Abstract
The concept of applying all active therapeutic agents in Total Therapy (TT) clinical trials for newly diagnosed multiple myeloma was pursued with the intent of developing curative treatment. The results of TT1 (n = 231), TT2 (n = 668) without or with thalidomide and TT3 with added bortezomib (n = 303) have been reported. An update with median follow-up times of 17.1, 8.7 and 5.5 years, respectively, is provided. Conditional overall survival (OS) analysis from a 4-year landmark was applied to account for earlier protocol failure owing to disease aggressiveness and toxicities. Cumulative relative survival was computed in the context of age- and gender-matched US population, and interval-specific relative survival ratios were estimated to determine times to normal survival expectation. Based on Cox model-adjusted statistics, OS, progression-free survival and complete-response duration all improved with the transitions from TT1 to TT2 to TT3; improvement was also evident from time-to-progression estimates, 4-year conditional survival data and cumulative relative survival. Interval-specific relative survival normalized progressively sooner, reaching near-normal levels with TT3 in patients who attained complete response. Thus, a strategy using all myeloma-effective agents up-front seems effective at preventing, in progressively larger patient cohorts over time, the outgrowth of resistant tumor cells that account for ongoing relapses.
Keywords: multiple myeloma, transplant, survival
INTRODUCTION
Despite major advances in therapy, multiple myeloma is still considered an incurable malignancy.1 Introduction of immunomodulatory drugs and bortezomib and advances in high-dose chemotherapy administration have improved progression-free survival (PFS) and overall survival (OS) for myeloma patients in general, but most patients suffer relapses and progressively shorter disease-free intervals with each relapse.2,3 We have reported our Total Therapy (TT) trials4–6 that use all active treatments up-front to achieve maximum tumor cytoreduction and thereby increase the frequency and duration of complete response (CR), with the goal of extending PFS and OS. With median follow-up times of 17.1 years for TT1, 8.7 years for TT2 and 5.5 years for TT3, we investigated, for each trial, the outcomes in relationship to baseline parameters. Results of these analyses demonstrate long-term outcomes that improve with each successive trial.
SUBJECTS AND METHODS
The details of trial design and dosing were reported previously for TT1, TT2 and TT3(refs 4–7) and are briefly described here. All three protocols used melphalan (200 mg/m2)-based tandem transplants. In TT1 (n = 231), a phase II trial, induction therapy included three cycles of VAD (vincristine, doxorubicine, dexamethasone), high-dose cyclophosphamide for collection of peripheral blood stem cells and etoposide, dexamethasone, cytarabine, cisplatin; interferon-α2b was used as maintenance therapy until relapse or intolerance. TT2 (n = 668) was a phase III trial that randomized patients to an experimental arm with thalidomide added from the outset and continuing throughout consolidation and maintenance. TT2 induction consisted of VAD followed by DCEP (dexamethasone and 4-day continuous infusions of cyclophosphamide, etoposide, cisplatin), cyclophosphamide, doxorubicin, dexamethasone with collection of peripheral blood stem cells, and a further cycle of DCEP. TT2 consolidation varied and eventually used DPACE (dexamethasone and 4-day infusions of cisplatin, doxorubicin, cyclophosphamide, etoposide) quarterly for 1 year. Maintenance therapy for TT2 consisted of dexamethasone pulsing in year 1 with interferon-α2B, which was then continued indefinitely until recurrence or intolerance. TT3 (n = 303), a phase II trial, used two cycles of VTD (bortezomib, thalidomide, dexamethasone)-PACE for induction before and consolidation after tandem transplants; this was followed by VTD maintenance therapy in year 1 and TD maintenance in years 2 and 3. All the TT patients received the induction, transplant and consolidation phases at the UAMS (University of Arkansas for Medical Sciences). The patients then were followed at least every 4 months during the maintenance phase and at least semi-annually after maintenance.
TT protocols were approved by the Institutional Review Board that received and approved annual follow-up reports. All patients had signed a written informed consent, in keeping with the institutional and Food and Drug Administration guidelines and in accordance with the Helsinki Declaration. An independent data-monitoring team audited >80% of clinical records every 6–8 months for toxicity and efficacy of TT protocols.
Endpoints and statistical methods
Data were compiled on 25 February 2011. The median follow-up times for TT1, TT2 and TT3 were 17.1, 8.7 and 5.5 years, respectively. Clinical endpoints8 included CR duration, time to progression (TTP), PFS and OS. CR duration was measured as the time from CR onset to disease progression or death from any cause. TTP was measured from the time of initiation of protocol therapy and also from onset of CR; events were restricted to disease progression and relapse. PFS was defined as the time from initiation of therapy until progression or death from any cause. OS was defined as the time from initiation of therapy until death from any cause.
OS, PFS and CR duration were estimated according to the method of Kaplan and Meier.9 Cumulative incidence curves for TTP or relapse were estimated as described by Gooley et al.10 For clinical endpoints, estimates were compared with the log-rank test and model-adjusted statistics derived from Cox regression.11,12
To assess outcomes after patient attrition due to treatment-related mortality or as a consequence of high-risk disease features, analyses of conditional survival were carried out with a 4-year landmark. We explored several landmarks, with similar conclusions. Four years was the longest time from the start of therapy with reasonable follow-up past the landmark for TT3.
To account for competing causes of death unrelated to myeloma, we also examined relative survival—patient outcomes in the context of the general age- and gender-adjusted population. Expected survival estimates based on age and gender were obtained for the general United States population from the Human Mortality Database (http://www.mortality.org). Relative survival was defined as the ratio of observed survival to that expected from the general population, adjusted for age and gender differences, and was estimated and compared as described by Dickman et al.13 Interval-specific relative survival (IRS) ratios were calculated at 1-year intervals. An IRS ratio <1 results from higher mortality in the observed population relative to the general population, while a ratio equal to 1 suggests the mortality rate has normalized or matched the mortality of the general population. Cumulative relative survival was calculated as the product of the IRS ratios.
RESULTS
Patient baseline characteristics were previously reported.4–6 Information on standard laboratory variables, including cytogenetics, was virtually complete (Supplementary Table 1). Among all 1202 patients, 20% were 65 years and older (those >75 years were ineligible), hypo-albuminemia <3.5 g/dl was present in 21%, β-2microglobulin ≥3.5 mg/l was present in 40, 50% had ISS stages II and III, renal function was impaired (creatinine ≥2 mg/dl) in 10, 28% had elevated serum levels of lactate dehydrogenease ≥190 U/l and 31% exhibited cytogenetic abnormalities (CA).
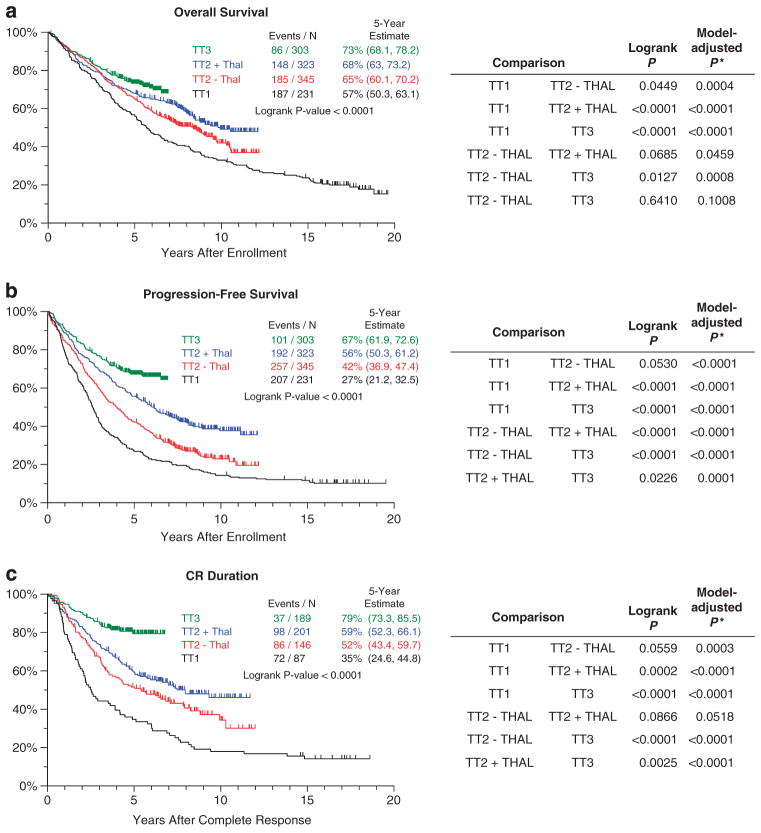
Clinical outcomes were analyzed for the three endpoints of OS, PFS and CR duration (Figure 1). Outcomes progressively improved with the transitions from TT1 to TT2 control arm (TT2 − Thal), TT2 thalidomide arm (TT2 + Thal) and TT3. Log-rank statistics applied for the three clinical endpoints indicated significant differences among the four treatment groups in terms of PFS (borderline for TT1 versus TT2 − Thal) and CR duration (borderline for TT1 versus TT2 − Thal, and for TT2 − Thal versus TT2 + Thal). OS also improved significantly with the transition from TT1 to TT2 − Thal. A trend toward significant improvement in OS was observed for TT2 − Thal versus TT2 + Thal, but no difference is yet documented for TT2 + Thal versus TT3. Model-adjusted comparisons were obtained with Cox regression analyses including standard baseline prognostic factors (age at registration, β-2microglobulin >5.5 mg/l, lactate dehydrogenease ≥190 U/l, the presence of metaphase-based CA) to control for disease-related and host risk-related features of TT populations. These comparisons revealed highly significant improvements with successive trials for all endpoints, with the exception of the comparisons of TT2 + Thal versus TT3 OS (P = 0.1008), and TT2 − Thal versus TT2 + Thal CR duration (P = 0.0518). Collectively, these data attest to the progressive improvements in patients’ outcomes with the application of newer TT trials.
Figure 1.
Kaplan–Meier plots of OS (a), PFS (b) and CR duration (c) for TT trials. Significant improvements were observed in OS, PFS and CR duration with the transitions from earlier trials to later trials that incorporated new agents, greater intensity of induction therapy and consolidation therapy after transplantation. This was especially apparent in the Cox model-derived comparisons adjusted for baseline prognostic factors. Black, TT1; red, TT2 − Thal; blue, TT2 + Thal; and green, TT3. *Model-adjusted P-value based on Cox regression model including terms for TT protocol, age, β-2microglobulin >5.5 mg/l, lactate dehydrogenease ≥190 U/l and the presence of CA.
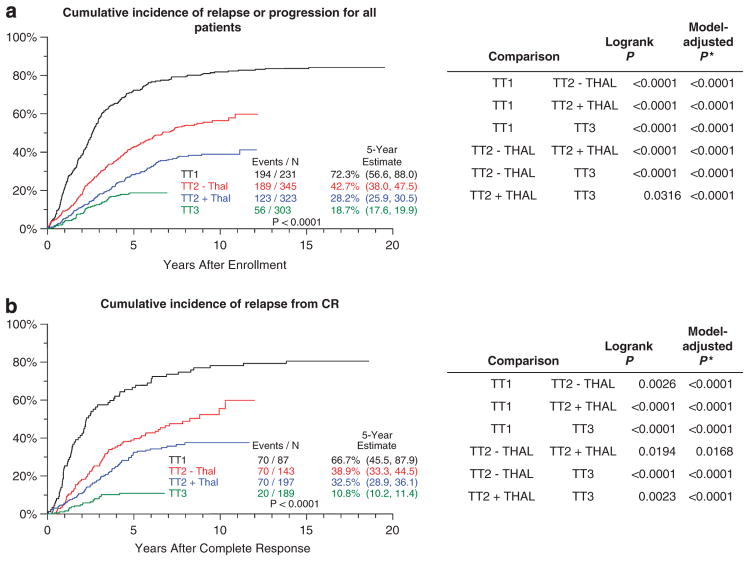
These observations were further supported by analyses of TTP, where deaths were considered censored (Figure 2). TTP comparisons of all successive TT trials (including TT2 − Thal versus TT2 + Thal) were highly significant, whether TTP was considered for all patients (Figure 2a) or limited to those who achieved CR status (Figure 2b). The 5-year cumulative incidence of progression or relapse among all patients was enormously reduced from 72.3% in TT1 to 42.7% in TT2 − Thal, 28.2% in TT2 + Thal and 18.7% in TT3; the corresponding values for those relapsing from CR were 66.7%, 38.9%, 32.5% and 10.8%, respectively.
Figure 2.
Cumulative incidence of progression or relapse for all patients (a) and for those who attained CR (b). (a) Cumulative incidences of relapse or progression consistently decreased over successive protocols. (b) Cumulative incidences of relapse following CR (for the subset of patients who achieved CR) show progressive improvements in outcome with transitions from early to later trials. Significant differences were observed with both log-rank statistics and Cox model-derived comparisons adjusted for baseline prognostic factors. Black, TT1; red, TT2 − Thal; blue, TT2 + Thal; and green, TT3. *Model-adjusted P-value based on Cox regression model including terms for TT protocol, age, β-2microglobulin >5.5 mg/l, lactate dehydrogenease ≥190 U/l and the presence of CA.
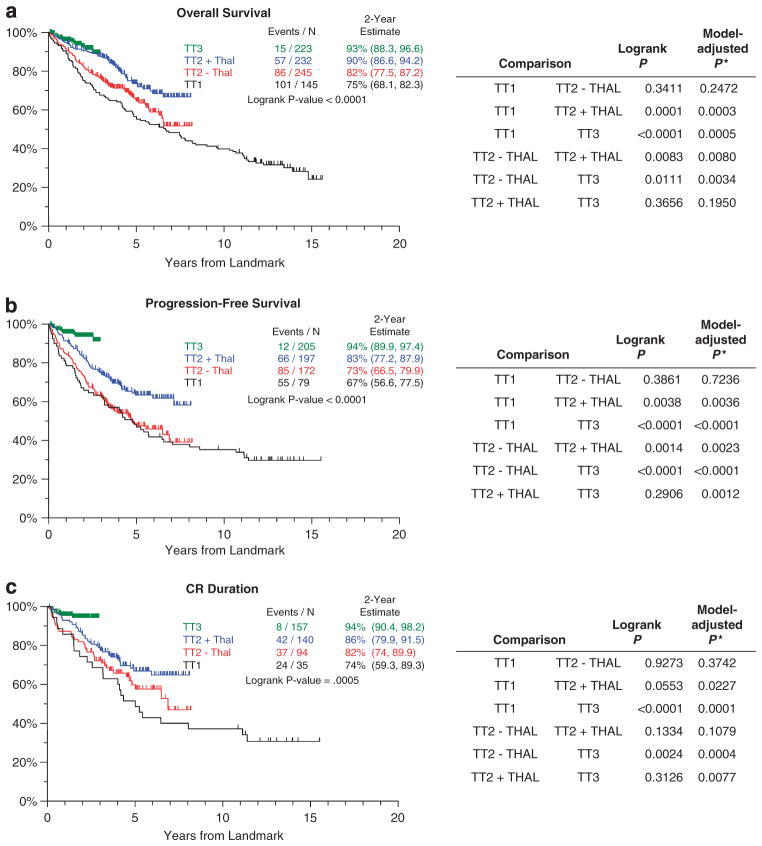
To adjust for early events related to disease aggressiveness or treatment-related toxicities, several conditional survival analyses were carried out to examine the long-term efficacy of TT trials. Representative data are shown for 4-year conditional survival analyses (Figure 3), which used model-adjusted statistics (β-2microglobulin >5.5 mg/l, CA and TT trial). OS significantly improved in four of the six comparisons (with the exceptions of TT1 versus TT2 − Thal, P = 0.2472, and TT2 + Thal versus TT3, P = 0.1950) (Figure 3a). For both PFS (Figure 3b) and CR duration (Figure 3c) significant improvements were noted for all comparisons except TT1 versus TT2 − Thal (PFS, P = 0.72; CR duration, P = 0.37) and TT2 + Thal versus TT2 − Thal (CR duration, P = 0.1079).
Figure 3.
Conditional survival outcomes from 4-year landmark in TT trials, shown for OS (a), PFS (b) and CR duration (c). Significant improvements in 4-year conditional survival were noted for all three endpoints with successive TT trials, especially when comparisons were adjusted for baseline prognostic factors. Black, TT1; red, TT2 − Thal; blue, TT2 + Thal; and green, TT3. *Model-adjusted P-value based on Cox regression model including terms for TT protocol, β-2microglobulin >5.5 mg/l and the presence of CA.
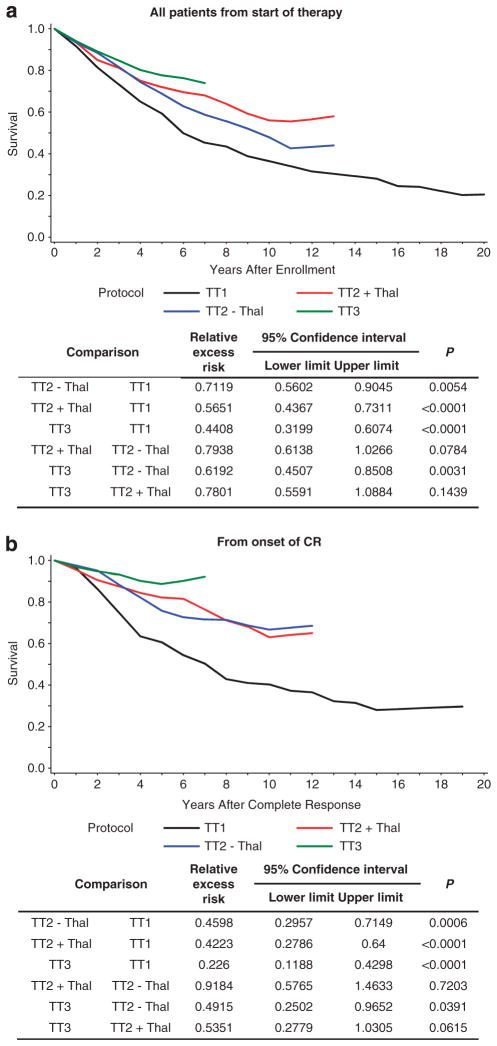
Considering the advanced age of the typical patient afflicted with myeloma, it appeared appropriate to examine survival outcomes in the context of a similar population (age- and gender-adjusted) within the general US population. Thus, cumulative relative survival was analyzed for all patients in TT1, TT2 and TT3 from the start of therapy. Cumulative relative survival improved when TT1 was compared with each arm of TT2 (TT2 − Thal P = 0.00054, TT2 + Thal P<0.0001) and with TT3 (P<0.0001); cumulative relative survival also improved when TT2 − Thal was compared with TT3 (P = 0.0031) (Figure 4a). When applied to survival from onset of CR (Figure 4b), cumulative relative survival comparisons showed significant benefits of newer TT trials, with the exception of borderline significance for TT2 + Thal versus TT3 (P = 0.06); TT2 − Thal outcomes were similar to those of TT2 + Thal (P = 0.72).
Figure 4.
Improvements in cumulative relative survival were observed in successive TT trials, whether considered from onset of therapy (a) or from onset of CR (b). OS was adjusted for the expected survival of the US population, stratified by gender and age at registration. Successive improvements were noted when comparing TT1 with TT2 and with TT3. No difference was detected between TT2 − Thal and TT2 + Thal. Black, TT1; blue, TT2 − Thal; red, TT2 + Thal; and green, TT3.
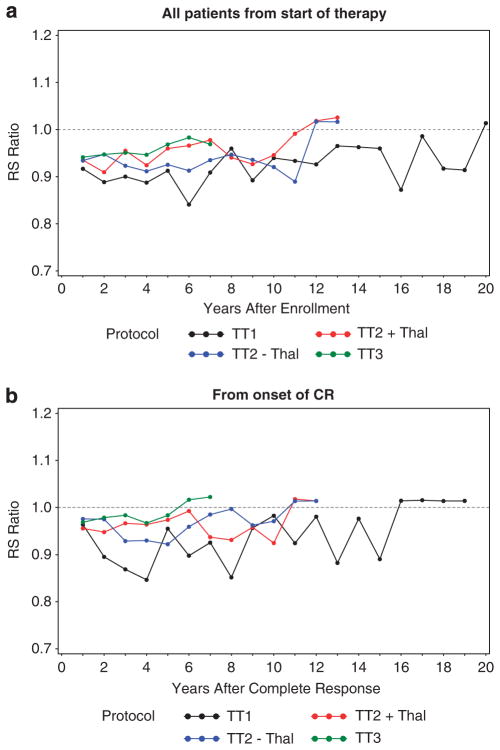
IRS ratios were computed to examine when near-normal survival expectations were reached during the course of each TT trial (Figure 5). When analyzed for all patients from the start of therapy, regardless of response status (Figure 5a), IRS ratios for TT1 remained near 90% in the first 10 years and increased to >95% thereafter; for the TT2 control arm, near-normal survival was reached at 10 years, and consistently superior estimates were observed in TT2 + Thal; for TT3, the IRS ratios virtually normalized at 6 years. When limited to subjects who achieved CR (Figure 5b), normal IRS ratios were reached at 16 years with TT1 and at 11 years with both arms of TT2 (transiently superior values were noted for TT2 + Thal in years 3–6); in contrast, patients treated with TT3 had near-normal IRS ratios almost from the outset of protocol therapy. The reduction of mortality to the level of the general US population in TT3 speaks to the efficacy of this treatment approach.
Figure 5.
IRS ratios from the start of therapy for all patients (a) or from the onset of CR for the subset of patients who attained CR (b). OS, adjusted for the expected survival of the US population, stratified by gender and age at registration, was calculated over 1-year intervals. With the transitions from TT1 to TT2 and TT3 trials, IRS ratios were successively higher from the outset, and improvements toward normal occurred earlier, whether examined for all patients or for those who attained CR. Black, TT1; blue, TT2 − Thal; red, TT2 + Thal; and green, TT3. (95% Confidence intervals provided in Supplementary Tables 2A–B).
DISCUSSION
We have demonstrated improvements in patient outcomes with successive TT protocols, which applied to most comparisons of OS, PFS, CR duration and TTP. The transition from TT1 to TT2 introduced more intensive induction therapy before tandem transplantation and consolidation chemotherapy after transplantation; the experimental arm of TT2 added thalidomide to this regimen. The transition to TT3 brought the addition of thalidomide and bortezomib for induction, consolidation and maintenance phases. The substantive improvements in patient outcomes were accounted for by reductions in relapses, not only in the subset of patients who achieved CR but also in the overall patient population. Our analyses used various statistical means of comparing clinical outcomes in successive clinical trials, including Cox model-adjusted comparisons, conditional 4-year clinical outcomes to account for earlier events due to myeloma aggressiveness and treatment-related toxicities and estimates of relative survival expectations of an age- and gender-adjusted subset of the general US population.
The data indicate that, in a closely followed population of patients with symptomatic/progressive myeloma treated at a single institution, long-term PFS and OS could be achieved with the TT1 protocol and that significant advances to earlier expectations of survival normalization occurred with addition of newer agents, particularly with incorporation of both bortezomib and thalidomide in TT3. The TTP curves plateaued at ~80% for TT1, regardless of CR status, and at about 20% for all patients in TT2 and at 10% for those in TT3 who achieved CR. This bodes well for long-term disease-free survivorship for the majority of patients. It is important to note that while the proportion patients achieving CR in the thalidomide arm of TT2 and TT3 are comparable, the CR duration is longer in TT3. This likely represents a better depth of response in TT3 with addition of bortezomib. It also appears that the patients who sustain CR status for over 7 years are less likely to relapse, regardless of the TT protocol.
Our data also show that Kaplan–Meier plots for OS and PFS are moving closer to each other with the transitions from TT1 to TT2 and, especially, to TT3, implying that salvage attempts are more difficult when the entire treatment armamentarium has been applied up-front in an effort to achieve durable disease control. However, this may not apply to late relapses where retreating with regimens that were already applied up-front has been effective (unpublished data). Thus, we anticipate that OS and PFS curves eventually will diverge as follow-up times increase. Patients have varying preferences for palliation versus cure objectives, and future trials should address these preferences as well as quality-of-life assessments.
We do not claim that results reported here can only be achieved with a TT-like treatment approach that applies all active treatments up-front; however, we believe that the enormous genomic chaos present at diagnosis, which is appreciated by most myeloma investigators, calls for an aggressive therapeutic regimen.14,15 We and others have drawn attention to shifts in tumor-cell subpopulations—‘clonal tides’—that can be observed on serial bone marrow examinations due to preferential treatment-related killing of tumor sub-clones.16,17 Such differential clonal sensitivity to different agents provided the rationale for combining all active myeloma agents up-front rather than in sequence, a strategy first advocated in the epochal discovery of curative combination chemotherapy for acute lymphoblastic leukemia by Frei et al.18 and extended in serial TT trials by the team at St. Jude Children’s Research Hospital.19 Our TT strategies, applying all myeloma-active agents and strategies up-front, were aimed at minimizing outgrowth or generation of further mutated tumor subpopulations, which were recognized as contributing to ultimate treatment failure.20
The analyses presented here are consistent with our previous suggestion that results of our TT trials7 and of GEMIMA and PETHEMA trials are consistent with cure of myeloma.21–23 The myeloma community, at present, is divided between those advocating cure and others advocating disease control.24 Randomized clinical trials are currently in progress to address this important issue,25 although median follow-up needs to be in the 10-year range for meaningful conclusions to be derived.
Although CR is an important objective of clinical trials, its durability is of paramount significance.26 Combinations of novel agents achieve CR rates that approach those achieved with transplants, but information is not yet available on the quality of response, which is reflected in CR duration, PFS and OS. The importance of this issue is highlighted by observations indicating that achieving CR does not always correlate with good long-term outcomes. We have reported that, for patients in TT3 who have myeloma that is defined by gene expression profiling (GEP) as high risk,16,27 CR rates match or exceed those seen for TT3 patients who have low-risk disease; however, the differences in CR duration, PFS and OS are stunning when TT3 patients with high-risk disease are compared to those with low-risk disease.6,28 On the other hand, Mayo investigators confirmed our observations that patients whose myeloma was preceded by smoldering myeloma achieved CR status less frequently but had comparable OS.29,30 These data indicate that simply achieving CR may not be an accurate indicator of long-term survival outcomes.
Several attempts at further refining CR have been undertaken to better quantify the depth of CR. Stringently defined CR relies on measuring myeloma secretory products and enumerating myeloma plasma cells in random bone marrow samples.31 Multi-parameter flow cytometry, introduced by Paiva et al.,32 and methods based on PCR33 both fail to account for non-secretory myeloma cells surviving in focal bone marrow sites that are readily detected by magnetic resonance imaging34 or positron emission tomography.35 Examination of fine-needle aspirates from such focal lesions revealed mitotically active myeloma cells that likely account for late relapses.36 Therefore, to further improve treatment outcomes, imaging-defined CR has become an objective of our TT4 trial for low-risk myeloma37 and TT5 trial for high-risk myeloma.38
As we anxiously await the results of prospective randomized trials comparing control- and cure-directed approaches, opportunities must be seized in the interim to target high-risk myeloma, as defined by the presence of CA, delTP53 (based on inter-phase fluorescence in situ examination39–42 or GEP43,44), high lactate dehydrogenease,45 primary plasma cell leukemia,46,47 GEP-based high-risk designation (~15% in newly diagnosed myeloma)27,48,49 and certain GEP- or fluorescence in situ hybridization-defined translocations.50–52 GEP-based high-risk myeloma is a common terminal pathway, and it also occurs in disease that begins as low risk53 (often with extramedullary manifestations54,55); therefore, a treatment focus on high-risk myeloma appears urgent. Clinical outcome results would be available within only 2–3 years, and promising agents can be included in trials for patients with lower-risk myeloma who carry some unfavorable prognostic stigmata. We and others are currently defining molecular pathways in high-risk myeloma that can be targeted therapeutically.
Supplementary Material
Acknowledgments
We recognize Mr Nathan Petty, Ms Susan Panozzo, Mr Doug Steward, Mr Clyde Bailey, the UAMS-MIRT data management team, the UAMS-MIRT nursing staff, referring physicians and our patients—without whom this body of work would not be possible. The manuscript was edited by Peggy Brenner, Office of Grants and Scientific Publications, University of Arkansas for Medical Sciences. This work has been supported by a grant from the National Cancer Institute, the National Institutes of Health (grant number CA 55813).
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
CONFLICT OF INTEREST
Dr Usmani is a consultant to Celgene, Millennium and Onyx and has received speaking honoraria from Celgene. Dr Barlogie has received research funding from Celgene and Novartis. He is a consultant to Celgene and Genzyme and has received speaking honoraria from Celgene and Millennium. Dr Barlogie is a co-inventor on patents and patent applications related to use of GEP in cancer medicine.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Barlogie B, Shaughnessy JD., Jr The molecular characterization and clinical management of multiple myeloma in the post-genome era. Leukemia. 2009;23:1941–1956. doi: 10.1038/leu.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlogie B, Tricot GJ, van Rhee F, Angtuaco E, Walker R, Epstein J, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006;135:158–164. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- 5.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 6.Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollmig K, Pineda-Roman M, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of Total Therapy 3. Br J Haematol. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 7.Nair B, van Rhee F, Shaughnessy JD, Jr, Anaissie E, Szymonifka J, Hoering A, et al. Superior results of Total Therapy 3 (2003–33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006–66 with VRD maintenance. Blood. 2010;115:4168–4173. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation using incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life-tables. J Royal Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 13.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson J, Zhan F, Sawyer J, Tricot G, Barlogie B, Shaughnessy J. Gene expression reflects changes in ploidy of some, but not all chromosomes, in multiple myeloma. Blood. 2002;100:316a. [Google Scholar]
- 15.Shaughnessy JD, Jr, Barlogie B. Integrating cytogenetics and gene expression profiling in the molecular analysis of multiple myeloma. Int J Hematol. 2002;76:59–64. doi: 10.1007/BF03165089. [DOI] [PubMed] [Google Scholar]
- 16.Shaughnessy JD, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca R. Risk-Adapted Therapy; Promises and Pitfalls. http://webcast.aacr.org/portal/p/2011annual/3577.
- 18.Frei E, III, Holland JF, Schneiderman MA, Pinkel D, Selkirk G, Freireich EJ, et al. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood. 1958;13:1126–1148. [PubMed] [Google Scholar]
- 19.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 20.Barlogie B, Hittelman W, Spitzer G, Trujilo JM, Hart JS, Smallwood L, et al. Correlation of DNA distribution abnormalities with cytogenetic findings in human adult leukemia and lymphoma. Cancer Res. 1977;37:4400–4407. [PubMed] [Google Scholar]
- 21.San-Miguel JF, Mateos MV. Can multiple myeloma become a curable disease? Haematologica. 2011;96:1246–1248. doi: 10.3324/haematol.2011.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Lopez J, Blade J, Mateos MV, Grande C, Alegre A, García-Laraña J, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118:529–534. doi: 10.1182/blood-2011-01-332320. [DOI] [PubMed] [Google Scholar]
- 23.Barlogie B, Crowley J. Could CR mean cure? Blood. 2011;118:483. doi: 10.1182/blood-2011-05-350322. [DOI] [PubMed] [Google Scholar]
- 24.Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: a clash of philosophies. Blood. 2011;118:3205–3211. doi: 10.1182/blood-2011-06-297853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson PG, Laubach J, Mitsiades CS, Schlossman R, Hideshima T, Redman K, et al. Managing multiple myeloma: the emerging role of novel therapies and adapting combination treatment for higher risk settings. Br J Haematol. 2011;154:755–762. doi: 10.1111/j.1365-2141.2011.08791.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoering A, Crowley J, Shaughnessy JD, Jr, Hollmig K, Alsayed Y, Szymonifka J, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114:1299–1305. doi: 10.1182/blood-2009-03-211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaughnessy JD, Jr, Qu P, Usmani S, Heuck CJ, Zhang Q, Zhou Y, et al. Pharmacogenomics of bortezomib test-dosing identifies hyperexpression of proteasome genes, especially PSMD4, as novel high-risk feature in myeloma treated with Total Therapy 3. Blood. 2011;118:3512–3524. doi: 10.1182/blood-2010-12-328252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haessler J, Shaughnessy JD, Zhan F, Crowley J, Epstein J, van Rhee F, et al. Benefit of complete response in multiple myeloma limited to high-risk subgroup identified by gene expression profiling. Clin Cancer Res. 2007;13:7073–7079. doi: 10.1158/1078-0432.CCR-07-0527. [DOI] [PubMed] [Google Scholar]
- 29.Zhan F, Barlogie B, Arzoumanian V, Huang Y, Williams DR, Hollmig K, et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109:1692–1700. doi: 10.1182/blood-2006-07-037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar SK, Dingli D, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Outcome after autologous stem cell transplantation for multiple myeloma in patients with preceding plasma cell disorders. Br J Haematol. 2008;141:205–211. doi: 10.1111/j.1365-2141.2008.07069.x. [DOI] [PubMed] [Google Scholar]
- 31.Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 32.Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- 33.Corradini P, Voena C, Omedé P, Astolfi M, Boccadoro M, Dalla-Favera R, et al. Detection of circulating tumor cells in multiple myeloma by a PCR-based method. Leukemia. 1993;7:1879–1882. [PubMed] [Google Scholar]
- 34.Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Jr, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25:1121–1128. doi: 10.1200/JCO.2006.08.5803. [DOI] [PubMed] [Google Scholar]
- 35.Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Nair B, Shaughnessy JD, Jr, Cartron MA, Haessler J, Anaissie E, et al. Cytogenetic abnormalities in multiple myeloma: poor prognosis linked to concomitant detection in random and focal lesion bone marrow samples and associated with high-risk gene expression profile. Br J Haematol. 2009;145:637–641. doi: 10.1111/j.1365-2141.2009.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anaissie EJ, van Rhee F, Hoering A, Waheed S, Alsayed Y, Petty N, et al. Comparing toxicities and survival outcomes with Total Therapy 4 (TT4) for 70-gene (R70)-defined low-risk multiple myeloma (MM) to results obtained with Total Therapy 3 protocols TT3A and TT3B. Blood. 2010;116:368. [Google Scholar]
- 38.Bladé J, Rosiñol L. Refining ‘total therapy’ for myeloma. Blood. 2010;115:4152–4153. doi: 10.1182/blood-2010-02-271338. [DOI] [PubMed] [Google Scholar]
- 39.Facon T, Avet-Loiseau H, Guillerm G, Moreau P, Geneviève F, Zandecki M, et al. Chromosome 13 abnormalities identified by FISH analysis and serum beta2-microglobulin produce a powerful myeloma staging system for patients receiving high-dose therapy. Blood. 2001;97:1566–1571. doi: 10.1182/blood.v97.6.1566. [DOI] [PubMed] [Google Scholar]
- 40.Gertz MA, Lacy MQ, Dispenzieri A, Greipp PR, Litzow MR, Henderson KJ, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106:2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avet-Loiseau H, Soulier J, Fermand JP, Yakoub-Agha I, Attal M, Hulin C, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia. 2010;24:623–628. doi: 10.1038/leu.2009.273. [DOI] [PubMed] [Google Scholar]
- 42.Shaughnessy JD, Jr, Haessler J, van Rhee F, Anaissie E, Pineda-Roman M, Cottler-Fox M, et al. Testing standard and genetic parameters in 220 patients with multiple myeloma with complete data sets: superiority of molecular genetics. Br J Haematol. 2007;137:530–536. doi: 10.1111/j.1365-2141.2007.06586.x. [DOI] [PubMed] [Google Scholar]
- 43.Shaughnessy JD, Zhou Y, Haessler J, van Rhee F, Anaissie E, Nair B, et al. TP53 deletion is not an adverse feature in multiple myeloma treated with total therapy 3. Br J Haematol. 2009;147:347–351. doi: 10.1111/j.1365-2141.2009.07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broyl A, Hose D, Lokhorst H, de Knegt Y, Peeters J, Jauch A, et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood. 2010;116:2543–2545. doi: 10.1182/blood-2009-12-261032. [DOI] [PubMed] [Google Scholar]
- 45.Barlogie B, Smallwood L, Smith T, Alexanian R. High serum levels of lactic dehydrogenase identify a high-grade lymphoma-like myeloma. Ann Intern Med. 1989;110:521–525. doi: 10.7326/0003-4819-110-7-521. [DOI] [PubMed] [Google Scholar]
- 46.Nair BP, Waheed S, Hoering A, VanRhee F, Anaissie EJ, Lorsbach R, et al. Primary plasma cell leukemia (PCL): clinical and laboratory presentation and clinical outcome with total therapy (TT) protocols. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 47.Avet-Loiseau H, Daviet A, Brigaudeau C, Callet-Bauchu E, Terré C, Lafage-Pochitaloff M, et al. Cytogenetic, interphase, and multicolor fluorescence in situ hybridization analyses in primary plasma cell leukemia: a study of 40 patients at diagnosis, on behalf of the Intergroupe Francophone du Myélome and the Groupe Français de Cytogénétique Hématologique. Blood. 2001;97:822–825. doi: 10.1182/blood.v97.3.822. [DOI] [PubMed] [Google Scholar]
- 48.Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 49.Broyl A, Hose D, Lokhorst H, de Knegt Y, Peeters J, Jauch A, et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood. 2010;116:2543–2553. doi: 10.1182/blood-2009-12-261032. [DOI] [PubMed] [Google Scholar]
- 50.Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magrangeas F, Nasser V, Avet-Loiseau H, Loriod B, Decaux O, Granjeaud S, et al. Gene expression profiling of multiple myeloma reveals molecular portraits in relation to the pathogenesis of the disease. Blood. 2003;101:4998–5006. doi: 10.1182/blood-2002-11-3385. [DOI] [PubMed] [Google Scholar]
- 52.Avet-Loiseau H, Mallard F, Campion L, Magrangeas F, Sebban C, Lioure B, et al. Translocation t(14;16) and multiple myeloma: is it really an independent prognostic factor? Blood. 2011;117:2009–2011. doi: 10.1182/blood-2010-07-295105. [DOI] [PubMed] [Google Scholar]
- 53.Nair B, Shaughnessy JD, Zhou Y, Astrid-Cartron M, Qu P, van Rhee F, et al. Gene expression profiling of plasma cells at myeloma relapse from tandem transplantation trial Total Therapy 2 predicts subsequent survival. Blood. 2009;113:6572–6575. doi: 10.1182/blood-2009-02-207803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bladé J, Fernández de Larrea C, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805–3812. doi: 10.1200/JCO.2011.34.9290. [DOI] [PubMed] [Google Scholar]
- 55.Usmani SZ, Mitchell A, Szymonifka J, Shaughnessy JD, Hoering A, Alsayed Y, et al. Extramedullary disease (EMD): a common terminal pathway in multiple myeloma (MM) progression. J Clin Oncol. 2011;29:8068. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.