Abstract
Chronic cholangiopathies have limited therapeutic options and represent an important indication for liver transplantation. The nuclear farnesoid X receptor (FXR) and the membrane G protein-coupled receptor, TGR5, regulate bile acid (BA) homeostasis and inflammation. Therefore, we hypothesized that activation of FXR and/or TGR5 could ameliorate liver injury in Mdr2−/− (Abcb4−/−) mice, a model of chronic cholangiopathy. Hepatic inflammation, fibrosis, as well as bile secretion and key genes of BA homeostasis were addressed in Mdr2−/− mice fed either a chow diet or a diet supplemented with the FXR agonist, INT-747, the TGR5 agonist, INT-777, or the dual FXR/TGR5 agonist, INT-767 (0.03% w/w). Only the dual FXR/TGR5 agonist, INT-767, significantly improved serum liver enzymes, hepatic inflammation, and biliary fibrosis in Mdr2−/− mice, whereas INT-747 and INT-777 had no hepatoprotective effects. In line with this, INT-767 significantly induced bile flow and biliary output, as well as gene expression of carbonic anhy-drase 14, an important enzyme able to enhance transport, in an Fxr-dependent manner. In addition, INT-767 dramatically reduced bile acid synthesis via the induction of ileal Fgf15 and hepatic Shp gene expression, thus resulting in significantly reduced biliary bile acid output in Mdr2−/− mice.
Conclusion
This study shows that FXR activation improves liver injury in a mouse model of chronic cholangiopathy by reduction of biliary BA output and promotion of -rich bile secretion.
Current pharmacological strategies for chronic cholangiopathies, such as primary sclerosing cholangitis (PSC), have limited efficacy,1,2 and novel therapies are eagerly awaited. Bile acids (BAs) are potent signaling molecules that, through activation of the nuclear receptor, farnesoid X receptor (FXR; NR1H4),3–5 and the membrane G protein-coupled receptor, TGR5 (also called GPBAR1 or M-BAR/ BG37),6,7 modulate BA homeostasis, inflammation, and lipid and glucose metabolism.8 In the liver, FXR is highly expressed in hepatocytes, whereas cholangiocytes show a weak expression.9 In contrast, TGR5 is highly expressed in the biliary epithelium, sinusoidal endothelial cells, and Kupffer cells.10–13 FXR activation inhibits BA synthesis14,15 and has anti-inflammatory effects in atherosclerosis,16 inflammatory bowel disease,17 and experimental cholestasis,18 whereas TGR5 activation, via cAMP-mediated pathways, reduces proinflammatory cytokine production in macrophages6 and Kupffer cells.11 In addition, FXR and TGR5 mutations have been identified in intrahepatic cholestasis of pregnancy19 and PSC,20 respectively, emphasizing that these receptors are attractive novel therapeutic targets.
We, therefore, hypothesized that selective FXR activation by INT-747,21 selective TGR5 stimulation by INT-777,22 and/or dual FXR/TGR5 activation by INT-7623 could exert beneficial therapeutic mechanisms on liver inflammation and fibrosis in mice lacking the phospholipid (PL) flippase multidrug resistance protein 2 (Mdr2) (Mdr2−/− or Abcb4−/−) with sclerosing cholangitis.24,25 In this study, we have identified the dual FXR/TGR5 agonist, INT-767, as a novel promising treatment in a mouse model of chronic cholangiopathy and characterized the underlying molecular and cellular mechanisms.
Materials and Methods
Animal Experiments
Mdr2−/− mice (FVB/N background) were obtained from Jackson Laboratory (Bar Harbor, ME) and housed in a 12-hour light-dark house facility with water and mouse chow diet (SSNIFF, Soest, Germany) ad libitum. Fxr+/+ (C57BL6/J background) mice were from Himberg, Austria. Fxr−/− mice were originally obtained from a colony at the National Institutes of Health (NIH; Bethesda, MD).26 Heterozygous Tgr5-Tg mice were described previously.27 Common bile duct ligation (CBDL) was performed on C57BL6/J mice, as previously described.28 Experimental protocols were approved by the local animal care and use committee, according to criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by U.S. National Academy of Sciences (NIH publication 86-23, revised 1985).
Feeding Protocols
INT-747 (selective FXR agonist), INT-777 (selective TGR5 agonist), and INT-767 (dual FXR/TGR5 agonist) (see Supporting Information) were synthesized by Intercept Pharmaceuticals (New York, NY) (Fig. 1A). Eight-week-old male Mdr2−/− mice received either a chow diet or a diet supplemented with INT-747, INT-777, and INT-767 (0.03% w/w, equaling 30 mg/kg) for 4 weeks. CBDL was performed after 5 days of prefeeding either a chow diet or a diet supplemented with INT-747, INT-777, or INT-767. Experimental feeding was continued for an additional 3 days, and mice were sacrificed on day 4 after CBDL.
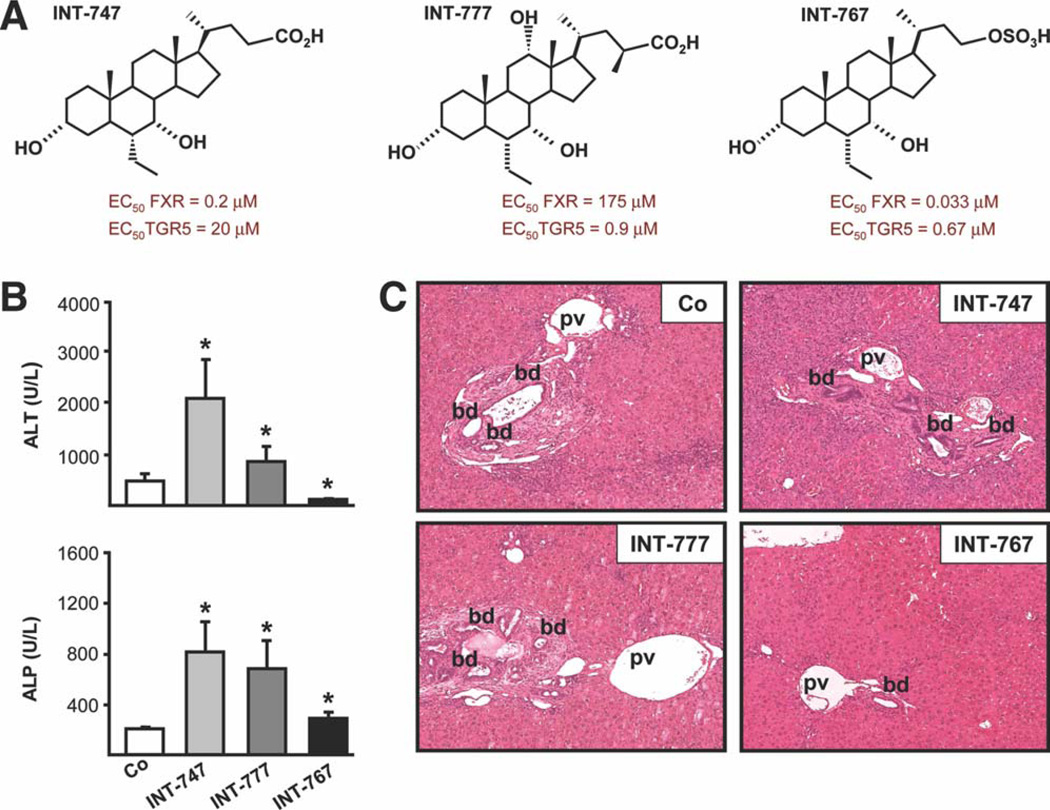
Fig. 1.
Dual FXR/TGR5 agonist INT-767 improves liver injury in Mdr2−/− mice. Mdr2−/− mice were either fed a chow diet or a diet supplemented with the FXR agonist, INT-747, the TGR5 agonist, INT-777, or the dual FXR/TGR5 agonist, INT-767 for 4 weeks. (A) Chemical structures of INT-747, INT-777, and INT-767 compounds with respective FXR and TGR5 EC50. (B) INT-747 and INT-777 increased serum ALT and ALP, whereas INT-767 significantly reduced serum ALT, but not ALP, levels. Means of 6 mice/group ± SD. *P < 0.05 INT-747, INT-767, and INT-777 versus controls. (C) Representative histological pictures of H&E-stained livers. Bile duct proliferation and portal infiltration of inflammatory cells was reduced by INT-767. The INT-747-fed Mdr2−/− mouse showed increased bile duct proliferation, expansion of biliary tract, and accumulation of inflammatory cells. No obvious alterations were detected in the INT-777-fed Mdr2−/− mouse liver. Co, chow-fed littermates; pv, portal vein; bd, bile duct.
Methods for further biochemical, molecular, and histological analysis and in vitro cell-culture experiments are described in the Supporting Information.
Statistical Analysis
Results were evaluated using SPSS software (Release 14.0, 2005; SPSS Inc., Chicago, IL). Statistical analysis was performed using the one-way analysis of variance test, followed by the Mann-Whitney U test. Data are reported as means of 5 animals per group (unless otherwise noted) ± standard deviation (SD). A P value ≤ 0.05 was considered significant.
Results
Dual FXR/TGR5 Agonist INT-767 Decreases Liver Injury in Mdr2−/− Mice
Treatment with INT-767 significantly reduced, whereas INT-747 and INT-777 increased serum alanine aminotransferase (ALT) levels in Mdr2−/− mice (Fig. 1B). However, all treatments increased serum alkaline phosphatase (ALP) levels (Fig. 1B) (modest increase by INT-767) and liver weight/body weight (LW/BW) ratio (Supporting Fig. 1A). Histological examination (i.e., hematoxylin and eosin [H&E] staining) of INT-767-treated Mdr2−/− mouse livers showed less portal inflammation and bile duct proliferation (Fig. 1C), compared with untreated mice. In contrast, INT-747 aggravated liver damage in Mdr2−/− mice, as reflected by increased bile duct proliferation, portal tract expansion (Fig. 1C), and single-cell necrosis with lobular inflammation (Supporting Fig. 1B), whereas no significant changes were detected after treatment with INT-777.
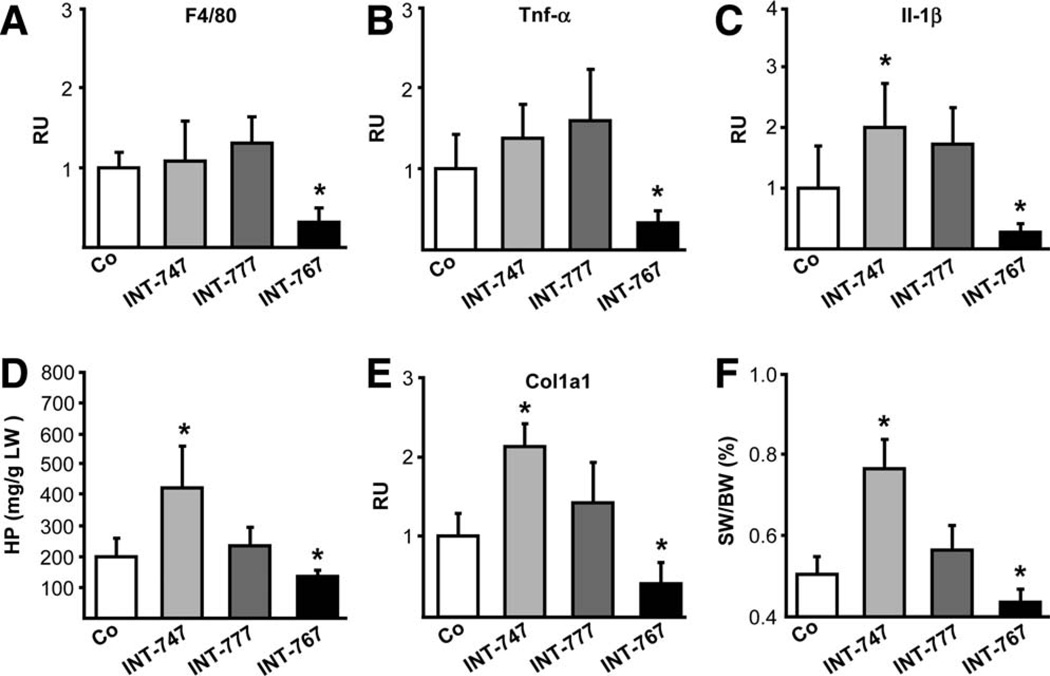
INT-767 Suppresses Hepatic Inflammation, Reactive Cholangiocyte Activation, and Fibrosis in Mdr2−/− Mice
INT-767 treatment reduced F4/80, tumor necrosis factor alpha (Tnf-α), and interleukin (Il)-1β messenger RNA (mRNA) levels (Fig. 2A–C) as well as the number of cluster of differentiation (CD)-11b- and F4/80-positive cells (Supporting Fig. 2A,B). In contrast, INT-747 increased Il-1β mRNA levels (Fig. 2C) and portal CD-11b-positive cell accumulation in Mdr2−/− mice (Supporting Fig. 2A). The reactive cholangiocyte phenotype was also reduced by INT-767, as reflected by significantly lowered K19 and vascular cell adhesion molecule-1 (Vcam-1) mRNA levels and by immunohistochemical staining (Supporting Fig. 3). INT-747 increased Vcam-1 and monocyte chemotactic protein 1 (Mcp-1) mRNA levels and induced Vcam-1 staining in cholangiocytes, inflammatory cell infiltrates, and periportal hepatocytes, whereas INT-777 increased only Mcp-1 mRNA levels (Supporting Fig. 3). Liver fibrosis was reduced in INT-767-treated Mdr2−/− mice, as reflected by hepatic hydroxyproline (HP) content, inhibition of collagen type 1 alpha 1 (Col1a1) gene expression, and reduced spleen weight (SW)/BW ratio (Fig. 2D–F). In contrast, HP, Col1a1 mRNA, as well as SW/BW ratio increased in INT-747-fed mice, but remained unchanged in INT-777-fed mice. These findings were also confirmed by Sirius red staining (Supporting Fig. 4). Ki-67 staining revealed increased hepatocyte proliferation by INT-767 and INT-747 in Mdr2−/− (data not shown) and Fxr+/+ mice, but not in Fxr−/− mice (Supporting Fig. 5).
Fig. 2.
INT-767 reduces hepatic inflammation and fibrosis in Mdr2−/− mice. INT-767 inhibited the gene expression of macrophage marker F4/80 (A) and proinflammatory cytokines Tnf-α (B) and Il-1β (C) in Mdr2−/− mice, whereas INT-747 induced Il-1β gene expression. INT-767 lowered hepatic HP levels (D) as well as Col1a1 gene expression (E) in Mdr2−/− mice, whereas INT-747 increased and INT-777 did not modify HP or Col1a1 gene expression. (F) Spleen weight was normalized to body weight, and percent ratio is presented (SW/BW). Means of 5–6 mice/ group ± SD are presented. Gene-expression levels are normalized to the 36b4 housekeeping gene and the mean expression value of untreated Mdr2−/− mice (Co) is accepted as 1. *P < 0.05 INT-747 and INT-767 versus Co.
Potential direct anti-inflammatory and antifibrotic effects of INT-767 were addressed in macrophage, cholangiocyte, and hepatocyte cell lines and isolated primary myofibroblasts (MFBs). Notably, despite the potent in vivo effects, INT-767 had only a modest or not statistically significant effect on lipopolysaccharide-induced Il-6 expression in RAW264.7 macrophages, Tnf-α-induced Vcam-1 gene expression in biliary epithelial cells (BEC), and TNF-α–induced TNF-α gene expression in HepG2 cells, despite pronounced inhibition of cholesterol 7 alpha-hydroxylase (CYP7A1) as a positive control (Supporting Fig. 6). Furthermore, INT-767 had no significant effect on 10% fetal calf serum (FCS)-induced Col1a1 gene expression in portal MFBs (Supporting Fig. 7).
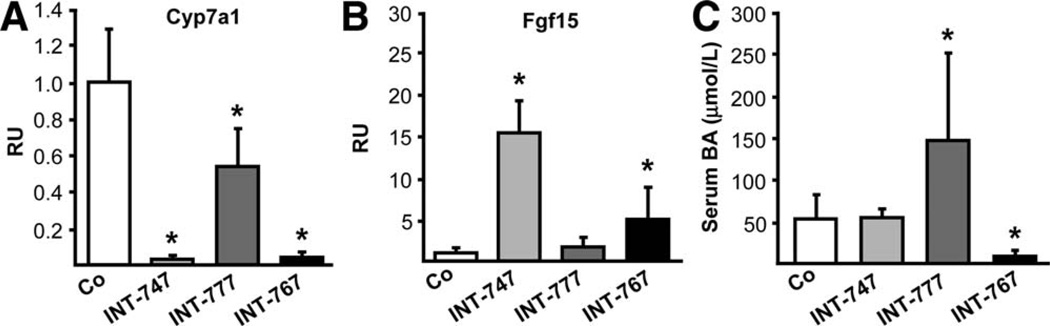
INT-767 Inhibits BA Synthesis by Induction of Ileal Fgf15 and Hepatic Shp Gene Expression
Importantly, both INT-747 and INT-767 dramatically inhibited Cyp7a1 (Fig. 3A) and Cyp8b1 (Supporting Fig. 8A) and stimulated Fgf15 gene expression (Fig. 3B). However, only INT-767 increased hepatic Shp gene expression (Supporting Fig. 8B). Ntcp was repressed by INT-747 and INT-767 at mRNA and protein levels, whereas only INT-767 increased bile salt export pump (Bsep) protein levels (Supporting Fig. 8C–E) and reduced serum BA levels in Mdr2−/− mice (Fig. 3C). No significant alterations of multidrug resistance-associated protein 2 (Mrp2), multidrug resistance-associated protein 3 (Mrp3), and multidrug resistance-associated protein 4 (Mrp4) were observed (Supporting Fig. 8E).
Fig. 3.
INT-747 and INT-767 inhibit BA synthesis in Mdr2−/− mice. Both INT-747 and INT-767 dramatically inhibited Cyp7a1 (A) while inducing ileal Fgf15 (B) gene expression in Mdr2−/− mice. (C) Only INT-767 significantly decreased, whereas INT-777 increased serum BA levels in Mdr2−/− mice. Means of 6 animals/group ± SD are presented. Gene-expression levels are normalized to the 36b4 housekeeping gene, and the mean expression value of untreated Mdr2−/− mice (Co) is accepted as 1. *P < 0.05 INT-747, INT-767, and INT-777 versus Co.
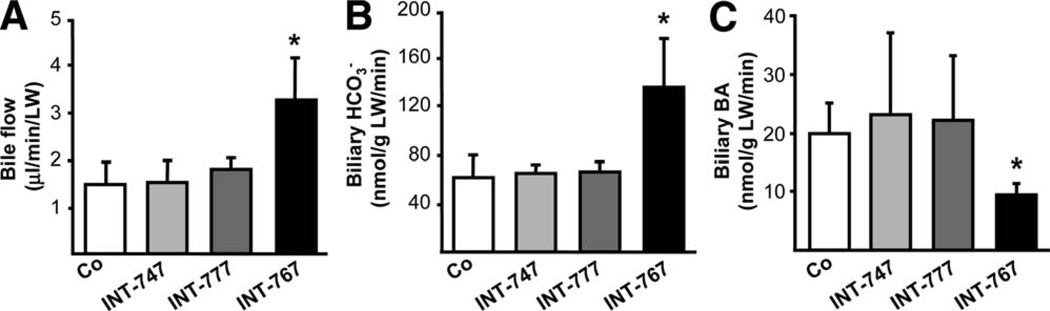
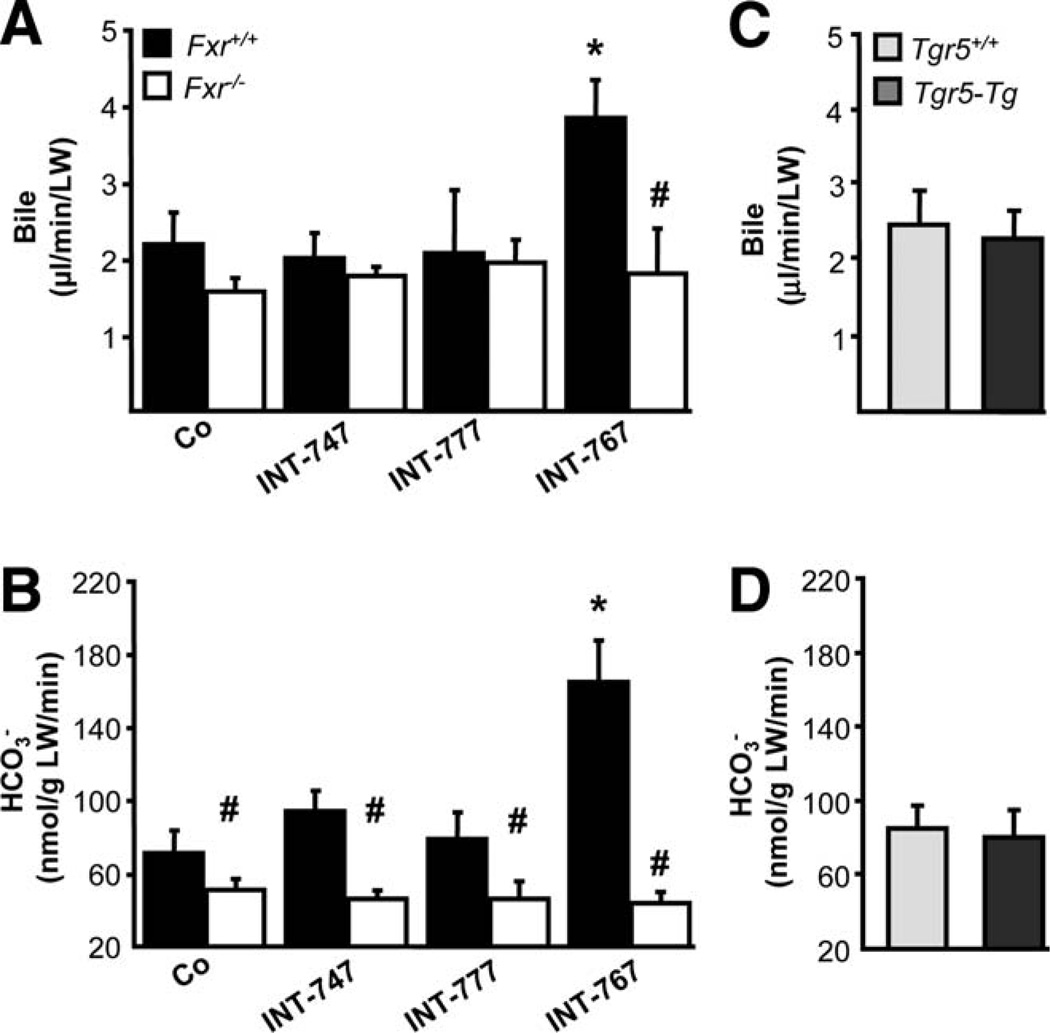
INT-767 Stimulates -rich Bile Flow and Inhibits BA Output via Fxr Activation
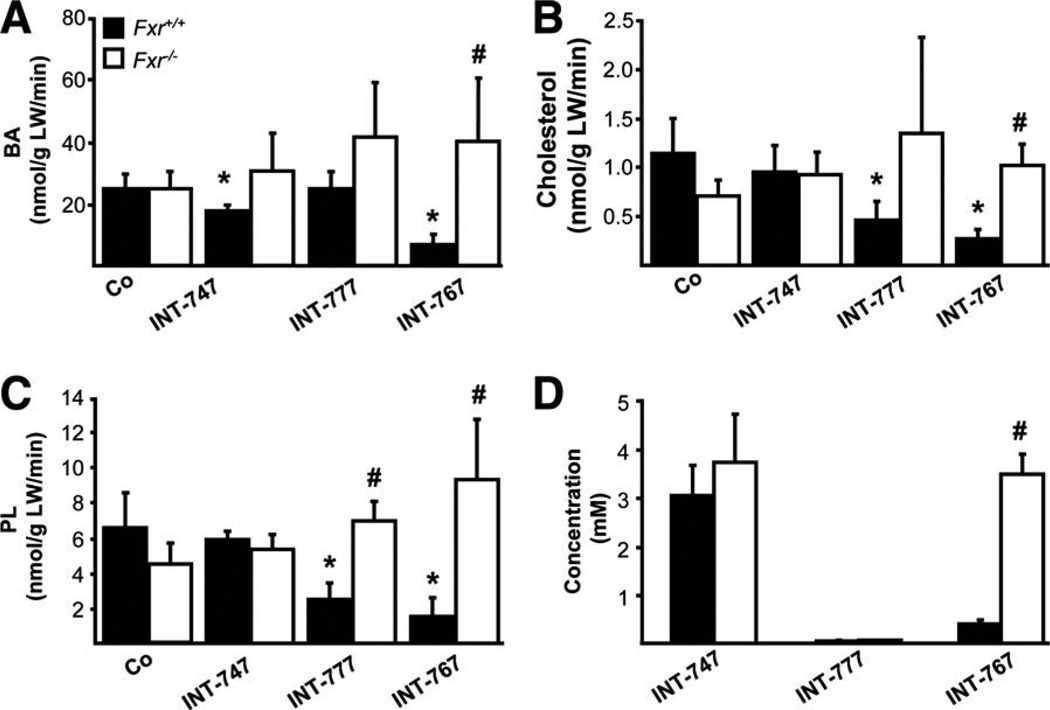
INT-767 significantly increased bile flow and output in Mdr2−/− mice, whereas biliary BA output was reduced (Fig. 4). In contrast, bile flow and bile composition remained unchanged in response to INT-747 and INT-777 feeding in Mdr2−/− mice. Because INT-767 represents a potent FXR, as well as TGR5 agonist, we next aimed to further discriminate the specific impact of each receptor in INT-767-induced choleresis with the aid of Fxr−/− mice. Bile flow and biliary output, increased by INT-767, were abolished in Fxr−/− mice (Fig. 5A,B), whereas INT-747 and INT-777 had no impact on bile flow or biliary output. By using a genetic model of Tgr5 overexpression (Tgr5-Tg mice), we further confirmed that bile flow and biliary secretion was independent of Tgr5 in vivo (Fig. 5C,D). In line with BA synthesis inhibition, INT-767 decreased biliary BA and, consequently, cholesterol and PL output (Fig. 6A–C) in an Fxr-dependent manner. INT-747 showed only modest reduction of BA output. Intriguingly, INT-777 decreased biliary PL and cholesterol output in Fxr+/+ mice (Fig. 6B,C), whereas glutathione output remained unchanged by all three compounds in both genotypes (Supporting Fig. 9). Biliary concentration of INT-767 was higher in Fxr−/−, compared with Fxr+/+ mice, whereas INT-747 and INT-777 concentrations did not differ between genotypes (Fig. 6D). However, INT-777 showed the lowest biliary enrichment.
Fig. 4.
INT-767 stimulates -rich bile flow in Mdr2−/− mice. Bile flow and composition were measured in Mdr2−/− mice after 7 days of feeding either a chow or a diet supplemented with INT-747, INT-777, or INT-767. (A) INT-767 increased bile flow and (B) biliary output. (C) Biliary BA output was significantly decreased by INT-767. Neither INT-747 nor INT-777 modified bile flow, biliary , or BA output in Mdr2−/− mice. Means of 4–5 mice/ group ± SD are presented. *P < 0.05 INT-767 versus chow-fed Mdr2−/− mice (Co).
Fig. 5.
Induction of -rich choleresis by INT-767 depends on Fxr, but not Tgr5. Bile flow and biliary output were measured in Fxr+/+ and Fxr−/− mice after 7 days of feeding on a chow or INT-747-, INT-777-, or INT-767-supplemented diet. (A) INT-767 induced bile flow only in Fxr+/+ mice, whereas INT-747 and INT-777 did not change bile flow in both Fxr+/+ and Fxr−/− mice. (B) Only INT-767 induced biliary output in Fxr+/+, but not in Fxr−/−, mice. Means of 4–5 mice/group ± SD are presented. *P < 0.05 INT-767-fed versus chow-fed Fxr+/+ mice (Co). #P < 0.05 Fxr−/− versus Fxr+/+ mice. Bile flow (C) and biliary (D) output were measured in age-matched Tgr5-Tg and Tgr5+/+ female mice. Bile flow, as well as biliary output, was undistinguishable between Tgr5-Tg and Tgr5+/+ mice. Means of 4–5 mice/group ± SD. are presented.
Fig. 6.
INT-767 reduces biliary output of endogenous BAs in an Fxr-dependent manner. Output of biliary BAs (A), cholesterol (B), and PLs (C) was reduced in Fxr+/+ mice by INT-767 while remaining unchanged in Fxr−/− mice. INT-747 modestly reduced biliary BA output (A), whereas INT-777 reduced PL (B) and Cholesterol (C) output in Fxr+/+ mice. (D) Biliary concentrations of respective INT compounds in bile samples of Fxr+/+ and Fxr−/− mice. Means of 4–5 animals/group ± SD are presented. *P < 0.05 INT-747, INT-767 and INT-777-fed versus chow-fed Fxr+/+ mice (Co). #P < 0.05 Fxr−/− versus Fxr+/+ mice.
INT-767 Induces Gene Expression of Hepatic Carbonic Anhydrase 14
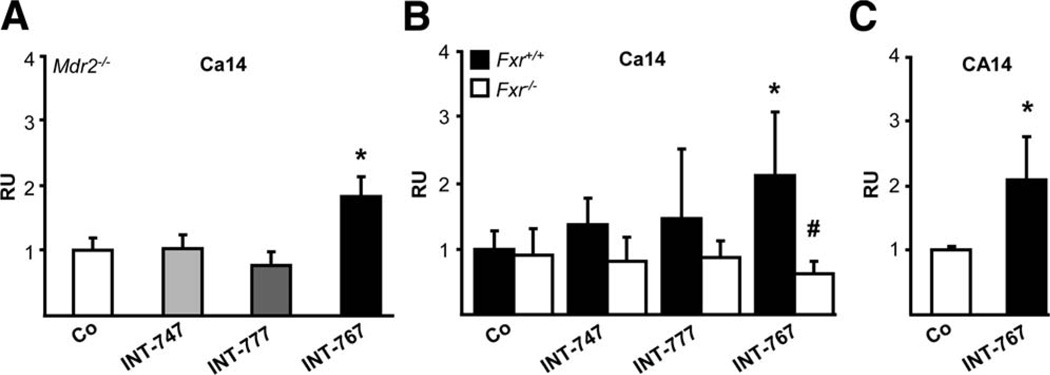
In human gallbladder epithelium, FXR was shown to induce -rich secretion30 via vasoactive intestinal peptide receptor (VPAC-1) induction. However, INT-767 even decreased hepatic Vpac-1 mRNA levels in Mdr2−/− as well as Fxr+/+ mice, (Supporting Fig. 10), indicating that Vpac-1 is unlikely to be responsible for -rich secretion in INT-767-treated mice. Gene expression of hepatocellular and cholangiocellular output transporter Ae231–33 as well as Slc4a4, an additional transporter in mouse cholangiocytes,34 remained unchanged in Mdr2−/−, Fxr+/+, and Fxr−/− mice (Supporting Fig. 11). Because none of the INT compounds altered gene expression of input transporter Slc4a5 in Mdr2−/− mice (data not shown), we studied the regulation of different carbonic anhydrases (Cas) by INT-767. INT-767 significantly increased the expression of hepatocellular membrane-bound Ca1435 in Mdr2−/− and Fxr+/+ mice and in human HepG2 hepatocytes, but not in Fxr−/− mice (Fig. 7A–C).
Fig. 7.
INT-767 significantly induces carbonic anhydrase 14 (Ca14) gene expression via Fxr. INT-767 significantly stimulated hepatic Ca14 gene expression in Mdr2−/− (A) and Fxr+/+ mice (B), whereas no alterations were detected in Fxr−/− mice. Means of 5–6 animals/group ± SD are presented. Gene-expression levels are normalized to the 36b4 housekeeping gene, and the mean expression value of chow-fed Mdr2−/−, Fxr+/+, and Fxr−/− mice is accepted as 1 (Co). *P < 0.05 INT-767-fed Fxr+/+ and Mdr2−/− mice versus respective controls. #P < 0.05 Fxr−/− versus Fxr+/+ mice. (C) INT-767 (10 µM) significantly induced CA14 gene expression in HepG2 cells. Values are presented as means of 3 samples per group ± SD. Expression levels are normalized to 36B4. *P < 0.05 INT-767- versus control medium-incubated (Co) cells. RU, relative units.
INT-767 Shows Moderate Protection Even in a Model of Total Biliary Obstruction
INT-747 and INT-767 increased the size and amount of bile infarcts, as well as LW/BW ratio, in CBDL mice (Supporting Fig. 12A,B), whereas only INT-767 significantly decreased SW/BW ratio (Supporting Fig. 12C) and showed a trend to reduction of serum ALT (Supporting Fig. 13A). Although histological examination of H&E-stained livers revealed bile infarcts in all the groups, only INT-747 increased infiltration of inflammatory cells within the portal fields (Supporting Fig. 13B). In line with serum ALT levels, INT-767-fed CBDL mice had reduced expression of proinflammatory genes Tnf-α and Il-1β and less CD-11b- and F4/80-positive cells around bile infarcts (Supporting Fig. 14A,B). However, keratin 19 (K19) and Vcam-1 gene expression remained unchanged in CBDL mice after INT-747, INT-777, and INT-767 feeding (Supporting Fig. 15).
Discussion
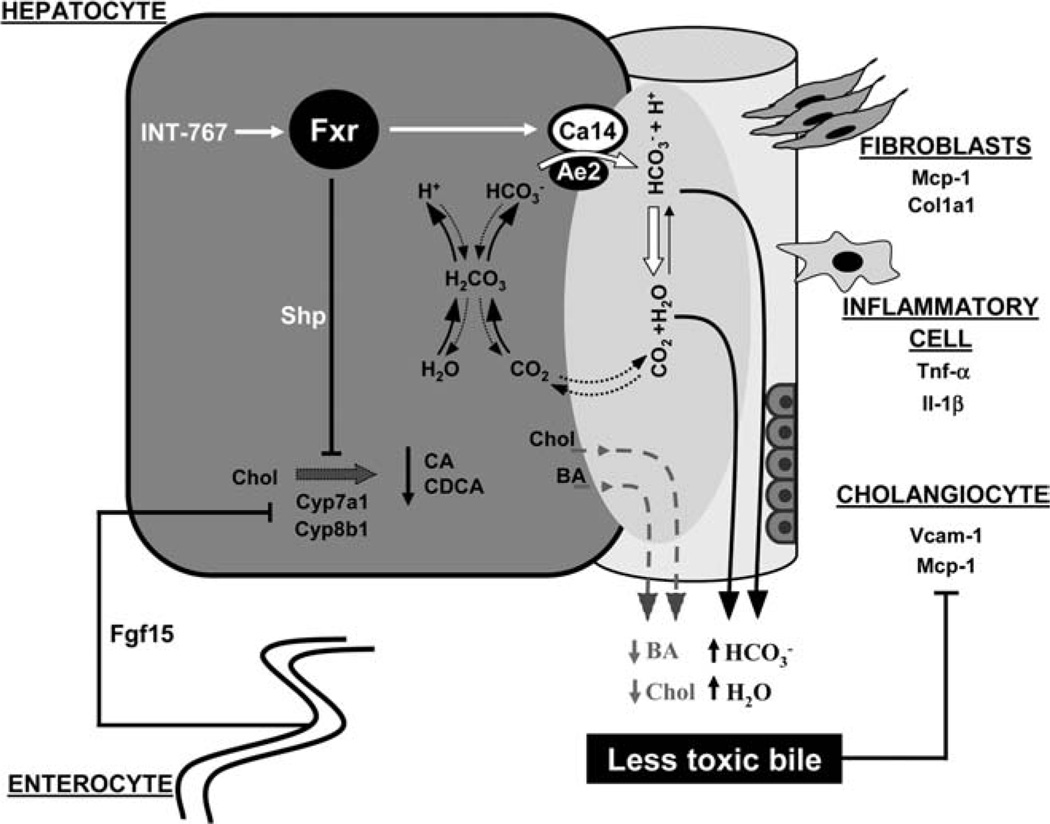
In this study, we have addressed the therapeutic mechanisms of BA receptor signaling through the nuclear BA receptor, FXR, and the G-protein-coupled membrane BA receptor, TGR5, in the Mdr2−/− mouse cholangiopathy model. We report herein that, in this model, the novel FXR/TGR5 agonist, INT-767, reduces bile toxicity by decreasing biliary BA output and inducing -rich choleresis in an FXR-dependent manner.
BAs are important signaling molecules with hormonal actions through dedicated nuclear and G-protein-coupled receptors, such as FXR and TGR5, respectively.8 TGR5 and FXR polymorphisms 19,20 further support the importance of BA signaling in human cholestastic diseases, such as PSC. Liver injury in Mdr2−/− mice is considered to evolve because of detergent properties of nonmicellar-bound free biliary BAs,29 leaving many open questions for the potential role of BA signaling in modulating biliary pathophysiology Only the dual FXR/TGR5 agonist, INT-767, was hepatoprotective in the Mdr2−/− model, as reflected by reduced serum ALT, decreased hepatic inflammation, improved reactive cholangiocyte phenotype, and reduced fibrosis. We could neither observe significant direct anti-inflammatory effects of INT-767 in RAW264.7 macrophages (with very low endogenous Fxr and Tgr5 expression), BEC cholangiocytes, or HepG2 hepatocytes (both with high levels of Fxr and very low Tgr5; data not shown) nor direct antifibrotic effects in primary MFBs (with very low endogenous Fxr and Tgr5 expression) as major fibrogenic cells in the Mdr2−/− model. Absent expression of FXR and TGR59,11 in hepatic stellate cells further indicates that FXR and TGR5 signaling may have no direct antifibrotic effects. These findings led us hypothesize that INT-767 might improve liver injury by directly impacting on bile formation and composition. Indeed, via Fxr activation, INT-767 inhibited BA synthesis (by ileal Fgf15 and hepatic Shp induction), thus resulting in decreased biliary BA output while significantly increasing bile flow and-unexpectedly- output.
formation and secretion into bile is considered to be protective by regulating intercellular pH, or bile alkalinization and sustaining BA-independent bile flow.36 Because INT-767 increased -rich choleresis via Fxr, we focused on the regulation of genes involved in transport and production. FXR was shown to increase biliary secretion in human gallbladder epithelium via VPAC-1 induction.30 However, in our experiments, Vpac-1 expression was even decreased by INT-767 in Mdr2−/− and Fxr+/+ mice, indicating that other mechanisms may contribute to the INT-767-stimulated secretion. Biliary export is mediated by anion exchanger 2 (Ae2) in hepatocytes31–33 and Ae2 and Slc4a4 in cholangiocytes.34 Impaired expression of Ae2 has been characterized in the pathogenesis of cholangiopathies,37 and induction of AE2 expression was found to be an important mechanism for the beneficial effects of combined therapy with UDCA and corticosteroids.38 Neither Ae2 nor Slc4a4 gene expression were altered by INT-767 in Mdr2−/− mice, showing that an alternative mechanism may be responsible. secretion can be facilitated by the induction of Cas which, via formation of functional complexes with Aes, form so-called ‘‘ transport metabolon’’39 to maximize flux.40,41 More specifically, the subgroup of membrane-bound or extracellular Cas facilitate Aes and transport to buffer the extracellular fluids.40,42–44 In addition, the role of membranebound Cas was suggested to propagate the umbrella at the apical surface of cholangiocytes.36 Expression of Ca4, an isoform expressed in apical membrane of cholangiocytes,45 was undetectable and remained unchanged in vivo and in vitro (BECs) by INT-767, whereas expression of cholangiocellular basolateral Ca946,47 increased by INT-767 in Fxr+/+, but not in Mdr2−/−, mice and remained unchanged in BECs (data not shown). However, INT-767 induced the gene expression of Ca14, a membrane-bound enzyme expressed in hepatocytes,35 in Mdr2−/− mice. The Fxr dependence of this finding was confirmed by showing an induction in Fxr+/+ and no increase in Fxr−/− mice after INT-767 administration and is further supported by the presence of inverted repeat 1, an FXR-responsive element,48 on the CA14/Ca14 promoter (identified in silico by Nuscan and Matinspector). Finally, we could show that INT-767 significantly induced CA14 mRNA levels in HepG2 cells, which show high FXR and undetectable TGR5 gene expression. The functional and physical interaction of Ca14 with exchanger anion exchanger 3 (Ae3) was proven to be an efficient mechanism to facilitate transport in the mouse brain.42 Therefore, it is tempting to speculate that INT-767 via Fxr-dependent induction of Ca14 expression in hepatocytes promotes the formation of transport metabolon involving Ca14 and Ae2. Whether INT-767 is also able to influence the insertion of Ae into plasma membrane as well as direct regulation of the Ca14 gene by Fxr remain to be established. Future studies will also have to address whether these compounds have direct effects on cholangiocellular bile secretion resulting from their BA-signaling properties.
In contrast to INT-767, the selective FXR agonist, INT-747, enhanced liver injury and fibrosis in the Mdr2−/− model. Although low-dose INT-1–747 had no impact on liver damage in Mdr2−/− mice (data not shown), a high dose (0.03% w/w) aggravated it, despite the induction of Fgf15 and inhibition of BA synthesis. Interestingly, INT-747 did not induce hepatic Shp gene expression, suggesting that in contrast to INT-767, which efficiently activates Fxr in the intestine and liver, INT-747 is less likely to have hepatic activity in Mdr2−/− mice. However, Ntcp gene expression was inhibited by INT-747, which may reflect direct repression by BAs or by proinflammatory cytokines caused by induced liver injury.49 In addition, INT-747 had no impact on Ca14 gene expression in vivo and in vitro (data not shown). Together, these findings point out to a differential regulation of Fxr-dependent genes by INT-747 and INT-767. Ligand binding to FXR can favor receptor conformations that, in turn, allow only specific cofactor recruitment, depending on the DNA-binding sequence, therefore resulting in selective modulation of gene expression.50 Our findings suggest that INT-767 acts as a specific FXR modulator in a way similar to other natural or synthetic FXR modulators.51–53
BA hydrophobicity is another important factor directly linked to BA detergent properties.54,55 Hydrophobic BAs are toxic to hepatocytes at micromolar concentrations.56 Endogenous BAs and INT-747 are hydrophobic BAs, whereas INT-767 is hydrophilic.23 INT-767 reduces bile toxicity and prevents further progression of liver injury via strong inhibition of endogenous BA synthesis, replacing hydrophobic BAs with the hydrophilic INT-767 and inducing -rich bile secretion. In contrast, accumulation of the hydrophobic INT-747 in the liver without stimulation of hepatoprotective mechanisms may act as an additional important factor for the promotion of liver damage in Mdr2−/− mice. Nevertheless, preliminary data from clinical phase II trials reported a beneficial effect of INT-747 on serum liver enzymes in patients with primary biliary cirrhosis.57 However, Fxr-mediated stimulation of bile flow may be deleterious in obstructive cholestasis.58 Importantly, despite promoting bile infarcts (because of increased bile flow) in the CBDL model, INT-767 even moderately reduced serum ALT levels and ameliorated proinflammatory cytokine expression, possibly because of low concentrations of endogenous hydrophobic BA and high content.
Because TGR5 signals through cAMP, an important regulator of chloride channel CFTR,10 we expected increased bile flow and output by the selective TGR5 agonist, INT-777. Surprisingly, INT-777 did not influence bile secretory function, as reflected by unchanged bile flow and biliary output in Mdr2−/−, Fxr+/+ and Fxr−/− mice, despite its ability to activate TGR5 in vitro and in vivo.22,27 Interestingly INT-777 showed the lowest biliary enrichment, indicating limited bioavailability and, subsequently, the lack of choleretic effect of this compound in mice. In addition to pharmacological TGR5 activation, by using Tgr5-Tg mice, we could confirm that Tgr5 over-expression also had no impact on bile secretion. However, INT-777 decreased biliary PL and cholesterol output in Fxr+/+ mice in the presence of unchanged BA concentrations. These findings are consistent with previous report showing that Tgr5−/− mice had higher biliary PL, compared with Tgr5+/+ mice, and were protected from gallstone development upon lithogenic diet feeding.59 Altogether, these data suggest that despite a beneficial effect of TGR5 activation in diabesity,8,27 TGR5 is unlikely to be benecial in cholangiopathies and diseases with impaired bile composition as well as gallbladder function. However, the failure of INT-777 to improve disease progression in Mdr2−/− does not rule out the possibility that other TGR5 activators might help to delay or cure cholestatic liver injury in humans.
In conclusion, our study demonstrates that FXR activation by INT-767, a novel, highly potent FXR/ TGR5 agonist, modifies bile flow and reduces bile toxicity by decreasing endogenous BA output and increasing output, resulting in the repression of hepatic inflammation as well as biliary fibrosis in Mdr2−/− mice.
Fig. 8.
Proposed model of INT-767-mediated beneficial effects in Mdr2−/− mice. INT-767 activates Fxr in the ileum and liver. By the induction of ileal Fgf15 and hepatic Shp, INT-767 profoundly inhibits endogenous BA synthesis, resulting in a significant reduction of biliary output of hydrophobic BAs and cholesterol. In addition, INT-767 via Fxr activation induces the expression of hepatocellular membrane-bound Ca14, which, in turn, promotes output and -rich choleresis because of (1) complex formation with transporter Ae2 and (2) hydration of with H+ into CO2 and H2O, thus increasing the local transmembrane gradient to further facilitate export. CO2, being easily permeable across the cell membrane, can enter cells where it is rehydrated to H+ and , thus contributing to recycling. Collectively, INT-767, by reducing the concentration of detergent BAs and increasing -rich choleresis, contributes to less reactive alterations of bile duct epithelium and results in decreased portal inflammation and fibrosis.
Acknowledgments
The authors gratefully acknowledge Dr. W. Erwa (Graz) and colleagues for performing the biochemical analyses of serum liver tests and A. Thüringer for help in primary myofibroblast isolation.
This work was supported by the Austrian Science Foundation (grant nos. P18613-B05, P19118, and SFB 3008; to M.T.), the Swiss National Science Foundation (grant no. SNF 31003A-125487/1; to K.S.), and by the PhD Program of the Medical University of Graz (to A.B.).
Abbreviations
- Ae2
anion exchanger 2
- Ae3
anion exchanger 3
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- BA
bile acid
- Bsep (Abcb11)
bile salt export pump
- BW
body weight
- Ca4
carbonic anhydrase 4
- Ca9
carbonic anhydrase 9
- CA14 (Ca14)
carbonic anhydrase 14
- CBDL
common bile duct ligation
- CD
cluster of differentiation
- Col1a1
collagen type I alpha 1
- Cyp7a1
cholesterol 7 alpha-hydroxylase
- FCS
fetal calf serum
- FXR (Fxr)
farnesoid X receptor
- H&E
hematoxylin and eosin staining
- HP
hydroxyproline
- Il
interleukin
- K19
keratin 19
- LW
liver weight
- Mdr2 (Abcb4)
multidrug resistance protein 2
- Mcp-1
monocyte chemotactic protein 1
- MFB
myofibroblast
- mRNA
messenger RNA
- Mrp2 (Abcc2)
multidrug resistance-associated protein 2
- Mrp3 (Abcc3)
multidrug resistance-associated protein 3
- Mrp 4 (Abcc4)
multidrug resistance-associated protein 4
- PL
phospholipid
- PSC
primary sclerosing cholangitis
- SD
standard deviation
- SW
spleen weight
- TNF-α (Tnf-α)
tumor necrosis factor alpha
- VPAC-1 (Vpac-1)
vasoactive intestinal peptide receptor-1
- Vcam-1
vascular cell adhesion molecule-1
Footnotes
Potential conflict of interest: Dr. Adornini owns stock in Intercept. Dr. Trauner is a consultant for Phenex. He is on the speakers’ bureau of Falk and received grants from Intercept.
Additional Supporting Information may be found in the onbline version of this article.
References
- 1.Ishibashi H, Komori A, Shimoda S, Gershwin ME. Guidelines for therapy of autoimmune liver disease. Semin Liver Dis. 2007;27:214–226. doi: 10.1055/s-2007-979472. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 4.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 6.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 8.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 9.Fickert P, Fuchsbichler A, Moustafa T, Wagner M, Zollner G, Halilbasic E, et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol. 2009;175:2392–2405. doi: 10.2353/ajpath.2009.090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Haussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50:861–870. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- 11.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 12.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 13.Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785–789. doi: 10.1515/BC.2010.077. [DOI] [PubMed] [Google Scholar]
- 14.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Guo GL, Santamarina-Fojo S, Akiyama TE, Amar MJ, Paigen BJ, Brewer B, Jr, Gonzalez FJ. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim Biophys Acta. 2006;1761:1401–1409. doi: 10.1016/j.bbalip.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, et al. Hepato-protection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Hov JR, Keitel V, Laerdahl JK, Spomer L, Ellinghaus E, ElSharawy A, et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 22.Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, Natalini B, et al. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem. 2009;52:7958–7961. doi: 10.1021/jm901390p. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 27.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 29.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine Mdr2 P-glyco-protein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 30.Chignard N, Mergey M, Barbu V, Finzi L, Tiret E, Paul A, Housset C. VPAC1 expression is regulated by FXR agonists in the human gallbladder epithelium. Hepatology. 2005;42:549–557. doi: 10.1002/hep.20806. [DOI] [PubMed] [Google Scholar]
- 31.Aranda V, Martinez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun. 2004;319:1040–1046. doi: 10.1016/j.bbrc.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 32.Benedetti A, Strazzabosco M, Ng OC, Boyer JL. Regulation of activity and apical targeting of the Cl-/HCO3- exchanger in rat hepatocytes. Proc Natl Acad Sci U S A. 1994;91:792–796. doi: 10.1073/pnas.91.2.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- 34.Uriarte I, Banales JM, Saez E, Arenas F, Oude Elferink RP, Prieto J, Medina JF. Bicarbonate secretion of mouse cholangiocytes involves Na(+)-HCO(3)(−) cotransport in addition to Na(+)-independent Cl(−)/HCO(3)(−) exchange. Hepatology. 2010;51:891–902. doi: 10.1002/hep.23403. [DOI] [PubMed] [Google Scholar]
- 35.Parkkila S, Kivela AJ, Kaunisto K, Parkkila AK, Hakkola J, Rajaniemi H, et al. The plasma membrane carbonic anhydrase in murine hepatocytes identified as isozyme XIV. BMC Gastroenterol. 2002;2:13–19. doi: 10.1186/1471-230X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. The biliary HCO(3)(–) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 37.Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, et al. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Arenas F, Hervias I, Uriz M, Joplin R, Prieto J, Medina JF. Combination of ursodeoxycholic acid and glucocorticoids upregulates the AE2 alternate promoter in human liver cells. J Clin Invest. 2008;118:695–709. doi: 10.1172/JCI33156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, et al. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- 40.Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl–/HCO3– exchanger binds carbonic anhydrase IV. J Biol Chem. 2002;277:25239–25246. doi: 10.1074/jbc.M202562200. [DOI] [PubMed] [Google Scholar]
- 41.Vince JW, Reithmeier RA. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J Biol Chem. 1998;273:28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 42.Casey JR, Sly WS, Shah GN, Alvarez BV. Bicarbonate homeostasis in excitable tissues: role of AE3 Cl-/HCO3- exchanger and carbonic anhydrase XIV interaction. Am J Physiol Cell Physiol. 2009;297:C1091–C1102. doi: 10.1152/ajpcell.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR. Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. Am J Physiol Cell Physiol. 2007;293:C738–C748. doi: 10.1152/ajpcell.00157.2007. [DOI] [PubMed] [Google Scholar]
- 44.Svichar N, Waheed A, Sly WS, Hennings JC, Hubner CA, Chesler M. Carbonic anhydrases CA4 and CA14 both enhance AE3-mediated Cl-HCO3- exchange in hippocampal neurons. J Neurosci. 2009;29:3252–3258. doi: 10.1523/JNEUROSCI.0036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parkkila S, Parkkila AK, Juvonen T, Waheed A, Sly WS, Saarnio J, et al. Membrane-bound carbonic anhydrase IV is expressed in the luminal plasma membrane of the human gallbladder epithelium. Hepato-logy. 1996;24:1104–1108. doi: 10.1002/hep.510240521. [DOI] [PubMed] [Google Scholar]
- 46.Pastorekova S, Parkkila S, Parkkila AK, Opavsky R, Zelnik V, Saarnio J, Pastorek J. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 47.Saarnio J, Parkkila S, Parkkila AK, Pastorekova S, Haukipuro K, Pastorek J, et al. Transmembrane carbonic anhydrase, MN/CA IX, is a potential biomarker for biliary tumours. J Hepatol. 2001;35:643–649. doi: 10.1016/s0168-8278(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 48.Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- 49.Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. J Biol Chem. 2006;281:15434–15440. doi: 10.1074/jbc.M508277200. [DOI] [PubMed] [Google Scholar]
- 50.Costantino G, Entrena-Guadix A, Macchiarulo A, Gioiello A, Pellicciari R. Molecular dynamics simulation of the ligand binding domain of farnesoid X receptor. Insights into helix-12 stability and coactivator peptide stabilization in response to agonist binding. J Med Chem. 2005;48:3251–3259. doi: 10.1021/jm049182o. [DOI] [PubMed] [Google Scholar]
- 51.Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dussault I, Beard R, Lin M, Hollister K, Chen J, Xiao JH, et al. Identification of gene-selective modulators of the bile acid receptor FXR. J Biol Chem. 2003;278:7027–7033. doi: 10.1074/jbc.M209863200. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, Lo JL, Huang L, Zhao A, Metzger E, Adams A, et al. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002;277:31441–31447. doi: 10.1074/jbc.M200474200. [DOI] [PubMed] [Google Scholar]
- 54.Heuman DM, Pandak WM, Hylemon PB, Vlahcevic ZR. Conjugates of ursodeoxycholate protect against cytotoxicity of more hydrophobic bile salts: in vitro studies in rat hepatocytes and human erythrocytes. Hepatology. 1991;14:920–926. doi: 10.1002/hep.1840140527. [DOI] [PubMed] [Google Scholar]
- 55.Moschetta A, vanBerge-Henegouwen GP, Portincasa P, Renooij WL, Groen AK, van Erpecum KJ. Hydrophilic bile salts enhance differential distribution of sphingomyelin and phosphatidylcholine between micellar and vesicular phases: potential implications for their effects in vivo . J Hepatol. 2001;34:492–499. doi: 10.1016/s0168-8278(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 56.Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem. 2009;284:2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
- 57.Kowdley KV, Jones D, Luketic VA, Chapman R, Burroughs A, Hirschfield GM, et al. An interantional study evaluating the farnesoid X receptor agonist obeticholic acid as monotherapy in PBC. J Hepatol. 2011:S13. [Google Scholar]
- 58.Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci USA. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398:423–430. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 61.Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Roda A, Gioacchini AM, Cerre C, Baraldini M. High-performance liquid chromatographic-electrospray mass spectrometric analysis of bile acids in biological fluids. J Chromatogr B Biomed Appl. 1995;665:281–294. doi: 10.1016/0378-4347(94)00544-f. [DOI] [PubMed] [Google Scholar]
- 63.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Pojer C, Zenz R, et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology. 2001;121:170–183. doi: 10.1053/gast.2001.25542. [DOI] [PubMed] [Google Scholar]
- 64.Yahagi K, Ishii M, Kobayashi K, Ueno Y, Mano Y, Niitsuma H, et al. Primary culture of cholangiocytes from normal mouse liver. In Vitro Cell Dev Biol Anim. 1998;34:512–514. doi: 10.1007/s11626-998-0106-x. [DOI] [PubMed] [Google Scholar]
- 65.Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thuringer A, et al. Curcumin improves sclerosing cholangitis in Mdr2−/− mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. doi: 10.1136/gut.2009.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]