Abstract
Exposure to commensal and pathogenic organisms strongly influences our immune system. Exposure to helminths was frequent before humans constructed their current highly hygienic environment. Today, in highly industrialized countries, contact between humans and helminths is rare. Congruent with the decline in helminth infections is an increase in the prevalence of autoimmune and inflammatory disease. It is possible that exclusion of helminths from the environment has permitted the emergence of immune-mediated disease. We review the protective effects of helminths on expression of inflammatory bowel disease, multiple sclerosis, and animal models of these and other inflammatory diseases. We also review the immune pathways altered by helminths that may afford protection from these illnesses. Helminth exposure tends to inhibit IFN-γ and IL-17 production, promote IL-4, IL-10, and TGF-β release, induce CD4+ T cell Foxp3 expression, and generate regulatory macrophages, dendritic cells, and B cells. Helminths enable protective pathways that may vary by specific species and disease model. Helminths or their products likely have therapeutic potential to control or prevent immune-mediated illness.
Keywords: helminths, dendritic cells, IBD, Treg, macrophage, autoimmunity
Introduction
The 20th century brought demise to Paul Ehrlich’s 1901 dictum of horror autotoxicus, that the body’s immune system would never attack host tissues to cause disease. In its place grew identification of more than 80 autoimmune or immune-mediated diseases. Most of these diseases emerged in the later half of the 20th century to become epidemic in highly developed industrialized countries. The National Institutes of Health now estimates that over 23.5 million Americans are afflicted with an autoimmune illness. Autoimmune disease ranks in the top 10 causes of death for women younger than 65 years of age. The dramatic emergence of these illnesses within two generations suggests that an environmental change has driven or permitted the immune dysregulation that results in autoimmunity, allergy, and inflammatory disease.
There are many striking environmental changes that have come with industrialization, but one that has immediate immunologic impact is loss of exposure to parasitic worms (helminths). Indoor plumbing, flush toilets, cement sidewalks, and well-regulated food industries conspire to prevent acquisition and transmission of helminths. In the United States, the prevalence of hookworm in Georgia schoolchildren dropped from 65% in the 1910s to less than 2% (mostly in recent immigrants) in the 1980s.1,2 Trichinosis, whipworm (Trichuris trichiura) and pinworm (Enterobius vermicularis) infections show similar declines in prevalence. Loss of helminth exposure also occurred in postwar Western Europe. In other regions, as socioeconomic conditions rise, the prevalence of helminth infection falls. Whipworm infections in South Korean schoolchildren fell from about 75% in 1969 to 0.02% in 2004.3 During this time span, the incidence of ulcerative colitis in Seoul, South Korea increased nearly sixfold.4 Prior to the 20th century, every individual was likely to have had a helminth infection. Now, a previously ubiquitous and universal exposure has become exceedingly rare.
This lack of helminth exposure could have a profound affect on our immune system that was shaped by exposure to commensal and pathogenic organisms. Immunologically, humans have battled helminths for millennia.5 This multigenerational, ubiquitous challenge has swayed genetic variation. Many of the polymorphisms in genetic loci associated with autoimmune diseases have been selected by parasite-driven adaptation.6 Helminths are complex multicellular organisms that have also adapted to their hosts. Helminths produce immune regulatory products and induce regulatory circuits to help maintain their niche. Loss of chronic helminth infection removes an “external immune governor” and leads to pathologic autoimmune and excessive inflammatory responses.
A common theme among the various dissimilar helminth species includes their capacity to strengthen both innate and adaptive immune regulatory circuitry. In addition, different helminths may influence different pathways. In the following sections, we will discuss the evidence for helminth–host interactions that prevent or control immune-mediated disease. We will approach this evidence according to the various helminthic effects on specific autoimmune and inflammatory diseases.
Helminths and inflammatory bowel disease
Ulcerative colitis and Crohn’s disease are collectively called inflammatory bowel disease (IBD). They are chronic inflammatory conditions of the gut that usually begin when people are in the second to third decade of life. Although the causes of these inflammatory diseases remain unknown, they are assumed to result from inappropriately aggressive mucosal immune responses to luminal substances. Identified are various genetic alterations that impart risk for, or protection from, acquiring these conditions. 7–9 Many of these genes have a role in mucosal barrier defense, innate immune, immune responsiveness, or immunoregulation. Yet, these gene alterations appear to function as factors that affect disease susceptibility, as the majority of patients with IBD display no particular genetic predisposition, and most patients bearing these “IBD genes” will never develop this condition.
IBD emerged as a growing health problem in highly developed countries in the latter half of the 20th century. The frequency of IBD is currently about 1 in 250 persons in some regions.10–12 Previously, these diseases were exceeding rare. IBD is now emerging rapidly in many underdeveloped countries.13–16 Poorly defined environmental factors are likely the cause for this rapid change in disease frequency worldwide. Hygiene associated with modern day living and causing alteration in intestinal flora and fauna is postulated to be a major risk factor.17 The IBD hygiene hypothesis proposes that modification in exposure to living organisms negatively affects development and maintenance of immune regulatory circuits that normally would afford protection from these diseases. Helminth infections are exceedingly strong inducers of regulatory circuits. Thus, loss of these infections in children and adults, a consequence of hygiene and strong public health measures, may be an important factor in disease causation.18 Several clinical and epidemiologic studies support this concept.19,20 Research exploring the use of helminths to treat Crohn’s disease and ulcerative colitis suggests that these agents may be useful therapeutic agents both to treat and prevent IBD.21–25
Animal models
Laboratories study various animal models that simulate human IBD26 to identify potential mechanisms through which helminths suppress disease. In one model, trinitrobenzene sulfonic acid (TNBS) is administered intrarectally into healthy mice to induce colitis. Another model is the IL-10–KO mouse, which develops chronic colitis spontaneously. In this model, brief exposure to a nonsteroidal anti-inflammatory drug (NSAID) triggers the disease quickly and more uniformly throughout the colon making the model more useful for experimentation.
In a variant of the IL-10–KO mouse model of IBD, Rag mice (T and B cell deficient) are reconstituted with IL-10–KO T cells, and the mice are fed an NSAID orally to induce severe Th1/Th17-type colitis.27 Human IBD is presumed to result from inappropriate T cell activation, in the gut lining, to luminal antigens. It is hard to study antigen-specific immunity in the intestines, since many poorly defined antigens provided by luminal organisms and orally consumed organic matter drive mucosal immune responses. To overcome this barrier, Rag mice also may receive, simultaneously with the IL-10–KO T cells, transgenic OT2 T cells bearing MHC class II-dependent TCR that recognize OVA. The OT2 cells subsequently appear in the gut allowing the study of how helminths work to regulation antigen-specific responses in the intestinal lamina propria.
Another valuable murine model of spontaneous IBD results from the reconstitution of Rag mice with CD25lo T cells. This model retains the capacity to make IL-10 and can be manipulated similarly to that of the IL-10–KO model to allow sophisticated analysis.
Mechanisms of regulation
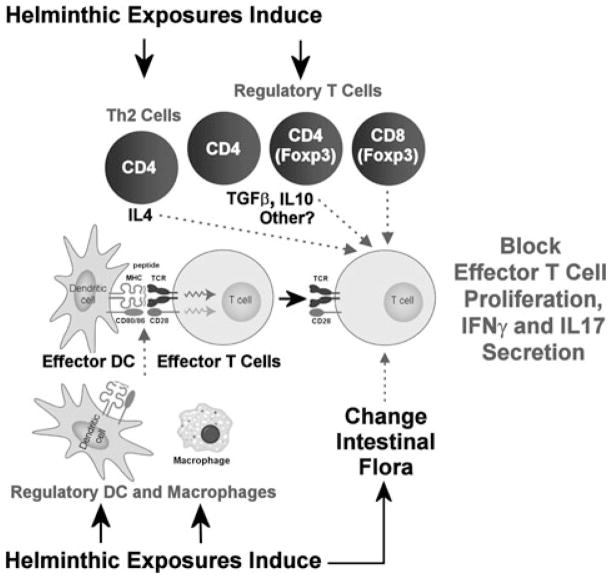
Helminths likely function to control IBD through induction of several independent regulatory pathways. They promote regulatory circuits involving cellular components of both innate and adaptive immunity, and stimulate release of several regulatory cytokines (Fig. 1).
Figure 1.
Helminth-induced regulatory circuits that limit inflammation.
Regulatory dendritic cells
Helmigmosomoides polygyrus bakeri is a murine intestinal nematode with some genetic similarity to pinworm and hookworm (Fig. 2). T cell- and B cell-deficient Rag mice infected with H. polygyrus bakeri and then dewormed with a pharmaceutical agent before reconstitution with colitogenic IL-10–KO T cells are protected from colitis.27 This implies that H. polygyrus bakeri does not require direct interactions with T or B cells to render animals resistant to this disease.
Figure 2.
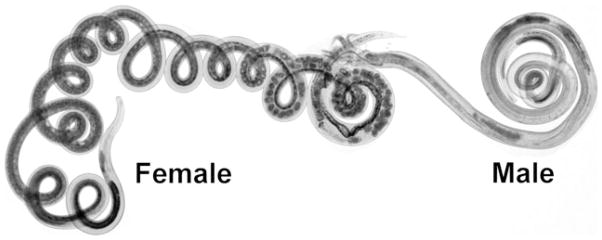
Male and female adult Heligmosomoides polygyrus bakeri. H. polygyrus bakeri is a nematode (round worm) that resides in the upper small intestine (duodenum and jejunum) of mice. The name of the parasite is changing from H. polygyrus to H. bakeri.
IFN-γ and IL-17 are proinflammatory cytokines implicated in driving colitis in both human and many murine models of IBD, including the ones described above.28 The intestinal mucosa makes less IFN-γ and IL-17 after H. polygyrus bakeri infection, whether the animals are primed for colitis or have been configured to remain free from disease. Thus, the decreased ability to produce these colitogenic cytokines is not simply secondary to the improvement in gut inflammation.
In the absence of adaptive immunity, exposing Rag mice to H. polygyrus bakeri greatly alters the function of the dendritic cells (DC) residing in the intestinal mucosa.27 After H. polygyrus bakeri infection, intestinal DC only weakly support antigen-driven IFN-γ secretion compared to DC from un-infected animals. More importantly, DC isolated from the MLN or intestines of H. polygyrus bakeri-infected Rag mice will block colitis and mucosal antigen-induced IFN-γ and IL-17 responses when the DC are transferred into colitis-susceptible animals. Thus, without the aid of T or B cells, the process of helminth infection alters the function of the DC in the gut and MLN of Rag mice, rendering them highly regulatory.
How the DC work to silence mucosal antigenic stimulation is partly understood. T cells populate the gut and MLN normally after DC transfer, but the T cell response is quelled by the surrounding non-T cell elements of the lamina propria and MLN, which have become strongly regulatory. The regulatory DC block IFN-γ and IL-17 production by interfering with the interaction between proinflammatory DC and effector T cells that drive the inflammation. The regulatory DC must physically contact the other cellular components to control the response. This interaction does not promote IL-4 secretion and is not associated with enhanced IL-10 or TGF-β production, suggesting that the mechanism of regulation is not dependent on increased production of these cytokines (Weinstock, et al., unpublished data).
There also are changes in expression of cell surface molecules on the intestinal DC.27 This includes decreased expression of the costimulatory molecules CD80 and CD86; more widely expressed are PDCA-1, a marker of plasmacytoid DC,29 and CD40. It is not yet determined if any of these changes in cell surface protein expression have importance for the capacity of these cells to block colitis or mucosal antigenic responses.
Regulatory macrophages
Macrophages populate the intestines in abundance. Macrophages occur in one of several possible states of cellular activation. Helminths induce the immune system of the host to produce IL-10 and Th2 cytokines like IL-4 and IL-5, which activate macrophages in ways distinct from macrophages exposed to Th1 cytokines. Such so-called alternatively activated macrophages display the mannose receptor and IL-4Ra on their outer membranes, and make some unique molecules like arginase-1, RELMa, Ym11, and some chitinases.30 While they produce little IL-12, alternatively activated macrophages can make IL-10, TGF-β, and other immunomodulatory factors notable for limiting Th1-type inflammation.31 Helminths may help protect from IBD through induction of alternatively activated macrophages.
Another frequently used model of IBD is dextran sodium sulfate (DSS)-induced enteritis. DSS administered orally to rodents damages the intestinal epithelial lining. This in turn induces gut inflammation that is relatively independent of adaptive immunity.
Infection of Balb/C mice with Schistosoma mansoni protects these animals from DSS-induced injury. In the DSS model of enteritis, it is the adult schistosome flukes living in the portal vein that shield the host from this inflammation, not their ova that lodge in the liver and intestinal wall. The mechanism of protection involves macrophages and functions independent of regulatory cytokines, such as IL-10 and TGF-β, and regulatory T cells.32
Cystatin, a secreted cysteine protease inhibitor of several species of filarial nematodes, protects mice from DSS colitis and an allergic-type airway hypersensitivity response. Macrophages and IL-10 are necessary for this protection,33 although this has thus far only been demonstrated for the lung Th2-type inflammatory model. Cystatin induces macrophages to make IL-10 and IL-12 p40 through activation of intracellular signaling pathways like ERK and p38, which are MAP kinases.34 This effect of cystatin on macrophages is the postulated mechanism of action.
In the IL-10–KO Rag model of IBD, exposure of Rag mice to H. polygyrus bakeri induces regulatory macrophages in the gut even before the mice are reconstituted with the colitogenic IL-10 deficient T cells. These intestinal macrophages inhibit antigen-induced, IL-17 and IFN-γ secretion, by a contact-dependent mechanism, when they are mixed with LPMC from mice with active colitis. The macrophages regulate with efficiency comparable to that of the H. polygyrus bakeri-induced intestinal regulatory DC described above. Also, when transferred into Rag mice along with the IL-10–KO T cells, they protect the mice from colitis and inhibit the intestinal antigenic response (Weinstock et. al., unpublished data). Thus, H. polygyrus bakeri activates two distinct cells of innate immunity (macrophages and DC) both of which can quell mucosal antigenic responses and colitis. It is not yet established if these two cell types function interdependently or work separately to provide overlapping protection. Profiling these regulatory macrophages using real-time PCR technology suggests that these cells do not have the molecular profile of classical alternatively activated macrophages.
In another study using dinitrobenzene sulfonic acid (DNBS) instead of TNBS to induce IBD, infection with the intestinal tapeworm H. diminuta protects the mice from colitis through a macrophage-dependent mechanism. The infection induces within the colon increased expression of markers of alternatively activated macrophages. Alternatively activated macrophages transferred into mice will protect the animals from DNBS-induced injury, suggesting that alternatively activated macrophages induced by the natural infection are the critical protecting factor. Extracts from H. diminuta adult worms injected intraperitoneally also provides disease protection and selectively suppresses macrophage function in vitro.35 Thus, this model, as well as the IL-10 transfer model of IBD, suggest that macrophages activated by helminth infection are sufficient to protect animals from IBD.36 Moreover, it is possible that some helminths make soluble factors that can mediate this process and substitute for the live agent.
Regulatory type T cells and cytokines
Various animal models of IBD suggest that regulatory-type T cells are important for maintaining mucosal immune homeostasis and for controlling enteritis.37 T cells that regulate immune responses are plentiful in the gut; most are Foxp3+ Tregs that express CD4. In the colon, more than 50% of the Foxp3+ T cells also make IL-10. There also are T cells that do not express Foxp3, but which are also major sources for immune regulatory molecules like IL-10 and/or TGF-β.
Helminths induce expansion of regulatory T cell subsets within the intestinal mucosa and mesenteric lymph nodes of their hosts. After H. polygyrus bakeri infection lamina propria T cells have a greater capacity to make IL-10 and TGF-β.38 A diverse array of helminths, such as H. polygyrus bakeri and Schistosoma mansoni, and the tapeworm Hymenolepis diminuta induce IL-10 secretion, which helps limit the colitogenic Th1 response and colitis in several animal models of IBD.39,40 However, helminths also prevent colitis or suppress ongoing disease in IL-10–KO mice, suggesting that IL-10 is not essential for this control.27,41
H. polygyrus bakeri infection stimulates Foxp3 mRNA expression in T cells 41 and expands the number of Foxp3+ T cells in the mesenteric lymph nodes 42 and intestinal lamina propria. T cells from the MLN of H. polygyrus bakeri–infected IL-10–deficient mice can be transferred into worm-naive animals and stop ongoing colitis, attesting to the importance of T cells in helping to control colitis.41 Also, colonic Foxp3+ CD4+ T cells, induced in the colon by H. polygyrus bakeri-infection, will prevent colitis when transferred into Rag mice reconstituted with CD25− T cells to make them susceptible to IBD. While most of the induced regulatory T cells are CD4+, some express CD8+ and can inhibit T lymphocyte proliferation via class I MHC interactions and cellular contact without the aid of IL-10 or TGF-β.43 CD8+ Tregs are implicated in the control of several diseases featuring immune dysregulation.44,45
Helminths can stimulate T cells, and other immune cell types, to make cytokines that impede development or function of T cell subtypes incriminated in IBD pathogenesis. Helminths, like H. polygyrus bakeri and Schistosoma mansoni, help protect mice from TNBS colitis by restraining the colonic Th1 IFN-γ response as well as IL-12 p40 secretion. Helminths promote the growth of IL-4–producing, Th2 cells. Abrogation of the Th2 pathway promotes persistence of disease and Th1 cell differentiation, showing the importance of Th2 cytokines for disease control in this murine model of IBD.39 IL-17 frequently comes from Th17 cells and often has an important role in driving colitis. H. polygyrus bakeri blocks IL-17 secretion in part through stimulating IL-4 production and, to a lesser extent, IL-10 production, which affects Th17 cell function.28 Disruption of Stat6 signaling specifically in T cells negates the ability of H. polygyrus bakeri infection to reverse established CD25lo T cell transfer colitis and inhibit IL-17 production (Elliott, unpublished data).
TGF-β is a critical cytokine in many immune reactions. Transgenic mice with T cells that do not signal correctly after TGF-β engagement cannot properly limit Th1 or Th2 responses in the gut and in other tissues, and thus these mice spontaneously develop colitis. In such transgenic mice, infection with H. polygyrus bakeri cannot prevent colitis or dampen mucosal Th1 responsiveness. This shows that H. polygyrus bakeri prevention of mucosal inflammation may require signaling of TGF-β through mucosal T cells.46
Overcoming the epithelial barrier
It is not entirely known how intestinal helminths bypass the intestinal barrier to interface with the gut mucosal immune system. Helminths like H. polygyrus bakeri live mostly in the proximal small bowel but reduce inflammation in the colon and distal terminal ileum. Transfer of protection using mesenteric lymph nodes from H. polygyrus bakeri-infected mice, however, emphasizes the importance of the immune system in this protective process.41
There are several possible mechanisms. DC extend dendrites across the epithelial barrier, which would permit sampling of molecules released from helminths living in the intestinal lumen. H. polygyrus bakeri and other helminths cultured in vitro release factors that can modify DC activation,47,48 impair DC-induced antibody responses,48 and promote regulatory T cell development. Thus, helminths produce substances that may affect the function of the DC that are sampling luminal contents.
The intestinal epithelial lining interfaces with the underlying immunocytes through release of regulatory molecules and direct cell contact. Interaction between intestinal helminths and the intestinal epithelium requires further consideration.
While some helminths swim freely in the gut lumen, others either mildly disrupt the mucosal barrier (e.g., hookworm) or attach to the intestinal wall by placing their heads beneath the epithelial lining (e.g., whipworm). This affords a further potential avenue for direct communication, for instance, with T cells to induce Tregs,42 or with other cells of host immunity to promote regulation.47
There is an enormous quantity of bacteria in the intestines. Intestinal bacteria are important for the health of the mucosal immune system and readily interact with intestinal DC and other cells.49 H. polygyrus bakeri infection rapidly shifts the abundance and distribution of some intestinal bacteria. There is a prominent increase in a family of bacteria called Lactobacillaceae. Various bacterial species within this group of organisms decrease intestinal inflammation in murine models of colitis.50
Helminths also can perturb the location and function of receptors of innate immunity. Bacteria often interact with host TLR and other receptors of innate immunity through release of LPS and other molecules. T cells in the intestinal lamina propria express TLR4 after H. polygyrus bakeri infection.51 LPS engagement with T cell TLR4 stimulates release of regulatory cytokines such as IL-10 and TGF-β, rather than the expected proinflammatory molecules. This may allow bacterial LPS to suppress adaptive immunity by provoking regulatory T cells that make IL-10 and TGF-β. Some helminth products also can bind to TLRs on DC to promote a Th2/regulatory T cell response.52
There also are helminth species that suppress colitis while living in the systemic circulation or regions of the host distant to the intestines. Their mode of communication with host immunity may be different than those discussed above.
Helminths and other immune-mediated diseases
Shared with IBD are the temporal and geographic prevalence patterns of multiple sclerosis (MS), type 1 diabetes (T1D), rheumatoid arthritis (RA), asthma, and many other autoimmune inflammatory diseases. This suggests that environmental factors which increase the risk for IBD, such as loss of helminth infections, also increase the risk for other immune-mediated illnesses. Investigators are studying the effect of helminth infection on development and expression of these inflammatory diseases using animal models (Table 1).
Table 1.
Animal modules of human disease that show improvement with helminths
| Animal model | Human disease | Helminth studied |
|---|---|---|
| TNBS and DNBS induced colitis | Crohn’s disease |
S. mansoni H. polygyrus bakeri T. spiralis H. diminuta |
| IL10−/− colitis | Crohn’s disease |
H. polygyrus bakeri S. mansoni T. muris |
| Transfer colitis | Crohn’s disease | H. polygyrus bakeri |
| Autoimmune encephalitis (EAE) | Multiple sclerosis |
S. mansoni T. spiralis H. polygyrus bakeri F. hepatica |
| NOD mouse | Type 1 diabetes |
S. mansoni T. spiralis H. polygyrus bakeri |
| Streptozotocin-induced diabetes | Type 1 diabetes | T. crassiceps |
| MRL/lpr arthritis | Rheumatoid arthritis |
H. polygyrus bakeri N. brasiliensis |
| Collagen-induced arthritis | Rheumatoid arthritis |
S. mansoni S. japonicum |
| CFA arthritis | Rheumatoid arthritis | H. diminuta |
| Reactive-airway disease | Asthma |
S. mansoni H. polygyrus bakeri T. spiralis |
Multiple sclerosis
Patients with MS that have helminthic infections (e.g., Trichuris trichiura and/or, Ascaris lumbricoides, Strongyloides stercoralis, and others) have a milder disease course compared to MS patients without helminths.53 Furthermore, in a case report of four patients, pharmacological eradication of helminthic infections resulted in worsening MS activity.54 Associated with helminth eradication was an increase in the number of PBMC making IFN-γ and IL-12, and a decrease in the number of cells producing IL-10 and TGF-β. There also was a decrease in circulating CD4+CD25+Foxp3+ T cells.54 An open label trial of therapeutic Trichuris suis exposure in five patients with relapsing-remitting MS showed that helminth exposure resulted in fewer neurological symptoms and development of fewer CNS lesions, as measured by magnetic resonance imaging.55 Lesion development recurred after discontinuation of T. suis administration. A Danish study showed similar results (personal communication).
Mice or rats immunized with myelin-associated peptides develop autoimmune encephalitis (EAE), which serves as a model of MS. Exposure of mice to Schistosoma mansoni, or even just to their dead eggs, protects mice from EAE.56,57 Schistosome exposure suppresses Th1-type cytokine (IL-12 p40, IFN-γ, and TNF-α) and augments regulatory and Th2-type cytokine (TGF-β, IL-10, and IL-4) production by splenocytes and CNS cells. Trichinella spiralis infection also affords protection in the agouti rat EAE animal model of MS.58 Trichinosis suppresses lymph node cell IFN-γ and IL-17 secretion while promoting IL-10 and IL-4 production, and it increases the number of CD4+CD25+Foxp3+ T cells in the spleen. Adoptive transfer of T cells from helminth-infected rats into helminth-naive rats protects these animals from developing EAE.58 This shows that the process of protection is immunologically-mediated, and that T cells are sufficient to control the disease. Infection with H. polygyrus bakeri 59 or Fasciola hepatica 60 also suppresses EAE. These studies show that helminths can suppress organ-specific inflammation beyond colitis through circuits likely similar to those that inhibit intestinal inflammation.
Type 1 diabetes (T1D)
Therapeutic trials using helminth exposure in patients with T1D are planned but not yet completed. For several years, laboratories have studied the effect of helminths on T1D using animal models of autoimmune diabetes such as the nonobese diabetic (NOD) mouse. Helminth exposure with Schistosoma mansoni, T. spiralis, or H. polygyrus bakeri61–64 protects NOD mice from insulitis. Protective schistosome exposure is associated with induction of IL-10 and expansion of NKT cells.62
Intraperitoneal injection of soluble proteins isolated from schistosome eggs (SEA) also protects NOD mice from diabetes and increases pancreatic mononuclear cell production of TGF-β, IL-4, and IL-10.65 In addition, SEA treatment increases the number of pancreatic and splenic CD4+CD25+Foxp3+ T cells 65 and induces alternatively activated peritoneal macrophages that make TGF-β.66 Transferring splenocytes from SEA-treated NOD mice into untreated NOD recipients protects these animals from disease. Splenocytes depleted of CD4+CD25+ T cells cannot provide this protection.65
The cytokine profile associated with a protective T. spiralis or H. polygyrus bakeri infection differs from that induced by schistosome worms or SEA. Characteristic of a protective, T. spiralis exposure increased splenic IL-4 secretion without inducing IL-10 production or inhibiting IFN-γ synthesis.63 Protection afforded by H. polygyrus bakeri infection associates with induction of alternatively activated macrophages, and IL-4 secretion and inhibition of IFN-γ production. However, this protection is independent of IL-10 and CD25+ T cells.64
Another murine model of T1D is low-dose streptozotocin-induced diabetes. Infection with Taenia crassiceps (a tape worm) decreases insulitis shielding Balb/C and C57BL/6 mice from streptozotocin-induced diabetes. Protection is associated with increase in IL-4 and alternatively activated macrophages, but not with induction of regulatory T cells.67 Thus, all of the classes of helminths (nematodes, cestodes, and trematodes) can confer protection in murine models of T1D, but different helminths may do so by triggering somewhat different regulatory pathways.
Rheumatoid arthritis
There are no published clinical trials of helminth exposure in patients with rheumatoid arthritis, but the effect of helminths on arthritis has been tested in animal models of this disease. Polyarticular arthritis develops spontaneously in MRL/lpr mice that have impaired Fas gene expression. Infection of these mice with bacteria aggravates arthritis, while infection with a helminth (H. polygyrus bakeri or Nippostrongylus brasiliensis) reduces the incidence of arthritis and the degree of synovial hyperplasia. 68
A more commonly used and well-described model of rheumatoid arthritis is collagen-induced arthritis. Mice infected with S. mansoni two weeks before sensitization with collagen in Freund’s complete adjuvant (FCA) do not develop the expected polyarticular arthritis.69 The reduction in arthritic score correlates with the number of worms per mouse. In protected animals, mitogen-stimulated splenocytes produce less IFN-γ, TNF-α, and IL-17, but more IL-4 and IL-10 than do splenocytes from mice without S. mansoni infection.69 Schistosoma japonicum, which is closely related to S. mansoni, also protects mice from collagen-induced arthritis. As with S. mansoni, this protection associates with inhibition of IFN-γ production and augmentation of IL-4 and IL-10 secretion by mitogen-stimulated splenocytes.70 However, there is no significant change in TNF-α production. Also, in contrast to protection afforded by S. mansoni exposure, infection with S. japonicum needs to advance to a patent (egg laying) stage at the time of collagen sensitization to inhibit arthritis development.70 If this difference is not due to technical artifacts, it suggests that timing of helminth exposure relative to disease challenge may be an important factor even for closely related worms.
The rodent filarial nematode Acanthocheilonema viteae, which resides in host lymphatics, secretes a 62 kD phosphocholine-containing glycoprotein (ES-62) that prevents and treats established collagen-induced arthritis.71 In mice protected from arthritis, their draining lymph node cells make less IFN-γ and TNF-α, and more IL-10, after collagen stimulation in vitro. Arthritis is inhibited even when ES-62 is administered subcutaneously after onset of collagen-induced inflammation.71 Recombinant ES-62 (expressed in yeast) does not inhibit arthritis because it lacks the phosphocholine moiety.72 These studies show that at least one systemically administered helminth-derived product can modulate arthritic disease activity but the molecule may require posttranslational modification to make it bioactive.
Another model of arthritis is inflammation provoked by intra-articular injection of CFA. This model allows assessment of joint discomfort by comparing CFA with saline-injected joints in the same animal. Exposure of mice to the tapeworm Hymenolepis diminuta before challenge reduces CFA-induced peak joint swelling and hastens resolution of inflammation in Balb/C and C57BL/6 mice.73 Exposure to the helminth after CFA injection does not ease peak swelling but continues to hasten recovery. CFA increases TNF-α mRNA expression in joints; H. diminuta infection blocks this induction and also alters splenocyte cytokine profiles, increasing IL-4 and IL-10 production. Infection of IL-10–deficient mice with the tapeworm does not afford protection from CFA arthritis.73 These experiments demonstrate that infection with a helminth residing in the intestinal lumen (H. diminuta) can protect from joint inflammation similar to a helminth (Schistosoma sp.) living in the mesenteric vasculature.
Allergy/asthma
IBD, MS, T1D, and rheumatoid arthritis likely result from dysregulated Th1/Th17 responses, while excessive Th2-type inflammation probably drives allergy and asthma. Helminth exposure stimulates strong Th2 responses and would be predicted to worsen allergic inflammation. However, helminths actually induce immune regulatory circuits that suppress Th2-driven atopic disease.
Helminth infection may influence the frequency of wheezing that is a sign of asthma. People with A. lumbricoides or hookworm (Necator) infections are less likely to experience wheezing than people without these infections.74 Also, inhabitants in areas endemic for S. mansoni report less wheezing compared to individuals living in nonendemic regions.75
Many epidemiological studies support the hypothesis that helminth exposure suppresses atopy, as measured by skin reaction to injected allergens. A study in Gabon, Africa showed that fewer children have atopic skin reactions to dust-mite allergens if they are infected with Schistosoma hematobium, compared to children without the helminth.76 Furthermore, children repeatedly treated for geo-helminths (e.g., T. trichiura) have increased skin reactions compared to untreated children.77–79 However, in other epidemiologic and interventional studies, helminthic infection either had no effect on, or increased the frequency of, atopic responses.79,80 Intensity and timing of helminth infection may explain some of theses differences in outcome. Individuals with the earliest and most sustained exposure may be the most protected against allergic inflammation.81 This requirement for prolonged exposure also may explain the limited results of a recent double-blind placebo-controlled therapeutic trial of T. suis for seasonal allergic rhinitis.82,83
A major model of allergic inflammation is airway hyper-responsiveness (AHR) induced by aerosol challenge with an antigen in previously sensitized mice. The model permits measurement of airway reactivity (respiratory resistance and compliance) and analysis of pulmonary inflammation. Colonization with H. polygyrus bakeri before or during antigen sensitization inhibits subsequent airway reactivity84 and inflammation84,85 upon aerosol antigen challenge. Helminth exposure decreases allergen-specific IL-5 production, an effect shown in people with S. mansoni infections.75
Exposure to other helminths, such as S. mansoni86 or T. spiralis,87 also affords protection from allergic airway reactivity and inflammation. In both of these models, helminth exposure is associated with decreased allergen-stimulated IL-5 release and increased IL-10 and TGF-β production.
Transfer of mesenteric lymph node cells or splenocytes from colonized mice into helminth naive animals inhibits airway inflammation, showing induction of regulatory cell activity. H. polygyrus bakeri exposure increases the percentage of CD4+ T cells in the mesenteric and thoracic lymph nodes that express CD25 and Foxp3,84,85 raising the possibility that Tregs mediate protection with cell transfer. In addition, H. polygyrus bakeri colonization induces a CD19+CD23+ regulatory B cell population that transfers suppression of allergen-kindled airway inflammation independent of IL-10 production.59 Like H. polygyrus bakeri, exposure to S. mansoni induces a population of CD19+CD23+ regulatory B cells that transfer protection from AHR.88 In this model the regulatory B cells express CD1d, require intact IL-10 production, and act in part by increasing the number of pulmonary CD4+CD25+Foxp3+ regulatory T cells in the lungs.88 These observations show that helminths activate multiple regulatory circuits that can work independently and at times in concert to inhibit aberrant inflammation.
How helminths communicate with the host immune system
It is conceivable that these organisms modulate host immunity through release of immune regulatory products or by display of such molecules on their integument. The previous paragraphs already described several molecules proposed to mediate such functions. These include cystatin and ES-62 both derived from various filarial species. Cystatin interacts with macrophages, whereas ES-62 is reported to modulate B cell, macrophage, and dendritic cell functions.89 Calreticulin is a secretory product of the murine helminth H. polygyrus bakeri 90 and the human hookworm Necator americanus.91 It binds scavenger receptor type A on dendritic cells, prompting a Th2 response. Other molecules and various other “excretory/secretory” factors have been described.47 To date, no one molecule has been ascribed to the majority of helminth species, and none display the broadly powerful immune regulatory properties shown by the whole organisms. This suggests that no single helminth molecule is responsible for the broad spectrum of immune regulatory activities of various helminth species.
It also remains possible that some helminths, like those which reside in the gut, release factors that indirectly modulate host immunity through altering the composition of our complex intestinal microflora 92 or through other indirect means.
Summary
Until very recently, helminth infection was ubiquitous. Helminth infection created a strong selective pressure on the human genome that outstrips that driven by bacteria and viruses.93 Indeed, many of the pathways identified by genome-wide association studies that confer risk for autoimmune and inflammatory disease show genetic variation influenced by helminths in the environment.6 Thus, it should come as no surprise that eradicating helminths can result in expression of diseases influenced by these pathways.
There are now many animal models representing a diverse range of diseases for which helminths either prevent and/or reverse ongoing pathology. The various animal models and epidemiological data suggest that many helminth species can mediate protection (Fig. 3). In some cases, different helminths may evoke dissimilar mechanisms to quell inflammation. This is not surprising since helminths have diverse evolutionary origins and inhabit unique niches in their host, which influences their access to the host’s immune system. The models also suggest that the mechanisms of action will not necessarily be the same for all diseases or mouse strains, or for out-bred individuals. Immunological diseases are caused by a vast array of gene interactions, environmental factors, and aberrant host immune responses. Thus, for helminths to modulate disease activity in a large number of patients with a wide range of diseases, one would expect that helminths possess the capacity to simultaneously, selectively, and/or sequentially modulate various immune regulatory pathways. At least in some diseases, helminths interface both with cells of innate and adaptive immunity to exert control. Their powerful stimulatory effect on regulatory dendritic cells, macrophages, T cells, B cells, and/or cytokines is particular noteworthy. While the mouse models hint at some of the potentially important immunologic mechanisms that protect from disease, mostly lacking are human studies validating these observations. Helminths are complex, multicellular animals that modulate and, at times, completely evade host immunity. In most circumstances, little is know regarding the molecular signals exchanged between helminth and host to mediate this process. Even less is known about which helminth products influence disease and how they work.
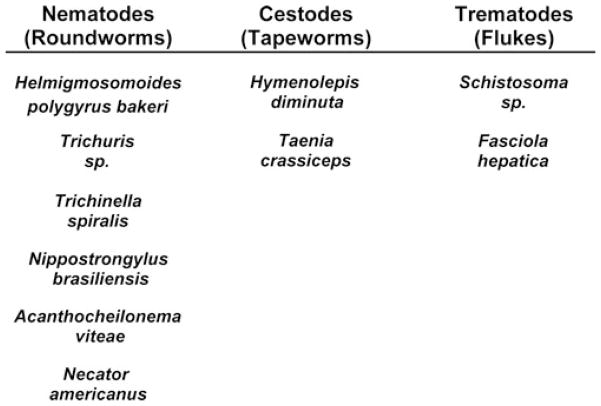
Figure 3.
Helminth types successfully used to control immunological diseases. Helminths are divided into two phyla. The Nemathelminthes phylum contains the roundworms (Nematodes). The Platyhelminthes phylum contains the tapeworms (Cestodes) and the flukes (Trematodes). Although they are all called worms, the genetic distance between Nemathelminthes and Platyhelminthes is vast. The ability to parasitize another organism developed independently within these groups.
Continued study of how helminths prevent and reverse inflammatory diseases should help should help elucidate the pathophysiology that led to the emergence of these illnesses in industrialized, highly hygienic countries and hopefully will identify targets for therapy. Furthermore, helminths or their products may prove useful as pharmaceutical agents to control or prevent immune-mediated illness.
Acknowledgments
This work was supported by DK38327, DK058755, the Broad Foundation, VAMC, the Schneider family, the Friedman family, and the Gilman family.
Footnotes
Conflicts of interest
The authors are named on patents held by the University of Iowa for the use of helminths in autoimmune and inflammatory disease.
References
- 1.Kappus KD, Lundgren RGJ, Juranek DD, et al. Intestinal parasitism in the United States: update on a continuing problem. Am J Trop Med Hyg. 1994;50:705–713. doi: 10.4269/ajtmh.1994.50.705. [DOI] [PubMed] [Google Scholar]
- 2.Wright WH. Current status of parasitic diseases. Pub Health Rep. 1955;70:966–975. [PMC free article] [PubMed] [Google Scholar]
- 3.Hong ST, Chai JY, Choi MH, et al. A successful experience of soil-transmitted helminth control in the Republic of Korea. Korean J Parasitol. 2006;44:177–185. doi: 10.3347/kjp.2006.44.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang SK, Hong WS, Min YI, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986–1997. J Gastroenterol Hepatol. 2000;15:1037–1042. doi: 10.1046/j.1440-1746.2000.02252.x. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves ML, Araujo A, Ferreira LF. Human intestinal parasites in the past: new findings and a review. Memorias do Instituto Oswaldo Cruz. 2003;98(Suppl 1):103–118. doi: 10.1590/s0074-02762003000900016. [DOI] [PubMed] [Google Scholar]
- 6.Fumagalli M, Pozzoli U, Cagliani R, et al. The landscape of human genes involved in the immune response to parasitic worms. BMC Evol Biol. 2010;10:264. doi: 10.1186/1471-2148-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renz H, von ME, Brandtzaeg P, et al. Gene-environment interactions in chronic inflammatory disease. Nat Immunol. 2011;12:273–277. doi: 10.1038/ni0411-273. [DOI] [PubMed] [Google Scholar]
- 8.Kaser A, Zeissig S, Blumberg RS. Genes and environment: how will our concepts on the pathophysiology of IBD develop in the future? Dig Dis. 2010;28:395–405. doi: 10.1159/000320393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenstiel P, Sina C, Franke A, Schreiber S. Towards a molecular risk map—recent advances on the etiology of inflammatory bowel disease. Semin Immunol. 2009;21:334–345. doi: 10.1016/j.smim.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Manninen P, Karvonen AL, Huhtala H, et al. The epidemiology of inflammatory bowel diseases in Finland. Scand J Gastroenterol. 2010;45:1063–1067. doi: 10.3109/00365521.2010.485323. [DOI] [PubMed] [Google Scholar]
- 11.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 13.Shin DH, Sinn DH, Kim YH, et al. Increasing incidence of inflammatory bowel disease among young men in Korea between 2003 and 2008. Dig Dis Sci. 2011;56:1154–1159. doi: 10.1007/s10620-010-1403-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang YF, Ouyang Q, Hu RW. Progression of inflammatory bowel disease in China. J Dig Dis. 2010;11:76–82. doi: 10.1111/j.1751-2980.2010.00421.x. [DOI] [PubMed] [Google Scholar]
- 15.Lakatos L, Mester G, Erdelyi Z, et al. Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977–2001. World J Gastroenterol. 2004;10:404–409. doi: 10.3748/wjg.v10.i3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang SK, Hong WS, Min YI, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986–1997. J Gastroenterol Hepatol. 2000;15:1037–1042. doi: 10.1046/j.1440-1746.2000.02252.x. [DOI] [PubMed] [Google Scholar]
- 17.Elliott DE, Urban JFJ, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J. 2000;14:1848–1855. doi: 10.1096/fj.99-0885hyp. [DOI] [PubMed] [Google Scholar]
- 18.Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- 19.Büning J, Homann N, von Smolinski D, et al. Helminths as governors of inflammatory bowel disease. Gut. 2008;57:1182–1183. doi: 10.1136/gut.2008.152355. [DOI] [PubMed] [Google Scholar]
- 20.Broadhurst MJ, Leung JM, Kashyap V, et al. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2010;2:60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- 21.Summers RW, Elliott DE, Qadir K, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 22.Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Summers RW, Elliott DE, Weinstock JV. Is there a role for helminths in the therapy of inflammatory bowel disease? Nat Clin Pract Gastroenterol Hepatology. 2005;2:62–63. doi: 10.1038/ncpgasthep0087. [DOI] [PubMed] [Google Scholar]
- 25.Croese J, O’neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136–137. doi: 10.1136/gut.2005.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 27.Hang L, Setiawan T, Blum AM, et al. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J Immunol. 2010;185:3184–3189. doi: 10.4049/jimmunol.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott DE, Metwali A, Leung J, et al. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes JL, Terrazas LI. The divergent roles of alternatively activated macrophages in helminthic infections. Parasite Immunol. 2007;29:609–619. doi: 10.1111/j.1365-3024.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith P, Mangan NE, Walsh CM, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 33.Schnoeller C, Rausch S, Pillai S, et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180:4265–4272. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- 34.Klotz C, Ziegler T, Figueiredo AS, et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog. 2011;7:e1001248. doi: 10.1371/journal.ppat.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston MJ, Wang A, Catarino ME, et al. Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infect Immun. 2010;78:1364–1375. doi: 10.1128/IAI.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter MM, Wang A, Parhar KS, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 37.Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Curr Opin Gastroenterol. 2008;24:733–741. doi: 10.1097/mog.0b013e328311f26e. [DOI] [PubMed] [Google Scholar]
- 38.Setiawan T, Metwali A, Blum AM, et al. Heligmosomoides polygyrus promotes regulatory T cell cytokine production in normal distal murine intestine. Infect Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott D, Li J, Blum A, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol. 2003;284:G385–G391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 40.Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol. 2005;174:7368–7375. doi: 10.4049/jimmunol.174.11.7368. [DOI] [PubMed] [Google Scholar]
- 41.Elliott DE, Setiawan T, Metwali A, et al. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 42.Grainger JR, Smith KA, Hewitson JP, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metwali A, Setiawan T, Blum AM, et al. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am J Physiol Gastrointest Liver Physiol. 2006;291:G253–G259. doi: 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- 44.Costantino CM, Baecher-Allan C, Hafler DA. Multiple sclerosis and regulatory T cells. J Clin Immunol. 2008;28:697–706. doi: 10.1007/s10875-008-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Ince MN, Elliott DE, Setiawan T, et al. Role of T cell TGF-beta signaling in intestinal cytokine responses and helminthic immune modulation. Eur J Immunol. 2009;39:1870–1878. doi: 10.1002/eji.200838956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol. 2009;167:1–11. doi: 10.1016/j.molbiopara.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 49.Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Walk ST, Blum AM, Ewing SA, et al. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ince MN, Elliott DE, Setiawan T, et al. Heligmosomoides polygyrus induces TLR4 on murine mucosal T cells that produce TGFbeta after lipopolysaccharide stimulation. J Immunol. 2006;176:726–729. doi: 10.4049/jimmunol.176.2.726. [DOI] [PubMed] [Google Scholar]
- 52.Carvalho L, Sun J, Kane C, Marshall F, et al. Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function. Immunology. 2009;126:28–34. doi: 10.1111/j.1365-2567.2008.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 54.Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Fleming J, Isaak A, Lee J, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Multiple Sclerosis. 2011;17:743–754. doi: 10.1177/1352458511398054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sewell D, Qing Z, Reinke E, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 57.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, et al. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol. 2010;32:450–459. doi: 10.1111/j.1365-3024.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 59.Wilson MS, Taylor MD, O’Gorman MT, et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh KP, Brady MT, Finlay CM, et al. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183:1577–1586. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 61.Cooke A, Tonks P, Jones FM, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 62.Zaccone P, Fehervari Z, Jones FM, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 63.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q, Sundar K, Mishra PK, et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun. 2009;77:5347–5358. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaccone P, Burton O, Miller N, et al. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 66.Zaccone P, Burton OT, Gibbs S, et al. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J Biomed Biotechnol. 2010:795210. doi: 10.1155/2010/795210. Epub; 2010 Mar 1.:795210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espinoza-Jimenez A, Rivera-Montoya I, Cardenas-Arreola R, et al. Taenia crassiceps infection attenuates multiple low-dose streptozotocin-induced diabetes. J Biomed Biotechnol. 2010:850541. doi: 10.1155/2010/850541. Epub; 2010 Jan 4.:850541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salinas-Carmona MC, de lC-G, Perez-Rivera I, et al. Spontaneous arthritis in MRL/lpr mice is aggravated by Staphylococcus aureus and ameliorated by Nippostrongylus brasiliensis infections. Autoimmunity. 2009;42:25–32. doi: 10.1080/08916930802228290. [DOI] [PubMed] [Google Scholar]
- 69.Osada Y, Shimizu S, Kumagai T, et al. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol. 2009;39:457–464. doi: 10.1016/j.ijpara.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 70.He Y, Li J, Zhuang W, et al. The inhibitory effect against collagen-induced arthritis by Schistosoma japonicum infection is infection stage-dependent. BMC Immunol. 2010;11:28. doi: 10.1186/1471-2172-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McInnes IB, Leung BP, Harnett M, et al. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–2133. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 72.Harnett MM, Kean DE, Boitelle A, et al. The phosphorycholine moiety of the filarial nematode immunomodulator ES-62 is responsible for its anti-inflammatory action in arthritis. Ann Rheum Dis. 2008;67:518–523. doi: 10.1136/ard.2007.073502. [DOI] [PubMed] [Google Scholar]
- 73.Shi M, Wang A, Prescott D, et al. Infection with an intestinal helminth parasite reduces Freund’s complete adjuvant-induced monoarthritis in mice. Arthritis Rheum. 2011;63:434–444. doi: 10.1002/art.30098. [DOI] [PubMed] [Google Scholar]
- 74.Scrivener S, Yemaneberhan H, Zebenigus M, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–1499. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 75.Araujo MI, Hoppe BS, Medeiros M, Jr, Carvalho EM. Schistosoma mansoni infection modulates the immune response against allergic and auto-immune diseases. Mem Inst Oswaldo Cruz. 2004;99(Suppl 1):27–32. doi: 10.1590/s0074-02762004000900005. [DOI] [PubMed] [Google Scholar]
- 76.van den Biggelaar AH, van Ree R, Rodrigues LC, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 77.van den Biggelaar AH, Rodrigues LC, van Ree R, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189:892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 78.Endara P, VacaVaca M, Chico ME, et al. Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin Exp Allergy. 2010;40:1669–1677. doi: 10.1111/j.1365-2222.2010.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flohr C, Tuyen LN, Quinnell RJ, et al. Reduced helminth burden increases allergen skin sensitization but not clinical allergy: a randomized, double-blind, placebo-controlled trial in Vietnam. Clin Exp Allergy. 2010;40:131–142. doi: 10.1111/j.1365-2222.2009.03346.x. [DOI] [PubMed] [Google Scholar]
- 80.Palmer LJ, Celedon JC, Weiss ST, et al. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002;165:1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 81.Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10:3–12. doi: 10.1007/s11882-009-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bager P, Arnved J, Ronborg S, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Summers RW, Elliott DE, Weinstock JV. Trichuris suis might be effective in treating allergic rhinitis. J Allergy Clin Immunol. 2010;125:766–767. doi: 10.1016/j.jaci.2009.12.937. [DOI] [PubMed] [Google Scholar]
- 84.Kitagaki K, Businga TR, Racila D, et al. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 85.Wilson MS, Taylor MD, Balic A, et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mangan NE, van RN, McKenzie AN, Fallon PG. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J Immunol. 2006;176:138–147. doi: 10.4049/jimmunol.176.1.138. [DOI] [PubMed] [Google Scholar]
- 87.Park HK, Cho MK, Choi SH, et al. Trichinella spiralis: infection reduces airway allergic inflammation in mice. Exp Parasitol. 2011;127:539–544. doi: 10.1016/j.exppara.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Amu S, Saunders SP, Kronenberg M, et al. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 89.Harnett MM, Melendez AJ, Harnett W. The therapeutic potential of the filarial nematode-derived immunodulator, ES-62 in inflammatory disease. Clin Exp Immunol. 2010;159:256–267. doi: 10.1111/j.1365-2249.2009.04064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rzepecka J, Rausch S, Klotz C, et al. Calreticulin from the intestinal nematode Heligmosomoides polygyrus is a Th2-skewing protein and interacts with murine scavenger receptor-A. Mol Immunol. 2009;46:1109–1119. doi: 10.1016/j.molimm.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 91.Kasper G, Brown A, Eberl M, et al. A calreticulin-like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins. Parasite Immunol. 2001;23:141–152. doi: 10.1046/j.1365-3024.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 92.Walk ST, Blum AM, Ewing SA, et al. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fumagalli M, Pozzoli U, Cagliani R, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]