Abstract
Objectives.
Discrepancy between self-report- and actigraphy-measured sleep, often considered an artifact of measurement error, has been well documented among insomnia patients. Sleep problems are common among older adults, and this discrepancy may represent meaningful sleep-related phenomenon, which could have clinical and research significance.
Method.
Sleep discrepancy was examined in 4 groups of older adults (N = 152, mean age = 71.93 years) based on sleep complaint versus no complaint and presence versus absence of insomnia symptoms. Participants completed the Beck Depression Inventory-second edition (BDI-II) and 14 nights of sleep diaries and actigraphy.
Results.
Controlling for covariates, group differences were found in the duration and frequency of discrepancy in sleep onset latency (SOLd) and wake after sleep onset (WASOd). Those with insomnia symptoms and complaints reported greater duration and frequency of WASOd than the other 3 groups. Quantities of SOLd and WASOd were related to BDI-II score and group status, indicating that sleep discrepancy has meaningful clinical correlates.
Discussion.
Discrepancy occurred across all groups but was pronounced among the group with both insomnia symptoms and complaints. This discrepancy may provide a means of quantifying and conceptualizing the transition from wake to sleep among older adults, particularly those with sleeping problems.
Key Words: Depression, Insomnia, Older adults, Sleep, Sleep discrepancy
ADULTS aged more than 65 years make up 13% of the total U.S. population and have the highest rates of sleep disturbance for any age group (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). Chronic insomnia, defined as difficulty initiating or maintaining sleep, waking prematurely, or feeling unrefreshed upon awakening accompanied by sleep complaints lasting at least 6 months (National Institutes of Health, 2005), is among the most common and costly sleep disturbance in late life. Disturbed sleep is related to daytime consequences in older adults including decreased quality of life, cognitive difficulties, increased depression symptoms, and functional limitations (Buysse, Germain, & Moul, 2005; Nebes, Buysse, Halligan, Houck, & Monk, 2009; Newman, Enright, Manolio, Haponik, & Wahl, 1997). Epidemiological studies that estimate the prevalence of insomnia among older adults (~30%) is up to twice that of younger populations (Nau, McCrae, Cook, & Lichstein, 2005). Even when insomnia is absent, aging is often accompanied by declines in indices of sleep quality including sleep continuity, depth, and night-to-night consistency (Boselli, Parrino, Smerieri, & Terzano, 1998; Nau et al., 2005). There is evidence that some changes in sleep quality are more related to declines in physical health than age specifically, suggesting a gradual decline in sleep quality from through middle age, stabilization around retirement, and a further decline in sleep quality related to physical health in older adulthood (Lemola & Richter, 2012).
There is intragroup variability in age-related sleep changes and variability in older adults’ perceptions of sleep. For example, some older individuals spend relatively little time trying to fall asleep and have minimal wake time during the night yet complain of poor sleep initiation or maintenance. Conversely, some individuals evidence considerable sleep initiation or maintenance problems consistent with an insomnia diagnosis (i.e., >30min trying to fall asleep or return to sleep) but report no complaints. This pattern is observed when comparing insomnia symptoms to insomnia complaints in older adult men and women. Interestingly, although older adult men experience greater age-related changes in objectively measured sleep onset latency (SOL), older adult women have higher rates of sleep complaints (Vitiello, Larsen, & Moe, 2004). It is possible that age-related changes in sleep predispose older adults to develop insomnia symptoms, but without a subjective sleep complaint, a diagnosis of insomnia is unwarranted. Therefore, the perception of age-related sleep changes may be a primary factor in determining which individuals have insomnia complaints and ultimately meet criteria for insomnia in late life.
Adding complexity is the emergence of decreased correspondence between subjective accounts of sleep and objective measures of sleep (Spiegel, 1981). Both objective and subjective measures support documented age-related sleep changes (Buysse et al., 1991), yet these variables tend to correlate poorly at the daily level (Haimov, Breznitz, & Shiloh, 2006; McCrae et al., 2005; Spiegel, 1981). These different methods of measurement may capture unique aspects of sleep with differential predictive value in relation to daytime functioning and health in aging individuals. Subjective methods of measuring sleep (i.e., daily sleep diaries) play a major role in sleep research but have been critiqued for their inability to replicate the findings of objective measures of sleep (i.e., PSG and actigraphy; Haimov et al., 2006).
The characterization of discrepancy between objective and subjective measures as error is unfortunate because discrepancy may represent meaningful phenomena. Indeed, the utility of sleep discrepancy between actigraphy and self-report has been previously documented among older adults (McCrae et al., 2005). A comparison of these measures demonstrated that sleep complaint and symptom severity were able to be distinguished among groups of older adults based on the correlation between actigraphy and self-report (McCrae et al., 2005). It was found that in predicting concordance between actigraphy- and self-report-measured sleep, self-reported complaint about sleep was more important than magnitude of insomnia symptoms. Sleep discrepancy, defined as the differences between objective and subjective measures of sleep, has been previously observed among older adults and is particularly common among older adults with sleep complaints (Kay, Dzierzewski, Rowe, & McCrae, 2013). In younger samples, sleep discrepancy is thought to predispose, precipitate, or perpetuate insomnia (Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997). However, the relationship between sleep discrepancy and insomnia remains understudied among older adults. This study seeks to advance the literature by investigating the patterns and clinical correlates of sleep discrepancy among older adults.
This study investigated sleep discrepancy in older adults during two distinct sleep periods: the initial sleep attempt, also called SOL, and also during unwanted awake time during the night, called wake after sleep onset (WASO). The primary aim was to characterize the magnitude and the frequency of discrepancy among older adults depending on whether insomnia symptoms in sleep initiation or maintenance were reported and whether there was an insomnia complaint. We hypothesized that older adults with insomnia symptoms (>30min of SOL or WASO) and a sleep complaint would have greater sleep discrepancy than older adults without a sleep complaint but with comparable levels of insomnia symptoms. We further predicted that older adults with a sleep complaint would have greater discrepancy particularly during WASO, as this aspect of sleep tends to be most disturbed for older adults (Foley et al., 1995). Identifying differences across complaint status and sleep onset or maintenance serves to determine whether sleep discrepancy is more related to the complaint aspect of insomnia or to quantifiable sleep differences among older adults.
Method
Participants
This study involved secondary analyses on a database on sleep patterns in community-dwelling older adults compiled at the University of Florida Sleep Research Laboratory. The sample consisted of 152 adults aged more than 60 years (Mage = 71.93 years, SDage = 5.24 years) who lived in her/his own homes in North Central Florida region. Participants were independently recruited over two studies: a clinical trial of Cognitive Behavioral Therapy for insomnia among patients with late-life insomnia and a normative sample of older adults, which included individuals both with (22%) and without sleep complaints (McCrae et al., 2005). The analyzed subsample consisted of 142 participants, a majority of whom were European Caucasian (95%), female (52%), and college educated (Meducation = 16.19 years, SDeducation = 2.92 years).
Measures
Mini-Mental Status Examination (MMSE).
—Participants were screened for significant cognitive impairment using the MMSE (Folstein, Folstein, & McHugh, 1975).
Demographics and health survey.
—This survey consists of 13 items designed to collect information on demographics, sleep disorder symptoms, physical health, and mental health (Lichstein, Durrence, Riedel, Taylor, & Bush, 2004). Six health survey variables were used in this study: age, gender, sleep complaint, length of time with sleep problems, total number of health complaints, and total number of medications. Self-report sleep questions on the survey contained information on whether the participant had a sleep problem or if a bed partner noticed heavy snoring, difficulty breathing or gasping for breath, frequent leg jerks, restlessness before sleep onset, sleep attacks during the day, or paralysis at sleep onset. Number of health conditions was calculated by summing the number of selected items including heart attack, other heart problems, cancer, AIDS, hypertension, neurological disorder, breathing disorder, urinary problems, diabetes, pain, and gastrointestinal disorders.
Actigraphy.
—Objective sleep was measured using the Actiwatch-L (Mini Mitter, a Respironics Company, Bend, OR). The Actiwatch-L sampled data 32 times per second for 30-s epochs using an omnidirectional, piezoelectric accelerometer with a sensitivity of greater than o equal to 0.01 g-force. A sum of the peak activity counts for each 30-s epoch was downloaded to a PC and then analyzed by Actiware-Sleep vol. 3.3. (Mini Mitter, 2001). A validated algorithm was used to identify the activity of each epoch as wake or sleep (Oakley, 1997) and utilized a high-sensitivity setting with the threshold for wake set at 20 activity counts. For a full description of the wake/sleep determination algorithm, please see McCrae and colleagues (2005).
Actigraphy has criterion validity when compared with PSG (0.80) and high test-retest reliability (0.92; Ancoli-Israel et al., 2003). The high-sensitivity setting was used as this setting provides high correlations with PSG-measured total sleep time (TST; 0.95) for healthy older adults (Colling et al., 2000) and for TST (0.73) and SOL (0.93) for individuals with insomnia (Cook et al., 2004). Using the Actiware-Sleep v. 3.3 software (Mini Mitter, a Respironics Company), a number of sleep parameters were derived from the data including SOL and WASO. Definitions of objective sleep variables used in this study are SOL (interval between bedtime and sleep start) and wake time after sleep onset (WASO; time spent awake after initial sleep onset until last awakening). The subscripts “o” was used in this document to denote objective sleep variables.
Actigraphy-measured SOL or TST corresponds to PSG more closely than WASO. In one study, actigraphy systematically underestimated WASO in healthy sleepers compared with PSG (Cole, Kripke, Gruen, Mullaney, & Gillin, 1992). This finding was explained by the poorer resolution of actigraphy in detecting wake compared with detecting sleep. Nevertheless, in field studies, actigraphy remains a good measure of detecting WASO in comparison to PSG, detecting 88% of WASO events longer than 15 s (Horne, Pankhurst, Reyner, Hume, & Diamond, 1994). Overall, the error associated with actigraphy is not sufficient to impair its ability to characterize sleep discrepancy (Tryon, 2004), and due to its utility and low invasiveness, actigraphy has advantages for prolonged measurement of sleep discrepancy (Ancoli-Israel et al., 2003). Although the discrepancy between actigraphy and PSG has been attributed to error in the actigraphic measurement among insomnia participants (Ancoli-Israel et al., 2003), the idea that actigraphy may measure valid, yet distinct, aspects of sleep than measured by PSG or sleep diaries is rarely considered.
Sleep diaries.
—Subjective sleep was measured using standard daily sleep diaries (Lichstein, Riedel, & Means, 1999) completed by participants each morning for 14 consecutive days. Sleep diaries are commonly used in research, have been validated in several studies, and are accepted as scientifically useful measures of the subjective sleep (Espie, Inglis, Tessier, & Harvey, 2001). The sleep diaries ask two questions about sleep, which can be directly compared with actigraphic measures: “Time to fall asleep?” and “Wake time (middle of the night)?” Two subjective sleep measures were used in this study: SOLs (time from lights out until sleep onset) and WASOs (time spent awake after sleep onset until last awakening).
Beck Depression Inventory-Second Edition (BDI-II).
—Depression was measured using the BDI-II (Beck, Steer, & Brown, 1996). This is a 21-item measure with a scale ranging from 0 to 3 measuring the severity of depressive symptoms (3 being the most severe). Scores range from 0 to 63, with higher scores indicating more depression symptoms. Participants were asked to respond to the questions based on the previous 2 weeks to match the 2-week sleep and actigraphy recording period. The BDI-II has demonstrated sufficient internal consistency reliability (0.90) and concurrent validity (0.69; Storch, Roberti, & Roth, 2004).
Procedures
At the first appointment, participants provided informed consent in accordance with the standards of the University of Florida Institutional Review Board. Individuals completed a demographic and health survey and were administrated the MMSE (Folstein et al., 1975). Individuals were excluded for (a) significant cognitive impairment defined as an MMSE score of less than 23, if ninth-grade education or higher, or an MMSE score of less than 18, if less than ninth-grade education (Folstein et al., 1975; Murden, McRae, Kaner, & Bucknam, 1991), (b) self-endorsement of any other sleep disorders (e.g., sleep apnea, periodic limb movements, narcolepsy), (c) self-endorsement of severe psychiatric disorders (e.g., thought disorders or BDI > 24), (d) use of psychotropic or other medications known to alter sleep (e.g., β-blockers), and (e) medical conditions linked to impaired independent daily functioning (McCrae et al., 2005). Each participant was provided with sleep diaries and an Actiwatch-L (Mini Mitter, a Respironics Company). Participants were instructed to complete the diaries each morning upon awakening and to wear the watch both day and night for 2 weeks on the nondominant wrist. Participants returned to the laboratory at the end of each week for collection of sleep data. At the end of the second week, the BDI-II (Beck, Steer, & Brown, 1996) was completed by each participant.
Study Variables
Sleep discrepancy variables.
—Sleep discrepancy is defined as the difference in the amount of time an individual reports being awake in bed during SOL or WASO during the night compared with objective measures. Daily values for actigraphically measured SOL and WASO were subtracted from respective sleep diary estimates to calculate two SOL and two WASO misperception variables: number of days overestimated and average amount overestimated.
Raw daily subjective reports of SOL and WASO were compared with respective daily actigraphy measures to compute daily SOLd and WASOd (subscript “d” denotes sleep discrepancy). The equation for each variable is listed below:
 |
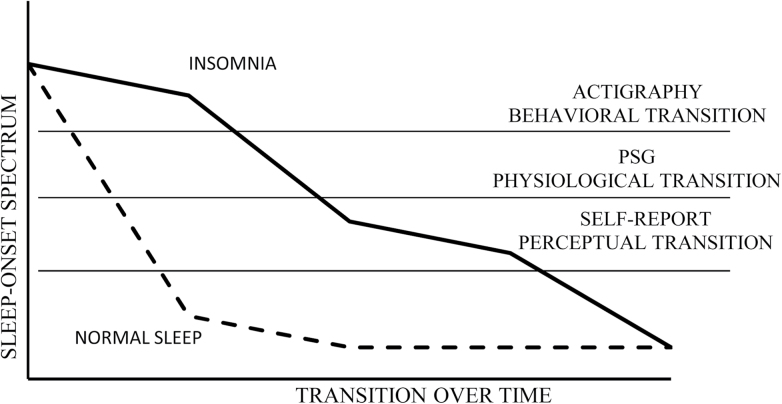
In this study, the term sleep discrepancy was used, rather than the construct of sleep misperception, based on the assertion that there is not necessarily a single valid measure of sleep. Thus, each measure captures different transitory stages from wakefulness to sleep, and differences between these measures are measurement discrepancy rather than incorrect perception on the part of the study subject (Figure 1). There are several sleep variables that may be interesting to investigate in terms of sleep discrepancy but were not included in this article. For example, discrepancy between self-reported TST and objective measures has been previously studied (Perlis et al., 1997; Perlis, Smith, Andrews, Orff, & Giles, 2001; Spiegel, 1981; Vanable, Aikens, Tadimeti, Caruana-Montaldo, & Mendelson, 2000). However, in this study, SOL and WASO discrepancy were specifically studied as these times of the night are particularly sensitive in older adults and have not been explored as often in previous research.
Figure 1.
The continuous sleep transition process during the beginning of the night among disordered and normal sleepers.
Sleep discrepancy frequency variables.
—The number of nights on which individuals’ subjective awake time estimates exceeded actigraphic measurements were used to calculate the number of nights with sleep onset latency (SOLf) or wake after sleep onset (WASOf) discrepancy.
Group variables.
—Participants were categorized as having insomnia symptoms if they reported greater than 30min of SOL or WASO on at least 6 out of 14 nights. These criteria are consistent with empirically supported experimental criteria for identifying insomnia symptoms (Lichstein, Durrence, Taylor, Bush, & Riedel, 2003). Participants were further categorized as complaining versus noncomplaining based on their responses to the question “Do you have a sleep problem? yes or no. These categorizations resulted in the following four groups: (a) insomnia symptoms with complaint, (b) no insomnia symptoms with complaint, (c) insomnia symptoms without complaint, and (d) no insomnia symptoms without complaint.
Data Analysis
All data were evaluated for normality and results showed relatively normally distributed data with some peaked kurtosis around the mean. Extreme outliers (>5 SD from the mean) were removed. Three individuals were excluded from analysis due to missing demographic information, three due to missing BDI-II data, two due to missing actigraphy data, and two individuals were found to be significant influential outliers (>5 SD) in sleep discrepancy variables. One-way analyses of covariance (ANCOVA) and chi-squared analyses were used to compare the four groups (insomnia symptoms with complaint, no insomnia symptoms with complaint, insomnia symptoms without complaint, and no insomnia symptoms without complaint) on demographic and health variables.
A one-way multivariate ANCOVA was conducted comparing the four groups in their average sleep discrepancy for more than 14 days in SOLd and WASOd along with subsequent pairwise comparisons. Bonferroni corrections were utilized to account for multiple comparisons in the multivariate analysis of variance and subsequent pairwise comparisons. In addition, the groups were compared in the average number of nights in which their subjective awake time estimates exceeded their actigraphic measurements the average number of nights when their sleep diary estimates were greater than actigraphic measurement on their sleep onset latency (SOLf) and wake after sleep onset (WASOf). The analysis controlled for depressive symptoms measured using the BDI-II, gender, and number of health problems. In preliminary analysis, number of health problems and number of medications were found to be strongly correlated and consequently, only the better predictor, number of health conditions, was retained in the final analysis.
Results
Preliminary analyses indicated significant group differences in gender, F(3, 139) = 3.29, p < .05), number of medical conditions, F(3, 139) = 11.87, p < .05, and BDI-II, F(3, 139) = 7.23, p < .05, and were included as covariates in subsequent analyses. See Table 1 for a summary of group demographic differences. Among those reporting sleep complaints, all participants self-reported chronic problems lasting 6 months and more (M = 10.8 years, SD = 13.1 years). A one-way multivariate analysis of covariance (using the Wilks’ criterion) of sleep complaint predicting frequency and intensity of sleep discrepancy, controlling for BDI-II score, gender, and number of medical conditions, revealed a significant group effect for sleep complaint, F(10, 132) = 9.07, p < .001, and for BDI-II score, F(10, 132) = 5.70, p < .001. Follow-up testing revealed significant group differences in the average discrepancy of SOL, F(7, 135) =9.43, p < .001, and WASO, F(7, 135) = 13.39, p < .001. In addition, there were group differences in the number of nights per week that individuals were discrepant on SOL, F(7, 135) = 3.95, p < .01, and WASO, F(7, 135) = 19.41, p < .001. See Table 2 for a summary. Individuals in the insomnia symptoms with complaint group reported more than three additional nights of WASO overestimation than the other groups. Individuals reporting insomnia symptoms, regardless of sleep complaint, had more nights of SOL overestimation (M = 9.01, SD = 3.13) than those not reporting symptoms (M = 6.63, SD = 3.19), t141 = −4.99, p < .001. In order to document the overlap between SOL and WASO discrepancy, correlations were calculated between SOL and WASO discrepancy among all groups. Only among individuals in the insomnia symptoms with complaint group did SOL discrepancy significantly correlate with WASO discrepancy, r(71) = .562, p < .001.
Table 1.
Demographic Variables Across Complaint and Insomnia Symptom Groups
| Gendera | Age (SD) | Number of medical conditions (SD)a | Number of medications (SD)a | BDI-II (SD)a | |
|---|---|---|---|---|---|
| No insomnia symptoms without complaint | 35 females and 20 males | 72.69 (6.75) | 1.58 (1.01) | 2.93 (2.19) | 4.38 (3.83) |
| No insomnia symptoms with complaint | 13 females and 7 males | 73.45(6.09) | 3.2 (1.85) | 4.15 (2.87) | 5.75 (4.64) |
| Insomnia symptoms without complaint | 12 females and 7 males | 71.84 (8.93) | 1.16 (1.21) | 1.95 (1.89) | 6.26 (6.045) |
| Insomnia symptoms with complaint | 18 females and 30 males | 69.62 (6.91) | 2.33 (1.49) | 2.95 (1.96) | 8.77 (7.81) |
Notes. BDI-II = Beck Depression Inventory-second edition; SD = standard deviation.
aIndicates significant overall group differences in demographic variable, p < .05.
Table 2.
Group Differences in Mean Sleep Discrepancy and Number of Nights Overestimating Awake Time
| N | SOLd (min) | SD | WASOd (min) | SD | SOLf | SD | WASOf | SD | |
|---|---|---|---|---|---|---|---|---|---|
| No insomnia symptoms without complaint | 55 | −2.54a | 10.82 | −34.74a | 21.56 | 6.72a | 3.29 | 0.89a | 1.34 |
| No insomnia symptoms with complaint | 20 | −2.67a | 12.68 | −23.66a | 20.13 | 6.85 | 3.29 | 1.34a | 2.41 |
| Insomnia symptoms without complaint | 19 | 8.92 | 8.81 | −32.73a | 34.22 | 9.94b,c | 1.99 | 2.00a | 2.64 |
| Insomnia symptoms with complaint | 42 | 16.84b | 21.17 | 11.87b | 36.92 | 9.1b | 3.03 | 6.43b | 3.53 |
Notes. BDI-II = Beck Depression Inventory-second edition; SD = standard deviation; SOLd indicates the mean amount of discrepancy in sleep onset latency; SOLf indicates the number of nights of subjective overestimation in sleep onset latency; WASOd indicates the mean amount of discrepancy in wake after sleep onset; WASOf indicates the number of nights of subjective overestimation of wake after sleep onset. Controlling for gender = 1.4485, number of medical conditions = 2.01, and BDI-II = 6.40
aSignifies significant difference from insomnia symptoms with complaint, p < .01.
bSignifies significant difference from no insomnia symptoms without complaint, p < .01.
cSignifies significant difference from no insomnia symptoms with complaint, p < .01.
As noted, mood was identified as a significant factor separating the four sleep groups, with the insomnia symptom with complaint group reporting the highest levels of depressive symptomatology. To further explore the role of mood in sleep discrepancy, a series of blocked multiple regressions were run by examining the discrepancy, demographic and health variables, sleep complaint/insomnia symptom group, BDI score, and the interaction of complaint/insomnia group and BDI score (see Tables 3 and 4). In the first model, variables were sequentially added to the regression equations to test for additional explanation of SOL discrepancy. In the first step, demographic and health variables were included to control for these variables and only age was significant. In the second step, sleep complaint/insomnia group was included and was a significant predictor of SOL discrepancy. In the third step of this model, BDI score was a significant predictor. However, in the final model after adding the interaction variable only, sleep complaint/insomnia group was a significant predictor, whereas neither BDI nor the interaction term were significant, F(6, 134) = 11.96, p < .001, r2 = .35. The loss of BDI as a significant predictor in the final step of the model suggests that the interaction between depressive symptoms and complaint group is a mediator of the relationship between BDI score and SOL discrepancy. A parallel model was built predicting WASO discrepancy. The first step revealed age and number of medical conditions as significant predictors. In the second step, complaint/insomnia group was a significant predictor. In the third step, complaint/insomnia group and BDI were significant predictors. Consistent with the previous model predicting SOL discrepancy, in the final step of the WASO discrepancy model, the complaint/insomnia group variable was significant, whereas the BDI score and the interaction term were not significant, F(6, 134) = 13.7, p < .001, r2 = .38. These results suggest that although complaint/insomnia group is the most predictive variable of sleep discrepancy, depression symptoms are also highly related. However, the combined effects of depression symptoms and complaint/insomnia group do not share a unique relationship with sleep discrepancy.
Table 3.
Multiple Regression Model Predicting Sleep Onset Latency (SOL) Discrepancy from Demographic, Mood, and Sleep Complaint Variables
| B | SE(B) | β | |
|---|---|---|---|
| Step 1 | |||
| Constant | 31.4 | 15.52 | |
| Age | −0.48 | 0.21 | −0.20* |
| Gender | 3.95 | 2.90 | 0.11 |
| Number of medical conditions | 1.54 | 1.01 | 0.13 |
| Step 2 | |||
| Constant | 7.01 | 14.32 | |
| Age | −0.27 | 0.19 | −0.11 |
| Gender | 122 | 2.31 | 0.03 |
| Number of medical conditions | 0.62 | 0.91 | 0.05 |
| Complaint/insomnia symptom group | 6.23 | 1.02 | 0.47* |
| Step 3 | |||
| Constant | 5.82 | 13.64 | |
| Age | −0.26 | 0.18 | −0.11 |
| Gender | 0.17 | 2.50 | 0.01 |
| Number of medical conditions | 0.15 | 0.88 | 0.01 |
| Complaint/insomnia symptom groups | 5.17 | 1.01 | 0.39* |
| BDI score | 0.81 | 0.21 | 0.29* |
| Step 4 | |||
| Constant | 5.59 | 13.63 | |
| Age | −0.20 | 0.18 | −0.09 |
| Gender | −0.29 | 2.54 | −0.01 |
| Number of medical conditions | 0.14 | 0.88 | 0.01 |
| Complaint/insomnia symptom group | 4.12 | 1.41 | 0.31* |
| BDI score | 0.17 | 0.63 | 0.06 |
| Interaction term | 0.20 | 0.19 | 0.28 |
Notes. BDI = Beck Depression Inventory-second edition.
Interaction term refers to the interaction between complaint/insomnia symptom group and BDI score.
R 2 for SOL Step 1 = 0.07, ΔR 2 = 0.2 for Step 2, ΔR 2 = 0.07 for Step 3, and ΔR 2 = 0.01 for Step 4 (all p < .05 except Step 4).
*p < .05.
Table 4.
Multiple Regression Model Predicting Wake After Sleep Onset (WASO) Discrepancy From Demographic, Mood, and Sleep Complaint Variables
| B | SE(B) | β | |
|---|---|---|---|
| Step 1 | |||
| Constant | 33.45 | 31.13 | |
| Age | −1.0 | 0.41 | −0.21* |
| Gender | 9.69 | 5.8 | 0.14 |
| Number of medical conditions | 4.09 | 1.99 | 0.17 |
| Step 2 | |||
| Constant | −20.15 | 27.77 | |
| Age | −0.54 | 0.36 | −0.11 |
| Gender | 3.23 | 5.06 | 0.05 |
| Number of medical conditions | 21.3 | 1.73 | 0.09 |
| Complaint/insomnia symptom group | 13.97 | 1.97 | 0.52* |
| Step 3 | |||
| Constant | −23.62 | 26.89 | |
| Age | −0.49 | 0.35 | −0.10 |
| Gender | 1.43 | 4.93 | 0.02 |
| Number of medical conditions | 1.37 | 1.69 | 0.06 |
| Complaint/insomnia symptom groups | 12.17 | 1.98 | 0.45* |
| BDI score | 1.33 | 0.41 | 0.23* |
| Step 4 | |||
| Constant | −23.86 | 26.98 | |
| Age | −0.46 | 0.37 | −0.10 |
| Gender | 1.15 | 5.02 | 0.02 |
| Number of medical conditions | 1.36 | 1.69 | 0.06 |
| Complaint/insomnia symptom group | 11.52 | 2.78 | 0.43* |
| BDI score | 0.93 | 1.26 | 0.17 |
| Interaction term | 0.13 | 0.37 | 0.09 |
Notes. BDI = Beck Depression Inventory.
Interaction term refers to the interaction between complaint/insomnia symptom group and BDI score.
R 2 for WASO Step 1 = 0.08, ΔR 2 = 0.25 for Step 2, ΔR 2 = 0.05 for Step 3, and ΔR 2 = 0.001 for Step 4 (all p < .05 except Step 4).
*p < .05.
Discussion
Sleep Discrepancy and Aging
Although aging is correlated with greater SOL and WASO discrepancy, the direction of this result was not as anticipated. Previous research has demonstrated that actigraphy identifies an increased quantity of WASO relative to self-report (Kushida et al., 2001). We found that this pattern significantly intensifies with increasing age and may generalize to SOL in older adults. Buysse and colleagues (1991) observed that healthy older adults adapt their subjective evaluations of sleep quality to objective age-related sleep changes. Although older adults in general may not perceive all events that would be defined as arousals based on objective measures (Boselli et al., 1998), older adults with self-reported insomnia may have increased awareness of objectively defined arousals and thus report increased insomnia symptoms.
Sleep Discrepancy, Insomnia Symptoms, and Sleep Complaints
A direct correlation between age and positive sleep discrepancy emerged, yet when group membership was added to the model, this association was no longer significant. This highlights the importance of considering interindividual differences in the association between objective and subjective sleep in late life. Although objective measures have been shown to be more related to chronological age (Foley et al. 1995), subjective measures are more predictive of health and insomnia in late life (Buysse et al., 1991; McCrae et al., 2008). In younger samples, overestimates of WASO and SOL by individuals relative to objective measures have been linked to insomnia complaints. Yet other studies suggest that this is not a generic feature of all individuals with insomnia (Means, Edinger, Glenn, & Fins, 2003). This is the first study to describe the frequency and severity of sleep discrepancy across insomnia symptoms and complaint status groups in older adults. These results suggest that sleep discrepancy is a defining feature of older adults who have both insomnia symptoms and who perceive this poor sleep as problematic (i.e., have a complaint). Our study provides evidence that late-life sleep complaint does not necessarily indicate a general propensity to misperceive sleep. This can be seen in the relatively low mean levels and frequency of discrepancy among the complaint without insomnia symptom group compared with the insomnia with complaint group.
Research in younger samples has identified considerable between-person differences in sleep discrepancy tendencies (Baker, Maloney, & Driver, 1999) largely explained by a moderately consistent tendency to overestimate SOL and underestimate TST (Means et al., 2003). In contrast, our sample of older individuals with insomnia symptoms and complaint, on average, had 5 of 14 days during which underestimates of SOL occurred and 7 of 14 during which underestimates of WASO occurred. Collectively, the results of this study do not support a consistent “trait-like” tendency for individuals with insomnia symptoms or complaint to overestimate sleep difficulties.
Possible Mechanisms of Sleep Discrepancy in Late Life
The neurocognitive model posits that relatively heightened brainwave activity during the transition to sleep may enhance information processing and contribute to greater sleep onset discrepancy and possibly WASO discrepancy (Perlis et al., 1997). In a younger adult sample, Perlis and colleagues (2001) found that more beta activity (characteristic of normal wakefulness) during non-REM sleep was significantly related to TST discrepancy and was trended toward greater SOL discrepancy but was not related to WASO discrepancy. Although these findings may suggest that SOL discrepancy and WASO discrepancy have unique mechanisms, very little variability in WASO discrepancy was present in their data. In this study, older adults with complaint and insomnia symptoms were most clearly differentiated from other groups by middle of the night events, including both the relative duration of WASO in a night and the number of nights that an increased duration of WASO was perceived. Given that WASO is far more common in older adults, additional research is needed to determine if beta activity is similarly related to SOL discrepancy and WASO discrepancy in older adults.
Depression Symptoms and Sleep Discrepancy
Prior research in younger adults has found mixed relationships between sleep discrepancy and depression (Edinger & Fins, 1995; Perlis et al., 2001; Vanable et al., 2000). We found that depressive symptoms were related to greater sleep discrepancy. One possible explanation for this pattern is that sleep discrepancy results from a distorted perception (Harvey, 2002) or bias to overreport sleep difficulties among depressed individuals (Vanable et al., 2000). Discrepancy was not limited to individuals with depressive symptoms in our study, and this finding adds to the argument that sleep discrepancy cannot be explained exclusively by a general exaggeration bias (Perlis, Gehrman, Pigeon, Findley, & Drummond, 2009). Nevertheless, the explanation that bias or distortions are specific only to periods of sleep that provide individuals with learned cues or markers (Sewitch, 1984), which lead to situation-specific sleep discrepancy, remains possible.
Another possibility is that sleep discrepancy reflects a true sleep disturbance localized to specific brain regions that has negative effects on mood. Consistent with the neurocognitive theory of insomnia (Riemann et al., 2010), recent functional neuroimaging studies have linked depression with heightened cortical arousal during sleep (reviewed in Drummond, Smith, Orff, Chengazi, & Perlis, 2004). As has been previously hypothesized (Merica, Blois, & Gaillard, 1998), we posit that sleep discrepancy reflects a localized sleep disturbance, which affects brain networks involved in perception of self, neurocognitive processing, and time awareness. This localized pattern may result from cortical arousal, desynchronized/low homeostatic sleep drive in localized areas of the brain, or impaired disengagement from the waking state specific to localized brain networks. From this perspective, sleep discrepancy can be conceptualized as a proxy measure for localized sleep deprivation that may lead to specific sleep-related impairments, such as depressed mood.
Clinical Relevance
Sleep discrepancy is an important consideration when assessing sleep disturbances in older adults. Older adults are more likely than younger individuals to be prescribed hypnotic medications for insomnia. These prescriptions are written and consumed based on subjective qualitative and quantitative sleep and not based on objective sleep (Haimov et al., 2006). Likewise sleep medications have been shown to improve self-reported insomnia symptoms greater than objective measures of sleep difficulty (reviewed in Perlis et al., 1997). It is possible that hypnotic medications have a more direct impact on sleep discrepancy and subjective reports than on objective sleep. Although some participants in this study used hypnotic medications, all participants were required to be on a stable dose for at least 6 months prior to participation. Thus, the impact of these medications on the current findings should be minimized. Future research could specifically examine the impact of sleeping medication on discrepancy between subjective and objective sleep.
Psychological treatment of insomnia enhances depression outcomes in patients with comorbid insomnia and major depression (Manber et al., 2008) and has a greater impact on subjective sleep compared with objective sleep (reviewed in McCrae et al., 2008). Conversely, treating depression does not always lead to improvements in perceptions of sleep (i.e., insomnia often persists after depression has been successfully treated). This differential effect supports the view that targeted treatment of sleep discrepancy may facilitate treatment and improve outcomes particularly among patients with depression. Although treatments targeting perceptions of sleep have shown promise in treating insomnia and its consequences (Tang & Harvey, 2004); these approaches have not been widely adopted and the long-term benefits of changing perceptions of sleep without changing sleep have not be demonstrated. It is possible that the elimination of the perception of a sleep problem may leave an underlying sleep disturbance intact. Clinical research is needed to determine if nondrug treatments, such as Cognitive Behavioral Therapy (CBT) for insomnia, have an impact on sleep discrepancy and to determine the impact improvements in sleep discrepancy may have on insomnia symptoms, sleep complaints, and mood.
Limitations
Because this study did not utilize EEG recording, we were unable to fully differentiate the transitory stages from wakefulness to sleep as has been described by other researchers (Tryon, 1996) and consequently direct comparison to previous studies using PSG is not possible. However, given the high correlations previously found between actigraphy and PSG, our study provides an approximation. The state thought to be measured by PSG would be hypothesized to fall between the events measured in this study (Figure 1). A comparison of life-span objective and subjective measures of sleep showed a nearly identical pattern using PSG, with average discrepancy values in the 20-min range (Spiegel, 1981). However, this relationship becomes less systematic with age. Although it is tempting to draw causal conclusions about the relationship between sleep discrepancy and depressive symptoms, it is not possible to determine the role that each plays in this study. Ultimately, the observed relationship is correlational, but it can be stated that there is a positive relationship between sleep discrepancy and depression symptoms. This observation warrants more detailed study that might also include the measurement of discrepancy, arousal, and more detailed assessment of mood states.
It should also be noted that the sample in study was predominately Caucasian and well-educated older adults. Whether the same patterns of discrepancy would be true of individuals identifying with minority groups or less educated individuals is unknown. However, given that sleep complaints have been found to be more common among African Americans (Lichstein et al., 2004), this group may experience higher rates of sleep discrepancy.
Conclusions
This study provides insight into how older adults perceive sleep and how this perception varies with the degree to which sleep is noted as a problem. These results highlight the promise of assessing underlying processes through the use of complementary measurement, rather than a single measure. Across designations of sleep complaint, older adults report varying degrees of correspondence between their subjective reports and behavioral measurement, with the greatest levels of complaint corresponding to the greatest amounts of measurement discrepancy. Increased levels of mood disturbance symptoms were found to correspond with increases in the observed measurement discrepancy. Although these results do not provide conclusive evidence for the perceptual factors involved in insomnia, they suggest that measurable difference do exist between different sleep complaint types and that meaningful clinical predictors are associated with these differences. These findings may have implications for the assessment and treatment of both mood and sleep problems in older adults. However, further research is needed to clarify the relationship between mood disturbances, sleep physiology, and sleep perception.
Funding
This work was supported by Award Number R21 AG02445 from the National Institute on Aging at the National Institutes of Health (C. S. McCrae, PhD, PI).
References
- Ancoli-Israel S., Cole R., Alessi C., Chambers M., Moorcroft W., Pollak C. P. (2003). The role of actigraphy in the study of sleep and circadian rhythms. Sleep, 26, 342–392 [DOI] [PubMed] [Google Scholar]
- Baker F. C., Maloney S., Driver H. S. (1999). A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. Journal of Psychosomatic Research, 47, 335–341.10.1016/S0022-3999(99)00017-3 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Brown G.K. (Eds.). (1996). Beck Depression Inventory-Second Edition. San Antonio, TX: The Psychological Corporation [Google Scholar]
- Boselli M., Parrino L., Smerieri A., Terzano M. G. (1998). Effect of age on EEG arousals in normal sleep. Sleep, 21, 351–357 [PubMed] [Google Scholar]
- Buysse D. J., Germain A., Moul D. E. (2005). Diagnosis, epidemiology and consequences of insomnia. Primary Psychiatry, 12, 37–44 [Google Scholar]
- Buysse D. J., Reynolds C. F., Monk T. H., Hoch C. C., Yeager A. L., Kupfer D. J. (1991). Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep, 14, 331–338 [PubMed] [Google Scholar]
- Cole R. J., Kripke D. F., Gruen W., Mullaney D. J., Gillin J. C. (1992). Automatic sleep/wake identification from wrist activity. Sleep, 15(5), 461–469 [DOI] [PubMed] [Google Scholar]
- Colling E., Wright M., Lahr S., Schmedlen L., DeJongh L., Singer C., Sack R. (2000). A comparison of wrist actigraphy with polysomnography as an instrument of sleep detection in elderly persons. Sleep, 23, A378
- Cook K. G., Lichstein K. L., Donaldson J., Nau S. D., Lester K. W., Aguillard R. N. (2004, June). An exploratory validation of actigraphic measures of insomnia. Paper presented at the meeting of the Associated Professional Sleep Societies; Philadelphia, PA: [Google Scholar]
- Drummond S. P., Smith M. T., Orff H. J., Chengazi V., Perlis M. L. (2004). Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Medicine Review, 8, 227–242.10.1016/j.smrv.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Edinger J. D., Fins A. I. (1995). The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep, 18(4), 232–239 [DOI] [PubMed] [Google Scholar]
- Espie C. A., Inglis S. J., Tessier S., Harvey L. (2001). The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: Implementation and evaluation of a sleep clinic in general medical practice. Behaviour Research and Therapy, 39, 45–60.10.1016/S0005-7967(99)00157-6 [DOI] [PubMed] [Google Scholar]
- Foley D. J., Monjan A. A., Brown S. L., Simonsick E. M., Wallace R. B., Blazer D. G. (1995). Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep, 18, 425–432 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198.10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Haimov I., Breznitz N., Shiloh S. (2006). Sleep in healthy elderly: Sources of discrepancy between self-report and recorded sleep. In: VM Kumar, HN Mallick, Eds. Clinical and neurophysiological aspects of sleep. Medimond: International Proceedings, 145–148 [Google Scholar]
- Harvey A. G. (2002). A cognitive model of insomnia. Behaviour Research and Therapy, 40, 869–893.10.1016/S0005-7967(01)00061-4 [DOI] [PubMed] [Google Scholar]
- Horne J. A., Pankhurst F. L., Reyner L. A., Hume K., Diamond I. D. (1994). A field study of sleep disturbance: Effects of aircraft noise and other factors on 5,742 nights of actimetrically monitored sleep in a large subject sample. Sleep, 17, 146–159 [DOI] [PubMed] [Google Scholar]
- Kay D. B., Dzierzewski J. M., Rowe M. A., McCrae C. S. (2013). Greater night-to-night variability in sleep discrepancy among older adults with a sleep complaint compared to noncomplaining older adults. Behavioral Sleep Medicine, 11(2), 76–90. 10.1080/ 15402002.2011.602775 [DOI] [PubMed] [Google Scholar]
- Kushida C. A., Chang A., Gadkary C., Guilleminault C., Carrillo O., Dement W. C. (2001). Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Medicine, 2, 389–396.10.1016/S1389-9457(00)00098-8 [DOI] [PubMed] [Google Scholar]
- Lemola S., Richter D. (2012). The course of subjective sleep quality in middle and old adulthood and its relation to physical health. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences.10.1093/geronb/gbs113 [DOI] [PubMed] [Google Scholar]
- Lichstein K. L., Durrence H. H., Riedel B. W., Taylor D. J., Bush A. J. (2004). Epidemiology of sleep: Age, gender, and ethnicity. Mahwah, NJ: Erlbaum; [Google Scholar]
- Lichstein K. L., Durrence H. H., Taylor D. J., Bush A. J., Riedel B. W. (2003). Quantitative criteria for insomnia. Behaviour Research and Therapy, 41, 427–445.10.1016/S0005-7967(02)00023-2 [DOI] [PubMed] [Google Scholar]
- Lichstein K. L., Riedel B. W., Means M. K. (1999). Psychological treatment of late-life insomnia. In Schulz R., Maddox G., Lawton M. P. (Eds.), Annual review of gerontology and geriatrics: Vol. 18. Focus on interventions research with older adults, (pp. 74–110). New York: Springer; [Google Scholar]
- Manber R., Edinger J. D., Gress J. L., San Pedro-Salcedo M. G., Kuo T. F., Kalista T. (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep, 31, 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae C. S., McNamara J. P., Rowe M. A., Dzierzewski J. M., Dirk J., Marsiske M., Craggs J. G. (2008). Sleep and affect in older adults: using multilevel modeling to examine daily associations. J Sleep Res, 17(1), 42–53.10.1111/j.1365-2869.2008.00621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae C. S., Rowe M. A., Tierney C. G., Dautovich N. D., Definis A. L., McNamara J. P. (2005). Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. The Journal of Gerontology Series B: Psychological Sciences and Social Sciences, 60, P182–P189.10.1093/geronb/60.4.P182 [DOI] [PubMed] [Google Scholar]
- Means M. K., Edinger J. D., Glenn D. M., Fins A. I. (2003). Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Medicine, 4, 285–296.10.1016/S1389- 9457(03)00057-1 [DOI] [PubMed] [Google Scholar]
- Merica H., Blois R., Gaillard J. M. (1998). Spectral characteristics of sleep EEG in chronic insomnia. European Journal of Neuroscience, 10, 1826–1834 [DOI] [PubMed] [Google Scholar]
- Murden R. A., McRae T. D., Kaner S., Bucknam M. E. (1991). Mini-Mental State exam scores vary with education in blacks and whites. Journal of the American Geriatrics Society, 39, 149–155 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (2005). State of the science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep, 28, 1049––1057 [DOI] [PubMed] [Google Scholar]
- Nau S. D., McCrae C. S., Cook K. G., Lichstein K. L. (2005). Treatment of insomnia in older adults. Clinical Psychology Review, 25, 645–672.10.1016/j.cpr.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Nebes R. D., Buysse D. J., Halligan E. M., Houck P. R., Monk T. H. (2009). Self-reported sleep quality predicts poor cognitive performance in healthy older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 64, 180–187.10.1093/geronb/gbn037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. B., Enright P. L., Manolio T. A., Haponik E. F., Wahl P. W. (1997). Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: The Cardiovascular Health Study. Journal of the American Geriatrics Society, 45, 1–7 [DOI] [PubMed] [Google Scholar]
- Oakley N. R. (1997). Validation with polysomnography of the sleep-watch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. [Google Scholar]
- Ohayon M. M., Carskadon M. A., Guilleminault C., Vitiello M. V. (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep, 27, 1255–1273 [DOI] [PubMed] [Google Scholar]
- Perlis M. L., Gehrman P., Pigeon W. R., Findley J., Drummond S. (2009). Neurobiologic mechanisms in chronic insomnia. Sleep Medicine Clinics, 4, 549–558.10.1016/j.jsmc.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis M. L., Giles D. E., Mendelson W. B., Bootzin R. R., Wyatt J. K. (1997). Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. Journal of Sleep Research, 6, 179–188.10.1046/j.1365-2869.1997.00045.x [DOI] [PubMed] [Google Scholar]
- Perlis M. L., Smith M. T., Andrews P. J., Orff H., Giles D. E. (2001). Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep, 24, 110–117 [DOI] [PubMed] [Google Scholar]
- Riemann D., Spiegelhalder K., Feige B., Voderholzer U., Berger M., Perlis M., Nissen C. (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Review, 14, 19–31.10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Sewitch D. E. (1984). The perceptual uncertainty of having slept: The inability to discriminate electroencephalographic sleep from wakefulness. Psychophysiology, 21, 243–259 [DOI] [PubMed] [Google Scholar]
- Spiegel R. (1981). Sleep and sleeplessness in advanced age. New York: Kluwer Academic Publishers; [Google Scholar]
- Storch E. A., Roberti J. W., Roth D. A. (2004). Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depression and Anxiety, 19, 187–189.10.1002/da.20002 [DOI] [PubMed] [Google Scholar]
- Tang N. K., Harvey A. G. (2004). Correcting distorted perception of sleep in insomnia: A novel behavioural experiment? Behaviour Research and Therapy, 42, 27–39.10.1016/S0005-7967(03)00068-8 [DOI] [PubMed] [Google Scholar]
- Tryon W. W. (1996). Nocturnal activity and sleep assessment. Clinical Psychology Review, 16, 197–213.10.1016/0272-7358(95) 00059-3 [Google Scholar]
- Tryon W. W. (2004). Issues of validity in actigraphic sleep assessment. Sleep, 27, 158–165 [DOI] [PubMed] [Google Scholar]
- Vanable P. A., Aikens J. E., Tadimeti L., Caruana-Montaldo B., Mendelson W. B. (2000). Sleep latency and duration estimates among sleep disorder patients: Variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep, 23, 71–79 [PubMed] [Google Scholar]
- Vitiello M., Larsen L., Moe K. (2004). Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. Journal of Psychosomatic Research, 56, 503–510.10.1016/S0022-3999(04)00023-6 [DOI] [PubMed] [Google Scholar]