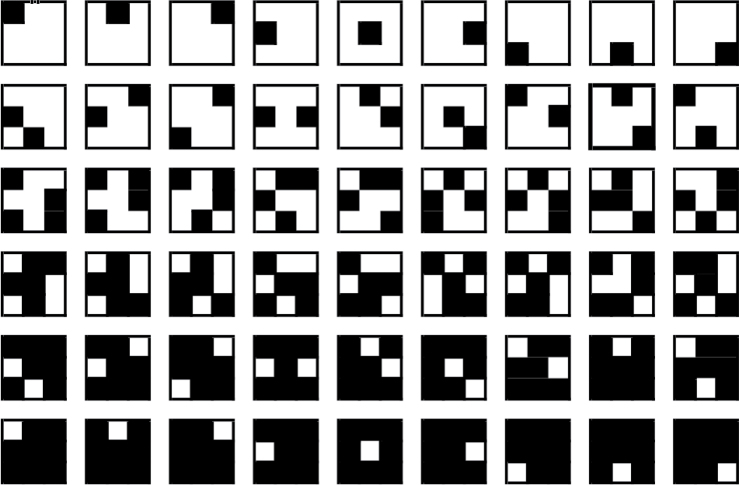
Figure 1.
The checkerboard stimuli as used in all both experiments. Each row represents all nine stimuli within each C value (C = 1, 2, 4, 5, 7, and 8, respectively).
It is the age sensitivity of this mechanism we wish to investigate here. More precisely, our research question is: Do older adults show a larger decrease in accuracy than younger adults do when items were committed to working memory in the dual-task situation of simultaneous access and transfer? We note here that the complexity manipulation allows us a rather high-resolution answer to this question: We have three complexity levels, and thus six data points (three levels of stimulus complexity by single-transfer vs. dual-task-transfer) per age group to test this hypothesis.
Additionally, our variant on the N-Back paradigm allows for a fresh investigation into a more traditional explanation for age-related changes in working memory, namely an age-related deficit in resistance to interference (often seen as the result of a breakdown in “inhibition”; Hasher & Zacks, 1988; possibly also a side effect of age-related deficits in maintaining goal-relevant information; De Jong, 2001). Given that items stored outside the focus of attention are by definition susceptible to interference, age-related differences in resistance to interference seem a natural candidate for a causal explanation for age-related differences in accuracy after a focus switch. The role of interference can easily be investigated in a paradigm where we now ask our participants to compare the stimuli in successive rows with those from row 1. In this scenario, the successive rows require access only, but the amount of intervening and potentially interfering stimuli increases with the number of probe items presented, likely driving down accuracy over successive rows. This was verified in Zhang and colleagues (2012), with younger adults and using three rows: Rows 2 and 3 yielded identical response times, unlike row 2 and row 3 of the experiment described previously, where row 2 (additionally implicating transfer) yielded longer response times than row 3. Accuracy did, however, decline significantly from row 2 to row 3. Because the accuracy decline from row 2 to row 3 in Zhang and colleagues, although significant, was not dramatic, we increased the number of rows from 3 to 6 in this study, in order to give this hypothesis a fair chance.
In sum, then, we report results from two experiments. In Experiment 1, a three-row N-Back paradigm was used to examine the influence of the dual-task requirement of access and transfer on age-related differences in accuracy. If such differences exist, they would be signaled by an age-by-row interaction, such that the expected drop in accuracy from row 2 to row 3 is larger in older adults than in younger adults. (This effect in turn might interact with stimulus complexity—one might expect that if older adults lose information at a higher rate, more complex information might be more susceptible to this effect.) In Experiment 2, we used a six-row N-Back experiment, probing access from row 1 only. If a purported age-related breakdown in resistance to interference were responsible for the age-related differences in accuracy after a focus switch, we would expect an age-by-row interaction such that later rows, where the build-up of interference is larger, yield larger age-related differences. (This effect in turn might interact with stimulus complexity—one might expect that if older adults suffer from a breakdown in resistance to interference, more complex information might be more susceptible to this effect.)
Experiment 1
Method
Participants
Twenty-two older adults (Mage = 71.0, SDage = 6.6; Meducation = 15.3, SDeducation = 2.2) participated in return for a $10hr−1 fee. The 29 young adults (Mage = 19.9, SDage = 2.1; Meducation = 14.1, SDeducation = 1.8) included in the experiment were those included in Experiment 2 in Zhang and colleagues (2012). None of our participants indicated being colorblind. Older adults had significantly lower Digit-Symbol Modalities scores than younger adults (40.86 vs. 66.93, t(49) = 7.27, p < .001), as well as lower Shipley Vocabulary scores (26.14 vs. 30.79, t(49) = 2.54, p < .014). This is somewhat unusual in samples of this sort (older adults generally tend to score higher on vocabulary tests that younger adults; Verhaeghen, Steitz, Sliwinski, & Cerella, 2003). Therefore, the odds are set against older adults performing well.
Stimuli and Complexity-Effect Pilot Experiment
Stimuli were black and white checkerboard patterns created by blackening C (with C = 1, 2, 4, 5, 7, or 8) cells of an invisible 3 × 3 matrix in a pseudorandom fashion. The number of possible stimuli is large; in this and the subsequent experiment, only nine patterns were used for each value of C, in analogy with the nine digits used in the standard digit versions of the N-Back task. Figure 1 depicts the full set of stimuli in black and white. Our earlier work (Zhang et al., 2012) examining response times for both a visual search and a 3-Back task confirmed that stimulus complexity increased from 1-cell to 2-cells and from 2-cells to 4-cells, and then decrease from 5 to 7 and from 7 to 8.
Procedure
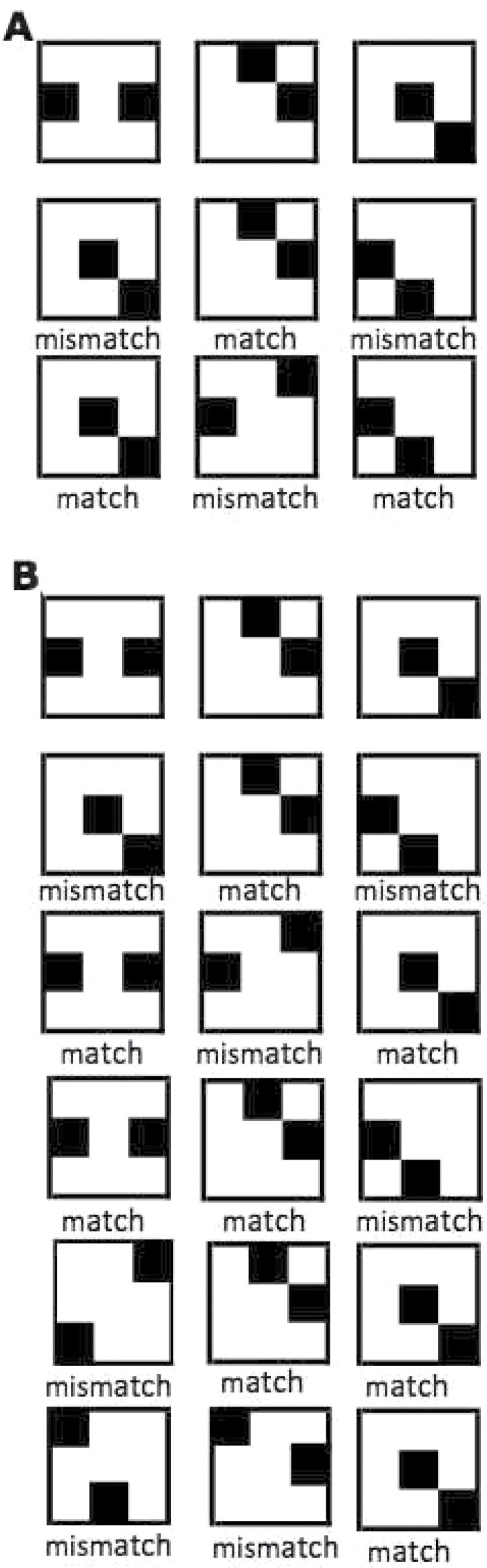
The WM task was an identity-judgment 3-Back procedure (Verhaeghen & Basak, 2005), with items presented in three rows of three columns. The first row was study-only. From the second row on, participants indicated whether the current item was identical to the item presented three positions back. In practice, this meant that participants indicated whether the item currently on screen matched the item presented in the same column as the current item but in the previous row. Each column was depicted in a different color. (The column/color scheme was meant to aid participants in keeping track, to emphasize the memory aspect of the task over the executive-control aspect.) Figure 2A shows a black-and-white rendition of a sample stimulus set for a single trial as it would appear on the computer screen if all the items remained visible. The size of each checkerboard was 3.2cm × 3.6cm; the checkerboards were separated by a horizontal gap of 4.7cm and a vertical gap of 1.3cm. In practice, only one pattern was shown at any time; the order of presentation was the conventional reading pattern for the English language: left to right, top to bottom. Presentation of successive stimuli in the top row was self-paced, initiated by pressing the space bar; from the second row on, participants pressed one of two keys to indicate their answer. The “/” key signified a match; the “z” key signified a mismatch. As soon as a key was pressed, presentation of the current stimulus was interrupted and the next stimulus appeared; presentation was thus self-paced. Viewing distance was not fixed; participants were allowed to choose the distance they felt most comfortable with. Participants were instructed to be both fast and accurate. All participants were tested in a single session of about 60–90min in duration. Participants were encouraged to take breaks between blocks. A total of 120 trials were presented, 20 trials for each value of C, yielding 1,080 responses (360 from the first row, 360 from the second row, and 360 from the third row).
Figure 2.
A sample trial from each of the experiments, at complexity level 2, if all the stimuli were to remain onscreen, with the correct answer added. Stimuli were shown one at a time, in a reading pattern (left to right, then on the next line, etc.); each column was depicted in a different color. Presentation times were subject paced. In Experiment 1 (panel A), stimuli for rows 2 and row 3 were compared with those in the row above it; in Experiment 3 (panel B), stimuli in rows 2–6 were compared with those in row 1.
Results and Discussion
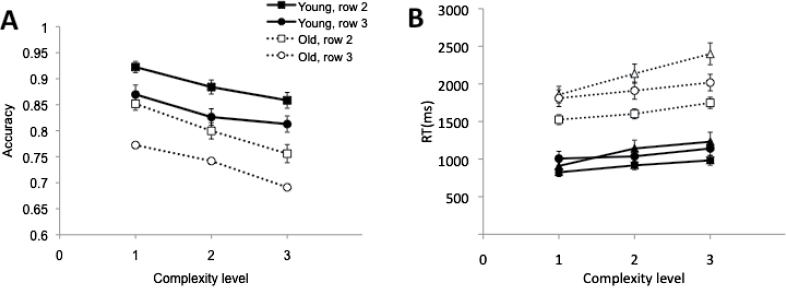
Accuracy
.—The main interest in the present experiment was on age-related differences in accuracy (Figure 3A). More specifically, we were interested in a potential row-by-age interaction, which would signify age-related difficulties with simultaneous memory access and memory transfer. In row 2, subjects access the memory representation of the stimuli remembered from row 1; they also transfer the new stimuli into the outer store. In row 3, they access the memory representation of the stimuli remembered from row 2. Thus, accuracy for row 2 reflects accuracy for items transferred into the outer store under full attention; accuracy for row 3 reflects accuracy for items transferred under divided attention (i.e., transfer in the presence of access). This result was not obtained: None of the interactions was significant, Fs < 2.27, ps > .11, 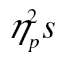 < 0.01; for the row-by-age interaction, F(1, 49) = 0.58, MSE = 0.01, p = .45,
< 0.01; for the row-by-age interaction, F(1, 49) = 0.58, MSE = 0.01, p = .45, 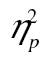 = 0.01. Thus, even with this complex stimulus set, older adults were not more susceptible to working memory dual-task effects than younger adults are.
= 0.01. Thus, even with this complex stimulus set, older adults were not more susceptible to working memory dual-task effects than younger adults are.
Figure 3.
Results from Experiment 1. Panels A and B depict accuracy and RT, respectively, as a function of stimulus complexity (1–3), separated by row and age group (young vs. old).
Analysis of variance (ANOVA) further revealed that, as expected, older adults were less accurate than younger adults, F(1, 49) = 30.51, MSE = 0.02, p < .001, 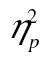 = 0.38, that accuracy was higher in row 2 than in row 3, F(1, 49) = 34.89, MSE = 0.01, p < .001,
= 0.38, that accuracy was higher in row 2 than in row 3, F(1, 49) = 34.89, MSE = 0.01, p < .001, 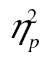 = 0.42, with a dual-task cost of 0.059 (0.845 vs. 0.786) on average, and that accuracy was lower for more complex shapes, F(2, 98) = 48.74, MSE < 0.01, p < .001,
= 0.42, with a dual-task cost of 0.059 (0.845 vs. 0.786) on average, and that accuracy was lower for more complex shapes, F(2, 98) = 48.74, MSE < 0.01, p < .001, 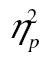 = 0.50.
= 0.50.
Response time.
—RT analyses were conducted on correct responses only. To remove outliers, RT distributions were truncated at three interquartile ranges above or below the median, to remove potential high-leverage points from the calculation of the mean RT. The percent of correct RTs which were removed as outliers was 2.4% in older adults and 2.1% in young adults.
Inspection of Figure 3B (RT as a function of age, complexity, and row) shows that older adults are generally slower than young adults. Additionally, for both young and older adults, the complexity effect appears larger in row 1 than in row 2 and row 3, which show a similar-sized effect. Additionally, row 2 RTs are generally elevated above row 3 RTs. These observations were confirmed in an ANOVA with stimulus complexity and row as within-subject factors and age group as the between-subject factor. The main effect of age was significant, F(1, 49) = 54.11, MSE = 1,569,955.03, p < .001, 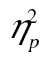 = 0.53. There was a significant main effect of row, F(2, 98) = 23.00, MSE = 214,257.16, p < .001,
= 0.53. There was a significant main effect of row, F(2, 98) = 23.00, MSE = 214,257.16, p < .001, 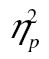 = 0.32, and of complexity, F(2, 98) = 38.85, MSE = 757,70.43, p < .001,
= 0.32, and of complexity, F(2, 98) = 38.85, MSE = 757,70.43, p < .001, 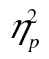 = 0.44, as well as a significant complexity-by-row interaction, F(4, 196) = 9.92, MSE = 46,124.99, p < .001,
= 0.44, as well as a significant complexity-by-row interaction, F(4, 196) = 9.92, MSE = 46,124.99, p < .001, 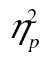 = 0.17. The figure suggests that rows 2 and 3 are parallel, and that row 1 has a steeper RT by complexity slope than rows 2 and 3. This was borne out in a formal analysis: The row-by-complexity effect was not significant for row 2 versus row 3, F(2, 98) = 0.22, MSE = 13,881.72, p = .80,
= 0.17. The figure suggests that rows 2 and 3 are parallel, and that row 1 has a steeper RT by complexity slope than rows 2 and 3. This was borne out in a formal analysis: The row-by-complexity effect was not significant for row 2 versus row 3, F(2, 98) = 0.22, MSE = 13,881.72, p = .80, 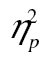 < 0.01; but it was for row 1 versus row 2, F(2, 98) = 13.86, MSE = 40,881.41, p < .001,
< 0.01; but it was for row 1 versus row 2, F(2, 98) = 13.86, MSE = 40,881.41, p < .001, 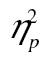 = 0.22, and row 1 versus row 3, F(2, 98) = 10.05, MSE = 44,650.54, p < .001,
= 0.22, and row 1 versus row 3, F(2, 98) = 10.05, MSE = 44,650.54, p < .001, 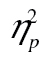 = 0.17. We also obtained a significant age-by-row interaction, F(2, 98) = 4.88, MSE = 214,257.16, p < .01,
= 0.17. We also obtained a significant age-by-row interaction, F(2, 98) = 4.88, MSE = 214,257.16, p < .01, 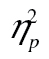 = 0.09. This interaction did not, however, survive logarithmic transformation, F(2, 98) = 0.96, MSE = 0.01, p = .39,
= 0.09. This interaction did not, however, survive logarithmic transformation, F(2, 98) = 0.96, MSE = 0.01, p = .39, 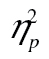 = 0.02. The three-way interaction was not significant, F(4, 196) = 1.25, MSE = 46,124.99,
= 0.02. The three-way interaction was not significant, F(4, 196) = 1.25, MSE = 46,124.99, 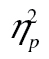 = 0.03.
= 0.03.
The elevated response time for row 1 replicates a result from Zhang and colleagues (2012). The finding could indicate one of two things: Either subjects are extra cautious while performing the transfer operation in row 1, where it is performed in isolation, or they move into an all-too-fast transferring mode in the subsequent row, where transfer occurs in the dual-task context of transfer plus access. We argue, as we did in Zhang and colleagues (2012), that the former interpretation is more likely. One indication of possible over-caution in row 1 is the lack of interaction between row and complexity in the accuracy data. That is, accuracy for the third row is determined (in part) by the complexity-insensitive transfer occurring in row 2, whereas accuracy for the second row is determined (in part) by the complexity-sensitive transfer occurring in row 1. If the longer transfer times for more complex stimuli observed in row 1 mattered, we would expect an accuracy drop for higher levels of complexity in row 3 compared with row 2. We did not observe this drop in either age group, suggesting that both groups are simply overly cautious when processing the row 1 stimuli.
Experiment 2
Method
Participants
.—Twenty-one older adults (Mage = 68.5, SDage = 7.0; Meducation = 15.5, SDeducation = 2.5) participated in return for a $10hr−1 fee. The 23 young adults (Mage = 20.4, SDage = 1.7; Meducation = 14.6, SDeducation = 2.0) participated in return for course credit or a $10hr−1 fee. None of our participants indicated being colorblind. As is usual for such samples, older adults had significantly lower Digit-Symbol Modalities scores than younger adults (45.57 vs. 59.91, t(42) = 2.54, p = .015), but higher Shipley Vocabulary scores (35.05 vs. 30.43, t(42) = −3.62, p < .001).
Procedure.
—Experiment 2 was identical to Experiment 1, with two exceptions: There were now six rows of three stimuli each, and comparisons in rows 2 to 6 were made to the stimuli from row 1 (Figure 2B).
Results and Discussion
Accuracy.
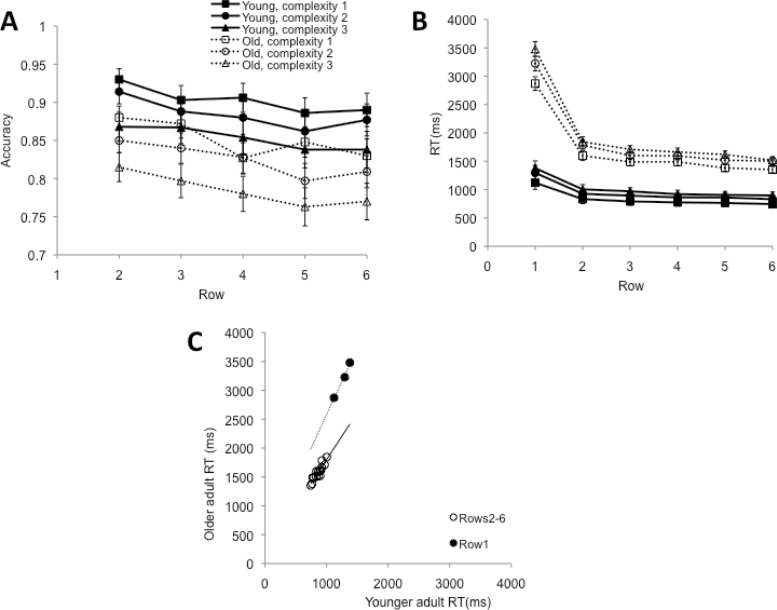
—The main interest in the present experiment was on age-related differences in accuracy (Figure 4A). More specifically, we were interested in the row-by-age interaction, which would signify age-related difficulties with resistance to interference. Subjects are required to retain and repeatedly access a memory representation of the initial row of three stimuli. As more and more stimuli intervene, interference is likely to build up, and if older adults would be more susceptible to the effects of such interference, we expect their performance to decline more steeply than that of younger adults.
Figure 4.
Results from Experiment 2. Panels A and B depict accuracy and RT, respectively, as a function of row (1–6), separated by stimulus complexity (1–3), and age group (young vs. old), respectively. Panel C is a Brinley plot, showing older adults’ RT as a function of young adults’ RT.
The data did show the expected interference effect—accuracy declines markedly over successive rows, F(4, 164) = 13.53, MSE < 0.01, p < .001, 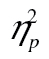 = 0.25. The size of the effect, however, is identical in younger and older adults, as signaled by the absence of a reliable age-by-row interaction and age by row-by-complexity level interaction, Fs < 1.09, ps > .37,
= 0.25. The size of the effect, however, is identical in younger and older adults, as signaled by the absence of a reliable age-by-row interaction and age by row-by-complexity level interaction, Fs < 1.09, ps > .37, 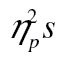 < 0.01. Note that the lines for younger and older adults in Figure 4A are very close to parallel; ours is indeed a null result not simply predicated on a lack of statistical power. Additionally, in accordance with Experiment 1, older adults were less accurate than younger adults, F(1, 41) = 5.78, MSE = 0.10, p = .02,
< 0.01. Note that the lines for younger and older adults in Figure 4A are very close to parallel; ours is indeed a null result not simply predicated on a lack of statistical power. Additionally, in accordance with Experiment 1, older adults were less accurate than younger adults, F(1, 41) = 5.78, MSE = 0.10, p = .02, 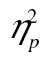 = 0.12, and more complex stimuli led to lower accuracy, F(2, 82) = 30.09, MSE = 0.01, p < .001,
= 0.12, and more complex stimuli led to lower accuracy, F(2, 82) = 30.09, MSE = 0.01, p < .001, 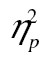 = 0.42.
= 0.42.
Response time
.—The percent of correct RTs removed as outliers was 2.2% for older adults and 1.5% for young adults (for the data, see Figure 4B and C). ANOVA revealed that older adults were slower, F(1, 41) = 100.00, MSE = 1.62 × 106, p < .001, 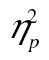 = 0.71, that more complex stimuli led to slower response time, F(2, 82) = 49.51, MSE = 97,373.28, p < .001,
= 0.71, that more complex stimuli led to slower response time, F(2, 82) = 49.51, MSE = 97,373.28, p < .001, 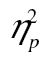 = 0.55, that response time decreased over rows, F(5, 205) = 155.26, MSE = 592,539.22, p < .001,
= 0.55, that response time decreased over rows, F(5, 205) = 155.26, MSE = 592,539.22, p < .001, 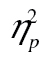 = 0.79, and that this decrease was larger for more complex stimuli, F(10, 410) = 8.32, MSE = 30,535.80, p < .001,
= 0.79, and that this decrease was larger for more complex stimuli, F(10, 410) = 8.32, MSE = 30,535.80, p < .001, 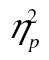 = 0.17. The complexity-by-age group interaction was not significant, F(2, 82) = 2.55, MSE = 97,373.28, p = .105,
= 0.17. The complexity-by-age group interaction was not significant, F(2, 82) = 2.55, MSE = 97,373.28, p = .105, 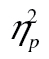 = 0.06, but the row-by-age group interaction was, F(5, 205) = 55.97, MSE = 592,539.22, p < .001,
= 0.06, but the row-by-age group interaction was, F(5, 205) = 55.97, MSE = 592,539.22, p < .001, 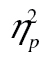 = 0.58, and so was the three-way interaction, F(10, 410) = 3.21, MSE = 30,535.80, p < .01,
= 0.58, and so was the three-way interaction, F(10, 410) = 3.21, MSE = 30,535.80, p < .01, 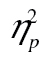 = 0.07. The row-by-age group interaction survived logarithmic transformation, F(5, 205) = 16.92, MSE = 0.11, p < .001,
= 0.07. The row-by-age group interaction survived logarithmic transformation, F(5, 205) = 16.92, MSE = 0.11, p < .001, 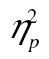 = 0.29, but neither the complexity-by-age group interaction, F(2, 82) = 0.95, MSE = 0.05, p = .39,
= 0.29, but neither the complexity-by-age group interaction, F(2, 82) = 0.95, MSE = 0.05, p = .39, 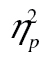 = 0.02, nor the three-way interaction, F(10, 410) = 0.61, MSE < 0.01, p = .80,
= 0.02, nor the three-way interaction, F(10, 410) = 0.61, MSE < 0.01, p = .80, 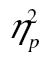 = 0.02, did. The age-by-row interaction was due to proportionally longer RTs in row 1, as illustrated in the Brinley plot in Figure 4C: When we restricted the analyses to rows 2–6, the row-by-age interaction on the logarithmic data was significant, F(4, 164) = 2.59, MSE = 0.01, p = .038,
= 0.02, did. The age-by-row interaction was due to proportionally longer RTs in row 1, as illustrated in the Brinley plot in Figure 4C: When we restricted the analyses to rows 2–6, the row-by-age interaction on the logarithmic data was significant, F(4, 164) = 2.59, MSE = 0.01, p = .038, 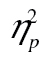 = 0.06, but the interaction now went against the hypothesis (i.e., a trend for smaller proportional age differences for later rows); other F values for interactions involving age < 1.00. Thus, older adults in this experiment took proportionally more time than younger adults to commit the stimuli of the first row to memory. This might be a strategic shift to ward off the effects of interference.
= 0.06, but the interaction now went against the hypothesis (i.e., a trend for smaller proportional age differences for later rows); other F values for interactions involving age < 1.00. Thus, older adults in this experiment took proportionally more time than younger adults to commit the stimuli of the first row to memory. This might be a strategic shift to ward off the effects of interference.
General Discussion
The present two experiments were motivated by a simple question, originating from a set of consistent findings from our lab, namely that compared with younger adults older adults show a marked accuracy decrease in retrieving items from working memory as soon as these items leave the focus of attention. More specifically, the study was motivated by our equally consistent failure in finding an external explanation for this deficit (Bopp & Verhaeghen, 2009; Lange & Verhaeghen, 2009; Vaughan et al., 2008; Verhaeghen & Basak, 2005). The list of failed accounts so far includes age-related slowing, age-related changes in retrieval dynamics, and age-related changes in the keeping-track (or source memory) requirement. The current experiments were designed to test two further hypotheses, namely (a) that the age-related drop in accuracy might be due to the dual-task requirement inherent in the kind of working memory tasks we (and many others) used in our previous work (viz., concurrent access and transfer vs. transfer or access only) and (b) that the age-related drop in accuracy might be due to an age-related decline in resistance to interference. Both of these mechanisms are plausible candidates for explaining the age-related deficit in working memory (e.g., dual-task performance is generally age-sensitive, e.g., Verhaeghen, 2011, and a decline in resistance to interference has been proposed as a root cause of working memory deficits, e.g., Hasher & Zacks, 1988), and their failure would thus be diagnostic.
Our results can be succinctly summarized by stating that both accounts were found lacking. Experiment 1 demonstrated that older adults’ dual-task cost were no larger than those of younger adults. Experiment 2 demonstrated that older adults were just as resistant to interference as younger adults. Experiment 2 additionally reconfirms the result from Experiment 1 that costs in accuracy can clearly arise when no dual-task situation is present, that is, when only access is required. Importantly, these two failures to obtain any age-related effects in the cognitive processes of interest were not failures to obtain the effects of interest themselves: In Experiment 1, we observed a clear dual-task cost, and in Experiment 2, we observed a clear build-up of interference. Neither were these failures to obtain age-related effects artifacts of low statistical power: In both instances, F values were low (around 1 or smaller), effect sizes were very small (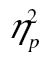 ≤ .01), and the figures show essentially parallel effects for both age groups. We also note that we gave the potential interactions plenty of opportunity to reveal themselves, using multiple levels of complexity in both experiments and a long series of interfering stimuli in the second.
≤ .01), and the figures show essentially parallel effects for both age groups. We also note that we gave the potential interactions plenty of opportunity to reveal themselves, using multiple levels of complexity in both experiments and a long series of interfering stimuli in the second.
We did observe one clear (and quite large) age effect in latency: In Experiment 2, older adults were disproportionately slower in their responses to the first row of stimuli. The most likely explanation is that older adults are overly cautious when transferring items into the outer store, taking much more time than is needed. This interpretation originates in Experiment 1, where the data suggested that the extra time taken by both younger and older adults in row 1 for transferring higher-complexity stimuli did not benefit performance for those stimuli compared with the performance for stimuli which were transferred without differential regard to complexity (i.e., row 3 accuracy). For a clear test of this hypothesis, however, we would need additional, targeted experiments, for instance one in which study time for the first row is restricted. The finding of larger age-related slowing in the first row of Experiment 1 also casts doubt on an age-differential speed-accuracy trade-off as a potential explanation for the lack of age effects in the dual-task cost: Older adults, compared with younger adults, speed up when transfer and access are combined, with apparently no ill results for the age-related difference in accuracy. At the very least, the data suggest a relative disconnect between accuracy data and response time data, an independence we have remarked on before (Verhaeghen & Basak, 2005).
If the lack of age-by-condition interactions in accuracy is the main result of our study, a second notable finding is the strong main effect of age on this measure. In both experiments, older adults performed strikingly less well than younger adults (Cohen’s d, or the mean standardized difference in accuracy between younger and older adults is 1.59 in Experiment 1 and 0.74 in Experiment 2). This result is remarkable, because the differences manifest themselves even when the task is relatively undemanding—simple retrieval of a stimulus encoded under full attention just a few seconds earlier, as in row 2 of either experiment. This initial age-related difference then remains constant both in the face of dual-task demands (Experiment 1) and in the face of increasing interference (Experiment 2).
Adding the latter result to our current and previous failures to find deeper explanations for the age-related decline in working memory accuracy makes it more and more tempting to ascribe these age-related differences to a fundamental, nonreducible deficit in a fundamental aspect of working memory functioning. We would like to reiterate that this was not a foregone conclusion: There were reasonable arguments to be made that each of the aspects we examined, here and in our previous work, could be at the heart of age-related deficits in working memory performance.
What do we now know about this purported fundamental deficit? At this point, we can only speculate, after exclusion of the five mechanisms described previously. It is clear that the age-related deficit has little to do with item accessibility—after general slowing is taken into account, older adults are typically just as fast as younger adults in accessing a memory representation (Lange & Verhaeghen, 2009), implying that they access the correct memory “slot.” Therefore, it must be due to item availability—how well or how much of the memory representation is preserved. The latter aspect of item maintenance has sometimes been labeled a representation’s “resolution” or clarity (Fukuda, Vogel, Mayr, & Awh, 2010). Perhaps, then, older adults have lower resolution, murkier, noisier working memory representations—a conjecture not out of line with venerable and mainstream ideas in cognitive aging (Ketcham and Stelmach, 2004; Li, Lindenberger, & Sikström, 2001; Welford, 1984). Testing the assertion that older adults’ working memory representations become noisier when items leave the focus of attention is precisely the subject of our current research. Additionally, if this age-related difference in the clarity of representations is indeed a fundamental cognitive deficit, one might expect it to cascade into more complex aspects of cognition. For instance, Kane, Conway, Miura, and Colflesh (2007) found that accuracy of retrieval from the outer store is correlated with performance on fluid intelligence tests; age-related differences in memory resolution might then explain some of the age-related variance in more assembled types of cognition (see also Verhaeghen, 2012). This too is on our laboratory’s list of future projects.
Funding
This work was supported by the National Institute of Health (AG-16201).
Acknowledgments
Parts of the research were presented at the 2012 Cognitive Aging Conference, Atlanta. We thank Patricia Hearons, Shivangi Jain, Saroja Malladi, and Didem Pehlivanoglu for help with the data collection.
References
- Baddeley A., Hitch G. J. (1974). Working memory. In Bower G. (Ed.), The psychology of learning and motivation, (Vol. 8, pp. 47–90). San Diego, CA: Academic Press; [Google Scholar]
- Bopp K. L., Verhaeghen P. (2005). Aging and verbal memory span: A meta-analysis. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, P223–P233.10.1093/geronb/60.5.P223 [DOI] [PubMed] [Google Scholar]
- Bopp K. L., Verhaeghen P. (2009). Working memory and aging: Separating the effects of content and context. Psychology and Aging, 24, 968–980.10.1037/a0017731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Li D. (2007). The roles of working memory updating and processing speed in mediating age-related differences in fluid intelligence. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 14, 631–646.10.1080/13825580600987660 [DOI] [PubMed] [Google Scholar]
- Cowan N. (1995). Memory theories from A to Z. Contemporary Psychology, 40, 552–555.10.1037/003723 [Google Scholar]
- Cowan N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. The Behavioral and Brain Sciences, 24, 87–185.10.1017/S0140525X01003922 [DOI] [PubMed] [Google Scholar]
- De Jong R. (2001). Adult age differences in goal activation and goal maintenance. European Journal of Cognitive Psychology, 13, 71–89.10.1080/09541440042000223 [Google Scholar]
- Fukuda K., Vogel E., Mayr U., Awh E. (2010). Quantity, not quality: The relationship between fluid intelligence and working memory capacity. Psychonomic Bulletin & Review, 17, 673–679.10.3758/17.5.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L. Zacks R, T. (1988). Working memory, comprehension, and aging: A review and new view. In G. H. Bower (Ed.), The psychology of learning and motivation: Advances in research and theory (Vol. 22, pp. 193–225). New York, NY: Academic Press.
- Kane M. J., Bleckley M. K., Conway A. R., Engle R. W. (2001). A controlled-attention view of working-memory capacity. Journal of Experimental Psychology. General, 130, 169–183.10.1037//0096-3445.130.2.169 [DOI] [PubMed] [Google Scholar]
- Kane M. J., Conway A. R., Miura T. K., Colflesh G. J. (2007). Working memory, attention control, and the N-back task: A question of construct validity. Journal of Experimental Psychology. Learning, Memory, and Cognition, 33, 615–622.10.1037/0278-7393.33.3.615 [DOI] [PubMed] [Google Scholar]
- Ketcham C. J., Stelmach G. E. (2004). Movement control in the older adult.. In Pew R. W., Van Hemel S. D. (Eds.), Technology for adaptive aging, (pp. 64–92). Washington DC: National Academies Press; [PubMed] [Google Scholar]
- Lange E. B., Verhaeghen P. (2009). No age differences in complex memory search: Older adults search as efficiently as younger adults. Psychology and Aging, 24, 105–115.10.1037/a0013751 [DOI] [PubMed] [Google Scholar]
- Li S.-C., Lindenberger U., Sikström S. (2001). Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences, 5, 479–486.10.1016/S1364-6613(00)01769-1 [DOI] [PubMed] [Google Scholar]
- Miyake A., Shah P. (1999). Toward unified theories of working memory: Emerging general consensus, unresolved theoretical issues, and future research directions.. In Miyake A., Shah P. (Eds.) Models of working memory: Mechanisms of active maintenance and executive control, New York, NY: Cambridge University Press; [Google Scholar]
- Oberauer K. (2002). Access to information in working memory: Exploring the focus of attention. Journal of Experimental Psychology. Learning, Memory, and Cognition, 28, 411–421.10.1037/0278-7393.28.3.411 [PubMed] [Google Scholar]
- Vaughan L., Basak C., Hartman M., Verhaeghen P. (2008). Aging and working memory inside and outside the focus of attention: Dissociations of availability and accessibility. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 15, 703–724.10.1080/13825580802061645 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. (2011). Aging and executive control: Reports of a demise greatly exaggerated. Current Directions in Psychological Sciences, 20, 174–180.10.1177/0963721411408772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P. (2012). Working memory still working: Age-related differences in working memory and executive control.. In: Ohta N., Naveh-Benjamin M. (Eds.), Memory and Aging, (pp. 3–30). New York, NY: Psychology Press; [Google Scholar]
- Verhaeghen P., Basak C. (2005). Ageing and switching of the focus of attention in working memory: Results from a modified N-back task. The Quarterly Journal of Experimental Psychology. A, Human Experimental Psychology, 58, 134–154.10.1080/ 02724980443000241 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P., Cerella J., Basak C. (2004). A working memory workout: How to expand the focus of serial attention from one to four items in 10 hours or less. Journal of Experimental Psychology. Learning, Memory, and Cognition, 30, 1322–1337.10.1037/0278-7393.30.6.1322 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P., Steitz D. W., Sliwinski M. J., Cerella J. (2003). Aging and dual-task performance: A meta-analysis. Psychology and Aging, 18, 332–339.10.1037/0882-7974.18.2.332 [DOI] [PubMed] [Google Scholar]
- Voigt S., Hagendorf H. (2002). The role of task context for component processes in focus switching. Psychologische Beiträge, 44, (S), 248–274 [Google Scholar]
- Welford A. T. (1984). Psychomotor performance. Annual Review of Gerontology & Geriatrics, 4, 237–273 [PubMed] [Google Scholar]
- Zhang Y., Verhaeghen P., Cerella J. (2012). Working memory at work: How the updating process alters the nature of working memory transfer. Acta Psychologica, 139, 77–83.10.1016/j.actpsy.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]