Abstract
The transposase (InsAB′) of the insertion element IS1 can create breaks in DNA that lead to induction of the SOS response. We have used the SOS response to InsAB′ to screen for host mutations that affect InsAB′ function and thus point to host functions that contribute to the IS1 transposition mechanism. Mutations in the hns gene, which codes for a DNA binding protein with wide-ranging effects on gene expression, abolish the InsAB′-induced SOS response. They also reduce transposition, whether by simple insertion or cointegrate formation, at least 100-fold compared with the frequency seen in hns+ cells. Examination of protein profiles revealed that in an hns-null mutant, InsAB′ is undetectable under conditions where it constitutes the most abundant protein in hns+ cells. Likewise, brief labeling of the hns cells with [35S]methionine revealed very small amounts of InsAB′, and this was undetectable after a short chase. Transcription from the promoters used to express insAB′ was essentially unaltered in hns cells, as was the level of insAB′ mRNA. A mutation in lon, but not in ftsH or clpP, restored InsAB′ synthesis in the hns strain, and a mutation in ssrA partially restored it, implying that the absence of H-NS leads to a problem in completing translation of insAB′ mRNA and/or degradation of nascent InsAB′ protein.
Active transposable elements appear to exhibit a high degree of autonomy. Typically, the transposase encoded by the element interacts specifically with the element's ends, locates a target sequence, and executes the cleavage and ligation reactions required to insert the element into the new site. Nevertheless, host functions can be intimately involved, most obviously in the case of the Mu prophage, where replication and other proteins of Escherichia coli are needed to transform the strand transfer intermediate into the transposition products (27). More generally, host factors appear to act as modulators of transposition (reviewed in reference 4). HU strongly stimulates formation of the Mu synaptic complex in vitro (3) and presumably in vivo. IHF maintains a bend in the IS10 ends which governs the choice of transposition pathway (41): it also stimulates transposase binding to Tn1000 ends (47). A strong IHF binding site is present in each of the inverted termini of IS1, though its function is unclear (15). Fis and DnaA modulate IS50 transposition (34). Acyl carrier protein and ribosomal protein L29 stimulate recognition of the specific Tn7 target sequence (38). Hns mutations influence the Mu transposition rate in a growth medium-dependent manner (13). Since these proteins act in general to mold and compact DNA and to influence gene expression, it is not hard to imagine how they might influence the precise geometry of interacting partners or serve as the agents through which transposition responds to cell physiology.
The small (768-bp) enterobacterial transposable element IS1 is an interesting example, because its transposition has several different outcomes: simple insertion of the element at new sites (2), formation of cointegrate molecules in which the donor replicon is fused to the target by flanking copies of the element (14, 28), deletion of DNA adjacent to the element (26, 33), inversion (6), and circle formation by precise excision (35, 45). Whether these products arise by branching of a common pathway or from distinct mechanisms is unknown.
The likelihood that accessory host proteins contribute to this diversity of transposition end products seemed high, since IS1 is essentially simple, being composed of two partly overlapping open reading frames, insA and insB, bounded by short, imperfect terminal repeats, IRL (left end) and IRR (right end). The transposase, InsAB′, is made as a result of a low-frequency −1 translational frameshift at the sequence A6C in the overlap region (11, 36). To identify host functions that regulate IS1 transposition, we used the SOS response induced by InsAB′ (25) as a screen for the inhibitory effects of mutations in candidate genes. Mutant alleles of most genes, including himD (IHF subunit) and fis, did not affect the SOS response to InsAB′, but a Tn10 insertion in the hns gene reduced it markedly (see Results). This paper is a report of our attempts to find out why.
H-NS is a small (15-kDa) abundant (∼20,000 molecules per cell) protein that plays a major role in compaction of the E. coli chromosome (43, 44). In binding to DNA, it shows a strong preference for curved regions (48). It modulates the transcription of many genes, usually as a repressor (1). In view of the precedents cited above, we expected that H-NS would affect IS1 transposition by directly modulating the transposition pathway. Our results show, however, that it intervenes at another point in IS1 transposition.
MATERIALS AND METHODS
Bacterial strains and plasmids
The strains used were derivatives of E. coli K-12 and were essentially as described previously, with genotypes (25). The transformation recipient for plasmid constructions and the host for InsAB′ production experiments was MC1061; the SOS reporter strain was BR293; the donor strain for transformation assays was C600 Δ(srl-recA)306::Tn10 carrying the conjugative transposon target plasmid, pOX38::dTn10 cat (Cmr), or when the donor carried the lon::Tn10 allele, C600 ΔrecA938::cat (Cmr) with pOX38::gen (Gmr). The mutations hns::Tn10, Δhns::kan, lon::Tn10, and ssrA::cat (kindly provided by C. Gutierrez, J.-Y. Bouet, O. Fayet, and E. Roche, respectively) were introduced into these strains by bacteriophage P1-mediated transduction.
Plasmids are listed in Table 1. pMET37, which expresses insAB′ under lacp control, was constructed by successive additions to a pBR322 origin fragment of restriction fragments carrying the Ωon-spc unit (see below), the lacIq gene, the insAB′ (GA2GA3C) sequence, and the lacp promoter (p1 and p2); details of construction are given elsewhere (10) and are available upon request. pMET35 was made from the immediate ancestor of pMET37 by deletion of most of the IS1 wild-type sequence to leave the last 57 nucleotides of IS1, including IRR.
TABLE 1.
Plasmids used and constructed
| Plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| pAPT1 | orip15Akan, lacp::c1857λ | 29 |
| pMET13 | oripMB1bla pLλ::insA+B+ Ωon-spc | 11 |
| pMET12 | As pMET13 but insAB′ (A7C) | 11 |
| pMET37 | oripMB1bla lacp::insAB′ (GA2GA3C) lacIq Ωon-spc | 10 |
| pMET35 | As pMET37 but ΔinsAB′ | 10 |
| pTemp1.1 | oripMB1bla pLλ::lacZ | D. Zerbib |
| pDAG98 | As pMET37 but insAB′ Ω(codon 127::lacZYA) | This work |
| pDAG99 | As pMET37 but insAB′ Ω(codon 206::lacZYA) | This work |
| pDAG92 | oripMB1bla araC araBADp::insA′::lacZ | This work |
| pMET8 | oripMB1bla Ωon-spc | 11 |
| pMP3 | oripMB1bla Ωon-spc insA+B+ | 30 |
| pCST200 | oripMB1bla araC araBADp::insA+B+ | 37 |
| pCST400 | As pCST200 but insAB′ (GA2GA3C) | 37 |
Ωon-spc is the artificial transposable element composed of two IRL ends flanking a spectinomycin resistance gene (aadA) and the transcription terminator of T4 gene 32 (31).
pTemp1.1 was constructed by Didier Zerbib. A PCR fragment containing bacteriophage lambda sequence from −229 to +3 relative to the pL transcriptional start site and flanked by synthetic HindIII sites, was cleaved with HindIII and inserted at a HindIII site upstream from a promoterless lacZ gene, which had replaced tetA in a pBR322-based vector, pAP201.
pDAG98 and -99 were made by insertion of the SmaI-StuI fragment of pRS591 (42), containing lacZYA, into pMET37 at the MluI site (made blunt ended by incubation with DNA polymerase I Klenow fragment and deoxynucleoside triphosphates) or the PshAI site in insB to fuse the first 127 and 206 codons, respectively, of insAB′ to codon 5 of lacZ.
pDAG92 was made by excising a fragment from pCST420 (37) containing araC, araBADp, and the first 20 bp of insA, using NsiI (and then blunt ending with T4 DNA polymerase) and PvuII and inserting it at the BamHI site (after blunt ending with Klenow fragment) in the proximal end of the lacZ gene of a pUC12-based vector, pFDX2561 (kindly provided by Caroline Welz).
pMET8 is pBR322 with Ωon-spc inserted at the PvuII site.
Media and growth conditions.
The medium for routine growth was Luria Bertani (LB) broth supplemented with 1.5% agar for solid medium and, as appropriate, with the antibiotics ampicillin (100 μg/ml), spectinomycin (100 μg/ml), kanamycin (30 μg/ml), chloramphenicol (20 μg/ml), tetracycline (12.5 μg/ml), and gentamicin (2.5 μg/ml). Cultures were grown at 37°C except where otherwise noted. The medium for l-[35S]methionine labeling of proteins was M9-mam (M9 salts with 1 μg of thiamine/ml, 0.2% glucose, and 0.5% Difco methionine assay medium). The medium for selection of Lon+ transductants was LB agar containing methyl methane sulfonate at 0.05%.
SOS induction.
Assay of the SOS response to induction of InsAB′ expression was essentially as described previously (25). In the case of the lacp-controlled insAB′, the conditions were the same, except that cultures were grown at 37°C and induced by addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 0.3 mM.
Promoter::lacZ activity.
Strains harboring plasmids that carried inducible promoters fused to lacZ were grown and induced under the same conditions as those used in the SOS induction assay (PL and lac promoters) or the InsAB′ production assay (araBAD promoter) and were sampled at intervals for determination of β-galactosidase specific activity (25).
Transposition assay.
The mating-out assay described by Chandler and Galas (5) was used, except for minor modifications noted in “Bacterial strains and plasmids” above. Care was taken to start donor cultures of hns mutants from small colonies and to monitor growth rates prior to mating so that faster-growing cultures could be eliminated. Transposition frequencies were calculated as the ratios of Spr Cmr recipients to Cmr recipients (simple insertions plus cointegrates) and of Apr Spr Cmr to Cmr (cointegrates). The efficiency of pOX38 conjugation was ∼10-fold lower from an hns donor than from an hns+ donor.
InsAB′ production.
Fresh pCST400 (or pCST200) transformants of MC1061 strains were grown overnight at 37°C in LB containing glucose (0.2%) and ampicillin, and the overnight cultures were diluted in fresh medium at an optical density at 600 nm (OD600) of 0.05 and incubated with aeration at 30°C. The cultures were maintained in logarithmic growth for about six generations by repeated dilution to monitor the growth rate and then induced by the addition of arabinose to 1% and incubated for one more hour. Samples of known OD600 were chilled by mixing with cold 10 mM sodium azide, centrifuged, and resuspended in sodium dodecyl sulfate (SDS)-mercaptoethanol buffer (24). Samples equivalent to 0.05 OD600 units were subjected to electrophoresis and Coomassie blue staining.
Pulse-chase analysis of InsAB′ stability.
Overnight cultures in M9-mam (plus ampicillin) of MC1061 hns+ and Δhns strains freshly transformed with pCST400 were diluted in fresh medium at an OD600 of ∼0.02, and the cultures were incubated at 37°C with aeration. At an OD600 of ∼0.25 (late log phase), a sample was withdrawn into another culture flask (uninduced control), and arabinose was added to the rest at 1% (final concentration). After incubation for a further one-half generation time (30 min for hns+; 42 min for Δhns), 4.0 ml of culture was added to 20 μCi of l-[35S]methionine (Amersham) (800 Ci/mmol) and incubated for 1 min before addition of unlabeled methionine to 1 mg/ml. Incubation was continued, and 0.5-ml samples were taken in ice-cold TESAz buffer (50 mM Tris, pH 8.0, 5 mM EDTA, 50 mM NaCl, 1 mM NaN3) at the time of unlabeled methionine addition and at intervals thereafter for 1 h. The uninduced culture, as well as an induced culture of the hns+ strain carrying pCST200, was quenched immediately after the 1-min labeling period. The chilled samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and exposure of the dried gel to a phosphorimager screen.
The proportion of the total 35S protein label represented by the InsAB′ band was determined using the Tina-PCBas program (Fuji). Quantitation of a small region containing the band was carried out for all samples; the uninduced sample provided the background from which the values for the InsAB′ band were calculated. These values were then normalized by dividing each of them by the value for the total protein in its lane.
Northern hybridization analysis.
The hns+ and Δhns strains of MC1061 carrying plasmids for induction of insAB′ expression were grown in LB at 37°C to an OD600 of ∼0.3 and then induced by addition of IPTG or arabinose. After further incubation for 30 min, the cultures were added to phenol-CHCl3 and RNA was extracted by the hot-phenol method, separated on formaldehyde agarose gels, transferred to a nylon membrane (Hybond N+; Amersham), and hybridized with a radioactively labeled DNA probe, as described previously (12). The probe was a PCR fragment composed of nucleotides 2 to 674 of the 699-bp insAB′ fused reading frames, labeled by random priming in the presence of [α-33P]dATP (3,000 Ci/mmol).
RESULTS
Loss of SOS response to InsAB′ in hns mutants.
To produce InsAB′ at levels that allow the SOS response to be readily detected, we used two systems, both of them employing insAB sequences in which single-basepair insertions in the frameshift motif A6C had fused the insA and insB sequences in phase, thus relieving InsAB′ production from dependence on inefficient frameshifting. The first system involves two compatible plasmids, one carrying the fused-frame (A7C) insAB′ sequence under the control of the λ PL promoter (pMET12) and the other carrying the gene for the temperature-sensitive repressor, CI857 (pAPT1); InsAB′ synthesis is induced by raising the growth temperature to 39°C. In the second system, a lacp-controlled fused-frame (GA2GA3C) insAB′ and the lacIq repressor gene are carried on a single plasmid (pMET37). An artificial IS1 element (omegon [Ωon]) (31) was present in both InsAB′ producer plasmids to provide ends for cleavage by transposase.
These plasmids were introduced into an SOS reporter strain that carries a λimm434 prophage with its PL promoter fused to the lacZ gene; cleavage of the 434 repressor following SOS induction results in β-galactosidase synthesis, which is detected on indicator medium or measured in samples of liquid cultures (9). Induction of the λ PL-controlled insAB′ (A7C) gene in this strain raised β-galactosidase specific activity above the background level of 149 U, to 859 U (Table 1). Most of this increase resulted from the presence of IS1 ends on the plasmid, since in their absence β-galactosidase rose only modestly, to 256 U (presumably through action on chromosomal IS1 ends). Derivatives of the reporter strain carrying hns::Tn10 and Δhns::kan alleles were constructed. The SOS response to InsAB′ induction in the hns::Tn10 mutant carrying pMET12 was much lower (264 U) than in the hns+ reporter. The difference was even more marked in the experiment, shown in Table 2, which employed the Δhns::kan derivative. Here, full induction of lac promoter activity with IPTG and an insA-insB joint sequence on which reverse frameshifting does not occur (GA2GA3C) (11) led to higher levels of InsAB′ and an increased SOS response in hns+ cells. Residual synthesis in the Δhns mutant, however, was as low as in the hns::Tn10 mutant.
TABLE 2.
Effects of hns mutations on induction of the SOS response by IS1 transposase
| Promoter and gene | hns | Presence of:
|
β-galactosidase sp act (Miller units) | |
|---|---|---|---|---|
| InsAB′ | IRL + IRR | |||
| PL::insAB′ (A7C) at 39°C | + | − | − | 149 |
| + | + | − | 256 | |
| + | + | + | 859 | |
| ::Tn10 | − | − | 150 | |
| ::Tn10 | + | − | 200 | |
| ::Tn10 | + | + | 264 | |
| lacp::insAB′ (GA2GA3C) + IPTG | + | − | + | 177 |
| + | + | + | 1,900 | |
| Δ::kan | − | + | 149 | |
| Δ::kan | + | + | 240 | |
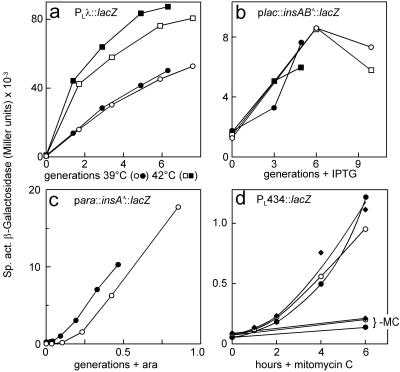
The PL and lacp promoters were just as active in the Δhns strain as in the wild type (Fig. 1a and b), and detection of SOS induction was just as responsive (Fig. 1d). The results imply that the absence of H-NS retards IS1 transposition. We next tested this suggestion directly.
FIG. 1.
Activities of promoters in hns mutant strains. Cultures of isogenic hns+ (open symbols) and hns (solid symbols) strains growing exponentially in LB medium were shifted to inducing conditions (0 generations) and sampled at intervals for assay of β-galactosidase. (a) BR293 strains carrying pAPT1 (cI857) and pTemp1.1 (PL::lacZ). (b) MC1061 strains carrying lacIq and lacp::insAB′::lacZ fusions on pDAG98 (circles) or pDAG99 (squares). (c) MC1061 strains carrying araC and araBADp::insA′::lacZ on pDAG92. (d) BR293 strains (○, hns+; •, Δhns; ⧫, hns::Tn10) carrying PL434::lacZ (SOS reporter fusion) at attλ incubated with or without (−MC) 20 ng of mitomycin C/ml. The culture doubling times (±2 min) in LB at 37°C for hns+ and Δhns strains were 30 and 51 min (BR293) and 26 and 58 min (MC1061), respectively. The cultures entered stationary phase after ∼4 generations (a and b) or 4 h (d). ara, arabinose.
IS1 transposition in hns mutants.
Transposition frequency was measured by the mating-out assay of Chandler and Galas (5). A ΔrecA derivative of strain C600 carrying the F′, pOX38-Cm, was transformed with the transposon donor plasmid pMET37, which carries bla (Apr), lacp::insAB′ (GA2GA3C), and the repressor gene, lacIq, as well an artificial IS1 element (Ωon-spc) composed of two IRLs flanking the aadA (spectinomycin resistance) gene. Transposition to the F′ was measured as the fraction of F+ (Cmr) exconjugant recipients that were also Apr Spr (cointegrates) or Spr (cointegrates plus simple insertions). The first two lines of Table 3 show the main results. Transposition in the Δhns strain was ∼3,000-fold lower than in the wild type, scarcely above background levels. When the donor cells had been grown in the presence of IPTG to induce InsAB′ production (Table 3, third and fourth lines), transposition in the wild-type was no higher than in the absence of IPTG; presumably, escape synthesis from lacp provided enough InsAB′ for the maximum rate of transposition in this system. In the Δhns strain, however, IPTG induction increased transposition ∼35-fold. The hns mutation did not significantly affect the frequency of cointegrates relative to direct insertion events.
TABLE 3.
Effects of Δhns allele on IS1 transposition frequency
| Transposon donor plasmid | hns | IPTG (1 mM)a | Transposition frequency (± SD) (Spcr/Camr) | n | Cointegrate fraction (Ampr/Spcr) | Δhns/hns+ |
|---|---|---|---|---|---|---|
| pMET37 (lacp::insAB′) | + | − | 1.4 × 10−3 (±0.5) | 3 | 0.62 | 0.00033 |
| Δ | − | 4.6 × 10−7 (±3.0) | 3 | 0.60 | ||
| + | + | 1.2 × 10−3 (±0.2) | 3 | 0.71 | 0.013 | |
| Δ | + | 1.6 × 10−5 (±0.7) | 3 | 0.50 | ||
| pMP3 (wild-type IS1) | + | 1.1 × 10−4 (±0.3) | 2 | 0.12 | 0.0032 | |
| Δ | 3.5 × 10−7 (±1.4) | 4 | 0.29 | |||
| pMET8 (no insAB′) | + | 2.7 × 10−7 (±0.1) | 2 | 0.93 | 0.56 | |
| Δ | 1.5 × 10−7 (±0.1) | 4 | 0.95 |
+, present; −, absent.
To examine the possibility that the H-NS effect we had seen might be an artifact resulting from very high levels of InsAB′, we measured transposition from Apr donor plasmids which carried a natural IS1 and Ωon-spc (pMP3) or Ωon-spc alone (pMET8); transposition from the latter plasmid depends on InsAB′ produced from chromosomal IS1 copies and can be taken as the background for the assay. Transposition from pMP3 in the Δhns strain was again close to background levels, 300-fold lower than in the hns+ strain. This result indicates that the disparity between transposition rates in hns+ and Δhns strains reflects an authentic involvement of H-NS in IS1 transposition. The frequencies of Aps Spr exconjugants from matings with the pMET8 strain were very low for both strains. The elevated levels of apparent cointegrates led us to suspect that a significant fraction of the Spcr F′s were formed by recombination events other than transposition, as reported previously (23).
The observation that leaky synthesis from lacp allowed maximal rates of transposition in the wild type whereas induction of lacp led to higher rates in the Δhns strain suggested that in the latter strain low transposition rates might result from limited quantities of InsAB′. We next investigated InsAB′ levels.
InsAB′ production in Δhns cells.
Cultures of strain DLT288 (Δhns) carrying pCST400, in which the insAB′ (GA2GA3C) sequence is controlled by the arap promoter, and of the equivalent hns+ strain (DLT286) were treated with arabinose, and samples were taken for analysis of proteins by SDS-PAGE. A Coomassie blue-stained gel is shown in Fig. 2. Extracts of hns+ cells induced with arabinose (lane 5) contain an abundant polypeptide of ∼27 kDa that is not seen in extracts of uninduced cells (lane 4) or those of induced cells carrying the wild-type insAB′ (A6C). The 27-kDa species corresponds to the predicted size of InsAB′, 26.6 kDa. No band of this size was detected in Δhns samples, even those taken from cultures grown in the presence of arabinose for 2 h (lane 8). The araBADp promoter, like lacp and PL, was just as active in Δhns cells as in hns+ cells (Fig. 1c). We conclude that in hns mutant cells, either the synthesis or the stability of InsAB′ is much reduced.
FIG. 2.
IS1 transposase (InsAB′) protein in hns+ and Δhns cells. Cultures of MC1061 strains carrying no IS1 sequence (−), wild-type insAB′ (A6C), or fused-frame insAB′ (GA2GA3C) under araC-araBADp control, on plasmids pDAD18, pCST200, and CST400, respectively, were grown in LB medium at 30°C to late log phase. Portions of the cultures were then added to arabinose (final concentration, 1%) or left untreated (−), and incubation was continued for 1 or 2 h, as shown, before sampling for analysis of the total protein content by SDS-PAGE (12% acrylamide) and Coomassie blue staining. The arrow indicates the position of the InsAB′ band.
To determine the rate of InsAB′ degradation in each strain, we performed a pulse-chase experiment. Arabinose was added to log-phase cultures of DLT286 and -288, as described above, to induce insAB′ transcription. Nascent protein was labeled by the addition of l-[35S]methionine, and labeling was terminated 1 min later by the addition of excess nonradioactive methionine. Samples were withdrawn at intervals for assay of 35S-labeled InsAB′ protein by SDS-PAGE and radioautography. We were obliged to observe InsAB′ against a background of labeled host polypeptides, because quantitative immunoprecipitation of InsAB′, using antibodies raised against InsA protein, was not successful. Nevertheless, the radioautograph shown in Fig. 3a reveals that a prominent band of 35S-InsAB′ is present in the sample from pulse-labeled hns+ cells and that this declines upon subsequent incubation. In contrast, an InsAB′ band in the pulse-labeled sample from Δhns cells is barely detectable and was not visible at all in chased samples. The contrast between the amounts of labeled InsAB′ in the two strains is more readily seen on the radioautograph of a gel in which equivalent samples from hns+ and Δhns cells were run side by side (Fig. 3b). We estimate the half-life of InsAB′ in hns+ cells to be ∼13 min (Fig. 3c); there was too little signal above background to allow calculation of the half-life in Δhns cells.
FIG. 3.
Pulse-chase analysis of InsAB′ synthesis and stability. (a) Arabinose-induced cultures of hns+ and Δhns strains carrying pCST400 were labeled with l-[35S]methionine for 1 min before the addition of excess unlabeled methionine. Samples removed at intervals (chase-mins) were subjected to SDS-PAGE and radioautography. −/0, uninduced hns+ culture, labeled as described above. The high- and low-molecular-weight regions of the gel are not shown. (b) As in panel a, with the hns+ and Δhns samples from the 0- to 4-min time points paired. (c) 35S-InsAB′ as a percentage of total labeled protein plotted against time after addition of unlabeled methionine. The half-life was calculated (see Materials and Methods) from the initial degradation rate, shown by the dotted line.
Without a measurement of InsAB′ stability in Δhns cells, we cannot eliminate the possibility that H-NS normally intervenes in the synthesis of the transposase. We next examined the various steps of InsAB′ synthesis.
InsAB′ mRNA synthesis in Δhns cells.
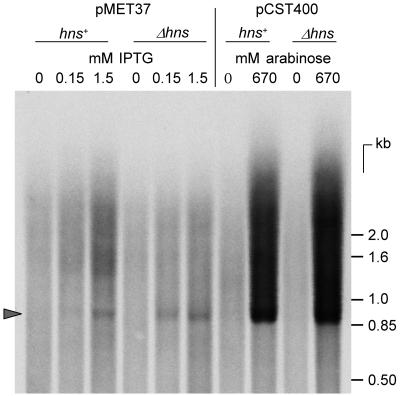
While the promoters used for expression of insAB′::lacZ fusions are just as active in hns mutants as in hns+ cells (Fig. 1), native insAB′ mRNA might be more sensitive to degradation in the mutants. This possibility was tested by Northern blot analysis of total RNA extracted from cultures of the same strains as those employed to test InsAB′ protein levels. Figure 4 shows the amounts of a labeled probe consisting of the entire IS1 sequence that hybridized with total RNA extracted from log-phase cultures of strains carrying IPTG-inducible and arabinose-inducible insAB′ genes. The amounts of insAB′ mRNA in induced Δhns cells were comparable to, or even higher than, those in the equivalent hns+ cells. Prentki et al. (32) reported the presence of a rho-dependent terminator in IS1; a higher efficiency of this terminator in hns mutants might reduce production of full-length mRNA. However, slot blot hybridization using a probe consisting of only the 3′ 286 nucleotides of insB revealed comparable amounts of IS1-specific RNA in the hns+ and Δhns total-RNA preparations (data not shown). Absence of H-NS, therefore, does not interfere with InsAB′ protein production at the level of transcription or messenger stability.
FIG. 4.
Northern analysis of insAB′ mRNA. Total RNAs extracted from cells growing exponentially in LB and induced as shown were resolved by formaldehyde-agarose gel electrophoresis, transferred to nylon membranes, and hybridized with 33P-labeled insAB′ DNA. The amount of Δhns RNA loaded was 0.66 times that of hns+ RNA (10 μg) to compensate for the lower proportion of rRNA present in hns mutant cells (20). The arrowhead indicates the position of the insAB′ mRNA. The scale on the right is derived from a 33P-labeled 1-kb DNA ladder (Gibco-BRL) denatured and processed with the RNAs.
InsAB′ mRNA translation in Δhns cells.
Yamashino et al. (49) reported that in an hns null mutant, production of the RpoS sigma factor was ∼15-fold higher from a given amount of mRNA than in the hns+ counterpart, while the rate of degradation of the RpoS protein was at least 10-fold lower. These observations provide a precedent for the involvement of H-NS in both translation efficiency and protein stability. The data in Fig. 1b indicate, however, that translation of most of the insAB′ mRNA is not significantly affected by the Δhns mutation. In plasmids pDAG98 and -99, the N-terminal 126 and 205 codons, respectively, of the 232-codon insAB′ reading frame are fused to the 5′ end of lacZ. If there is a problem with translation of insAB′ mRNA, it must occur during reading of the last 27 codons or, conceivably, be suppressed as a result of the lacZ mRNA extension.
Mutation in lon suppresses InsAB′ deficiency in Δhns cells.
We examined the abilities of mutant alleles of known proteolysis-related genes to restore InsAB′ synthesis in the Δhns strain. Figure 5a shows a Coomassie blue-stained SDS-polyacrylamide gel on which the proteins of lon and ssrA mutant derivatives of strains DLT288 (Δhns) have been resolved. A strong InsAB′ band is present in the extract of the lon mutant, and this band can also be detected in the ssrA mutant sample. No restoration of InsAB′ synthesis (or of SOS response) was seen upon introduction of ftsH, clpP, or clpX mutations into Δhns or hns::Tn10 strains (data not shown). These results suggest that the InsAB′ protein is subject to specific degradation by Lon protease and the C-terminal proteolysis-marking mechanism governed by ssrA.
FIG. 5.
InsAB′ protein in mutant derivatives of the Δhns strain. (a) Cultures of the MC1061-based strains shown, carrying pCST400, were induced (+) with arabinose (ara) or not induced (−) and were analyzed as for Fig. 2. (b) As in panel a, arabinose-induced cells of four Lon+ Tets transductants of the Δhns lon::Tn10 strain. The arrowhead indicates the position of the InsAB′ band.
Extragenic suppressors of slow growth arise frequently in Δhns strains (20). It was therefore important to test whether the observed restoration of InsAB′ synthesis in the lon::Tn10 derivative was indeed due to the allele introduced and not to overgrowth by mutants carrying unknown suppressors. The lon+ allele was substituted for the mutant allele in the Δhns lon::Tn10 strain by P1 transduction, using selection for resistance to methyl methane sulfonate. Four transductants carrying pCST400 were tested for InsAB′ production following the addition of arabinose. All showed the absence of InsAB′ characteristic of the original Δhns strain (Fig. 5b).
To determine whether restoration of InsAB′ synthesis by the lon mutation is accompanied by the return of InsAB′ function, transposition frequency in the Δhns lon::Tn10 strain was measured, using the mating-out system described above. The transposition frequency in the Δhns strain relative to that in hns+ did increase as a consequence of the introduction of the lon mutation (Table 4), though not to the hns+ level. However, inspection of the transposition frequency column reveals that the apparent shortfall results from a significant stimulation of transposition frequency that the lon mutation also causes in the hns+ strain. Transposition frequencies in the Δhns lon::Tn10 strain were actually comparable to those in the wild type (hns+ lon+): 0.23 × 10−3 (compared to 0.73 × 10−3) without IPTG induction and 2.6 × 10−3 (compared to 2.1 × 10−3) with induction. It is nevertheless possible that other functions affected by H-NS or Lon protease prevent transposition from reaching the very high level observed in the hns+ lon::Tn10 mutant.
TABLE 4.
Effect of lon mutation on transposition frequency in an hns mutant
| lon | hns | IPTG (1 mM)a | Transposition frequency (Spcr/Gmr) (10−3) | Cointegrate fraction (Ampr/Spcr) | Δhns/hns+ |
|---|---|---|---|---|---|
| + | + | − | 0.73 | 0.64 | 0.0062 |
| Δ | − | 0.0045 | 0.75 | ||
| + | + | 2.1 | 0.81 | 0.022 | |
| Δ | + | 0.047 | 0.71 | ||
| ::Tn10 | + | − | 8.9 | 0.77 | 0.026 |
| Δ | − | 0.23 | 0.64 | ||
| + | + | 8.7 | 0.73 | ||
| Δ | + | 2.6 | 0.94 | 0.30 |
+, present; −, absent.
DISCUSSION
We have shown that in hns mutants, the inability of IS1 transposase to induce the SOS response or to stimulate its element to transpose at high rates results primarily from the cell's failure to accumulate it in sufficient quantities. The transposase deficit appears to be created at or very soon after completion of translation, since the Δhns strain maintained normal levels of insAB′ transcription, messenger stability, and translation initiation but allowed amounts of InsAB′ protein production that were barely detectable by pulse-labeling.
Suppression of the InsAB′ deficit by mutations in ssrA and lon also highlight this stage in transposase production as the point at which a lack of H-NS is sensed. Nevertheless, it is not clear how these two functions might be related in InsAB′ degradation. The SsrA peptide tag elicits degradation by the ClpAP and ClpXP proteases (17), whereas we found that clpX and clpP mutations did not reduce the level of InsAB′ protein below that in wild-type cells. The spectrum of proteases to which SsrA-tagged proteins are sensitive has been extended to FtsH (18), but not to Lon. Hence, even if the SsrA peptide were often fused to InsAB′ near the latter's 3′ terminus, it is unlikely that it would act as a direct target of Lon. An example of the more subtle interactions of proteolytic pathways is the degradation of the UmuD/UmuD′ heterodimer reported by Gonzalez et al. (16). Close to the Lon degradation signal in the N terminus of UmuD is a short peptide patch needed for ClpXP attack of UmuD′, while degradation of UmuD′ by ClpXP is necessary to expose the UmuD protein to Lon. It is possible that an interaction of nascent InsAB′ with another protein might account for the involvement of the SsrA tagging system in its stability.
Our finding that Δhns cells fail to make or maintain the InsAB′ protein does not exclude the possibility that H-NS also participates directly in the transposition process through bending of IS1 and target DNA or by other means. It does mean, however, that there is no evidence for the claim by Shiga et al. (39, 40) that low IS1 transposition frequencies in hns cells imply direct involvement of H-NS in a transposition complex. The experiments on which these authors based their conclusion did not include a control for the presence of the InsAB′ protein.
The observation that IS1 transposase is subject to proteolysis in vivo is hardly surprising, in view of its susceptibility to protease attack in vitro (M.-C. Serre, unpublished data) and the reported instability of other transposases (e.g., that of IS903, also degraded by Lon [7]). What is unusual is the apparent protective effect of H-NS. How might H-NS help ensure the survival of InsAB′ or the completion of its synthesis? Among the multitude of genes subject to repression by H-NS (19), one or more might encode functions inimical to InsAB′ accumulation. Alternatively, more general physiological changes associated with H-NS deficiency, such as those stemming from diminished transcription from stringently regulated promoters (20), might activate such functions. It is also possible that H-NS acts at a posttranscriptional level. Hns mutations have been reported to affect the translation of mRNA both positively (rpoS [49]) and negatively (malT [21]) and to enhance the stability of the sigma factor RpoS (49). H-NS protein interacts directly with an RNA chaperone, StpA, protecting it from degradation by Lon protease (22), as well as with a flagellar motor protein, FliG (8). Distinct domains of H-NS specify repression, DNA binding, and dimerization functions (46); the observation of Shiga et al. (39) that hns mutations that abolish DNA binding and repression do not reduce IS1 transposition while a mutation that prevents dimerization does argues in favor of H-NS action at the posttranscriptional level. Simple working hypotheses include an ability of H-NS to recognize nascent InsAB′ on the ribosome and chaperone it through the folding process, protecting it from Lon protease, and an ability of H-NS to counteract chaperones which modify InsAB′ to promote its degradation. Further experiments are needed to assess the validity of these ideas.
Although the lon::Tn10 mutation returned transposition frequency in the Δhns strain to that of wild-type E. coli, it did not allow the frequency to rise to that seen in the hns+ lon strain (Table 4). It is possible that in the strain used as a donor in the mating-out assay of transposition frequency, the absence of H-NS exposes the InsAB′ protein to proteases other than Lon. Alternatively, secondary effects of the combination of hns and lon mutations may interfere in some way with transposition and mask the full extent of restoration of InsAB′ activity. Involvement of H-NS in the transposition process itself also might explain why transposition falls short of wild-type levels in the Δhns lon strain. The latter possibility will be best examined by analysis of the IS1 transposition mechanism in vitro using purified components.
Acknowledgments
We thank Claude Gutierrez, Jean-Yves Bouet, Olivier Fayet, and Eric Roche for providing the marked hns, lon, and ssrA mutations used here and Jean-Michel Escoubas, Didier Zerbib, and Caroline Welz for plasmids. We are grateful to Mick Chandler and the Eléments Génétiques Mobiles group for plasmids and useful discussions and to Ton-Hoang Bao for her comments on the manuscript.
This work was supported by grants from l'Association pour la Recherche sur le Cancer (l'ARC; no. 2400) and la Région Midi-Pyrénées.
REFERENCES
- 1.Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. [DOI] [PubMed] [Google Scholar]
- 2.Calos, M. P., L. Johnsrud, and J. H. Miller. 1978. DNA sequence at the integration sites of the insertion element IS1. Cell 13:411-418. [DOI] [PubMed] [Google Scholar]
- 3.Chaconas, G., B. D. Lavoie, and M. A. Watson. 1996. DNA transposition: jumping gene machine, some assembly required. Curr. Biol. 6:817-820. [DOI] [PubMed] [Google Scholar]
- 4.Chandler, M., and J. Mahillon. 2002. Insertion sequences revisited, p. 305-365. In E. A. N. L. Craig (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 5.Chandler, M., and D. J. Galas. 1983. Cointegrate formation mediated by Tn9. II. Activity of IS1 is modulated by external DNA sequences. J. Mol. Biol. 170:61-91. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis, G., and H. Saedler. 1980. Deletions and an inversion induced by a resident IS1 of the lactose transposon Tn951. Mol. Gen. Genet. 178:367-374. [DOI] [PubMed] [Google Scholar]
- 7.Derbyshire, K. M., M. Kramer, and N. D. Grindley. 1990. Role of instability in the cis action of the insertion sequence IS903 transposase. Proc. Natl. Acad. Sci. USA 87:4048-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donato, G. M., and T. H. Kawula. 1998. Enhanced binding of altered H-NS protein to flagellar rotor protein FliG causes increased flagellar rotational speed and hypermotility in Escherichia coli. J. Biol. Chem. 273:24030-24036. [DOI] [PubMed] [Google Scholar]
- 9.Elespuru, R. K., and M. B. Yarmolinsky. 1979. A colorimetric assay of lysogenic induction designed for screening potential carcinogenic and carcinostatic agents. Environ. Mutagen. 1:65-78. [DOI] [PubMed] [Google Scholar]
- 10.Escoubas, J.-M. 1992. Etude de l'élément transposable IS1: mise en évidence de la protéine impliquée dans la réaction de transposition. Ph.D. thesis. L'université Paul Sabatier, Toulouse, France.
- 11.Escoubas, J. M., M. F. Prere, O. Fayet, I. Salvignol, D. Galas, D. Zerbib, and M. Chandler. 1991. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 10:705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estevenon, A. M., M. Lemonnier, C. Rouquette, and D. Lane. 1997. Genetic basis of the MbrC “ploidy” phenotype in Escherichia coli. Mol. Gen. Genet. 256:291-297. [DOI] [PubMed] [Google Scholar]
- 13.Falconi, M., V. McGovern, C. Gualerzi, D. Hillyard, and N. P. Higgins. 1991. Mutations altering chromosomal protein H-NS induce mini-Mu transposition. New Biol. 3:615-625. [PubMed] [Google Scholar]
- 14.Galas, D. J., and M. Chandler. 1982. Structure and stability of Tn9-mediated cointegrates. Evidence for two pathways of transposition. J. Mol. Biol. 154:245-272. [DOI] [PubMed] [Google Scholar]
- 15.Gamas, P., M. G. Chandler, P. Prentki, and D. J. Galas. 1987. Escherichia coli integration host factor binds specifically to the ends of the insertion sequence IS1 and to its major insertion hot-spot in pBR322. J. Mol. Biol. 195:261-272. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez, M., F. Rasulova, M. R. Maurizi, and R. Woodgate. 2000. Subunit-specific degradation of the UmuD/D′ heterodimer by the ClpXP protease: the role of trans recognition in UmuD′ stability. EMBO J. 19:5251-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman, S., E. Roche, Y. Zhou, and R. T. Sauer. 1998. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 12:1338-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman, C., D. Thevenet, P. Bouloc, G. C. Walker, and R. D'Ari. 1998. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH). Genes Dev. 12:1348-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 20.Johansson, J., C. Balsalobre, S. Y. Wang, J. Urbonaviciene, D. J. Jin, B. Sonden, and B. E. Uhlin. 2000. Nucleoid proteins stimulate stringently controlled bacterial promoters: a link between the cAMP-CRP and the (p)ppGpp regulons in Escherichia coli. Cell 102:475-485. [DOI] [PubMed] [Google Scholar]
- 21.Johansson, J., B. Dagberg, E. Richet, and B. E. Uhlin. 1998. H-NS and StpA proteins stimulate expression of the maltose regulon in Escherichia coli. J. Bacteriol. 180:6117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson, J., and B. E. Uhlin. 1999. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary-phase survival of Escherichia coli. Proc. Natl. Acad. Sci. USA 96:10776-10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilbane, J. J., and M. H. Malamy. 1980. F factor mobilization of non-conjugative chimeric plasmids in Escherichia coli: general mechanisms and a role for site-specific recA-independent recombination at orV1. J. Mol. Biol. 143:73-93. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Lane, D., J. Cavaille, and M. Chandler. 1994. Induction of the SOS response by IS1 transposase. J. Mol. Biol. 242:339-350. [DOI] [PubMed] [Google Scholar]
- 26.Mickel, S., E. Ohtsubo, and W. Bauer. 1977. Heteroduplex mapping of small plasmids derived from R-factor R12: in vivo recombination occurs at IS1 insertion sequences. Gene 2:193-210. [DOI] [PubMed] [Google Scholar]
- 27.Nakai, H., V. Doseeva, and J. M. Jones. 2001. Handoff from recombinase to replisome: insights from transposition. Proc. Natl. Acad. Sci. USA 98:8247-8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohtsubo, H., and E. Ohtsubo. 1978. Nucleotide sequence of an insertion element, IS1. Proc. Natl. Acad. Sci. USA 75:615-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polard, P., M. F. Prere, M. Chandler, and O. Fayet. 1991. Programmed translational frameshifting and initiation at an AUU codon in gene expression of bacterial insertion sequence IS911. J. Mol. Biol. 222:465-477. [DOI] [PubMed] [Google Scholar]
- 30.Prentki, P., P. Gamas, D. J. Galas, and M. Chandler. 1987. Functions of the ends of IS1, p. 719-734. In T. Kelley et al. (ed.), DNA replication and recombination. Alan R. Liss, Inc., New York, N.Y.
- 31.Prentki, P., M. H. Pham, P. Gamas, M. Chandler, and D. J. Galas. 1987. Artificial transposable elements in the study of the ends of IS1. Gene 61:91-101. [DOI] [PubMed] [Google Scholar]
- 32.Prentki, P., B. Teter, M. Chandler, and D. J. Galas. 1986. Functional promoters created by the insertion of transposable element IS1. J. Mol. Biol. 191:383-393. [DOI] [PubMed] [Google Scholar]
- 33.Reif, H. J., and H. Saedler. 1975. IS1 is involved in deletion formation in the gal region of E. coli K12. Mol. Gen. Genet. 137:17-28. [DOI] [PubMed] [Google Scholar]
- 34.Reznikoff, W. S. 1993. The Tn5 transposon. Annu. Rev. Microbiol. 47:945-963. [DOI] [PubMed] [Google Scholar]
- 35.Sekine, Y., N. Eisaki, K. Kobayashi, and E. Ohtsubo. 1997. Isolation and characterization of IS1 circles. Gene 191:183-190. [DOI] [PubMed] [Google Scholar]
- 36.Sekine, Y., and E. Ohtsubo. 1989. Frameshifting is required for production of the transposase encoded by insertion sequence 1. Proc. Natl. Acad. Sci. USA 86:4609-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serre, M. C., C. Turlan, M. Bortolin, and M. Chandler. 1995. Mutagenesis of the IS1 transposase: importance of a His-Arg-Tyr triad for activity. J. Bacteriol. 177:5070-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharpe, P. L., and N. L. Craig. 1998. Host proteins can stimulate Tn7 transposition: a novel role for the ribosomal protein L29 and the acyl carrier protein. EMBO J. 17:5822-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiga, Y., Y. Sekine, Y. Kano, and E. Ohtsubo. 2001. Involvement of H-NS in transpositional recombination mediated by IS1. J. Bacteriol. 183:2476-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiga, Y., Y. Sekine, and E. Ohtsubo. 1999. Transposition of IS1 circles. Genes Cells 4:551-561. [DOI] [PubMed] [Google Scholar]
- 41.Signon, L., and N. Kleckner. 1995. Negative and positive regulation of Tn10/IS10-promoted recombination by IHF: two distinguishable processes inhibit transposition off of multicopy plasmid replicons and activate chromosomal events that favor evolution of new transposons. Genes Dev. 9:1123-1136. [DOI] [PubMed] [Google Scholar]
- 42.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 43.Spassky, A., S. Rimsky, H. Garreau, and H. Buc. 1984. H1a, an E. coli DNA-binding protein which accumulates in stationary phase, strongly compacts DNA in vitro. Nucleic Acids Res. 12:5321-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spurio, R., M. Durrenberger, M. Falconi, A. La Teana, C. L. Pon, and C. O. Gualerzi. 1992. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol. Gen. Genet. 231:201-211. [DOI] [PubMed] [Google Scholar]
- 45.Turlan, C., and M. Chandler. 1995. IS1-mediated intramolecular rearrangements: formation of excised transposon circles and replicative deletions. EMBO J. 14:5410-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueguchi, C., T. Suzuki, T. Yoshida, K. Tanaka, and T. Mizuno. 1996. Systematic mutational analysis revealing the functional domain organization of Escherichia coli nucleoid protein H-NS. J. Mol. Biol. 263:149-162. [DOI] [PubMed] [Google Scholar]
- 47.Wiater, L. A., and N. D. Grindley. 1988. Gamma delta transposase and integration host factor bind cooperatively at both ends of gamma delta. EMBO J. 7:1907-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada, H., T. Yoshida, K. Tanaka, C. Sasakawa, and T. Mizuno. 1991. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol. Gen. Genet. 230:332-336. [DOI] [PubMed] [Google Scholar]
- 49.Yamashino, T., C. Ueguchi, and T. Mizuno. 1995. Quantitative control of the stationary phase-specific sigma factor, sigma S, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO J. 14:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]