Abstract
The Arc two-component system, comprising the ArcB sensor kinase and the ArcA response regulator, modulates the expression of numerous genes in response to the respiratory growth conditions. Under anoxic growth conditions ArcB autophosphorylates and transphosphorylates ArcA, which in turn represses or activates its target operons. The anaerobic metabolite d-lactate has been shown to stimulate the in vitro autophosphorylating activity of ArcB. In this study, the in vivo effect of d-lactate on the kinase activity of ArcB was assessed. The results demonstrate that d-lactate does not act as a direct signal for activation of ArcB, as previously proposed, but acts as a physiologically significant effector that amplifies ArcB kinase activity.
The Arc (anoxic redox control) two-component system is an important element in the complex transcriptional regulatory network that allows facultative anaerobic bacteria, such as Escherichia coli, to sense various respiratory growth conditions and adapt their gene expression accordingly (13, 15, 22). This system consists of the transmembrane sensor kinase ArcB and the cognate response regulator ArcA. The ArcB protein belongs to a subfamily of tripartite hybrid kinases, because it contains three catalytic domains: an N-terminal transmitter domain (H1) with a conserved His292 residue, a central receiver domain (D1) with a conserved Asp576 residue, and a C-terminal phosphotransfer domain (H2) with a conserved His717 residue (10, 15). Under reducing conditions, ArcB autophosphorylates at the expense of ATP and transphosphorylates ArcA via a His292→Asp576→His717→Asp54 phosphorelay (8, 19). Phosphorylated ArcA (ArcA-P), in turn, represses the expression of many operons involved in respiratory metabolism and activates a few operons encoding proteins involved in fermentative metabolism (22, 23). Under oxidizing conditions ArcB autophosphorylation is inhibited by the quinone electron carriers (7), and ArcA-P dephosphorylates via a reverse Asp54→His717→Asp576 phosphorelay (8). Signal transduction by phosphorelay has also been reported for the Kin/Spo system of Bacillus subtilis (4), the BvgS/BvgA system of Bordetella pertussis (29), the TorS/TorR system of E. coli (16), and the Sln1p/Ypd1p/Ssk1p system of Saccharomyces cerevisiae (28). This complex phosphotransfer mechanism is believed to allow multiple levels of control for fine-tuning.
It has been reported previously that the presence of certain fermentation intermediates, such as d-lactate, acetate, and pyruvate, accelerates the autophosphorylation activity of ArcB and enhances the subsequent transphosphorylation of ArcA (6, 11). However, no in vivo evidence has been obtained to support the physiological significance of such an effect. Furthermore, because the cellular metabolites mentioned above accumulate during anaerobiosis, it has been proposed that these compounds might act as the actual signals through which ArcB senses anaerobic environments in vivo (2, 11).
Here we present the results of experiments designed to probe the in vivo effect of d-lactate on Arc signaling and to test whether this compound acts as a primary signal or as an allosteric effector. We limited our study to d-lactate, which was reported previously to have the strongest effect on the activity of ArcB (6).
MATERIALS AND METHODS
Construction of strains.
To construct a ΔldhA strain, a 5′ flanking sequence (0.8 kb) of ldhA was PCR amplified from the chromosomal DNA of strain MC4100 with primers Ldh-5N (5′-CCAGCGGCCGCGACGACATCCAGTTC-3′) and Ldh-5C (5′-CCAGGATCCAGGTACTTCTTGTCG-3′). The PCR product was digested with NotI and BamHI and cloned between the corresponding sites of pKO3 (21), resulting in pLdh1. A 3′ flanking sequence (0.8 kb) of ldhA was then PCR amplified with primers Ldh-3N (5′CCTGGATCCCTGCCCGAACGAACTGG-3′) and Ldh-3C (5′CACGTCGACTTGGGTAGGGTGTGC-3′). The PCR product was digested with BamHI and SalI and cloned between the corresponding sites of pLdh1, resulting in pLdh2. Plasmid pLdh2 was transformed into strain ECL 5001 (20) (Table 1) to delete the ldhA gene by homologous recombination as described previously (21), yielding strain ECL 5201. Plasmid pDld2 (27) was then transformed into strain ECL 5201 to delete the dld gene by homologues recombination, yielding strain ECL 5202. Deletion of the ldhA and dld genes was confirmed by PCR. Subsequently, a Δfnr::Tn9(Cmr) allele was P1 transduced into strain ECL 5202 from ECL 5003, yielding strain ECL 5203. To construct strain ECL 5204, a ΔarcB::Kanr allele was P1 transduced into strain ECL 5203 from ECL 5005.
TABLE 1.
E. coli K-12 strains, bacteriophage, and plasmids used in this study
| Strain, phage, or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| Strains | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC ptsF25 rbsR | 20 |
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′ traD36 proAB+lacIqlacZΔM15 | Promega |
| ECL 5001 | MC4100 but Φ(cydA′-lacZ) | 20 |
| ECL 5003 | MC4100 but Δfnr::Tn9(Cmr) Φ(cydA′-lacZ) | 20 |
| ECL 5005 | φ(cydA′-lacZ) Δfnr::Tn9(Cmr) ΔarcB::Kanr | 20 |
| ECL 5201 | φ(cydA′-lacZ) ΔldhA | This study |
| ECL 5202 | φ(cydA′-lacZ) ΔldhA Δdld | This study |
| ECL 5203 | φ(cydA′-lacZ) ΔldhA Δdld Δfnr::Tn9(Cmr) | This study |
| ECL 5204 | φ(cydA′-lacZ) ΔldhA Δdld Δfnr::Tn9(Cmr) ΔarcB::Kanr | This study |
| ECL 5205 | φ(cydA′-lacZ) ΔldhA Δdld Δfnr::Tn9(Cmr) arcBcyt::Kanr | This study |
| P1vir | Laboratory stock | |
| Plasmids | ||
| pKO3 | 21 | |
| pLDH2 | ΔldhA in pKO3 | This study |
| pDLD2 | Δdld in pKO3 | 26 |
| pABW | arcB+ in pBluescript KS II(+) | 20 |
| pABS | arcB78-778 in pBluescript KS II(+) | 20 |
| pIBW | arcB+::Kanr in pIB3 | 20 |
| pIBcyt | arcBΔ23-77::Kanr in pIB3 | This study |
To construct strain ECL 5205, a mutant arcB allele expressing cytosolic protein was created by deleting sequences encoding the transmembrane region of ArcB (amino acid residues of 23 to 77) by a two-step fusion PCR. Briefly, a DNA fragment encoding amino acid residues 1 to 22 of ArcB was amplified by PCR by using primers B5NDE (5′-CCCGGATCCCATATGAAGCAAATTCGTCTGCTGGCGC-3′) and BDT (5′-GTCGTGACTCCTCCAGTTGCTCGCGCACCAGACCTAACTTCATC-3′) with pABW (20) as the template. The product of this reaction was purified and used as a megaprimer for PCR in combination with primer B3NRU (5′-GTAATGTCGCGACCAAAGCCCATCAAACCG-3′) and with pABS (20) as the template. Finally, the product of the second PCR was digested with NdeI and NruI and cloned between the corresponding sites of pIBW, generating plasmid pIBcyt. This plasmid was used to replace the wild-type arcB gene by homologous recombination as described previously (20), yielding strain ECL 5205 [arcBcyt::Kanr Δdld ΔldhA Φ(cydA′-lacZ) Δfnr::Tn9(Cmr)].
Growth conditions.
Luria-Bertani (LB) broth and LB agar (15 g/liter) were used for routine growth. Ampicillin, tetracycline, kanamycin, and chloramphenicol were provided at final concentrations of 50, 12, 40, and 20 μg/ml, respectively. For the β-galactosidase activity assay, the Φ(cydA′-lacZ)-bearing strains were cultured in buffered LB broth containing 0.1 M MOPS (morpholinepropanesulfonic acid) (pH 7.4) and 20 mM d-xylose.
β-Galactosidase activity assay.
Aerobic cultures (5 ml) were grown in 250-ml baffled flasks at 37°C with shaking (300 rpm), whereas anaerobic cultures were grown in closed 5-ml test tubes which were filled to the brim and stirred with a small magnetic bar. β-Galactosidase activity, expressed in Miller units, was assayed with exponentially growing cultures as described previously (26).
Permease assays.
Exponentially growing cells were collected by centrifugation at 4,000 × g and washed twice with buffer A (0.1 M MOPS, 0.5 mM MgCl2; pH 7.0). The bacterial pellet was suspended in buffer A at a final density of 0.5 mg (dry weight)/ml and kept at 15°C. The rate of uptake was assayed by diluting the 14C-labeled substrate 10-fold with the cell suspension to obtain a final concentration of 4 μM. d-[14C]lactate (56 mCi/mmol) was purchased from ICN. Since it has been reported previously that d-lactate uptake by E. coli cell suspensions reaches the steady state within 2 min of the start of incubation (17, 27), samples (100 ml) were withdrawn at 0.5 and 5 min and filtered through 0.65-μm-pore-size cellulose nitrate filters. The filters were washed with 4 ml of buffer A, placed in plastic vials, and counted in the presence of Emulsifier-safe (Packard, Meriden, Conn.). To calculate the concentration of d-lactate inside the cells, the following conversion factor was used: 0.63 μl of intracellular H2O per ml of cell suspension at an optical density at 600 nm (OD600) of 1.
Enzyme assays.
For d-lactate dehydrogenase assays, cells were harvested by centrifugation at 5,000 × g for 15 min and washed once in cold 10 mM potassium phosphate buffer (pH 7.0). The pellet was weighed and suspended in 4 volumes of the same buffer. The suspended cells were lysed for 1 min/ml with a model 60W ultrasonic disintegrator at 1.5 A while they were chilled in a dry ice-ethanol bath. Lysates were cleared by centrifugation for 30 min at 10,000 × g. Enzyme assays were performed by a method similar to the method used for glycerol-3-phosphate dehydrogenase assays by measuring the reduction of 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl-tetrazolium bromide mediated by phenazine methasulfate (18), but with 0.1 M d-lactate as a substrate. The specific activity of d-lactate dehydrogenase was expressed in nanomoles per minute per milligram of protein at 30°C. The Coomassie Plus protein assay reagent (Pierce) was employed to estimate protein concentrations, and bovine serum albumin was used as the standard.
RESULTS
Construction of a mutant strain able to accumulate but not metabolize d-lactate.
d-Lactate has been shown to accelerate the autophosphorylating activity of ArcB and to enhance the transphosphorylation of ArcA in vitro (6, 11). To test the effect of d-lactate on the kinase activity of ArcB under physiological conditions, we constructed a mutant strain in which the intracellular concentration of d-lactate depended solely on the exogenous supply. The following aspects of d-lactate metabolism were considered in the design of the mutant strain. During glucose fermentation, d-lactate is formed by an NAD-linked oxidoreductase encoded by the monocistronic operon ldhA at min 30.5 (3). Under aerobic conditions, however, d-lactate can be scavenged from the medium by the membrane transport carriers LldP (l-lactate permease) and GlcA (glycolate permease) (27). The captured d-lactate is then converted back to pyruvate by a flavin adenine dinucleotide-dependent dehydrogenase encoded by the dld monocistronic operon at min 47.9. Hence, strain ECL 5203 with the genotype Δdld ΔldhA was constructed as described in Materials and Methods. This strain also harbors a λΦ(cydA′-lacZ) operon fusion as the reporter and a Δfnr::Tn9(Cmr) allele to avoid repression of the reporter by Fnr (5).
d-Lactate uptake by the Δdld ΔldhA mutant strain.
The ability of the constructed Δdld ΔldhA mutant strain to accumulate but not metabolize d-lactate was verified. ECL 5203 was grown aerobically or anaerobically, and at mid-exponential phase the cells were incubated with d-[14C]lactate. After 0.5 and 5 min of incubation the intracellular amount of d-lactate was determined (Table 2). It was found that the mutant strain was able to accumulate radiolabeled d-lactate to a concentration gradient (intracellular concentration/extracellular concentration) greater than 16. The calculations were based on the intracellular volume of water determined by the method of Maloney et al. (24). It was also observed that the d-lactate uptake by anaerobically growing cells was about three times lower than that by aerobically growing cells. This might be explained by the fact that the expression of the l-lactate permease gene (lldP) is inhibited by the ArcA/ArcB system under anaerobic growth conditions (12) and therefore d-lactate uptake relies solely on the glycolate permease (GlcA). The inability of the constructed strain to metabolize d-lactate was verified by comparing the d-lactate dehydrogenase activities in the cellular extracts of strain ECL 5203 and the isogenic wild-type strain (Fig. 1). It was found that the specific d-lactate dehydrogenase activity for the wild-type strain was 57 ± 5 nmol/mg of protein per min, in agreement with a previously reported value (17). In contrast, no specific activity was detected for the mutant strain. Moreover, the growth of ECL 5203 on M9 minimal media with d-lactate as the sole carbon source was monitored. No growth was detected (data not shown). Taken together, these results suggest that strain ECL 5203 is unable to metabolize d-lactate.
TABLE 2.
Accumulation of d-lactate against concentration gradient by the ΔldhA Δdld mutant strain ECL 5203
Cells were grown in xylose minimal media to an OD600 of ∼0.3.
d-Lactate uptake was assayed with 4 μM d-[14C]lactate as the substrate as previously described (27).
FIG. 1.
Specific activity of d-lactate dehydrogenase. Strain ECL 5003 and the isogenic strain ECL 5203 (ΔldhA Δdld) were grown aerobically in buffered LB medium containing 0.1 M MOPS (pH 7.4), 20 mM d-xylose, and 10 mM d-lactate. d-Lactate dehydrogenase activity was measured as described in Materials and Methods with about 60 μg of protein. The reaction was allowed to proceed for 2 min, and the OD570 was monitored. Open bars, reaction mixture without substrate (control); solid bars, reaction mixture with substrate.
Effect of exogenous d-lactate on the aerobic and anaerobic activities of ArcB.
The constructed Δdld ΔldhA mutant strain was used to test the effect of d-lactate on the in vivo signaling activity of ArcB by monitoring changes in the levels of phosphorylated ArcA, as indicated by the expression pattern of the λΦ(cydA′-lacZ) operon fusion. To do this, strains ECL 5203 [Δdld ΔldhA Φ(cydA′-lacZ) Δfnr::Tn9(Cmr)] and ECL 5204, the isogenic ΔarcB strain, were cultured aerobically or anaerobically in the presence or absence of 10 mM d-lactate, and at the mid-exponential growth phase the β-galactosidase activity levels were determined (Fig. 2A). It was found that during aerobic growth d-lactate had no effect on the expression of the reporter in both the arcB+ strain and the ΔarcB strain. In contrast, during anaerobic growth the presence of d-lactate in the growth medium resulted in a significant increase in Φ(cydA′-lacZ) expression in the arcB+ strain but not in the ΔarcB strain. Thus, d-lactate appears to amplify the ArcB kinase activity under anaerobic growth conditions, as judged by the increased level of reporter expression.
FIG. 2.
Effect of d-lactate on expression of λΦ(cydA′-lacZ). (A) Strain ECL 5203 (arcB+) and the isogenic strain ECL 5204 (ΔarcB) were grown aerobically or anaerobically in LB medium containing 0.1 M MOPS (pH 7.4) and 20 mM d-xylose with or without 10 mM d-lactate. (B) Strain ECL 5003 and the isogenic strain ECL 5203 (ΔldhA Δdld) were grown anaerobically in the medium described above with or without 10 mM d-lactate. β-Galactosidase activity was assayed and was expressed in Miller units (26). Open bars, growth with no d-lactate; solid bars, growth with d-lactate. The data are averages from four experiments (the variations were less than 10% of the means).
To exclude the possibility that deletion of dld and/or ldhA has a direct influence on the expression of Φ(cydA′-lacZ), the expression levels in the Δdld ΔldhA mutant strain and the isogenic dld+ ldhA+ strain (ECL 5003) were compared. It was found that the aerobic expression levels of the Φ(cydA′-lacZ) reporter were very low and nearly indistinguishable in the two strains. In contrast, the anaerobic expression level of the reporter in the Δdld ΔldhA mutant strain was only half of that in the wild-type strain (Fig. 2B), indicating that ArcB was less active in the mutant strain. Since no d-lactate is formed in this strain because of the ldhA deletion, a less active ArcB kinase would expected only if d-lactate is a physiological modulator of ArcB. In such a case, addition of d-lactate to the growth medium should restore the kinase activity of ArcB. To test this reasoning, 10 mM d-lactate was added to the growth medium of the mutant strain. It was found that the level of reporter expression was restored to almost wild-type levels (Fig. 2B).
Thus, the results described above provide strong support for the conclusion that d-lactate does not act as a direct signal, because it has no influence on the activity of ArcB under aerobic growth conditions, but it is required for full activity under anaerobic conditions.
Enhancement of the DTT-dependent activity of ArcB by d-lactate.
In an independent study, we noticed that the reducing agent dithiothreitol (DTT) activates ArcB under aerobic growth conditions. Because DTT is a strong reductant, we argued that it might affect the redox state of the quinone pool and thereby result in aerobic activation of ArcB. We therefore tested whether the quinone electron carriers can be reduced by DTT. To do this, we incubated ubiquinone 0 (Q0), a soluble analog of ubiquinone 8, with DTT and monitored its spectroscopic characteristics. As shown in Fig. 3, the maximum absorbance for the oxidized Q0 was ∼265 nm. However, upon incubation with DTT the maximum absorbance was shifted to ∼295 nm, which is characteristic for the reduced form of Q0. Thus, it seems likely that DTT exerts its effect on ArcB through reduction of the quinone electron carriers.
FIG. 3.
Spectroscopic characteristics of Q0. The UV spectrum of oxidized Q0 (∼0.1 mM) was recorded from 220 to 380 nm in 50 mM sodium phosphate, pH 7.0 (solid line). DTT was added to the cuvette, and the spectrum of the reduced Q0 (dashed line) was recorded after 10 min of incubation at room temperature.
We then hypothesized that if d-lactate is a physiologically significant modulator of ArcB, it may have an augmenting effect on the aerobic DTT-dependent ArcB activity, mimicking the effect exerted during anaerobiosis. To test this hypothesis, strains ECL 5203 (arcB+) and ECL 5204 (ΔarcB) were grown aerobically in four parallel cultures for each strain to an OD600 of 0.3. One of the cultures served as the control, whereas either DTT (5 mM), d-lactate (10 mM), or both DTT and d-lactate were added to the other cultures (Fig. 4). It was found that addition of DTT to the growth medium of strain ECL 5203 led to immediate activation of λΦ(cydA′-lacZ) expression, in agreement with our previous observation. Moreover, simultaneous addition of DTT and d-lactate to the culture medium had an even stronger effect on the expression of the reporter, which reached a level significantly higher than the level in the culture with only DTT (Fig. 4). On the other hand, addition of d-lactate alone had no effect on expression of the reporter. Finally, addition of DTT alone or in combination with d-lactate to the growth medium of the ΔarcB strain had no effect on the expression level of the reporter. Thus, d-lactate is able to amplify the in vivo kinase activity of ArcB under aerobic growth conditions when ArcB is active as a kinase.
FIG. 4.
Effect of DTT and/or d-lactate on the aerobic expression of λΦ(cydA′-lacZ). Four parallel cultures of strain ECL 5203 (arcB+) and ECL 5204 (ΔarcB) were grown aerobically in LB medium containing 0.1 M MOPS (pH 7.4) and 20 mM d-xylose. At an OD600 of 0.3 one aliquot was withdrawn from each culture and used to measure the β-galactosidase activity (−15 min). At time zero either 5 mM DTT (▴), 20 mM d-lactate (⧫), both DTT and d-lactate (•), or nothing (○) was added to the cultures, and the β-galactosidase activity was monitored for 1 h at 15-min intervals. Left panel, ECL 5203; right panel, ECL 5204 (ΔarcB). The data are the averages from four experiments (the variations were less than 10% of the means).
Effect of d-lactate on the aerobic activity of ArcBcyt.
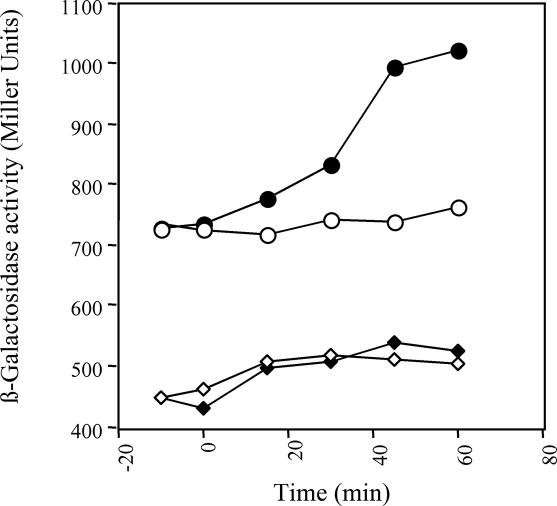
To obtain independent support for our conclusion, we took advantage of the previous finding that liberation of ArcB from the plasma membrane results in a partially constitutive kinase activity in vivo (20). We therefore tested the effect of d-lactate on the aerobic signaling activity of a truncated version of ArcB, ArcBcyt, which was devoid of the transmembrane segments (amino acids 23 to 77) and therefore was not anchored to the membrane. For this experiment, strains ECL 5205 (in which the chromosomal wild-type arcB allele was replaced with arcBcyt) and ECL 5203 (arcB+), both harboring the λΦ(cydA′-lacZ) operon fusion, were grown aerobically in buffered LB medium (Fig. 5). At an OD600 of ∼0.3 a sample for the β-galactosidase assay was withdrawn (at −15 min in Fig. 5). As expected, the expression of the reporter was significantly higher in the mutant strain than in the wild-type strain, confirming that ArcBcyt is a partially active kinase under aerobic growth conditions. After an additional 15 min of growth, the cultures were each divided into two aliquots; for each pair, one aliquot served as the control, while 10 mM d-lactate was added to the other, and the β-galactosidase activity was monitored (Fig. 5). It was found that addition of d-lactate to the growth medium of the wild-type strain had no effect. However, addition of d-lactate to the growth medium of the strain carrying ArcBcyt resulted in an immediate increase in the expression level of the reporter, supporting our conclusion that d-lactate accelerates the in vivo kinase activity of the active ArcB protein.
FIG. 5.
Effect of d-lactate on the aerobic activity of ArcBcyt. Two parallel cultures of strains ECL 5203 (arcB+) and ECL 5205 (in which the chromosomal wild-type arcB allele was replaced by arcBcyt) were grown aerobically in LB medium containing 0.1 M MOPS (pH 7.4) and 20 mM d-xylose. At an OD600 of 0.3 one aliquot was withdrawn from each culture and used to measure the β-galactosidase activity (−15 min). At time zero 20 mM d-lactate was added to one of the cultures, and the β-galactosidase activity was monitored for 1 h at 15-min intervals. Symbols: ⋄, ECL 5203 without d-lactate; ⧫, ECL 5203 with d-lactate; ○, ECL 5205 without d-lactate; •, ECL 5205 with d-lactate. The data are the averages from four experiments (the variations were less than 10% of the means).
Therefore, it seems reasonable to conclude that d-lactate acts as an allosteric effector, which plays a significant role in modulating the activity of ArcB under physiological conditions.
DISCUSSION
In a previous in vitro study, it was reported that the level of autophosphorylation of the primary transmitter domain of ArcB was up to three times greater than the basal level of autophosphorylation in the presence of the fermentative metabolite d-lactate (6). In this paper, we present results of in vivo experiments demonstrating that the enhancement of the kinase activity of ArcB by this metabolic intermediate also occurs under physiological conditions. Moreover, our results rule out the previously proposed possibility that d-lactate might serve as an actual signal through which ArcB senses anaerobic environments in vivo (2, 11).
Our conclusions are based on the following findings. First, under nonstimulating (aerobic) growth conditions the presence of d-lactate has no influence on the expression of the Φ(cydA′-lacZ) reporter, suggesting that ArcB remains inactive. Because the kinase activity of ArcB does not respond to d-lactate under aerobic growth conditions, despite the fact that d-lactate reaches an intracellular concentration that is threefold higher than that under anaerobic conditions (Table 2), it is not plausible that this metabolite serves as a direct signal that activates ArcB. In contrast, under stimulating conditions (anaerobiosis) the presence of d-lactate causes a significant increase in reporter expression, indicating that ArcB operates with enhanced activity. Therefore, it appears that d-lactate serves as an allosteric effector that acts on the active ArcB.
Second, although d-lactate has no influence on the activity of ArcB under aerobic growth conditions, it does enhance the aerobic DTT-dependent activity of ArcB. Thus, the d-lactate effect on ArcB can be observed even under aerobic growth conditions, with the prerequisite that ArcB be active. Interestingly, the DTT-dependent activation of ArcB under aerobic conditions seems to be a result of reduction of the quinone pool. In fact, because of its low redox potential (−380 mV), DTT should be able to readily reduce the pool of quinones, as the redox potentials are 45 mV for ubiquinone and −75 mV for menaquinone. An alternative might be that DTT, which is known to affect protein folding and in particular disulfide bond formation, acts by reducing either or both cysteine residues present in the linker region of ArcB. However, mutating Cys241 of ArcB to Ala has been shown to have no effect on the in vivo regulation of ArcB (14, 25).
Third, addition of d-lactate to an aerobic culture of a strain carrying the arcBcyt mutant gene, which encodes a partially active ArcB kinase protein, results in an immediate increase in the level of the Φ(cydA′-lacZ) reporter, supporting the conclusion that an active ArcB protein is required for the d-lactate effect to be observed. Finally, the function of this metabolic intermediate as an allosteric activator is further supported by the fact that its cellular level depends not only on the respiratory state of the cell but also on the oxygen/hydrogen ratio of the carbohydrate being fermented (1).
Thus, three independent lines of evidence support the conclusion that d-lactate has a physiological role as an allosteric effector that accelerates the kinase activity of ArcB under stimulating growth conditions.
Although the reaction mechanism by which d-lactate modulates the activity of ArcB remains unknown, it is worth mentioning that the ArcB receiver domain (D1), even when it is catalytically inactive (e.g., D1Asp576→Ala) is indispensable for d-lactate to exert its effect in vitro (6). It thus seems that either the effector binding site is located in D1 or the structural presence of D1 enables the effector to bind to the primary transmitter domain of ArcB (H1). Because Asp576 is the actual residue that receives the ∼P from His292 (9), the site of autophosphorylation, and is also indispensable for the phosphatase activity of ArcB (8), d-lactate must act upon the first step of the Arc phosphorelay, which is the autophosphorylation reaction. It is therefore tempting to speculate that effector binding to ArcB may cause a conformational change that results in an increase in the Vmax of this reaction. If H1 autophosphorylation is the rate-limiting step in the Arc signal transduction cascade and the phosphorelay is the sole means of ArcA phosphorylation by ArcB, then increasing the Vmax of the autophosphorylation reaction with d-lactate would be sufficient to raise the level of ArcA-P. Such a process should be advantageous to the cell, because as d-lactate accumulates under anaerobic growth conditions, maximal activity of the ArcB kinase is ensured. Indeed, acceleration of the ArcB kinase activity by allosteric effectors such as d-lactate should result in a faster response to changes in redox conditions and thereby faster adaptation to the new environment. Also, a faster response should guarantee a more economic mode of growth, as the expression of many unneeded enzymes should be immediately repressed.
ArcB belongs to a subfamily of tripartite sensor kinases that possess three catalytic domains that participate in complex phosphorelay reactions for signal transmission and signal decay. It has been widely believed that the elaborate structure and complex phosphotransfer mechanism of such tripartite sensor kinases allow various inputs to be integrated in a multilevel control mechanism that fine-tunes their signaling activity. Here, ArcB provides the first example of an at least dual-level control mechanism, as illustrated by the orchestrated action of the quinone electron carriers and the fermentative metabolite d-lactate. The oxidized forms of ubiquinone and menaquinone have previously been shown to serve as direct negative signals that silence the kinase activity of ArcB and thereby act on the first level of control (7). Now, we show that d-lactate serves as an allosteric effector that amplifies the kinase activity of ArcB but is unable to override the quinone-dependent inhibition of ArcB and therefore acts on a secondary level of control.
Two challenges that remain are to pinpoint the structural requirement and reaction mechanism of the effectors and to determine whether there are additional elements that influence signal transmission and signal decay in the Arc system.
Acknowledgments
We thank E. C. C. Lin for advice and D. González-Halphen, B. Michel, and R. Kadner for critically reading the manuscript.
This work was supported by grant 37342-N from the Consejo Nacional de Ciencia y Tecnología (CONACyT), by NIH research grant R03 TW06003, and by Molecular and Cellular BioDiscovery Research Program grant M1-0311-00-0081 from the Korean Ministry of Science and Technology.
REFERENCES
- 1.Alam, K. Y., and D. P. Clark. 1989. Anaerobic fermentation balance of Escherichia coli as observed by in vivo nuclear magnetic resonance spectroscopy. J. Bacteriol. 171:6213-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. [DOI] [PubMed] [Google Scholar]
- 3.Bunch, P. K., F. Mat-Jan, N. Lee, and D. P. Clark. 1997. The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Burbulys, D., K. A. Trach, and J. A. Hoch. 1991. The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell 64:545-552. [DOI] [PubMed] [Google Scholar]
- 5.Cotter, P. A., S. B. Melville, J. A. Albrecht, and R. P. Gunsalus. 1997. Aerobic regulation of cytochrome d oxidase (cydAB) operon expression in Escherichia coli: roles of Fnr and ArcA in repression and activation. Mol. Microbiol. 25:605-615. [DOI] [PubMed] [Google Scholar]
- 6.Georgellis, D., O. Kwon, and E. C. Lin. 1999. Amplification of signaling activity of the Arc two-component system of Escherichia coli by anaerobic metabolites. An in vitro study with different protein modules. J. Biol. Chem. 274:35950-35954. [DOI] [PubMed] [Google Scholar]
- 7.Georgellis, D., O. Kwon, and E. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 8.Georgellis, D., O. Kwon, P. De Wulf, and E. C. Lin. 1998. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J. Biol. Chem. 273:32864-32869. [DOI] [PubMed] [Google Scholar]
- 9.Georgellis, D., A. S. Lynch, and E. C. Lin. 1997. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J. Bacteriol. 179:5429-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishige, K., S. Nagasawa, S. Tokishita, and T. Mizuno. 1994. A novel device of bacterial signal transducers. EMBO J. 13:5195-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iuchi, S. 1993. Phosphorylation/dephosphorylation of the receiver module at the conserved aspartate residue controls transphosphorylation activity of histidine kinase in sensor protein ArcB of Escherichia coli. J. Biol. Chem. 268:23972-23980. [PubMed] [Google Scholar]
- 12.Iuchi, S., A. Aristarkhov, J. M. Dong, J. S. Taylor, and E. C. C. Lin. 1994. Effects of nitrate respiration on expression of the Arc-controlled operons encoding succinate dehydrogenase and flavin-linked l-lactate dehydrogenase. J. Bacteriol. 176:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iuchi, S., and E. C. C. Lin. 1988. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc. Natl. Acad. Sci. USA 85:1888-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iuchi, S., and E. C. C. Lin. 1992. Mutational analysis of signal transduction by ArcB, a membrane sensor protein responsible for anaerobic repression of operons involved in the central aerobic pathways in Escherichia coli. J. Bacteriol. 174:3972-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iuchi, S., Z. Matzuda, T. Fujiwara, and E. C. Lin. 1990. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol. Microbiol. 4:715-727. [DOI] [PubMed] [Google Scholar]
- 16.Jourlin, C., M. Ansaldi, and V. Mejean. 1997. Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS unorthodox sensor in Escherichia coli. J. Mol. Biol. 267:770-777. [DOI] [PubMed] [Google Scholar]
- 17.Kang, S. Y. 1978. Mechanism of autoenergized transport and nature of energy coupling for d-lactate in Escherichia coli. J. Bacteriol. 136:867-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuritzkes, D. R., X. Y. Zhang. And E. C. C. Lin. 1984. Use of Φ(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J. Bacteriol. 157: 591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon, O., D. Georgellis, and E. C. Lin. 2000. Phosphorelay as the sole physiological route of signal transmission by the Arc two-component system of Escherichia coli. J. Bacteriol. 182:3858-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon, O., D. Georgellis, A. S. Lynch, D. Boyd, and E. C. Lin. 2000. The ArcB sensor kinase of Escherichia coli: genetic exploration of the transmembrane region. J. Bacteriol. 182:2960-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch, A. S., and E. C. Lin. 1996. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J. Bacteriol. 178:6238-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch, A. S., and E. C. Lin. 1996. Responses to molecular oxygen, p. 1526-1538. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 24.Maloney, P. C., E. R. Kashket, and T. H. Wilson. 1996. Methods for studying transport in bacteria, p. 1-49. In E. D. Korn (ed.), Methods in membrane biology, vol. 5. Plenum, New York, N.Y. [Google Scholar]
- 25.Matsushika, A., and T. Mizuno. 2000. Characterization of three putative sub-domains in the signal-input domain of the ArcB hybrid sensor in Escherichia coli. J. Biochem (Tokyo) 127:855-860. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Núñez, M. F., O. Kwon, T. H. Wilson, J. Aguilar, L. Baldoma, and E. C. Lin. 2002. Transport of l-lactate, d-lactate, and glycolate by the LldP and GlcA membrane carriers of Escherichia coli. Biochem. Biophys. Res. Commun. 290:824-829. [DOI] [PubMed] [Google Scholar]
- 28.Posas, F., S. M. Wurgler-Murphy, T. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 29.Uhl, M. A., and J. F. Miller. 1996. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 15:1028-1036. [PMC free article] [PubMed] [Google Scholar]