Abstract
The symptoms of Autism Spectrum Disorder (ASD) have been suggested to manifest from atypical functioning of the autonomic nervous system (ANS), leading to altered arousal and atypical processing of salient stimuli. Coherent with this, persons with ASD show heightened autonomic activity, sleep difficulties, and structural and neurochemical alterations within the ANS. Recently, we observed decreased pupil responses to human faces in children with ASD. In the current study, we found differences in baseline (tonic) pupil size, with the ASD group exhibiting a larger pupil size than age-matched controls. Pupil responses are sensitive and reliable measures of ANS functioning, thus, this finding highlights the role of the ANS, and may provide clues about underlying neuropathology.
Keywords: autism spectrum disorder, pupillometry, autonomic nervous system, arousal, eye-tracking, early identification
Since the initial description of Autism Spectrum Disorder (ASD) by Kanner (1943), it has been hypothesized that ASD symptomology may manifest from a more basic neuropsychological deficit in arousal or alertness (Dawson & Lewy, 1989; Ornitz, 1969), which may themselves be traced to atypical functioning of the autonomic nervous system (ANS) (Hirstein, Iversen, & Ramachandran, 2001). Consistent with this hypothesis are reports of reduced amount and quality of sleep (Daoust, Limoges, Boldue, Mottron, & Godbout, 2004; Williams, Sears, & Allard, 2004), and heightened autonomic responses at rest in persons with ASD, relative to controls. These ANS responses include higher skin conductance (SC) (Hirstein et al., 2001; Zahn, Rumsey, & Van Kemmen, 1987), heart-rate (HR) (Hirstein et al., 2001; Ming, Julu, Brimacombe, Connor, & Daniels, 2005) blood pressure (Ming et al., 2005), and respiratory rate (Zahn et al., 1987). In addition, persons with ASD show a lack of HR increase to potentially stressful stimuli (Goodwin et al., 2006), and a lack of SC increase to human presence (Hirstein et al., 2001). Furthermore, we recently found children with ASD to have a decreased pupil size to human faces, particularly while inspecting the internal features of the face, while age-matched controls showed an increase in pupil size (Anderson, Colombo, & Shaddy, 2006). A balance of inhibitory and excitatory activity within the sympathetic and parasympathetic divisions of the ANS determines the level of autonomic activity in multiple organs systems (Shields, 1993) and prepares an individual to respond appropriately to incoming information (Aston-Jones & Cohen, 2005). Thus, structural and/or neurochemical impairment within the ANS would lead to an altered balance, and could play a major role in the heightened tonic (baseline or at-rest) autonomic responses, atypical sleep patterns, and differential processing of salient information seen in aberrant task-specific ANS responses in ASD.
Evaluation of pupil size has long been used to assess neurological functioning, and has been used in both clinical and typical populations to assess alertness and cognitive processing (Loewenfeld, 1999). This is because pupillary responses are more reliable and sensitive to small-scale changes and small sample sizes than other autonomic measures (Kahneman, Tursky, Shapiro, & Crider, 1969). Thus, the measurement of pupil size in ASD could prove to be valuable in early detection of ASD and understanding its underlying pathology. The parasympathetic division of the pupillary system is primarily mediated by acetycholine (ACh) and reduction in cholinergic receptor number has been found in persons with ASD (Bauman & Kemper, 1994; Martin-Ruiz et al., 2004). The sympathetic division of the pupillary system is primarily mediated by norepinephrine (NE) (Hou, Langley, Szabadi, & Bradshaw, 2007; Stenberg, 2007; Szabadi & Bradshaw, 1996) and increases in plasma levels of NE have also been found in those with ASD (e.g., Cook et al., 1990; Lake, Ziegler, & Murphy, 1977). These facts suggest the pupillary system as a potentially powerful marker for ASD (e.g., Anderson et al., 2006). Here we sought to analyze tonic (baseline) pupillary responses, obtained from our previous investigation, since many other autonomic indicators of ASD are evident at baseline or rest. To our knowledge, examination of tonic pupil size in ASD has not been previously reported.
Participants consisted of 22 children (23–70 months of age) whose data were obtained from our previous investigation (Anderson et al., 2006). Participants were divided among three groups: ASD (n = 7), mental age-match (MA; n = 6), or chronological age-match (CA; n = 9). The ASD group consisted of children who had a previous diagnosis of Autism or Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) (mental age, M = 32; chronological age, M = 48). The MA group was matched with the ASD group on both mental (M = 38) and chronological age (M = 46), and the CA group was matched with the ASD group on chronological age (M = 49). All participants were free of impairments in vision, hearing, and/or motor function, and were healthy and medication-free. Additional information on group composition, descriptives, matching strategies, and inclusion/exclusion criteria for the current participants have been described elsewhere (Anderson et al., 2006).
The experimental paradigm, stimuli, and eye-tracking procedures have also been described (Anderson et al., 2006). Briefly, we presented the participants with eight face (human and animal) and non-face (toys and land-scapes) static photographs (2 from each category) for 15 s each, using an Applied Science Laboratory (ASL) eye-tracking system (Applied Science Laboratory, 2001), with GazeTracker interface software (Eye-Gaze Response Interface Computer Aid, 2001). A blank gray interstimulus slide was presented before and after each stimulus for 3, 5, 7, 9, 11, 13, 15, and 17 s; these times were randomly dispersed within each stimulus set. The interstimulus slides were matched for luminance (5.1 lx) and size (25.8 cm2; visual angle = 5.45°).
To examine tonic pupil size, we derived pupillary data from each of the nine interstimulus slides for all participants. Only pupil responses that occurred while the subject was looking at the interstimulus slide were used in the analysis. Pupil data was inspected and corrected for artifacts using methods previously described (Anderson et al., 2006), and a mean pupil size and pupil waveform (i.e., pupil size changes across time; an average pupil size was computed every .12 s then plotted over time to obtain a pupil waveform) was obtained for each of the nine interstimulus slides.
A repeated-measures analysis of variance (ANOVA) revealed no differences as a function of the ordinal position of the nine interstimulus slides [F (8, 64) = 1.18, p = .328]; thus, we averaged pupil size and pupil wave-forms across these slides to form an overall average tonic pupil size and average pupil waveform. We examined between-group differences in tonic pupil size using a oneway ANOVA, revealing a significant difference between the groups [F (2,19) = 3.863, p = .039], with a large effect size (z2 = .29; d = 1.359; Hedges’ g = 1.30) even when the sample size is accounted for (this is adjusted in the Hedges’s g estimate; Hedges & Olkin, 1985). More specifically, the ASD group had a significantly larger tonic pupil size (n = 7; M = 2.79; SD= 0.54) than either the MA (n = 6; M = 3.10; SD = 0.64) (p = .035) or CA (n = 9; M = 3.10; SD = 0.48) (p = .020) groups, who did not differ from each other (p = .987) (Fig. 1).
FIGURE 1.
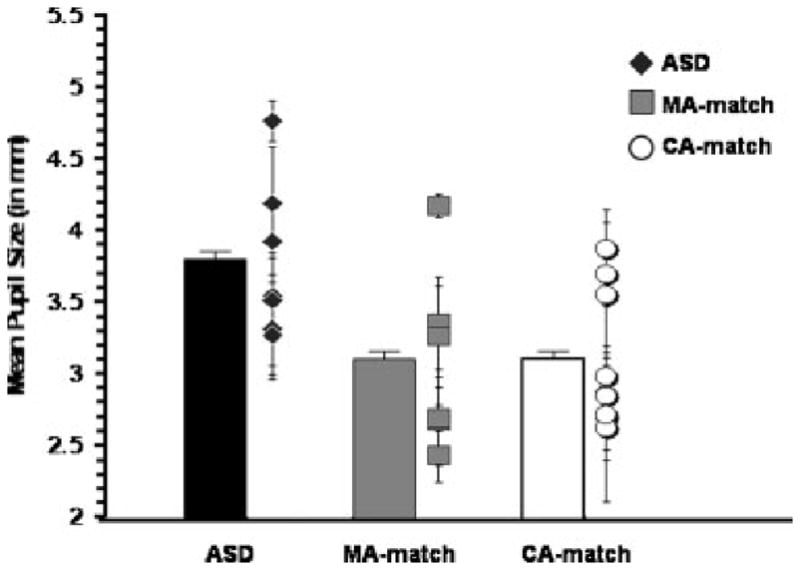
Mean pupil size during the nine interstimulus slides for the Autism Spectrum Disorder (ASD), mental age-match (MA), and chronological age-match (CA) groups, presented in the bar graph. Mean pupil size and standard deviation during the nine interstimulus slide for each individual subject, organized by group, presented in the scatterplot
We examined between-group differences in pupil waveforms using a repeated-measures ANOVA, revealing a significant main effect of pupil size [A = .378, F (6, 13) = 3.563, p = .026, z2 = .622], with a significant linear decrease across time [F (1, 18) = 12.995, p = .002, Z2 = .419]. More specifically, pupil size decreased significantly between .24 (M = 3.354) and .36 s (M = 3.263, p = .001), and between .36 and .48 s (M = 3.214, p = .042). As revealed in the previous analysis, there was also a significant main effect of group [F (2, 18) = 4.099, p = .034, z2 = .313], with the same follow-up results. However, the group by pupil interaction was not significant [A = .546, F (12, 26) = .765, p = .679, Z2 = .261], indicating that the decrease in pupil size across time does not vary as a function of diagnostic group. Thus, average tonic pupilsize is the only measure, from these analyses, that significantly distinguishes the ASD group from age-matched controls (Fig. 2).
FIGURE 2.
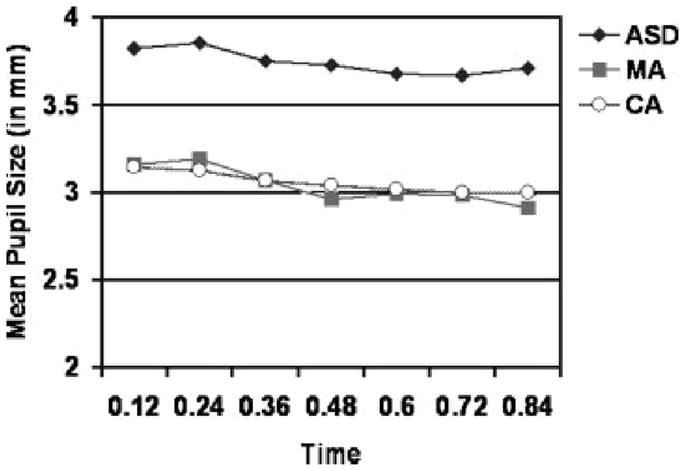
Mean pupil waveforms from. 12 to .84 s across the nine interstimulus slides for the Autism Spectrum Disorder (ASD), mental age-match (MA), and chronological age-match (CA) groups.
We were concerned that tonic pupil size might have mediated phasic pupil responses to human faces, found in our previous study (Anderson et al., 2006). To address this question, we partialled tonic pupil size from the previously observed decrease to faces; the previous effect remained significant [rp (16) = .65, p = .003], indicating that tonic pupil size was in fact independent of the differential phasic pupil responses to human faces that we have previously reported (Anderson et al., 2006). In addition, analyses also indicate that the stimulus presented prior to the interstimulus period, which tonic pupil size was derived, had no effect on tonic pupil size. These analyses suggest that tonic and phasic pupil responses may reflect independent cognitive and neurological processes, which are both separately implicated in ASD.
A discriminant analysis conducted to determine whether tonic pupil size successfully identified group membership was significant [A = .71, j2 (2, N = 22) = 6.48, p = .039]. The analysis successfully predicted group membership for 59% of the participants, with a cross-validated classification using the “leave-one-out” technique correctly classifying 23% of the cases. In particular, group membership classification using tonic pupil size as a predictor yielded 71% correct classification (71% cross-validated) for the ASD group, 33% (0% cross-validated) for the MA group, and 67% (0% cross-validated) for the CA group; thus, the ability of tonic pupil size to predict group membership warrants further investigation (Table 1).
Table 1.
Classification Results of Group Membership
| Actual group | Predicted group
|
||
|---|---|---|---|
| ASD | MA | CA | |
| ASD | 5 (71%) | 2 (29%) | 0 (0%) |
| MA | 1 (17%) | 2 (33%) | 3 (50%) |
| CA | 3 (33%) | 0 (0%) | 6 (67%) |
Numbers represent the number of subjects predicted to be in group based on tonic pupil size. The number in parentheses is the percentage of the actual group.
ASD, autism spectrum disorder; MA, mental age-match; CA, chronological age-match.
The results of this study are consistent with previous reports indicating a heightened level of ANS functioning in ASD. Pupil size is symptomatic of ANS balance, and this balance can be altered by age, level of arousal or alertness, and/or neural impairment within the pupillary system (Loewenfeld, 1999). Because the ASD group was age-matched with controls, however, the larger tonic pupil size cannot be attributed to age effects among our participants. Given the large effect size of this phenomenon, our small sample size is acceptable; however, replication with a larger sample would enable additional assessment of age effects through group-by-age interactions. It is possible that heightened autonomic responses in ASD are reflective of increased arousal, which may be the result of increased stress caused by the testing environment. However, based on neurochemical (Bauman & Kemper, 1994; Cook et al., 1990; Lake et al., 1977; Martin-Ruiz et al., 2004) and neuroanatomical studies in ASD (Bailey et al., 1998; Bauman & Kemper, 1994; Rodier, Ingram, Tisdale, Nelson, & Romano, 1996; Weidenheim et al., 2001), it is also plausible that the larger tonic pupil size is reflective of pathology within the pupillary system. Thus, future studies examining the relation between pupil size and neural structure and function will help to determine the source (neural or environmental) of these heightened tonic pupil responses. In addition, because our MA group was heterogeneous, in terms of delay area, replication of this study with more homogeneous clinical controls (e.g., Down syndrome) will be necessary to determine whether this response is specific to ASD. Finally, some autonomic responses such as blood pressure, respiration rate, and HR have been found to be with correlated body mass index (BMI) (e.g., Gelber, Pfeifer, Dawson, & Schumer, 1997; Nagai & Moritani, 2004; Pitzalis et al., 2000). However, no such relationship has been found with pupillary responses (Filipe, Falcao-Reis, Castro-Correia, & Barros, 2003; Hendriksen, Oey, Wieneke, Bravenboer, & Banga, 1992; Piha, Rommemaa, & Koskenvuo, 1994), and thus BMI was not measured in the current study. However, because persons with ASD have been found to have a larger BMI compared to control groups and normative values (e.g., Mraz, Green, Dumont-Matbieu, Makin, & Fein, 2007; Torrey, Dhavale, Lawlor, & Yolken, 2004; Webb et al.,2007; Whiteley, Dodou, Todd, & Shattock, 2004), it may be prudent to include BMI in future studies to ensure that pupil size is not confounded by the larger BMI of those with ASD.
In summary, the results of this report indicate a larger tonic pupil size in children with ASD. This may implicate atypical processes in cognitive and/or neurological function. Thus, future investigations aimed at replicating and extending these findings to examine the neurological basis of both tonic and phasic pupil responses in ASD is warranted, and may help to clarify the neuropathology of the disorder. In addition, because the preliminary results of the discriminant analysis indicate that tonic pupil size correctly classified 71% of the ASD group, further investigation into the ability of tonic pupil size to predict group membership is warranted and may lead to further examination of this measure as an early indicator of ASD.
Acknowledgments
We are obliged to the children and their parents in Kansas and Missouri who participated in this study. Christa J. Anderson was supported by NSF grants 0318072 and 0521860. The current study is based on and uses the same participants as the Anderson, Colombo, and Shaddy (2006) study.
Contributor Information
Christa J. Anderson, Schiefelbusch Institute for Life Span Studies, Department of Psychology, University of Kansas
John Colombo, Schiefelbusch Institute for Life Span Studies, Department of Psychology, University of Kansas.
References
- Anderson CJ, Colombo J, Shaddy DJ. Visual scanning and pupillary responses in young children with autism spectrum disorder. Journal of Clinical and Experimental Neuropsychology. 2006;28:1238–1256. doi: 10.1080/13803390500376790. [DOI] [PubMed] [Google Scholar]
- Applied Science Laboratory. Eye-tracking system Model 504 with Pan/tilt optics [Computer software, hardware, and manual] Bedford, MA: Applied Science Laboratory; 2001. [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism. In: Bauman ML, Kemper TL, editors. The neurobiology of autism. Baltimore: Johns Hopkins University Press; 1994. pp. 119–169. [Google Scholar]
- Cook EH, Leventhal BL, Heller W, Metz J, Wainwright M, Freedman DX. Autistic children and their first-degree relatives: Relationships between serotonin and norepinephrine levels and intelligence. Journal of Neuro-psychiatry. 1990;2(3):268–274. doi: 10.1176/jnp.2.3.268. [DOI] [PubMed] [Google Scholar]
- Daoust AM, Limoges E, Boldue C, Mottron L, Godbout R. EEG spectral analysis of wakefulness and REM sleep in high functioning autistic spectrum disorders. Clinical Neurophysiology. 2004;115:1368–1373. doi: 10.1016/j.clinph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Dawson G, Lewy A. Arousal, attention, and the socioemotional impairments of individuals with autism. In: Dawson G, editor. Autism: Nature, diagnosis, and treatment. New York: Guilford Press; 1989. pp. 49–74. [Google Scholar]
- Eye-Gaze Response Interface Computer Aid [ERICA], Inc. GazeTracker [Computer software and manual] Charlottesville, VA: ERICA; 2001. [Google Scholar]
- Filipe JAC, Falcao-Reis F, Castro-Correia J, Barros H. Assessment of autonomic function in high level athletes by pupillometry. Autonomic Neuroscience: Basic and Clinical. 2003;104:66–72. doi: 10.1016/s1566-0702(02)00268-0. [DOI] [PubMed] [Google Scholar]
- Gelber DA, Pfeifer M, Dawson B, Schumer M. Cardiovascular autonomic nervous system tests: Determination of normative values and effect of confounding variables. Journal of the Autonomic Nervous System. 1997;62:40–44. doi: 10.1016/s0165-1838(96)00107-5. [DOI] [PubMed] [Google Scholar]
- Goodwin MS, Groden J, Velicer WF, Lipsitt LP, Baron MG, Hofmann SG, et al. Cardiovascular arousal in individuals with autism. Focus on Autism and Other Developmental Disabilities. 2006;21(2):100–123. [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- Hendriksen PH, Oey PL, Wieneke GH, Bravenboer B, Banga JD. Subclinical diabetic neuropathy: Similarities between electrophysiological results of patients with Type 1 (insulin-dependent) and Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:690–695. doi: 10.1007/BF00400264. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proceedings of Biological Sciences. 2001;268:1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou RH, Langley RW, Szabadi E, Bradshaw CM. Comparison of diphenhydramine and modafinil on arousal and autonomic functions in healthy volunteers. Journal of Psychopharmacology. 2007;21(6):567–578. doi: 10.1177/0269881106071022. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tursky B, Shapiro D, Crider A. Pupillary, heart rate, and skin resistance changes during a mental task. Journal of Experimental Psychology. 1969;79:164–167. doi: 10.1037/h0026952. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Lake CR, Ziegler MG, Murphy DL. Increased norepinephrine levels and decreased dopamine-fi-hydroxylase activity in primary autism. Archives of General Psychiatry. 1977;34:553–556. doi: 10.1001/archpsyc.1977.01770170063005. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. The pupil: Anatomy, physiology, and clinical applications. Detroit: Wayne State University Press; 1999. [Google Scholar]
- Martin-Ruiz CM, Lee M, Perry RH, Bauman M, Court JA, Perry EK. Molecular analysis of nicotinic receptor expression in autism. Molecular Brain Research. 2004;123:81–90. doi: 10.1016/j.molbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ming X, Julu POO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain and Development. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Mraz KD, Green J, Dumont-Mathieu T, Makin S, Fein D. Correlates of head circumference growth in infants later diagnosed with autism spectrum disorders. Journal of Child Neurology. 2007;22(6):700–713. doi: 10.1177/0883073807304005. [DOI] [PubMed] [Google Scholar]
- Nagai N, Moritani T. Effect of physical activity on autonomic nervous system function in lean and obese children. International Journal of Obesity. 2004;28:27–33. doi: 10.1038/sj.ijo.0802470. [DOI] [PubMed] [Google Scholar]
- Ornitz EM. Disorders of perception common to early infantile autism and schizophrenia. Comprehensive Psychiatry. 1969;10(4):259–274. doi: 10.1016/0010-440x(69)90002-9. [DOI] [PubMed] [Google Scholar]
- Piha SJ, Ronnemaa T, Koskenvuo M. Autonomic nervous system function in identical twins discordant for obesity. International Journal of Obesity. 1994;18(8):547–550. [PubMed] [Google Scholar]
- Pitzalis MV, Massari F, Mastropasqua F, Fioretti A, Guida P, Colombo R, et al. Age effect on phase relations between respiratory oscillations of the RR interval and systolic pressure. PACE. 2000;23:847–853. doi: 10.1111/j.1540-8159.2000.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: Developmental anomalies of the cranial nerve motor nuclei. The Journal of Comparative Neurology. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Shields RW. Functional anatomy of the autonomic nervous system. Journal of Clinical Neurophysiology. 1993;10(1):2–13. doi: 10.1097/00004691-199301000-00002. [DOI] [PubMed] [Google Scholar]
- Stenberg D. Neuroanatomy and neurochemistry of sleep. Cellular and Molecular Life Sciences. 2007;64(10):1187–1204. doi: 10.1007/s00018-007-6530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadi E, Bradshaw CM. Autonomic pharmacology of a2-adrenoceptors. Journal of Psychopharmacology. 1996;10(3):6–18. [Google Scholar]
- Torrey EF, Dhavale D, Lawlor JP, Yolken RH. Autism and head circumference in the first year of life. Biological Psychiatry. 2004;56:892–894. doi: 10.1016/j.biopsych.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Nalty T, Munson J, Brock C, Abbott R, Dawson G. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. Journal of Child Neurology. 2007;22(10):1182–1190. doi: 10.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenheim KM, Goodman L, Dickson DW, Gillberg C, Rastam M, Rapin I. Etiology and pathophysiology of autistic behavior: Clues from two cases with an unusual variant of neuroaxonal dystrophy. Journal of Child Neurology. 2001;16(11):809–819. doi: 10.1177/08830738010160110601. [DOI] [PubMed] [Google Scholar]
- Whiteley P, Dodou K, Todd L, Shattock P. Body mass index of children from the United Kingdom diagnosed with pervasive developmental disorders. Pediatrics Inter-national. 2004;46:531–533. doi: 10.1111/j.1442-200x.2004.01946.x. [DOI] [PubMed] [Google Scholar]
- Williams PG, Sears LL, Allard A. Sleep problems in children with autism. Journal of Sleep Research. 2004;13:265–268. doi: 10.1111/j.1365-2869.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- Zahn TP, Rumsey JM, Van Kemmen DP. Autonomic nervous system activity in autistic, schizophrenic, and normal men: Effects of stimulus significance. Journal of Abnormal Psychology. 1987;96(2):135–144. doi: 10.1037//0021-843x.96.2.135. [DOI] [PubMed] [Google Scholar]


