Abstract
Aims
Endothelin (ET) receptor A antagonism decreases neuronal damage in experimental models of stroke. Since large arteries like basilar artery contribute significantly to total cerebrovascular resistance and are major determinants of microvascular pressure, dysregulation of basilar artery function may worsen stroke injury. ET-1 is involved in the regulation of basilar constriction. However, whether stroke influences vasoreactivity of basilar artery and to what extent ET-1 contributes to basilar vascular dysfunction after stroke remained unknown. The goal of this study was to test the hypothesis that ET-1 impairs basilar artery vasorelaxation after ischemia/reperfusion (I/R) injury via activation of ETA receptor.
Main methods
Male Wistar rats were subjected to 3/21 h middle cerebral artery occlusion (MCAO)/reperfusion surgery and one group received ETA receptor antagonist atrasentan (5 mg/kg) ip at reperfusion. At 24 h, basilar arteries were isolated from control non-stroked, stroked and stroked + atrasentan treated animals for vascular reactivity measurements using pressurized arteriograph.
Key findings
Acetylcholine (Ach)-induced maximum relaxation (Rmax) was decreased in stroked animals as compared to non-stroked group and ETA antagonism partially restored it. There was also a trend for decreased EC50 value for the antagonist treatment group indicating improved Ach sensitivity.
Significance
These findings suggest that ischemia/reperfusion not only affects vessels distal to the occlusion but also impairs relaxation of proximal large vessels. ET-1-mediated basilar artery dysfunction may contribute to neurovascular damage after stroke and early restoration of vascular function by ET receptor antagonism after I/R injury may offer a therapeutic strategy.
Keywords: Ischemia/reperfusion, Basilar artery (Rat), Vascular reactivity, Endothelin-1, Receptor antagonist
Introduction
Vascular endothelium plays an important role in regulating vascular tone, coagulation and smooth muscle growth. Endothelin-1 (ET-1) generated by vascular endothelial cells and vascular smooth cells is a potent vasoconstrictor with mitogenic and profibrotic properties (Inoue et al. 1989). ET-1 can exert opposing vascular effects via two G protein-coupled receptor subtypes, ETA and ETB. Activation of ETA receptors on vascular smooth muscle cells lead to vasoconstriction, while stimulation of ETB receptors on endothelial cells promote vasodilation via stimulation of nitric oxide production (Edvinsson and Povlsen 2011; Ergul 2002; Haynes and Webb 1998). Therefore abnormal regulation of ET-1 system contributes to the pathophysiology of various cardiovascular diseases including ischemia reperfusion injury (Ergul 2002). Many studies showed increased ET levels after ischemic stroke both in patients and experimental models (Barone et al. 1994; Thampatty et al. 2011; Ziv et al. 1992) and that ETA receptor antagonists have neuroprotective effects in both mechanical and embolic models of stroke (Matsuo et al. 2001; Patel et al. 1996). Whether this effect is due to direct neuronal protection and/or improvement of vascular function is not fully understood.
Cerebrovascular autoregulation is crucial for maintaining constant blood flow during changes of perfusion pressure (Owens 2011). In the brain, large extracranial and intracranial arteries e.g. basilar arteries contribute significantly to vascular resistance which attenuates changes in downstream microvascular pressure during increases in systemic arterial pressure (Cipolla 2009). Therefore any alterations in the reactivity of those vessels may lead to disturbance of this protective mechanism and could contribute to cerebrovascular disease. Accordingly, the goals of the current study were to determine: 1) the impact of reperfusion injury on ET-1-mediated vasoconstriction and endothelium- dependent relaxation of basilar arteries, and 2) the role of ETA receptors in this response in an experimental model of stroke.
Materials and methods
Animals and experimental groups
This study was conducted in accordance with National Instituted of Health guidelines for the care and use of animals in research and under protocols approved by the Division of Laboratory Animal Services at the Georgia Health Sciences University. Male Wistar rats (Harlan Laboratories, Indianapolis, IN) were housed four per cage on a 12-h light/dark cycle.
Model of ischemia and drug treatment
Experimental groups included 1. Control (C, no stroke, n=8), 2. Stroke (S, stroke +vehicle, n=10), and 3. Atrasentan (A, stroke+ atrasentan, n=8). Transient middle cerebral artery occlusion (MCAO) was induced as previously described (Ergul et al. 2007). The cerebral perfusion was measured with a PIM3 laser Doppler scanning system (Perimed, Stockholm, Sweden) to evaluate basal perfusion as well as perfusion changes following MCAO to confirm successful occlusion. At the end of ischemia (3 hr MCAO), the animal was briefly anesthetized, atrasentan (5 mg/kg) was given by intraperitoneal injection, and reperfusion was initiated by filament withdrawal.
Pressurized arteriograph system and basilar artery preparation
At 24 hours after ischemia, the animals were anesthesized and perfused with 100 ml of 0.01 M phosphate buffered saline (PBS). Basilar arteries isolated from control, stroked or stroked treated rats were mounted on glass cannulas in an arteriograph chamber containing 5 ml HEPES buffer (NaCl, 130mM; KCl 4 mM; MgSO4 1.2 mM; NaHCO3 4 mM; HEPES 10 mM; KH2PO4 1.18 mM; Glucose 5.5 mM) and the vessels were pressurized at 60 mmHg for 1 hour. The Living Systems arteriograph chamber was connected to a temperature controller that kept HEPES buffer at a constant temperature 37±0.5 during the whole experiment. Lumen diameter was measured with a video dimension analyzer both at the beginning and at the end of the 1 hour equilibration.
Vascular reactivity studies
Basilar lumen diameters of control, stroked and stroked rats treated with atrasentan were measured after 2 min of exposure to increasing concentrations of ET-1 (2*10−9, 10−8, 2*10−8, 10−7, 1.5*10−7, 2*10−7 and 5*10−7 M) to determine the impact of ischemia and the treatment on the contractility of the basilar artery. At the end of ET dose response, the endothelium dependent relaxation was assessed by measuring the lumen diameter in response to increasing concentrations of acetylcholine (Ach) (2*10−10– 2*10−5 M). In preliminary experiments, when higher doses of ET-1 were used to reach a plateau, vessels were either unresponsive or did not relax in response to the following Ach treatment. Therefore, dose-response curve to ET-1 was performed up to 5*10−7 M. Maximum response (Rmax) and EC50 values were calculated.
Infarct and edema analysis
At 24 hours after ischemia, after the isolation of basilar arteries for vascular function studies, brains were cut into 2-mm slices and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 5 minutes at 37°C. The image of the slices was scanned and the infarct volume was analyzed as previously described (Ergul et al. 2007). Total infarct volume was calculated as percent volume of the total ischemic hemisphere. Edema is reported as the percent increase in ischemic hemisphere size to the contralateral hemisphere.
Statistical analysis
A repeated measures analysis of variances was used to determine group differences (control versus stroked versus stroked treated) across ET-1 or Ach concentrations. Post hoc group comparisons at each concentration used a Tukey’s adjustment for the multiple comparisons. Unpaired two-tailed t test was used to compare infarct size and edema between groups. Effects were considered statistically significant at p< 0.05. Graphpad Prism 5 was used for all statistical tests performed. Results are expressed as the means ± SEM.
Results
Vascular reactivity
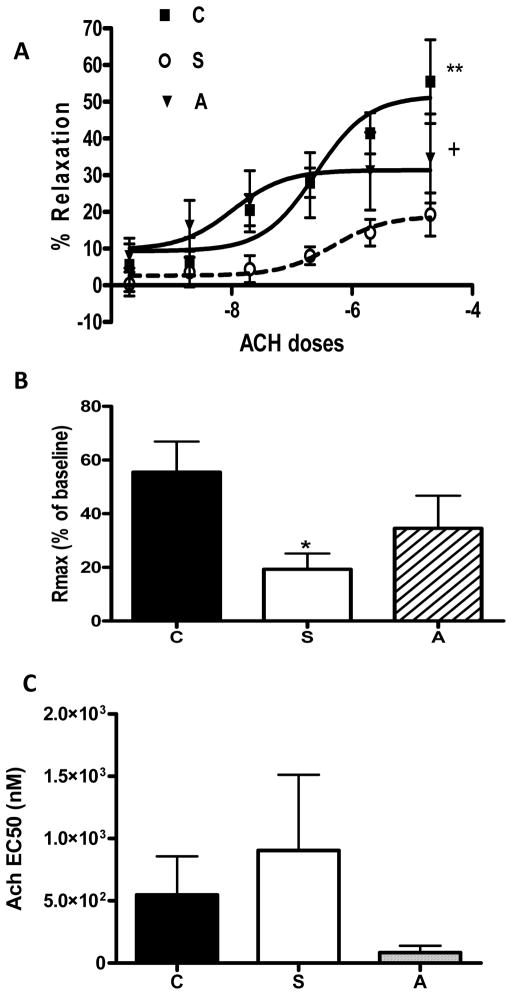
The sensitivity (EC50) and the contractile response to at 500 nm ET-1 (maximum dose studied) were similar among the three groups (Fig 1 A, B). Stroked rats exhibited impaired endothelium relaxation following preconstriction with ET-1. Atrasentan treatment caused a leftward shift and improved relaxation compared to the stroked rats (Fig 2 A). Treatment with atrasentan did not only improve endothelium dependent relaxation but also increased the maximum response (Fig 2 B). There was also a trend for decreased EC50 value for the antagonist treatment group indicating improved Ach sensitivity (Fig 2 C).
Fig. 1.
Effects of acute endothelin receptor antagonism on endothelin-1 mediated contractility in stroked rats (S). A) Dose response curves to ET-1 demonstrated no change in the contractility between control (C), stroked (S) and stroked rats treated with atrasentan (A). B) Sensitivity to ET-1 (EC50) did not differ among the groups.
Fig. 2.
Effects of acute endothelin receptor antagonism on endothelium-dependent relaxation in stroked rats (S). in stroked rats. A) Dose response curves to Ach in ET-1-preconstricted vessels demonstrated impaired relaxation in antagonism with atrasentan (A) partially restored it. B) stroked rats (S) and ETA The magnitude of dilation was less in stroked rats and atrasentan treatment improved relaxation. C) There was a trend for improved sensitivity (EC50) to acetylcholine with ET receptor antagonism. (*p<0.05 vs control, +p<0.05 and **p<0.01 vs stroked rats).
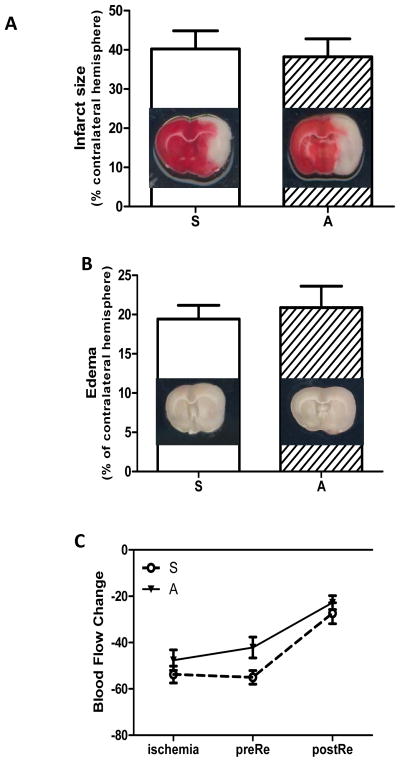
Infarct volume and edema
Treatment with atrasentan had no effect on infarct size or edema as both parameters were similar in both groups (Fig 3 A, B). To ensure that the drop in cerebral blood flow (CBF) at MCAO is similar across the study groups and also to determine whether there are differences in CBF in the post-reperfusion period, the cerebral perfusion was measured with a scanning Doppler imaging system at different time points (ischemia, right before reperfusion when antagonist was administered and after reperfusion). Compared to baseline, there was approximately a 50% decrease in cerebral perfusions in both vehicle and atrasentan-treated rats and recovery after reperfusion was similar across the groups (Fig 3 C).
Fig. 3.
Effects of acute endothelin receptor antagonism on stroke outcomes. A) Infarct size measured by TTC staining was similar in stroked and stroked rats treated with atrasentan. B) There was no difference in edema between the two groups. C) Cerebral blood flow was significantly decreased at ischemia and was partially restored at early reperfusion in both groups.
Discussion
The present study demonstrates that while temporary focal brain ischemia does not affect ET-1-mediated vasoconstriction of basilar arteries, it impairs the ability of cerebral arteries to relax. Moreover, acute treatment with atrasentan, an ETA receptor antagonist, given right before reperfusion restored endothelium- dependent vasodilation. The current study also provides evidence that acute short term use of atrasentan is not sufficient to reduce infarct size or edema. Taken together, these findings suggest that I/R injury causes cerebrovascular dysfunction of vessels upstream of occlusion in an ET-1-dependent manner and that the role of ET-1 in the pathogenesis of cerebral ischemic injury is complex.
Regulation of cerebrovascular resistance and ultimately CBF is critical for proper brain function (Faraci and Heistad 1990). Temporary interruption of CBF as in stroke has important implications. The current treatment strategies focus on the reestablishment of CBF as soon as possible to prevent infarct expansion but it is also known that reperfusion causes significant tissue damage (Li et al. 2010; Takahashi et al. 1997). Early hyperperfusion following ischemia contributes to blood brain barrier disruption and edema (Sage et al. 1984). This period is usually followed by postischemic hypoperfusion and multiple mechanisms including increased vascular smooth muscle tone of downstream microvessels (Cipolla and Bullinger 2008; Cipolla et al. 2004; Takahashi et al. 1997). In an early study, Takahashi et al elegantly demonstrated that vascular smooth muscle of the microvasculature in particular is highly influenced by I/R injury (Takahashi et al. 1997). It is also known that both microvasculature and macrovasculature are equally involved in control of cerebrovascular resistance (Cipolla and Bullinger 2008; Faraci and Heistad 1990). While significant effort was directed towards how ischemia induced by occlusion of a vessel modifies structure and function of the downstream microvasculature and ultimately cerebral perfusion (Cipolla and Bullinger 2008; Coulson et al. 2002), the impact on upstream vessels is relatively understudied. Therefore in the current study we investigated the vascular reactivity of basilar arteries after I/R. Given that ET-1 is one of the most potent vasoconstrictor identified to date and that ET receptor antagonism decreases ischemic injury (Gupta et al. 2005; Matsuo et al. 2001; Patel et al. 1996; Zhang et al. 2008), we focused on ET-1-mediated vascular reactivity.
While there was no difference in ET-1-mediated vasoconstriction among control (no stroke) and stroked (vehicle or atrasentan-treated) rats, the endothelium- dependent vascular relaxation was significantly impaired by I/R and ETA receptor antagonism partially prevented this effect. We studied vascular reactivity 21 h after the antagonist administration. Thus, our data suggests that acute ET receptor antagonism has a long lasting effect on vascular function. It is highly possible that at early time points after drug administration the restoration of vascular relaxation can be greater. Another possibility is the upregulation of ETB receptors after ischemia. While we did not investigate the effect of ETB receptor blockade on basilar artery reactivity, several studies demonstrated that ischemia upregulates ETB receptors on vascular smooth muscle cells (Henriksson et al. 2003; Stenman et al. 2002). Therefore, it is highly possible that combined ETA and ETB receptor blockade may fully restore large artery dysfunction following I/R injury and that needs to be tested.
Several lines of evidence suggest that ET-1 contributes to ischemic brain injury. First, direct administration of ET-1 into the brain causes severe vasoconstriction and significant reductions in CBF inducing stroke (Macrae et al. 1993). Second, both experimental and clinical studies reported increased plasma, cerebrospinal fluid and tissue ET-1 levels following I/R (Barone et al. 1994; Lampl et al. 1997; Loo et al. 2002). Not only vasculature but also neuronal and glial ET-1 and ET receptors are altered following ischemia (Loo et al. 2002). Third, gain and loss of function approaches indicated an important role of ET-1 in ischemic brain injury.
While endothelial overexpression of ET-1 exacerbates infarct size and edema (Leung et al. 2009), with the exception of one study (Chuquet et al. 2002), pharmacological blockade of ET receptors in various experimental models of stroke reduces neurovascular injury and improves outcomes. Chuquet and colleagues demonstrated that selective ETB receptor blockade by direct injection into the brain, reduces CBF and exacerbates neuronal injury (Chuquet et al. 2002). Gupta et al., however, reported that dual ETA and ETB antagonist TAK-044 pretreatment reduces ischemic injury and this is associated with reduced oxidative markers and improved functional outcomes (Gupta et al. 2005). Another study demonstrated that selective ETA blockade given for 24 h starting 10 min, 1 or 3 hours after middle cerebral artery occlusion decreases ischemic injury (Matsuo et al. 2001). A more recent study showed that when given with the tissue plasminogen activator (tPA), the only FDA-approved therapeutic for stroke treatment, ETA antagonism reduces the risk of bleeding and provides neurovascular protection in an embolic model of stroke (Zhang et al. 2008). In all these past studies ET receptor blockade was administered either before stroke as a preventive measure or continuously during the reperfusion period varying from 22 to 72 hours. The current study aimed to determine the acute use of ET receptor blockade and ETA antagonist was given once right before perfusion with neurovascular injury being evaluated 21 h later. We did not find any differences in infarct size or edema between the groups. This may be due to the acute and the one time administration of the antagonist. Use of the receptor antagonist prior to reperfusion may reduce neuronal injury. It is may also be due to the greater magnitude of injury induced by 3 h MCAO used in the current study. In a less severe model of ischemia, differences between vehicle and antagonist-treated groups may be easier to detect.
There are several limitations that need to be addressed. It has to be recognized that the current study used healthy and young animals. Even in this group, I/R injury influenced vascular function. We and others have reported under disease states like diabetes, ET-1 causes hyperactivity of basilar arteries and impairs endothelial function (Harris et al. 2008; Matsumoto et al. 2004). Given that diabetes is a risk factor for stroke, the impact of ischemic injury on cerebrovascular function in diabetes needs to be further investigated. We also reported that diabetes alters ET receptor expression (Kelly-Cobbs et al. 2011). As such, role of both ETA and ETB receptors in the regulation of vascular reactivity should be studied. The current study did not include functional outcome of stroke but based on the infarct sizes we did not anticipate any differences in neurological function. We also acknowledge that we did not study the effect of ET receptor blockade on microvascular responses but rather focused on basilar arteries that significantly contribute to the regulation of cerebrovascular resistance. Due to the shorter viability of basilar arteries after ischemic injury, we were able to conduct only ET-1 and Ach dose response curves and could not assess endothelium-independent vascular responsiveness in limited number of vascular segments we can isolate from one animal. Nevertheless, this study provides important information for the use of ET receptor antagonists in the setting of stroke. While the acute use of ETA receptor blockade is able to restore vascular dysfunction, this does not translate into reduction of neuronal injury suggesting that the role of ET-1 in ischemic injury is more complex. Therefore, extended use of ETA receptor antagonist in the hours following stroke is more desirable to reduce injury and improve outcomes.
Conclusion
The present study provides evidence that, in this model of I/R injury, ET-1 plays an important role in the impairment of endothelium dependent relaxation of cerebral vessels and that acute treatment with ETA receptor antagonist could improve vascular function.
Acknowledgments
Adviye Ergul is a research pharmacologist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. This work was supported in part by VA Merit Award (BX000347), NIH award (NS054688), and American Heart Association Established Investigator Award to (0740002N) to Dr. Ergul.
Footnotes
Conflict of interest
There are no conflicts of interest.
References
- Barone FC, Globus MY, Price WJ, White RF, Storer BL, Feuerstein GZ, et al. Endothelin levels increase in rat focal and global ischemia. J Cereb Blood Flow Metab. 1994;14:337–42. doi: 10.1038/jcbfm.1994.41. [DOI] [PubMed] [Google Scholar]
- Chuquet J, Benchenane K, Toutain J, MacKenzie ET, Roussel S, Touzani O. Selective blockade of endothelin-B receptors exacerbates ischemic brain damage in the rat. Stroke. 2002;33:3019–25. doi: 10.1161/01.str.0000039401.48915.9f. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ. The Cerebral Circulation. 2009 [PubMed] [Google Scholar]
- Cipolla MJ, Bullinger LV. Reactivity of Brain Parenchymal Arterioles after Ischemia and Reperfusion. Microcirculation. 2008:1. doi: 10.1080/10739680801986742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol. 2004;44:1–8. doi: 10.1097/00005344-200407000-00001. [DOI] [PubMed] [Google Scholar]
- Coulson RJ, Chesler NC, Vitullo L, Cipolla MJ. Effects of ischemia and myogenic activity on active and passive mechanical properties of rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2268–75. doi: 10.1152/ajpheart.00542.2002. [DOI] [PubMed] [Google Scholar]
- Edvinsson LI, Povlsen GK. Vascular plasticity in cerebrovascular disorders. J Cereb Blood Flow Metab. 2011;31:1554–71. doi: 10.1038/jcbfm.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A. Endothelin-1 and endothelin receptor antagonists as potential cardiovascular therapeutic agents. Pharmacotherapy. 2002;22:54–65. doi: 10.1592/phco.22.1.54.33505. [DOI] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Briyal S, Sharma U, Jagannathan NR, Gulati A. Effect of endothelin antagonist (TAK-044) on cerebral ischemic volume, oxidative stress markers and neurobehavioral parameters in the middle cerebral artery occlusion model of stroke in rats. Life Sci. 2005;77:15–27. doi: 10.1016/j.lfs.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1213–9. doi: 10.1152/ajpregu.00885.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16:1081–98. doi: 10.1097/00004872-199816080-00001. [DOI] [PubMed] [Google Scholar]
- Henriksson M, Stenman E, Edvinsson L. Intracellular pathways involved in upregulation of vascular endothelin type B receptors in cerebral arteries of the rat. Stroke. 2003;34:1479–83. doi: 10.1161/01.STR.0000072984.79136.79. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–7. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Cobbs AI, Harris AK, Elgebaly MM, Li W, Sachidanandam K, Portik-Dobos V, et al. Endothelial endothelin B receptor-mediated prevention of cerebrovascular remodeling is attenuated in diabetes because of up-regulation of smooth muscle endothelin receptors. J Pharmacol Exp Ther. 2011;337:9–15. doi: 10.1124/jpet.110.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl Y, Fleminger G, Gilad R, Galron R, Sarova-Pinhas I, Sokolovsky M. Endothelin in cerebrospinal fluid and plasma of patients in the early stage of ischemic stroke. Stroke. 1997;28:1951–5. doi: 10.1161/01.str.28.10.1951. [DOI] [PubMed] [Google Scholar]
- Leung JW, Chung SS, Chung SK. Endothelial endothelin-1 over-expression using receptor tyrosine kinase tie-1 promoter leads to more severe vascular permeability and blood brain barrier breakdown after transient middle cerebral artery occlusion. Brain Res. 2009;1266:121–9. doi: 10.1016/j.brainres.2009.01.070. [DOI] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, et al. Adaptive Cerebral Neovascularization in a Model of Type 2 Diabetes: Relevance to Focal Cerebral Ischemia. Diabetes. 2010;59:228–35. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo LS, Ng YK, Zhu YZ, Lee HS, Wong PT. Cortical expression of endothelin receptor subtypes A and B following middle cerebral artery occlusion in rats. Neuroscience. 2002;112:993–1000. doi: 10.1016/s0306-4522(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Macrae IM, Robinson MJ, Graham DI, Reid JL, McCulloch J. Endothelin-1-induced reductions in cerebral blood flow: dose dependency, time course, and neuropathological consequences. J Cereb Blood Flow Metab. 1993;13:276–84. doi: 10.1038/jcbfm.1993.34. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yoshiyama S, Kobayashi T, Kamata K. Mechanisms underlying enhanced contractile response to endothelin-1 in diabetic rat basilar artery. Peptides. 2004;25:1985–94. doi: 10.1016/j.peptides.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Mihara S, Ninomiya M, Fujimoto M. Protective effect of endothelin type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke. 2001;32:2143–8. doi: 10.1161/hs0901.94259. [DOI] [PubMed] [Google Scholar]
- Owens WB. Blood pressure control in acute cerebrovascular disease. J Clin Hypertens (Greenwich) 2011;13:205–11. doi: 10.1111/j.1751-7176.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TR, Galbraith S, Graham DI, Hallak H, Doherty AM, McCulloch J. Endothelin receptor antagonist increases cerebral perfusion and reduces ischaemic damage in feline focal cerebral ischaemia. J Cereb Blood Flow Metab. 1996;16:950–8. doi: 10.1097/00004647-199609000-00019. [DOI] [PubMed] [Google Scholar]
- Sage JI, Van Uitert RL, Duffy TE. Early changes in blood brain barrier permeability to small molecules after transient cerebral ischemia. Stroke. 1984;15:46–50. doi: 10.1161/01.str.15.1.46. [DOI] [PubMed] [Google Scholar]
- Stenman E, Malmsjo M, Uddman E, Gido G, Wieloch T, Edvinsson L. Cerebral ischemia upregulates vascular endothelin ETB receptors in rat. Stroke. 2002;33:2311–6. doi: 10.1161/01.str.0000028183.04277.32. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Park HK, Melgar MA, Alcocer L, Pinto J, Lenzi T, et al. Cerebral cortex blood flow and vascular smooth muscle contractility in a rat model of ischemia: a correlative laser Doppler flowmetric and scanning electron microscopic study. Acta Neuropathol. 1997;93:354–68. doi: 10.1007/s004010050627. [DOI] [PubMed] [Google Scholar]
- Thampatty BP, Sherwood PR, Gallek MJ, Crago EA, Ren D, Hricik AJ, et al. Role of endothelin-1 in human aneurysmal subarachnoid hemorrhage: associations with vasospasm and delayed cerebral ischemia. Neurocrit Care. 2011;15:19–27. doi: 10.1007/s12028-011-9508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang C, Zhang L, Roberts C, Lu M, Kapke A, et al. Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke. Stroke. 2008;39:2830–6. doi: 10.1161/STROKEAHA.108.515684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv I, Fleminger G, Djaldetti R, Achiron A, Melamed E, Sokolovsky M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke. 1992;23:1014–6. doi: 10.1161/01.str.23.7.1014. [DOI] [PubMed] [Google Scholar]